Abstract
Mercury accumulation in fish is a global public health concern, because fish are the primary source of toxic methylmercury to humans. Fish from all lakes do not pose the same level of risk to consumers. One of the most intriguing patterns is that potentially dangerous mercury concentrations can be found in fish from clear, oligotrophic lakes whereas fish from greener, eutrophic lakes often carry less mercury. In this study, we experimentally tested the hypothesis that increasing algal biomass reduces mercury accumulation at higher trophic levels through the dilution of mercury in consumed algal cells. Under bloom dilution, as algal biomass increases, the concentration of mercury per cell decreases, resulting in a lower dietary input to grazers and reduced bioaccumulation in algal-rich eutrophic systems. To test this hypothesis, we added enriched stable isotopes of Hg to experimental mesocosms and measured the uptake of toxic methylmercury (CH3200Hg+) and inorganic 201Hg2+ by biota at several algal concentrations. We reduced absolute spike detection limits by 50–100 times compared with previous techniques, which allowed us to conduct experiments at the extremely low aqueous Hg concentrations that are typical of natural systems. We found that increasing algae reduced CH3Hg+ concentrations in zooplankton 2–3-fold. Bloom dilution may provide a mechanistic explanation for lower CH3Hg+ accumulation by zooplankton and fish in algal-rich relative to algal-poor systems.
Nutrient enrichment with subsequent eutrophication is one of the most important problems impacting lakes worldwide (1, 2). Increased nutrient concentrations produce algal blooms, which in turn alter concentrations of nutrients, gases, pH, and metal ions in the water (3). It is our hypothesis that by increasing algal abundance, nutrient enrichment also alters Hg inputs to lake food webs. Mercury concentrations in fish have been related to metal burdens in their zooplankton prey (4–8), but the connection between Hg accumulation by zooplankton and increasing algal density under nutrient enrichment has not been established. It is critical to discern this association because algae can concentrate Hg from the aqueous phase (e.g., by 100–10,000+ times) and thus provide the greatest inputs of Hg to the food chain (9, 10). Here we report how an induced algal bloom affects the accumulation of methyl and inorganic Hg in the cladoceran Daphnia after 2 and 3 weeks of grazing on algae labeled with stable isotopes of Hg. Daphnia is a common zooplankton herbivore and known to be a major food for planktivorous fish (11), therefore factors affecting Hg burdens in this “keystone” (12, 13) prey taxon may have important ramifications for predicting CH3Hg+ burdens in fish across lakes of varying trophic status.
We experimentally tested the hypothesis that at equal initial concentrations of aqueous Hg, an increase in algae will result in a decrease in Hg uptake—by zooplankton grazers. Our rationale for this hypothesis was that the concentration of metal per cell would be lower in dense algal blooms (hereafter, bloom dilution) because the same amount of metal would be distributed among a greater number of algal cells. A related but different phenomenon, growth biodilution of trace metals, is observed in rapidly growing phytoplankton, whereby biomass-specific concentrations of metal diminish as cells divide (14). How either process of dilution with the phytoplankton affects the zooplankton, however, is not known. Possible bloom dilution has been observed for polychlorinated biphenyls (15, 16), As (17), Po, Cd, and Co (18) but has not been reported for Hg. To our knowledge, this is the first experimental manipulation to test bloom dilution in freshwater plankton.
Materials and Methods
Preparation of Algal Density Gradient.
