Abstract
The vocal control system of zebra finches shows auditory gating in which neuronal responses to the individual bird's own song vary with behavioral states such as sleep and wakefulness. However, we know neither the source of gating signals nor the anatomical connections that could link the modulatory centers of the brain with the song system. Two of the song-control nuclei in the forebrain, the HVC (used as the proper name) and the interfacial nucleus of the nidopallium, both show auditory gating, and they receive input from the uvaeform nucleus (Uva) in the thalamus. We used a combination of anterograde and retrograde tracing methods to show that the dorsal part of the reticular formation and the medial habenula (MHb) project to the Uva. We also show by choline acetyl transferase immunohistochemistry that the MHb is cholinergic and sends cholinergic fibers to the Uva. Our findings suggest that the Uva might serve as a hub to coordinate neuromodulatory input into the song system.
Keywords: auditory gating, cholinergic, song system, thalamus
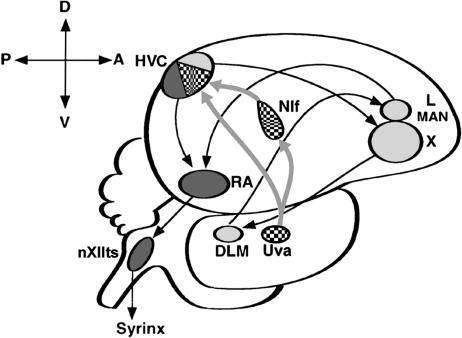
The song system is a discrete, interconnected series of brain nuclei in songbirds that controls singing, which is a learned vocal behavior (Fig. 1) (1). In addition to displaying song motor activity, neurons of most song nuclei selectively respond to playback of the individual bird's own song (BOS) (2). Responses to the BOS are not created de novo in each song nucleus (3) but are relayed from the HVC to a vocal motor pathway that includes the robust nucleus of the arcopallium (RA) and the tracheosyringeal part of the hypoglossal nucleus. Auditory information also is transmitted from the HVC to an anterior forebrain pathway that includes area X within the songbird medial striatum; the nucleus dorsolateralis anterior thalami, pars medialis; the lateral magnocellular nucleus of the anterior nidopallium; and the RA. The source of auditory input to the HVC is thought to be the interfacial nucleus of the nidopallium (NIf), in which selectivity for the BOS is not as exclusive as in the HVC (4). Both the degree and selectivity of BOS-evoked responses in the HVC vary with natural and induced behavioral states such as sleeping, wakefulness, and anesthesia. This phenomenon is called “auditory gating” (5) and provides evidence that links the modulation of auditory input with the vocal control system. The first site of gating in the chain of song nuclei is thought to be the NIf (6). However, little is known about the source of signals that control gating in the NIf. Although several candidate sources for neural modulation have been postulated (7, 8), the connections between these areas and the song system remain tenuous. The present study provides anatomical evidence that the uvaeform nucleus (Uva), which projects to both the NIf and HVC, receives cholinergic fibers from neurons in the medial habenula (MHb) and also afferents from the nucleus reticularis superior, pars dorsalis (RSd). These thalamic areas are neuromodulatory centers in other vertebrates, suggesting that the Uva in songbirds may play an important role in the gating of auditory information in the song system.
Fig. 1.
Schematic representation of the zebra finch brain in sagittal view. The song system can be subdivided into the anterior forebrain circuit (light gray) and the motor pathway (dark gray) and is bridged by their common connection to the HVC, which receives efferent projections from both the Uva and NIf (patterned). nXIIts, tracheosyringeal part of the hypoglossal nucleus; X, area X within the songbird medial striatum; LMAN, lateral magnocellular nucleus of the anterior nidopallium; RA, robust nucleus of the arcopallium; DLM, nucleus dorsolateralis anterior thalami, pars medialis
Materials and Methods
We used a total of 20 adult male zebra finches (Taeniopygia guttata) from a breeding colony in our animal facilities. All experiments involving these birds were conducted by using protocols approved by California Institute of Technology's Office of Laboratory Animal Resources.
Surgery. Birds were anesthetized with an intramuscular injection of ketamine (50 mg/kg) and xylazine (15 mg/kg) and held in a stereotaxic apparatus. The Uva was located by a combination of stereotaxic coordinates, responses to visual stimuli in the contralateral eye (9), and characteristic firing properties learned through preliminary studies. Neural activity was recorded through a finely pulled glass pipette with a tip diameter of 10 μm, which was filled with a 0.2% solution of KCl connected with a silver wire. Spike activity was amplified, filtered, displayed on an oscilloscope, and acoustically monitored.
