Abstract
Atmospheric transport and deposition of nutrients, especially nitrogen, is a global environmental problem with well-documented consequences for ecosystem dynamics. However, monitoring nitrogen deposition is relatively expensive, monitoring stations are widely spaced, and estimates and predicted impacts of nitrogen deposition are currently derived from spatial modeling and interpolation of limited data. Ombrotrophic (“rain-fed”) bogs are nutrient-poor ecosystems that are especially sensitive to increasing nutrient input, and carnivorous plants, which are characteristic of these widespread ecosystem types, may be especially sensitive indicators of N deposition. Botanical carnivory is thought to have evolved in nutrient-poor and well-lit habitats such as bogs because the marginal benefits accruing from carnivory exceed the marginal photosynthetic costs associated with the maintenance of carnivorous organs. However, the production of carnivorous organs can be a phenotypically plastic trait. The northern pitcher plant, Sarracenia purpurea, produces leaves specialized for prey capture and nutrient uptake (pitchers) and leaves that are more efficient at photosynthesis (phyllodia). We hypothesized that relative allocation to these two types of leaves reflects ambient nitrogen availability. We manipulated nutrient availability to plants with leaf enrichment and whole-plot fertilization experiments. Increased nitrogen, but not phosphorus, reduced production of pitchers relative to phyllodia; this result provided empirical support for the cost–benefit model of the evolution of botanical carnivory. Because this phenotypic shift in leaf production occurs in ecological time, our results suggest that S. purpurea could be a reliable and inexpensive biological indicator of nitrogen deposition rates. This suggestion is supported by field observations across a geographic gradient of nitrogen deposition.
Humans currently use nearly 40% of the world's primary production (1, 2), and in doing so have doubled the amount of nitrogen (N) released to terrestrial ecosystems (2, 3). The atmospheric transport and deposition of nitrogenous pollutants is a widespread environmental problem with well-documented consequences for ecosystem dynamics (4). Deposition rates of SO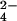 , the main component of “acid rain,” have been reduced dramatically since the early 1980s (5). However, deposition rates of nitrogenous compounds (N2O, NO
, the main component of “acid rain,” have been reduced dramatically since the early 1980s (5). However, deposition rates of nitrogenous compounds (N2O, NO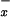 , and NH
, and NH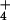 ) have not declined in parallel (5), and in fact continue to increase at ≈0.3% per year (6), in large part because the anthropogenic sources of N are not strongly localized and easily regulated. Rather, they are principally by-products of agriculture (3) and dispersed fossil-fuel combustion (7). Because N2O and NO
) have not declined in parallel (5), and in fact continue to increase at ≈0.3% per year (6), in large part because the anthropogenic sources of N are not strongly localized and easily regulated. Rather, they are principally by-products of agriculture (3) and dispersed fossil-fuel combustion (7). Because N2O and NO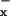 are significant greenhouse gases, their effects are often analyzed at a global scale (3, 6). Yet many of the effects of N deposition occur at local and regional scales, in between the widely spaced existing network of monitoring stations, such as those of the United States National Atmospheric Deposition Program (http://nadp.sws.uiuc.edu/). There is a critical need, therefore, to identify effective fine-scale indicators of N deposition and N saturation, which can provide data that can complement data obtained from national monitoring networks.
are significant greenhouse gases, their effects are often analyzed at a global scale (3, 6). Yet many of the effects of N deposition occur at local and regional scales, in between the widely spaced existing network of monitoring stations, such as those of the United States National Atmospheric Deposition Program (http://nadp.sws.uiuc.edu/). There is a critical need, therefore, to identify effective fine-scale indicators of N deposition and N saturation, which can provide data that can complement data obtained from national monitoring networks.