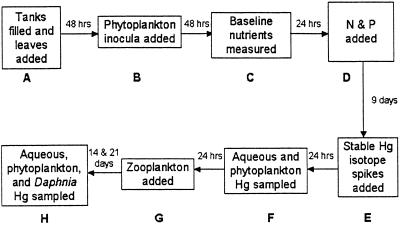
To test for effects of algal density on mercury accumulation in algae and on Daphnia subsequently grazing on those algae, 12 mesocosm stock tanks were used. The 550-liter resin tanks were scrubbed clean with a low detergent, low trace metal soap, rinsed, and then filled with approximately 450 liters of low ionic-strength water from a crystalline bedrock well. Samples of well water were first analyzed for trace metals by means of magnetic sector inductively coupled plasma-MS to ensure that the well water was low in metals and there were no significant differences between tanks (P.C.P., unpublished data). To buffer the systems from fluctuations in pH and to provide an adequate microbial community, 50 g (wet weight) of leaves (locally collected Fagus grandifolia, Betula papyrifera, Acer saccharum, and Quercus rubra) were added to each tank (Fig. 1A). Tanks were covered securely with fiberglass window screening to reduce unwanted colonization by invertebrates and to minimize airborne nutrient inputs. Water in the tanks was equilibrated with the atmosphere for 48 h before further additions. Each tank was then inoculated with phytoplankton and microzooplankton, by adding 3 liters of 48 μm of filtered Post Pond (Lyme, NH) water (Fig. 1B). Baseline nitrogen and phosphorus were measured after phytoplankton had been in the tanks for 48 h (Fig. 1C). Twenty-four hours after baseline nutrient measurements, tanks were randomly assigned to one of six nutrient levels with two tanks at each level. The lowest phosphorus level was 7.4 μg of P⋅liter−1 with inorganic nutrients doubling at each of the subsequent nutrient levels to a maximum of 44.6 μg of P⋅liter−1 at level six. Additions of nitrogen and phosphorus in the form of dissolved NaNO3 and K2HPO4 (2.51 and 36.72 g⋅liter−1, respectively) were made so as to achieve the desired atomic ratio of 30:1 (N:P) (Fig. 1D). Phosphorus concentrations added to the tanks corresponded to concentrations found routinely in lakes in the northeastern U.S. (19). Standing stocks of phytoplankton within the 12 tanks were left to develop for 9 days after the application of the 6 inorganic nutrient levels (Fig. 1 D and E).
Figure 1.
Chronology of mesocosm tank experiments. Time intervals given between boxes indicate time elapsed between the respective procedures. Note that the sampling described in H was conducted at two separate periods after zooplankton addition (G).
Adding Hg Isotopes and Zooplankton.
On day 14 (Fig. 1E), stable isotopes were added to the tanks. A stock solution of 50 mg⋅liter−1 enriched 201Hg (Oakridge National Laboratory, 98.11% 201Hg) was prepared in 0.01 M HCl. Enriched monomethylmercury, CH3200Hg+ (Oakridge National Laboratory, 96.41% 200Hg), was synthesized by methylating 200HgCl2 with methylcobalamin (20). After extraction with CH2Cl2 and back extraction into dilute HCl, a stock solution of 8 mg⋅liter−1 CH3200HgCl in 0.01 M HCl was made. Of the 201HgCl2 and CH3200HgCl stock solutions, 1.00 and 1.25 ml, respectively, were added and thoroughly mixed with a wooden paddle to each of the 12 tanks to achieve an initial tank water concentration of 100 ng⋅liter−1 201Hg and 20 ng⋅liter−1 CH3200Hg (Fig. 1E). Forty-eight hours after the stable isotope spikes, macrozooplankton collected from Post Pond with an 80-μm net were added at approximately 2 times the natural density to allow for mortality in transition.
Tank Monitoring.
Physical conditions in all of the tanks were monitored throughout the experiments. Specific conductivity, dissolved oxygen, temperature, and tank water pH were measured every 48 h between 13:00 and 15:00. By the addition of small volumes of dilute (2.0 M) H2SO4, the pH was maintained between 7.8 and 8.2 for all tanks. Samples for phytoplankton biomass (by means of chlorophyll a samples) were collected 24 h after mercury spike additions (Fig. 1F) and at the two zooplankton sampling periods (Fig. 1H). Samples for zooplankton taxonomy, density, length, and biomass were also collected when zooplankton were sampled for Hg (Fig. 1H).