Tract-Tracing Experiments. Anterograde or retrograde labeling from the Uva was conducted in eight birds by iontophoresing either 10,000 molecular-weight (MW) biotinylated dextran amine (BDA) (Molecular Probes) [10% in 25 mM phosphate buffer (PB), pH 7.5] or Alexa Fluor 488-dextran amine (Molecular Probes) (10,000 MW in a 10% solution of PB). The tracers were iontophoresed with positive-current 5-μA pulses (7 sec on/7 sec off) for 10 min through a glass pipette (15 μm in diameter). Survival times varied from 3 days to >1 week. Animals were given a lethal dose of pentobarbital (Abbott) and perfused through the left ventricle of the heart with 0.9% NaCl until clearing, followed by 1 hr of 2% paraformaldehyde (PF) in 25 mM PB. After 1 day of postfixing at 4°C, the brains were cryoprotected by immersing them in a solution of 30% sucrose in 2% PF in PB for 2 days (4°C.). The brains were cut on a freezing microtome (30 μm), and the sections were serially collected in cold PB. Fluorescent-labeled sections were mounted as soon as possible from PB onto precleaned slides, dried for 30 min in the dark, and coverslipped with Vectashield (Vector Laboratories). Brain sections with BDA microinjections were similarly cut and collected in PB. After a 15-min wash in PB, the sections were incubated in the ABC Elite kit (Vector Laboratories) for 1 hr at room temperature, followed by three washes in PB with 0.1% Triton X-100 (TX) (Sigma) for 15 min each. The reaction product was visualized by incubating the sections in 0.02% 3–3′-diaminobenzidine tetrahydrochloride (Sigma) and 0.009% hydrogen peroxide in PB with or without cobalt chloride intensification (10). The sections were washed twice for 15 min in PB, mounted onto subbed slides, and dried. The slides were either coverslipped unstained in Permount (Sigma) or counter-stained first in a 1% solution of neutral red (Sigma) before coverslipping. Injections of the tracer that missed their target provided us with ample controls.
Immunohistochemistry. Ab to choline acetyl transferase (ChAT) was donated to us by M. Epstein (University of Wisconsin, Madison) (11). Six birds were perfused, their brains were cut, and sections were collected as in the tract-tracing experiments. After washing the fixed sections in three changes of PB for 15 min each at room temperature, the sections were blocked for nonspecific binding sites by immersing them for 30 min in 5% normal goat serum (NGS) in 0.1% TX with gentle rocking. The sections were then incubated for 24–48 hr (at 4°C) in the rabbit anti-chicken ChAT diluted 1:500 in PB with 0.1% NGS and 0.1% TX. The excess unbound Ab was washed off in three changes of PB with 0.1% TX for 30 min each. The sections were incubated in a biotinylated secondary Ab to rabbit IgG (Vector Laboratories) for 1 hr (1:1,000 dilution) or processed for immunofluorescence by reacting the sections in Alexa Fluor 594 anti-rabbit IgG (1:200 dilution in PB) for 2–3 hr in the dark. All sections were subsequently washed three times in PB for 15 min each wash, and the fluorescent sections were immediately mounted onto slides and coverslipped with Vectashield. All fluorescent slides were stored in light-tight boxes kept in the refrigerator. The biotinylated secondary Ab-reacted sections were processed with the Vector Elite kit as described earlier. Control sections were processed without the primary or secondary Ab and did not produce any specific staining.
Double-Labeling Experiments. Tract-tracing and ChAT immunohistochemistry experiments were combined to double-label brain areas of interest. Six birds were first iontophoresed in the Uva with a 10% solution of Alexa Fluor 488-dextran amine (10,000 molecular weight, Molecular Probes) for 20 min at 5 μA pulsed on/off for 7 sec through a glass micropipette with a tip diameter of 15 μm. After a 7- to 10-day survival period, the birds were perfused with 0.9% NaCl followed by 2% paraformaldehyde for 1 hr as described above. After postfixing, cryoprotection, and cutting at 30 μm, the sections were processed for fluorescent anti-ChAT immunoreactivity (ChAT-IR) (Alexa Fluor 594 anti-rabbit IgG secondary Ab) and examined with a Zeiss laser confocal microscope.