Nutrient-poor ecosystems are likely to be especially sensitive to increasing nutrient input. Nonforested peatlands (bogs and fens) are widespread in northern latitudes (above 40° N), and despite their relatively small total aereal extent, comprise nearly one-third of the global pool of soil carbon (8). Atmospheric deposition is the primary pathway for long-term accumulation and maintenance of nutrient stocks in ombrotrophic (“rain-fed”) bogs (9). Carnivorous plants, which are characteristic of such ecosystems (10), are likely to be particularly sensitive to changes in N availability because of their reliance on nonsoil sources of N (11). Botanical carnivory is thought to have evolved in nutrient-poor and well-lit habitats such as bogs because the marginal benefits accruing from carnivory exceed the marginal photosynthetic costs associated with the maintenance of carnivorous organs (12). However, the production of carnivorous organs can be a phenotypically variable trait (13–15). For example, pitcher plants in the genus Sarracenia produce both carnivorous pitchers and noncarnivorous but photosynthetically functional phyllodia (16, 17). We determined experimentally that in S. purpurea, the production of phyllodia is a direct response to N availability. This result provides empirical support for the cost–benefit model for the evolution of carnivory (12). Because the production of phyllodia occurs rapidly in response to short-term ecological changes, we hypothesized that the morphology of this plant could be used as a reliable fine-scale indicator of N deposition rates throughout its wide geographic range. We tested this hypothesis by simulating N deposition in two manipulative field experiments, and by surveying N availability and pitcher morphology across 26 New England bogs.
Materials and Methods
S. purpurea.
The northern pitcher plant, S. purpurea, is a long-lived (>50 years) rosette-forming perennial that ranges throughout Canada and in the eastern United States from Maine to Georgia, where it grows in nutrient-poor bogs, fens, and seepage swamps (17). This species also has been introduced to bogs in California, Scotland, Ireland, England, Switzerland, and Japan (18). This carnivorous plant collects rainwater in its pitcher-shaped leaves (10), which are derived developmentally through adaxial folding of the leaf primordia (19). Prey attracted to the brightly colored, water-filled pitchers fall in and drown, whereupon the prey are processed by a suite of consumers and decomposers, including bacteria, protists, rotifers, and fly larvae (20). Because S. purpurea lacks digestive glands or enzymes, these decomposers mineralize nutrients in the prey and make them available for absorption by the plant (10, 21). However, northern pitcher plants are somewhat inefficient predators (22), and insects account for only 10% of their nutrient budget (11). Because S. purpurea (and other carnivorous plants) typically grows in ombrotrophic bogs that receive nutrient inputs to the plant rooting zone solely from precipitation (9, 23), the balance of this plant's N budget comes from nutrients dissolved in rainwater that collects in its pitchers with some additional N contributed through mineralization by rotifers inhabiting the pitchers (24). Consequently the growth and morphology of S. purpurea should be particularly responsive to changes in local N deposition occurring through acidified precipitation.
Direct Effects of N Additions on Morphology of S. purpurea.
We first tested the hypothesis that the morphology and performance of S. purpurea is responsive to N availability by directly adding N into pitchers. This experiment also tested the cost–benefit model for the evolution of carnivory (12) by focusing on the ecophysiological pathway responsible for the shift in morphology. In May 1998, we randomly selected 90 adult (rosette diameter ≥ 10 cm) S. purpurea plants at Hawley Bog, Massachusetts (42° N, 72° W, elevation 543 m above sea level), an ≈3-hectare ombrotrophic, stream headwaters bog (25). We randomly assigned each of these plants to one of nine different nutrient treatments: two control treatments (distilled H2O or 10% concentration of micronutrients only from Hoaglands solution); two N treatments (0.1 mg or 1.0 mg NH4-N/liter as NH4Cl); two phosphorus (P) treatments (0.025 mg or 0.25 mg PO4-P/liter as NaH2PO4); and three treatments in which we altered the N/P ratio [low N/low P ≡ 1:2.5 (NP1 on Fig. 1), high N/high P ≡ 4:1 (NP2 on Fig. 1), and high N/low P ≡ 40:1 (NP3 on Fig. 1)]. All N and P treatments also received micronutrients as 10% Hoaglands solution. N concentrations were chosen to bracket measured rates of annual deposition (determined from United States National Atmospheric Deposition Program data). P concentrations also were varied to determine whether plants responded to N limitation directly or to P limitation caused by increased N availability (26). Growth of bog plants may be limited by either N or P (26); growth of S. purpurea appears to be colimited by N and P (median N/P = 15.5; range = 10.15–39.32; n = 31 adult plants; N and P concentrations of dried, ground plant tissue determined with a Perkin–Elmer Autoanalyzer). Sample size was determined as that needed to give statistical power of 80% with a nominal type I error rate of 0.05, given expected effect sizes published previously (27).