Collection and Inductively Coupled Plasma (ICP)-MS Analyses of Isotope Samples.
The isotope spike analyses were performed by continuous-flow cold-vapor generation magnetic sector-ICP-MS (8, 21–23). Collection and digestion of samples for CH3200Hg+ and 201Hg2+ in water, particulates, and zooplankton were conducted as follows. Sampling equipment and sample vials were acid-cleaned in sequential 1 M nitric acid, 1:5 hydrochloric acid, and trace metal-grade (distilled) dilute nitric acid with ultra-pure water rinses before and after each acid bath (8). Aqueous mercury samples were collected in borosilicate glass vials with Teflon septa and preserved to ≈pH 1 with Seastar Baseline HNO3 (Seastar Chemicals, Sidney, BC, Canada). Particulate samples (particles >0.45 μm and <45 μm) were collected by filtering 100 ml of tank water on to cellulose acetate filters that had been rinsed with dilute (≈0.33 M) distilled nitric acid and ultra-clean water. Cellulose acetate filters with sample were immediately transferred to Teflon vials. Aqueous and particulate samples were collected 24 h after metal spike additions (Fig. 1F) and again when live zooplankton were sampled (Fig. 1H). Live zooplankton were field-sorted into Teflon vials under a dissecting scope 2 and 3 weeks after metal spike additions (Fig. 1H). Daphnia mercury burdens were calculated for two tanks at each respective treatment level with two samples (10–20 Daphnia mendotae) from each tank. All samples were stored in the dark at ≈4°C before digestion and analysis. Particulate and zooplankton samples were digested for 10–12 h at 70°C with a mixture of HNO3 and HCl (2:1; Seastar Baseline acids). Acidified water samples were not digested further (8).
The quantification of the enriched isotope spikes of 200Hg and 201Hg was performed by standard-sample-standard bracketing with certified external Hg standards of natural isotopic abundance. The natural background of 200Hg and 201Hg was subtracted based on the measured 198Hg/200Hg and 198Hg/201Hg ratio of the bracketing standards. The external calibration of the 200Hg and 201Hg spike concentrations was based on the atomic mass fraction of 200Hg and 201Hg in the natural abundance standards (46.24 g 200Hg⋅mol−1 Hg and 26.54 g 201Hg⋅mol−1 Hg). The procedural detection limits by isotope dilution were a function of the precision of the isotope ratio measurements (about 0.1%) and the background concentrations. Our method allows for the unambiguous tracking of picograms/femtomols of CH3Hg+ and Hg(II) from aqueous spikes into algae and zooplankton.
Detection Limits.
Twenty-four hours after the stable isotope additions, aqueous Hg concentrations were close to our method detection limits for water samples (0.5 ng⋅liter−1 for 200Hg and 201Hg). These extremely low aqueous Hg concentrations met our goal of conducting experiments at dilute concentrations typical of most lakes (8). We achieved detection limits of the isotopically labeled Hg species for the particulate and zooplankton samples for 200Hg and 201Hg of 1 ng⋅liter−1 or 0.5 pg, respectively, which is a 50–100-fold improvement over traditional analytical techniques using additions of isotopically unlabeled Hg or radioactive Hg tracers (8, 24).
Statistical Analyses.
We adopted a gradient approach with our mesocosm experiments wherein we traded off lower replication at each treatment level (n = 2) in favor of increasing the number of treatment levels (n = 6). This design is intended for regression analysis and allows for a more robust examination of trends and overall effects of a treatment in the face of high variation within treatments. This gradient approach was ideal for our goal to identify the general direction and magnitude of nutrient addition and increasing algal biomass effects on mercury uptake by grazers. The strength and generality afforded by this approach to ascertain the overall effect of treatments on specific dependent variables has made it a common approach for experiments involving ecological gradients (25, 26). Treatment effects were assessed by means of regression analysis [F test comparison of model mean squares divided by error mean squares, jmp (version 4.04, SAS Institute, Cary, NC)]. Least squares regression lines and 95% confidence intervals are plotted for variables only when the relationship is significant at the P ≤ 0.05 level.