Imaging. California Institute of Technology's Biological Imaging Center provided us with the digital imaging microscopes and related computer software for both the light microscope (Axio-Cam and axiovision 3.0.6) and the laser scanning confocal microscope (Pascal) (all from Zeiss).
Results
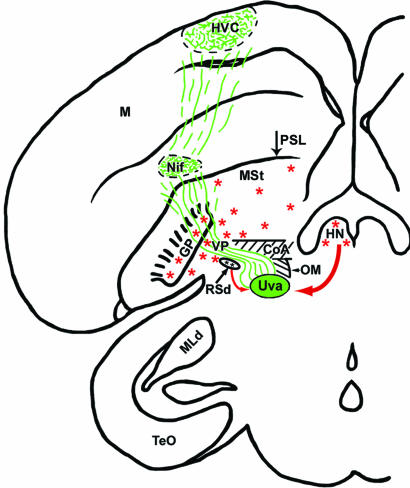
Projections from the Uva into the Telencephalon. Localized iontophoretic injections of either BDA or fluorescent dextran amines in the Uva show two major labeled tracts emanating from the nucleus. One tract courses in a rostroventral direction from the Uva and heads in the direction of the ventral midbrain, tectal, and hindbrain regions. Although some of these connections have been previously documented (9), they are not relevant to the present study. The other labeled tract splits off from the previous one just after leaving the Uva and courses rostrally to enter the fasciculus prosencephalus lateralis, just lateral to the occipitomesencephalic tract. The labeled fibers from the Uva then make a sharp upward turn to enter the telencephalon just lateral to the anterior commisure and medial to the globus pallidus (Fig. 2). The once-tight bundle of labeled fibers then spreads out significantly as they ramify dorsally into the ventral forebrain and innervate NIf, just dorsal to the pallial-subpallial lamina (PSL). The labeled fibers from and around the NIf continue to spread out into the adjacent nidopallium until they innervate the HVC.
Fig. 2.
A coronal composite rendition summarizing the results of a BDA tracer injection into the Uva. Anterograde-labeled axons (green) from the Uva are shown entering the telencephalon just above the RSd, overlapping with the VP area, and spreading out to innervate both the NIf and HVC. Red arrows show the input areas to the Uva (RSd and HN), and the red asterisks denote the general location of ChAT-IR cells that were relevant to the present study. TeO, optic tectum; MLd, nucleus mesencephalicus lateralis, pars dorsalis; M, mesopallium; HN, habenula nuclei, which include both the lateral habenula and MHb, but only the latter contains ChAT-IR cells; PSL, pallial-subpallial lamina; GP, globus pallidus; OM, occipitomesencephalic tract; MSt, medial striatum; CoA, anterior commisure
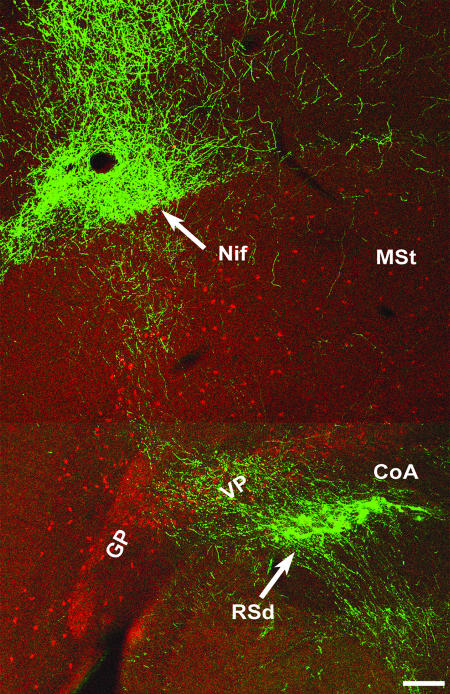
We also investigated the relationship between labeled fibers exiting the Uva and ChAT-IR, because cholinergic mechanisms have been implicated in the modulation of auditory responses in songbirds (8). The ChAT-IR we performed confirms previous reports of many immunopositive cells in the ventral pallidum (VP) region of the basal forebrain (BF) (12). Although there are many scattered and widely separated ChAT-stained cells throughout most of the medial striatum region, there is a sharp division of both somal and extrasomal staining delineated by the PSL. We did not find any evidence of ChAT-IR cell bodies or fibers in any brain areas dorsal to the PSL, including the NIf and HVC. We also did not find any labeled cells or fibers in the robust nucleus of the arcopallium, which is located ventral to the lamina arcopallialis dorsalis, although there could occasionally be a few immunopositive cells located outside of this nucleus. Experiments combining both ChAT-IR and anterograde labeling from the Uva (Fig. 3) reveal that most, if not all, of the BDA-labeled axons innervating both the NIf and HVC traverse the BF area and overlap with the ChAT-IR cells in the VP region as described by Li and Sakaguchi (12). Under high-power magnification, the BDA-labeled fibers from Uva injections do not seem to make synaptic contacts with any VP cells, compared with the significant boutons and fine branching networks seen in both the NIf and HVC. Control anterograde tracer injections (n = 3) that missed the Uva, except those placed in the rostral tract leaving the nucleus, failed to label fibers in the fasciculus prosencephalus lateralis, NIf, or HVC.