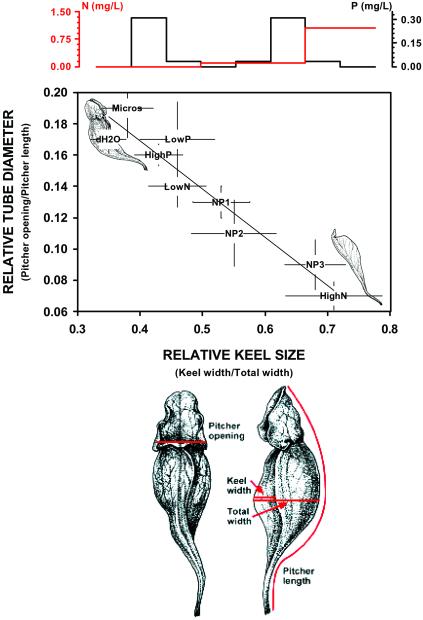
Figure 1.
Morphological response of S. purpurea to direct nutrient additions. Morphology is expressed as two correlated characters: relative tube diameter (an indication of how large an opening the leaf has in which it captures prey) and relative keel size (an indication of the relative size of the pitcher tube itself, in which prey are digested). Pitcher measurements are illustrated on the drawings of the pitchers (by E. J. Farnsworth) below the main plot. The joint changes in morphology are illustrated with line drawings (by E. J. Farnsworth) of a normal pitcher with a relatively large mouth and small keel (top left) and a phyllode with a relatively small mouth and large keel (bottom right). On the main plot, each point represents the joint mean of the two morphological variables and their 95% confidence intervals. Means are labeled with the nutrient addition treatment. The plot at Top illustrates the N and P concentrations added at each treatment. The red line illustrates the N concentration and the black line the P concentration in each treatment as ordered from left to right along the regression line.
Every two weeks during the growing seasons (June 1 to September 30) of 1998, 1999, and 2000, 5 ml of the assigned nutrient solution was added directly to each open pitcher on each plant. pH of pitcher water in the experimental plants during the growing season remained within the range (3.5–5.5) observed in control pitchers. After each nutrient addition, leaves were plugged with glass wool to prevent colonization by common pitcher inhabitants and capture of prey. All leaves were measured in mid-September every year, before senescence. Pitcher height was measured with a flexible vinyl tape (±1 mm), and mouth diameter, tube width, and keel width were measured with calipers (±0.1 mm). Mortality was <10% in all treatments, and was not concentrated in any given treatment. Further details of the experimental design are provided elsewhere (28).
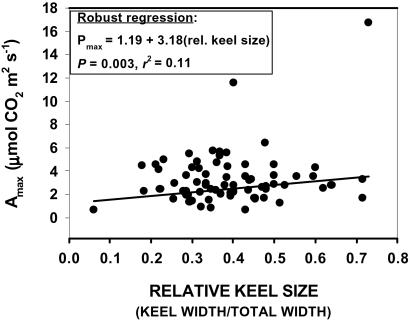
Photosynthetic rates (μmol of CO2 per m2 per s) of the largest leaf on all surviving plants (n = 73) in the nutrient addition experiment were measured between 20 June and 3 July, 2000, by using a Li-Cor Li-6200 photosynthesis system (Li-Cor, Lincoln, NE) and custom-built 4-liter chamber. All measurements were made between 0900 and 1400 h, under ambient solar radiation that exceeded the light saturation point for S. purpurea (photosynthetic photon flux density >800 μmol per m2 per s). We regressed photosynthetic rates on relative keel size by using robust regression (MM method) in s-plus for windows, version 6.0 (Insightful, Seattle). This robust regression reduced the influence of outliers by minimizing the maximum possible bias of the estimates of the regression coefficients.
Simulating N Deposition.