Results and Discussion
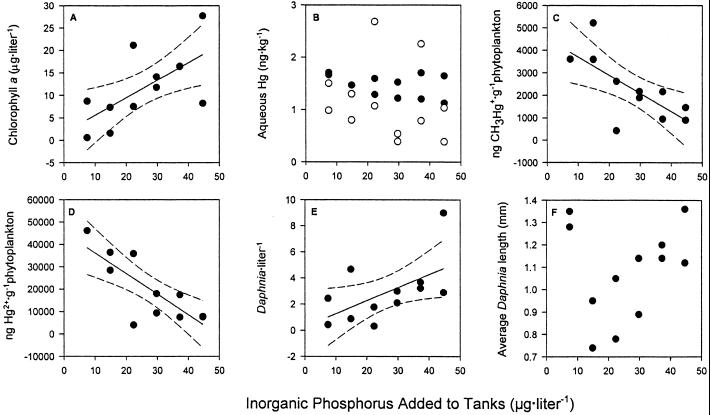
As expected, 9 days after the inorganic nutrient gradient was applied to the mesocosms there were significant differences in standing algal biomass measured as chlorophyll a (Fig. 2A) across tanks. Temperature, conductivity, and pH did not vary across treatments although there was a significant increase (R2 = 0.17, P < 0.0001) in dissolved oxygen at higher nutrient concentrations as expected with increased algal density (P.C.P., unpublished data). The conditions in the tanks at the time of zooplankton addition (11 days after inorganic nutrient additions as per Fig. 1) were well within the range of conditions experienced in the pelagic water of oligotrophic to mesotrophic lakes in temperate North America (7, 19).
Figure 2.
Effect of added phosphorus on chlorophyll a concentrations at the time of the metal spike additions (A). n = 12, chlorophyll a = 0.389(μg P added⋅liter−1) + 1.69, R2 = 0.431, P < 0.021. Aqueous concentrations of CH3200Hg+ (●) and 201Hg2+ (○) 24 h after additions to experimental tanks (B). For aqueous CH3200Hg+: n = 12, CH3Hg+ = −0.005(μg P added⋅liter−1) + 1.59, R2 = 0.102, P > 0.311. For 201Hg2+: n = 12, Hg2+ = −0.010(μg P added⋅liter−1) + 1.41, R2 = 0.035, P > 0.558. Effect of nutrient additions to CH3Hg+ (C) and Hg2+ (D) associated with algal biomass 24 h after metal spike additions. For CH3Hg+ (C): n = 11, CH3Hg+ = −80.14(μg P added⋅liter−1) + 4502, R2 = 0.499, P < 0.016. For Hg2+ (D): n = 11, Hg2+ = −917(μg P added⋅liter−1) + 45290, R2 = 0.623, P < 0.004. Algal biomass derived from chlorophyll a concentrations at time of metal spikes, assumes 1.25% of algal biomass is chlorophyll a (27). For both C and D an outlier from the lowest phosphorus addition level was excluded from regression analyses—in each case, the measured value exceeded 1 SD from a 0.99 confidence interval. Effect of nutrient additions on adult Daphnia density 3 weeks after metal spikes (E): n = 12, Daphnia⋅liter−1 = 0.74(μg P added⋅liter−1) + 0.27, R2 = 0.323, P < 0.054. Effect of nutrient additions on the mean length of adult Daphnia 3 weeks after metal spikes (F): n = 12, mean Daphnia length = 0.02(μg P added⋅liter−1) + 1.01, R2 = 0.030, P > 0.59. Total nitrogen and total phosphorus addition were kept at the atomic ratio of 30:1 as described in Materials and Methods. The 95% confidence intervals (---) are plotted for significant regressions.