Fig. 3.
Double fluorescent-labeled anterograde fibers from the Uva (green) and ChAT-IR cells (red) in coronal section. The low-power image shows the general trajectory of the pathway from the Uva, which sends projections to the NIf and keeps coursing upward to ultimately innervate the HVC (compare with Fig. 2). (Scale bar, 200 μm.)
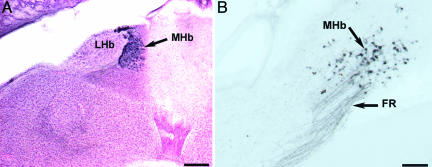
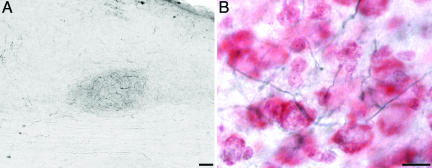
The Uva Receives Efferent Projections from both the MHb and the Reticular Nucleus. Injections of BDA or fluorescent dextran amines into the Uva show intensely retrograde-filled cells in the ipsilateral MHb and RSd. In coronal sections, the MHb is located adjacent to the midline and is the most dorsal structure in the diencephalon (Fig. 4A). Labeled fibers from the Uva can be seen to enter the fasciculus retroflexus (FR) and course dorsomedially to label cells in the MHb (Fig. 4B). Although relatively wide (200 μm), in sagittal sections, the MHb is very thin and could easily be missed during routine histological examination. Anterograde tracers into the MHb confirm that it sends efferents to innervate the ipsilateral Uva (Fig. 5). Control injections that were just outside of the Uva did not retrogradely label any cells in the MHb. However, injections that missed the nucleus and were located just dorsolateral to it did label some cells in the MHb, presumably by hitting the FR. Injections of tracers in the Uva also yielded retrograde labeling of cells in the RSd, just above the occipitomesencephalic tract and intercalcated within the fasciculus prosencephalus lateralis plexus, just before the ventral part of the telencephalic–diencephalic junction. This group of cells, therefore, can be seen to lie right in the pathway of the Uva axons going toward the NIf and HVC (Figs. 2 and 3). Because of the diffuse and precarious location of the RSd within the projection pathway of the Uva, tracer injections were not attempted here. However, control injections (n = 3) just outside of the Uva consistently did not label any cells in the RSd.
Fig. 4.
The MHb in coronal sections. (A) Neutral red and ChAT-IR (dark blue) cells show the location of the MHb between the lateral habenula on the left (lateral) and the anterior-most portion of the cerebellum on the right. Dorsal is up. (Scale bar, 200 μm.) (B) Retrograde-labeled cells from tracer injections into the ipsilateral Uva show labeled axons in the fasciculus retroflexus and cells in the MHb. Dorsal is up, and medial is to the right. (Scale bar, 50 μm.)
Fig. 5.
Anterograde transport to the Uva after a BDA injection into the MHb. (A) Low-power coronal section reveals labeled fibers from the MHb innervating the Uva. (Scale bar, 50 μm.) (B) Higher magnification of the same section, which was later counterstained with neutral red. Dorsal is up, and lateral is to the right. (Scale bar, 10 μm.)