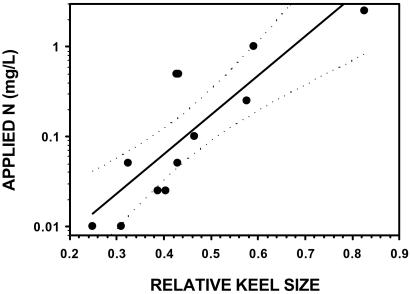
Precipitation deposits nutrients not only into pitchers but also onto the peat surface and into the pore water. To determine whether leaf morphology predicts deposition rates at the whole-plot level, we conducted a second experiment in which we established experimental plots that contained S. purpurea, and sprayed each plot with solutions of NH4NO3. Nine 2 × 2 m plots, each with 1–3 S. purpurea individuals, were established at Molly Bog, a 0.8-hectare ombrotrophic pond-margin bog in northwest Vermont (44° N, 72° W, elevation 236 m above sea level). Each plot was randomly assigned, in a regression design (29), to a different treatment: 0, 0.01, 0.025, 0.05, 0.1, 0.25, 0.5, 1.0, or 2.5 g N/m2 to be added over the growing season. We applied N as NH4NO3 in solution by using a backpack sprayer every 3 weeks from May 8 to September 11, 2000. P and K also were added to each plot as KH2PO4 to produce an application of 5 and 6.3 g/m2, respectively, over the growing season, to ensure that our results were caused by N-limitation, not to P- or K-limitation brought on by excess N (30, 31). In mid-September 2000, we measured the morphology of the largest S. purpurea leaf on each plant. We calculated a morphological index for each leaf (the relative keel size, see Results, and Fig. 1) and regressed it on the amount of N applied to each plot. To avoid measurement bias, this was a “double-blind” experiment. The applicators did not know the concentration of the treatment they were applying, and we did not know the treatment applied to the plants we were measuring.
S. purpurea as a Biological Indicator of N Deposition.
Pitcher plant bogs are scattered across the landscape, and occur in a region of North America that receives significant acid rain (5, 32). However, the monitoring stations of the National Atmospheric Deposition Program are few and far between; for example, in our home states of Massachusetts and Vermont, there are only three and two stations, respectively. Consequently, local estimates of N deposition currently are derived from spatial modeling and interpolation of limited data (33). Based on the results of the two field experiments, we hypothesized that the observed leaf morphology of pitcher plants in bogs could be used as a convenient biological indicator of local N deposition.
To test this hypothesis, we extensively surveyed 26 bogs across Massachusetts and Vermont during the summer of 2000. These bogs are classified (34) as acidic peatlands (mean pH = 4.3, range 3.27–5.5; mean Ca2+ = 11.2 mg/liter, range = 1.4–23; with a well-developed Sphagnum mat and dominated by shrubs in the family Ericaceae). Details of the geography and vegetation of these sites are published elsewhere (35). In this region, N deposition generally increases from west to east, and from south to north (33), and ranges from 0.75 to 2.25 mg NO3-N/liter and 0.1 to 0.4 mg NH4-N/liter. In bogs, most of the NO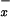 deposited is transformed into NH
deposited is transformed into NH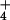 in the pore water (36, 37). Therefore, we used pore-water NH4 concentration as a proxy for NO
in the pore water (36, 37). Therefore, we used pore-water NH4 concentration as a proxy for NO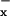 deposition, while recognizing that concentrations of NH
deposition, while recognizing that concentrations of NH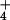 in the pore water reflect a balance between deposition rates (which we infer) and rates of absorption by the plants. At each bog, we measured the largest leaf of 25 randomly selected S. purpurea individuals, and collected 5 samples of pore water by pressing a 50-ml sterile centrifuge tube into the Sphagnum mat. Tubes filled with water within 10 s. Concentration of NH
in the pore water reflect a balance between deposition rates (which we infer) and rates of absorption by the plants. At each bog, we measured the largest leaf of 25 randomly selected S. purpurea individuals, and collected 5 samples of pore water by pressing a 50-ml sterile centrifuge tube into the Sphagnum mat. Tubes filled with water within 10 s. Concentration of NH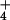 and NO
and NO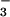 in pore water was determined by using salicylate and cadmium reduction spectrophotometry, respectively (38). In general, NO
in pore water was determined by using salicylate and cadmium reduction spectrophotometry, respectively (38). In general, NO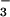 was not detectable, so we regressed only NH
was not detectable, so we regressed only NH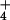 concentrations on plant morphology.
concentrations on plant morphology.
Results
Direct Effects of N Additions on Morphology of S. purpurea.