Our first important finding was that at the time of zooplankton addition there was considerable bloom dilution of the Hg spikes under reasonable levels of nutrient enrichment. Twenty-four hours after the mercury spikes were added there was no detectable difference in aqueous Hg concentrations across tanks (Fig. 2B). Yet organic CH3Hg+ and inorganic Hg2+spike concentrations in particulates were 103-104 times greater than in the water after 24 h of exposure to the isotope spikes (Fig. 2 C and D), demonstrating the rapid and successful incorporation of the isotope spikes into algal biomass. Moreover, there were significant differences in total algal Hg measured across the nutrient gradient after 24 h (Fig. 2 C and D). In general, tanks with greater nutrient enrichment had greater algal biomass and lower Hg per gram of algal material (Fig. 2 A, C, and D). This evidence is a sound demonstration of bloom dilution.
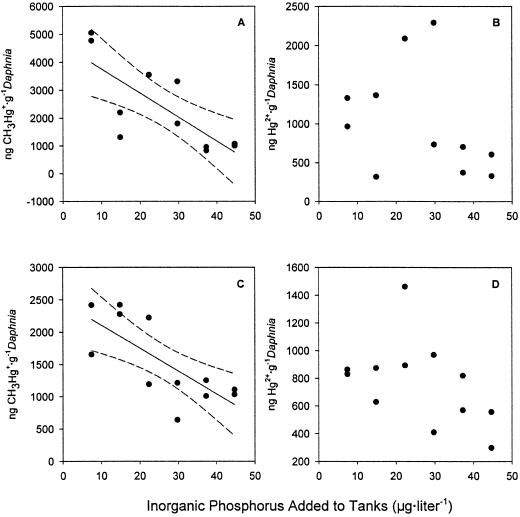
Our second major finding was that as hypothesized, bloom dilution of Hg in algae initiated different mercury uptake dynamics in the zooplankton under high- vs. low-nutrient enrichment. Specifically, methylmercury concentrations were consistently and significantly lower in Daphnia from the high nutrient, high initial algal biomass tanks compared with Daphnia from the low nutrient, and low initial algal biomass tanks at 2 and 3 weeks after zooplankton additions (Fig. 3 A and C). Correspondingly, low algal abundances resulted in a 2–3-fold increase in the accumulation of CH3Hg+ in Daphnia from low-nutrient tanks (Fig. 3 A and C). From these results, we infer that the concentration of CH3Hg+ in Daphnia across treatments was related to the concentration of CH3Hg+ (Fig. 2C) in the algal cells they ingested, which was in turn affected by algal biomass; e.g., that bloom dilution drives a diminution of metal in the zooplankton. We also observed similar results for effects of bloom dilution on calanoid and cyclopoid copepods (P.C.P., unpublished data). This result has important implications for trophic transfer of toxic CH3Hg+ to fish in oligotrophic lakes.
Figure 3.
Mean CH3Hg+ (A) and Hg2+ (B) concentration in Daphnia (g−1 dry weight Daphnia) against phosphorus addition (as per Materials and Methods) 2 weeks after zooplankton were added to the tanks. For CH3Hg+: n = 12, CH3Hg+ g−1 = −643(μg P added⋅liter−1) + 4630, R2 = 0.583, P < 0.0063. For Hg2+: n = 12, Hg2+ g−1 = −125.4(μg P added⋅liter−1) + 1455, R2 = 0.115, P > 0.306. Mean CH3Hg+ (C) and Hg2+ (D) concentration in Daphnia with nutrient addition 3 weeks after zooplankton were added to the tanks. For CH3Hg+: n = 12, CH3Hg+ g−1 = −265(μg P added⋅liter−1) + 2465, R2 = 0.554, P < 0.0056. For Hg2+: n = 12, Hg2+ g−1 = −78.7(μg P added⋅liter−1) + 1041, R2 = 0.213, P > 0.130. The 95% confidence intervals (---) are plotted for significant regressions.