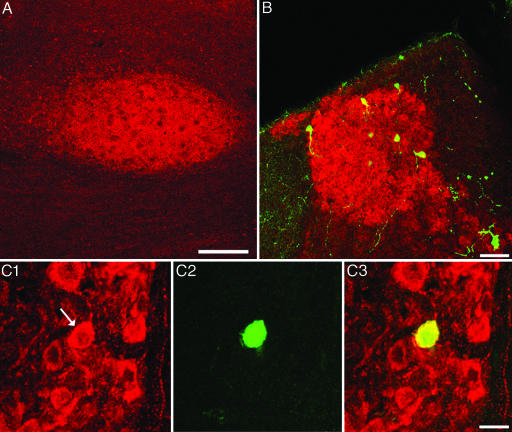
The MHb Provides Cholinergic Input to the Uva. Anti-ChAT immunohistochemistry in five birds showed intense and specific labeled cells confined to the medial part of the habenula area (Fig. 4A). No labeled cells were found in the lateral habenula. In sections where the reaction product was cobalt chloride-intensified, many fine immunopositive fibers could be seen connecting the MHb with the ipsilateral Uva by means of the fasciculus retroflexus. Although these extremely fine fibers are difficult to photograph, low-power immunofluorescence clearly shows that the Uva is cholinoceptive (Fig. 6A). Retrograde tracer injections into the Uva followed by ChAT staining confirm that the ChAT-IR cells in the MHb project to the ipsilateral Uva (Fig. 6 B and C). Control experiments in which either the primary or secondary Ab was eliminated did not show any ChAT-IR anywhere in the brain.
Fig. 6.
The MHb provides cholinergic input to the Uva. (A) Coronal section of an adult male zebra finch shows ChaT-IR in the Uva. Dorsal is up, and lateral is to the left. (Scale bar, 200 μm.) (B) Low-power view of the MHb double labeled with ChAT-IR (red) and retrograde cells from an ipsilateral Uva injection of a fluorescent green tracer. Dorsal is up, and medial is to the right. (Scale bar, 50 μm.) (C) High magnification of cells double labeled in the MHb. (C1) ChaT-IR cells in the MHb; arrow points to a cell of interest. (C2) The same cell is retrogradely labeled from the Uva with a green tracer. (C3) An overlay of C1 and C2 confirms that this cell is double labeled. Dorsal is up, and medial is to the right. (Scale bar, 20 μm.)
Discussion
The results of the present study point to the Uva as a nexus by which thalamic modulatory areas can affect the NIf, HVC, or both. The possible role of the Uva in auditory gating derives from an earlier study by Williams (13), who showed that electrical stimulation of the Uva in anesthetized finches greatly diminished the auditory responses in HVC neurons. Consistent with the above observation, M. J. Coleman and E. T. Vu (personal communication) found that Uva lesions disrupt the normal gating of BOS responses in the HVC of behaving finches. Although the connections to and from the Uva are by no means simple (9), we concentrated on finding relevant connections to the Uva that could mediate auditory gating in both the NIf and HVC. We combined anatomical tracing methods and ChAT immunohistochemistry to identify the MHb as a rich source of cholinergic input to the Uva. The MHb has been reported to be cholinergic in a number of other species, including rats (14), chickens (15), and humans (16), but, to our knowledge, our results are the first to show a direct cholinergic input into the Uva from the MHb in the songbird brain. Evidence for the cholinergic basis of auditory gating in the HVC has been reported by Shea and Margoliash (8). Both direct injections of cholinergic agonists in the HVC and electrical or chemical stimulations of the BF induced auditory gating in the HVC of anesthetized birds. The researchers concluded that auditory gating in this nucleus involves direct cholinergic input from cells in the BF. The effects of electrical stimulation or lesions of the Uva on auditory gating in the HVC need to be considered against the background of these observations. Evidence for the possible involvement of the Uva and the conditions necessary for our arguments are as follows. (i) Electrical stimulation of the BF caused auditory gating in the HVC by exciting the fibers of passage from the Uva. (ii) Chemical stimulation of the VP region by microinjections of glutamate could have stimulated the RSd, which is located right next to the cells in the VP area and is known to receive excitatory glutamatergic inputs (17). Stimulation of the RSd could have then influenced the Uva, to which it projects. In both (i) and (ii), we must assume that the HVC receives cholinergic input from the Uva to account for the observation that the effects of electrical stimulation of the BF could be prevented if it is preceded by injections of nicotinic or muscarinic antagonists into the HVC (8). (iii) Electrical stimulation or lesions of the Uva affected the BF fibers projecting to the HVC. This possibility is unlikely, because the Uva is outside the BF.