Within a single growing season, direct nutrient additions dramatically altered leaf morphology (Fig. 1) and elevated plant photosynthetic rates (Fig. 2). Those plants that received high concentrations of N alone or solutions with high N/P ratios produced leaves with large noncarnivorous keels and small carnivorous tubes (phyllodia). In extreme cases, no tubes were produced and leaves were entirely noncarnivorous. Plants that received only distilled water, micronutrients, P alone, or low concentrations of N produced normal carnivorous pitchers with small keels and large tubes (Fig. 1). This result supported a prediction of the cost–benefit model for the evolution of carnivory (12), that carnivory would not be favored when there is an excess supply of N. However, the production of carnivorous organs was phenotypically plastic, and we observed this morphological change in ecological, rather than evolutionary, time. A second prediction of the cost–benefit model is that there should be a photosynthetic cost to producing carnivorous structures. This prediction was also supported. When the experimental plants were ordered along the morphological continuum from pitcher to phyllode, maximum whole-leaf photosynthetic rate increased as leaves became more flattened and less carnivorous (Fig. 2).
Figure 2.
Maximum photosynthetic rates of plants of different shapes. Relative keel size increased (from left to right) with increasing N addition (see Fig. 1).
Can Plant Morphology Predict N Deposition Rate?
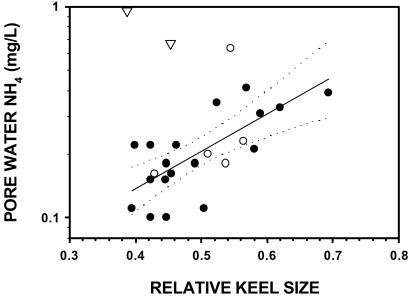
We found a direct linear relationship between plant morphology and N applied as aerial spray. The relative sizes of pitcher keels and tubes in the manipulated plots at Molly Bog were, in fact, accurate predictors of the amount of N applied (Fig. 3). Similarly, across New England, pitcher morphology was an accurate predictor of pore-water NH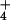 concentrations up to 0.5 mg/liter (Fig. 4). However, plant morphology did not accurately predict the extremely high pore-water NH
concentrations up to 0.5 mg/liter (Fig. 4). However, plant morphology did not accurately predict the extremely high pore-water NH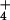 concentrations we found at two bogs in northern Vermont (Fig. 4). These two bogs are not geographically unique, however. One of these two bogs was in the west-central part of the state and the other in the northeast corner. Pore-water concentrations of NH
concentrations we found at two bogs in northern Vermont (Fig. 4). These two bogs are not geographically unique, however. One of these two bogs was in the west-central part of the state and the other in the northeast corner. Pore-water concentrations of NH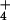 of bogs to the east, west, north, and south of these two bogs were predicted well by plant morphology.
of bogs to the east, west, north, and south of these two bogs were predicted well by plant morphology.
Figure 3.
N concentration added as aerial spray in large plots at Molly Bog as a function of relative keel size. Dotted lines indicate 95% confidence intervals. The fitted model was log([N]) = −2.94 + 4.36 × (relative keel size), and explained 72% of the variation in N concentration applied.
Figure 4.
Pore water NH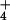 concentration at 26 bogs in Massachusetts (●) and Vermont (○ and ▿). The fitted model to all but the two Vermont outliers (▿) was log([NH
concentration at 26 bogs in Massachusetts (●) and Vermont (○ and ▿). The fitted model to all but the two Vermont outliers (▿) was log([NH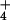 ]) = −1.57 + 1.78 × relative keel size, and explained 45% of the variation in pore-water NH
]) = −1.57 + 1.78 × relative keel size, and explained 45% of the variation in pore-water NH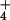 concentration. Dotted lines indicate the 95% confidence interval on the regression.
concentration. Dotted lines indicate the 95% confidence interval on the regression.
Discussion
Nitrogen deposition is a global problem that requires accurate monitoring at a variety of spatial scales. Although S. purpurea is a long-lived perennial plant, our experiments showed that it is phenotypically plastic. Within a single growing season, individual rosettes responded to local increases in N availability by shifting rapidly from production of carnivorous pitchers to production of more photosynthetically efficient phyllodia. Because S. purpurea commonly occurs in bogs and other wetlands throughout Canada and eastern North America, and has been introduced in western Europe and Japan, we propose that it can be used as a ready biological indicator of local N deposition, based on simple measurements taken only with field calipers.