The main issues are the presence of BF fibers innervating the HVC and the cholinergic or cholinoceptive nature of the HVC itself. Different studies appear to disagree on both issues. Histochemical staining for acetylcholinesterase (AChE) by other researchers suggests that the HVC is cholinoceptive, and autoradiographic-binding assays show a high level of muscarinic cholinergic receptors in this nucleus as well (18). However, our results, as well as others using the Ab from the same source (19), show that very little, if any, ChAT-IR occurs in any telencephalic song system nuclei, including the HVC, to which the VP cells of the BF are supposed to send cholinergic fibers (12). Previous studies in a wide variety of animals, including zebra finches (19), pigeons (20), and zebra fish (21), have shown that there can be considerable mismatches between ChAT-IR and AChE histochemistry, as well as between transmitter levels and their receptors (20). AChE has also been seen in noncholinergic areas of the brain and has been shown to be important in degrading other neuropeptides as well as AChE (22, 23, 24). Another fundamental issue concerns the initial site of auditory gating, which is now recognized to be the NIf, from which the HVC receives most or all of its auditory input (6). If so, then from where are the cholinergic inputs to the NIf coming? There is no anatomical evidence that we know of to show that the NIf receives any projection, cholinergic or otherwise, from cells in the BF, including the VP subregion.
The cholinergic circuits are, however, not the only putative modulatory input to the song system. Cardin and Schmidt (7) demonstrated that norepinephrine (NE) infusions into the NIf and HVC can affect the auditory responses in both of these nuclei. Their conclusions were that NE is the physiological neuromodulatory agent for auditory gating in these brain areas and that the likely source of NE is the locus coeruleus (LC), although the connections between the NIf and the LC have not yet been demonstrated.
The habenular complex is located in the epithalamus and is reported to be a phylogenetically highly conserved structure in all vertebrates (25). In the zebra finch, Nissl-stained coronal sections show a distinct medial and lateral part made even more obvious with concomitant ChAT staining (Fig. 4A). More detailed studies in the rat, using a combination of morphological, hodological, and cytochemical criteria, reveal that the MHb can be further divided into 5 subnuclei and the lateral habenula into 10 nuclei (25). The MHb is involved in a diverse variety of biological functions, many of which are associated with highly emotional and motivated behaviors (26). Those that are particularly relevant to birdsong include mating behavior (27, 28), learning (29), endocrine functions (30), and sleep–wake cycles (31). The habenulointerpeduncular pathway has also been shown to play a major role in modulating the levels of circulating adrenal hormones (32). When a lightly anesthetized zebra finch is startled into a transitory alert state, the response of the HVC and NIf to the BOS decreases (7). Also, BOS responses in the HVC and robust nucleus of the arcopallium increased under natural sleep conditions (33, 34). Hence, auditory gating is closely associated with sleep/wake states, and it is thus reasonable to infer a functional link to brain areas that could influence these changes. Because many studies have shown that the MHb is such a sleep/wake modulatory brain area (31, 35, 36), we propose that the MHb may play an important part in the auditory gating of the HVC and NIf by means of the Uva.
Tracer injections into the Uva also strongly retrogradely labeled cells in an area of the diencephalon that can be identified with reference to published avian atlases (37, 38) and previous work by Wild (9) and Medina and Reiner (20) as the RSd. Previous anatomical tracing studies in the pigeon (39) have determined that the avian RSd is comparable to the mammalian thalamic reticular nucleus in location, connectivity, and histochemistry. The RSd is a thin lamina of cells that is located near the interface of the dorsal thalamus and the ventral-most portion of the telencephalon. Like the MHb, the thalamic reticular nucleus is considered to be highly conserved among vertebrates (40). In rats, this brain area has been described as a shield between the thalamus and cortex, such that all of the fibers passing either way must first pass through the reticular nucleus. But it is generally known that the reticular nucleus is not merely a relay center to the telencephalon. In rat studies, for example, the thalamic reticular formation was found to be an “attentional gate” that regulates the flow of information between the thalamus and cortex by an inhibitory interface. By this model, thalamic cells can fire in either a “tonic” or “burst” fashion, which is either permissive or restrictive, respectively, to the flow of information into the cortex (41). In their review, Guillery et al. (17) describe the mammalian reticular nucleus as being subdivided into distinct visual-, somatosensory-, and auditory-specific sectors. Relevant to our study, a recent electrophysiological experiment using paired tones in anesthetized rats has clearly shown auditory gating in the auditory portion of the reticular formation (42). In the zebra finch, control injections that missed the Uva (but also lay within the thalamus) also did not retrogradely label the RSd. In this respect, therefore, this connection appears to be highly specific. However, we do not yet know whether the RSd in the zebra finch is homologous to that part of thalamic reticular nucleus in rats that has been shown to exhibit auditory gating. Because our results show that the RSd innervates the Uva, future electrophysiological experiments in the Uva are necessary to determine whether auditory gating occurs in the Uva itself.
In conclusion, our results show that the Uva occupies a strategically relevant position to integrate and coordinate several brain areas that can provide the behavioral state-dependent inputs that have been shown to influence auditory gating in both the NIf and HVC. Although future electrophysiological, pharmacological, or other experimental procedures are obviously needed to provide greater insights into how these areas work in the zebra finch, our results cast light on previously overlooked but important pathways into the song system.
Acknowledgments
We thank Gregory Ball, Richard Mooney, and Martin Wild for reviewing drafts of the manuscript. This work was supported by National Institutes of Health Grant MH55984.
Author contributions: M.K. designed research; E.A. performed research; E.A. analyzed data; and E.A. and M.K. wrote the paper.
Abbreviations: BDA, biotinylated dextran amine; BF, basal forebrain; BOS, bird's own song; ChAT, choline acetyl transferase; ChAT-IR, ChAT immunoreactivity; MHb, medial habenula; NIf, interfacial nucleus of the nidopallium; RSd, nucleus reticularis superior, pars dorsalis; Uva, uvaeform nucleus; VP, ventral pallidum.
References
- 1.Nottebohm, F., Stokes, T. M. & Leonard, C. M. (1976) J. Comp. Neurol. 165, 457-486. [DOI] [PubMed] [Google Scholar]
- 2.Margoliash, D. M. (1983) J. Neurosci. 3, 1039-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doupe, A. J. & Konishi, M. (1991) Proc. Natl. Acad. Sci. USA 88, 11339-11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman, M. J. & Mooney, R. (2004) J. Neurosci. 24, 7251-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt, M. F. & Konishi, M. (1998) Nat. Neurosci. 1, 513-518. [DOI] [PubMed] [Google Scholar]
- 6.Cardin, J. A. & Schmidt, M. F. (2004) J. Neurosci. 24, 7745-7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardin, J. A. & Schmidt, M. F. (2004) J. Neurophysiol. 91, 2148-2163. [DOI] [PubMed] [Google Scholar]
- 8.Shea, S. D. & Margoliash, D. (2003) Neuron 40, 1213-1226. [DOI] [PubMed] [Google Scholar]
- 9.Wild, J. M. (1994) J. Comp. Neurol. 349, 512-535. [DOI] [PubMed] [Google Scholar]
- 10.Adams, J. C. (1981) J. Histochem. Cytochem. 29, 775. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, C. D. & Epstein, M. L. (1986) J. Neurochem. 46, 968-976. [DOI] [PubMed] [Google Scholar]
- 12.Li, R. & Sakaguchi, H. (1997) Brain Res. 763, 239-246. [DOI] [PubMed] [Google Scholar]
- 13.Williams, H. (1989) Ann. N.Y. Acad. Sci. 563, 148-164. [Google Scholar]
- 14.Kawaja, M. D., Flumerfelt, B. A. & Hrycyshyn, A. W. (1990) Synapse 6, 45-54. [DOI] [PubMed] [Google Scholar]
- 15.Sorenson, E. M., Parkinson, D., Dahl, J. L. & Chiappinelli, V. A. (1989) J. Comp. Neurol. 281, 641-657. [DOI] [PubMed] [Google Scholar]
- 16.Heckers, S., Geula, C. & Mesulam, M. M. (1992) J. Comp Neurol. 325, 68-82. [DOI] [PubMed] [Google Scholar]
- 17.Guillery, R. W., Feig, S. L. & Lozsádi, D. A. (1998) Trends Neurosci. 21, 28-32. [DOI] [PubMed] [Google Scholar]