The ecophysiological mechanism underlying this biological indicator also suggests that other carnivorous plants could be used as similar biological indicators of nutrient accumulation and saturation. The cost–benefit model for the evolution of carnivory (12) predicted that carnivory will not be favored when there is an excess supply of nutrients in the environment. Although this model was developed to predict the evolution of botanical carnivory, the production of carnivorous organs is phenotypically plastic (Fig. 1), and occurs in ecological time. The cost–benefit model also predicted that there should be a photosynthetic cost to producing carnivorous structures, and we observed this cost as well (Fig. 2). Plants do not respond to nutrients in isolation, however. For example, S. purpurea produces pitchers at extremely high levels of pore-water NH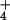 , such as those observed at two bogs in Vermont, possibly as another nutrient (e.g., P) becomes limiting. This hypothesis is supported inferentially by observation of N/P ratios >15 in these two Vermont bogs. Although the dominant nutrient contributed by insect prey to pitcher plants is N (39), insect prey also are a source of P for pitcher plants (21).
, such as those observed at two bogs in Vermont, possibly as another nutrient (e.g., P) becomes limiting. This hypothesis is supported inferentially by observation of N/P ratios >15 in these two Vermont bogs. Although the dominant nutrient contributed by insect prey to pitcher plants is N (39), insect prey also are a source of P for pitcher plants (21).
Other carnivorous plants also have been shown to vary their production of carnivorous structures in response to nutrient availability, including species in the globally widespread genera Utricularia (13–15) and Pinguicula (40, 41). Carnivorous plants occur in nutrient-poor habitats on every continent except Antarctica (42); further research on the morphological responses of these unique plants to nutrients could lead to the development of inexpensive biological indicators for a broad spectrum of anthropogenic nutrients.
Acknowledgments
This research was permitted by the Massachusetts chapter of The Nature Conservancy and the University of Vermont. Leszek Bledzki, Elizabeth Farnsworth, and Kirsten McKnight assisted with field work in Massachusetts, and Stephen Hudman, Krista Matthews, and Justine Sears assisted with field work in Vermont. Barbara Bedford, Leszek Bledzki, and Elizabeth Farnsworth commented on early drafts of the manuscript. This work was supported by National Science Foundation Grants DEB 98-05722 and 98-08504.
References
- 1.Vitousek P M. Science. 1997;277:485–525. [Google Scholar]
- 2.Vitousek P M, Aber J D, Howarth R W, Likens G E, Matson P A, Schindler D W, Schlesinger W H, Tilman D G. Ecol Appl. 1997;7:737–750. [Google Scholar]
- 3.Tilman D, Faragione J, Wolff B, D'Antonio C, Dobson A, Woarth R, Schindler D, Schlesinger W H, Simberloff D, Swackhamer S. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- 4.Fenn M E, Poth M A, Aber J D, Baron J S, Bormann B T, Johnson D W, Lemly A D, McNulty S G, Ryan D F, Stottlemyer R. Ecol Appl. 1998;8:706–733. [Google Scholar]
- 5.Sirois A, Vet R, MacTavish D. Ecosystems. 2001;4:503–513. [Google Scholar]
- 6.Olsen S C, McLinden C A, Prather M J. J Geophys Res Atmos. 2001;106:28771–28784. [Google Scholar]
- 7.Horowitz L W, Jacob D J. J Geophys Res Atmos. 1999;104:23823–23840. [Google Scholar]
- 8.Gorham E. Ecol Appl. 1991;1:182–195. doi: 10.2307/1941811. [DOI] [PubMed] [Google Scholar]
- 9.Damman A W H. Aquilo Ser Bot. 1990;28:5–14. [Google Scholar]
- 10.Juniper B E, Robins R J, Joel D M. The Carnivorous Plants. New York: Academic; 1989. [Google Scholar]
- 11.Ellison A M, Gotelli N J. Trends Ecol Evol. 2001;16:623–629. [Google Scholar]
- 12.Givnish T J, Burkhardt E L, Happel R E, Weintraub J D. Am Nat. 1984;124:479–497. [Google Scholar]
- 13.Sorenson D R, Jackson W T. Planta. 1968;83:166–170. doi: 10.1007/BF00385021. [DOI] [PubMed] [Google Scholar]
- 14.Knight S E. Ecology. 1991;72:728–734. [Google Scholar]
- 15.Guisande C, Andrade C, Granado-Lorencio C, Duque S R, Núñez-Avellaneda M. Aquat Ecol. 2000;34:137–142. [Google Scholar]
- 16.Mandossian A J. Mich Bot. 1966;5:26–35. [Google Scholar]
- 17.Schnell D E. Carnivorous Plants of the United States and Canada. Winston-Salem, NC: John F. Blair; 1976. [Google Scholar]
- 18.Taggart J B, McNally S F, Sharp P M. Heredity. 1990;64:177–184. [Google Scholar]
- 19.Arber A. Ann Bot. 1941;5:563–578. [Google Scholar]
- 20.Addicott J F. Ecology. 1974;55:475–492. [Google Scholar]
- 21.Plummer G, Kethley J B. Bot Gaz. 1964;125:245–260. [Google Scholar]
- 22.Newell S J, Nastase A J. Am J Bot. 1998;85:88–91. [PubMed] [Google Scholar]
- 23.Siegel D I, Glaser P H. J Ecol. 1987;75:743–754. [Google Scholar]
- 24.Bledzki L A, Ellison A M. Hydrobiologia. 1998;385:193–200. [Google Scholar]
- 25.Moizuk G A, Livingston R B. Ecology. 1966;47:942–950. [Google Scholar]
- 26.Bedford B L, Walbridge M R, Aldous A. Ecology. 1999;80:2151–2169. [Google Scholar]
- 27.Chapin C T, Pastor J. Can J Bot. 1995;73:728–734. [Google Scholar]
- 28. Gotelli, N. J. & Ellison, A. M. (2002) Ecology82.
- 29.Trinca L A, Gilmour S G. Comp Stat Data Anal. 2000;33:25–43. [Google Scholar]
- 30.Aerts R, Berendse F, Caluwe H, Schmitz M. Oikos. 1990;57:310–318. [Google Scholar]
- 31.Johnson L C, Shaver G R, Cades D H, Rastetter E, Nadelhoffer K, Giblin A, Laundre J, Stanley A. Ecology. 2000;81:453–469. [Google Scholar]
- 32.Schwartz S E. Science. 1989;243:753–763. doi: 10.1126/science.243.4892.753. [DOI] [PubMed] [Google Scholar]
- 33.Ollinger S V, Aber J D, Lovett G M, Millham S E, Lathrop R G, Ellis J M. Ecol Appl. 1993;3:459–472. doi: 10.2307/1941915. [DOI] [PubMed] [Google Scholar]
- 34.Kearsley J. Non-Forested Acidic Peatlands of Massachusetts: A Statewide Inventory and Vegetation Classification. Westborough, MA: Natural Heritage and Endangered Species Program; 1999. [Google Scholar]
- 35. Gotelli, N. J. & Ellison, A. M. (2002) Ecology82.
- 36.Gorham E, Eisenreich S J, Ford J, Santelman M V. In: in Chemical Processes in Lakes. Stumm W, editor. New York: Wiley; 1985. pp. 339–363. [Google Scholar]
- 37.Stumm W, Morgan J J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. New York: Wiley; 1996. [Google Scholar]
- 38.APHA. Standard Methods for the Examination of Water and Wastewater. Washington, DC: American Public Health Association; 1985. [Google Scholar]
- 39.Schulze W, Schulze E-D, Pate J S, Gillison A N. Oecologia. 1997;112:464–471. doi: 10.1007/s004420050333. [DOI] [PubMed] [Google Scholar]
- 40.Zamora R, Gomez J M, Hodar J A. Oecologia. 1997;111:443–451. doi: 10.1007/s004420050257. [DOI] [PubMed] [Google Scholar]
- 41.Mendéz M, Karlsson P S. Oikos. 1999;86:105–112. [Google Scholar]
- 42.Givnish T J. In: Plant–Animal Interactions. Abramson W G, editor. Toronto: McGraw-Hill; 1989. pp. 243–290. [Google Scholar]