- 18.Ryan, S. M. & Arnold, A. P. (1981) J. Comp. Neurol. 202, 211-219. [DOI] [PubMed] [Google Scholar]
- 19.Zuschratter, W. & Scheich, H. (1990) Brain Res. 513, 193-201. [DOI] [PubMed] [Google Scholar]
- 20.Medina, L. & Reiner, A. (1994) J. Comp. Neurol. 342, 497-537. [DOI] [PubMed] [Google Scholar]
- 21.Clemente, D., Porteros, A., Weruaga, E., Alonso, J. R., Arenzana, F. J., Aijon, J. & Arevalo, R. (2004) J. Comp. Neurol. 474, 75-107. [DOI] [PubMed] [Google Scholar]
- 22.Emson, P. C. & Lindvall, O. (1979) Neuroscience 4, 1-30. [DOI] [PubMed] [Google Scholar]
- 23.Chubb, I. W., Hodgson, A. J. & White, G. H. (1980) Neuroscience 5, 2065-2072. [DOI] [PubMed] [Google Scholar]
- 24.Chubb, I. W., Ranieri, E., Hodgson, A. J. & White, G. H. (1982) Neurosci. Lett. 8, Suppl., S39. [Google Scholar]
- 25.Andres, K. H., von During, M. & Veh, D. W. (1999) J. Comp. Neurol. 407, 130-150. [DOI] [PubMed] [Google Scholar]
- 26.Sutherland, R. J. (1982) Neurosci. Biobehav. Rev. 6, 1-13. [DOI] [PubMed] [Google Scholar]
- 27.Corodimas, K. P., Rosenblatt, J. S. & Moirell, J. I. (1992) Behav. Neurosci. 106, 853-865. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang, X., Silverman, A. J. & Silver, R. (1993) Horm. Behav. 27, 283-295. [DOI] [PubMed] [Google Scholar]
- 29.Thornton, E. W. & Davies, C. (1991) Physiol. Behav. 49, 819-822. [DOI] [PubMed] [Google Scholar]
- 30.Wilhelm, M., King, B., Silverman, A. J. & Silver, R. (2000) Endocrinology 141, 1178-1186. [DOI] [PubMed] [Google Scholar]
- 31.Sugama, S., Cho, B. P., Baker, H., Joh, T. H., Lucero, J. & Conti, B. (2002) Brain. Res. 958, 1-9. [DOI] [PubMed] [Google Scholar]
- 32.Murray, M., Murphy, C. A., Ross, L. L. & Haun, F. (1994) Res. Neurol. Neurosci. 6, 301-307. [DOI] [PubMed] [Google Scholar]
- 33.Dave, A. S., Yu, A. C. & Margoliash, D. M. (1998) Science 282, 2250-2254. [DOI] [PubMed] [Google Scholar]
- 34.Nick, T. A. & Konishi, M. (2001) Proc. Natl. Acad. Sci. USA 98, 14012-14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haun, F., Eckenroder, T. C. & Murray, M. (1992) J. Neurosci. 12, 3282-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valjakka, A., Vartiainen, J., Tuomisto, L., Tuomisto, J. T., Olkkonen, H. & Airaksinem, M. M. (1998) Brain Res. Bull. 47, 171-184. [DOI] [PubMed] [Google Scholar]
- 37.Karten, H. J. & Hodos, W. (1967) in A Stereotaxic Atlas of the Brain of the Pigeon (Columba livia) (Johns Hopkins Univ. Press, Baltimore), pp. 78-85.
- 38.Stokes, T. M., Leonard, C. M. & Nottebohm, F. (1974) J. Comp. Neurol. 156, 255-374. [DOI] [PubMed] [Google Scholar]
- 39.Medina, L. & Reiner, A. (1997) Neurosci. 8, 773-802. [DOI] [PubMed] [Google Scholar]
- 40.Jones, E. G. (1975) J. Comp. Neurol. 162, 285-308. [DOI] [PubMed] [Google Scholar]
- 41.McAlonan, K. & Brown, V. J. (2002) Neuroscientist 8, 302-305. [DOI] [PubMed] [Google Scholar]
- 42.Krause, M., Hoffman, W. E. & Hajós, M. (2003) Biol. Psychiatry 53, 244-253. [DOI] [PubMed] [Google Scholar]