Despite the highly significant relationships measured in Daphnia CH3Hg+ burdens across the nutrient gradient, there is a substantial amount of unexplained variation in our data. Varying Daphnia ages, feeding rates, the number of developing embryos in Daphnia brood pouches, or possible genetic differences are possible factors contributing to this unaccounted variance. Moreover, there are other possible explanations for our finding. For example, as hypothesized for rapidly growing algae [e.g., growth biodilution (14)], a diminution of the mass-specific metal spike in animals could result whenever there are rapid increases in zooplankton density or biomass (i.e., when the production of new tissue outpaces the uptake of metal). Growth biodilution cannot explain our results at 2 weeks because there were no differences in zooplankton density across treatments even though marked differences in methylmercury levels of individuals were evident. Growth biodilution did not occur by means of increases in body size either, because there were no significant body-size differences in Daphnia with increasing nutrient addition 2 and 3 weeks after spike additions (see Fig. 2F for lengths at week 3). However, 3 weeks after the zooplankton additions there was a marginally significant trend for lower methylmercury concentrations in treatments with higher Daphnia densities (Fig. 2E). This pattern provides some support for the hypothesis that growth biodilution leads to lower mass-specific CH3Hg+ in Daphnia at high density over time.
Finally our third significant finding was that unlike CH3Hg+, bloom dilution of inorganic Hg2+ concentrations in the algae (Fig. 2D) had no measurable influence on the accumulation of inorganic Hg2+ in Daphnia (Fig. 3 B and D). To our knowledge, this is the first study to experimentally demonstrate the preferential accumulation of CH3Hg+ relative to inorganic Hg2+ in grazing invertebrates feeding on an intact phytoplankton assemblage. Preferential accumulation of CH3Hg+ in zooplankton is reasonable to expect because zooplankton show the greatest assimilation rates of Hg from algal cytoplasm (28), where CH3Hg+ is concentrated in algal cells (6, 9, 10). In contrast, inorganic mercury tends to remain surface-bound and thus is less likely to be assimilated (10).
Our study did not include data for mercury accumulation by nonalgal particulate matter, which is known to be a significant Hg source to nonselective grazers such as Daphnia in some natural systems (29). In these experiments, the tanks were low in nonalgal particulates. Another important determinant of mercury cycling in aquatic systems that we did not quantify was the scavenging of mercury compounds by suspended particulate matter and detritus (30).
We conclude that CH3Hg+ transferred to grazing zooplankton, and eventually to fish and other vertebrates, will be influenced by nutrient pulses and algal blooms. More specifically, algae effectively and rapidly concentrate both inorganic and organic Hg, but the metal burden per cell decreases in algal blooms. Bloom dilution of CH3Hg+ in algae results in a substantial reduction of CH3Hg+ uptake by cladocerans in high-nutrient, high-algae conditions. Conversely, cladocerans feeding within low-nutrient, low-algae treatments accumulate more CH3Hg+. Further, zooplankton that graze on algae preferentially accumulate CH3Hg+ relative to inorganic Hg2+. This difference is instrumental in the efficient trophic transfer of CH3Hg+ relative to inorganic Hg to vertebrates. A final, unique feature of this research is demonstration of the value of using specific, stable isotope spikes of Hg to unambiguously track mercury through the food web near ambient concentrations. In particular, we tracked spikes of CH3Hg+ and inorganic Hg2+ and obtained exceptionally low absolute detection limits of those isotopic spikes (0.5–1 pg), which represents a significant improvement over traditional natural Hg or radioisotope methods.
Acknowledgments
We thank M. Kelley for assistance with mesocosm tank monitoring, zooplankton measurement, and acid washing laboratory and sampling gear. Additional thanks to L. Aucoin, K. Kronlein, B. Kennedy, C. Otto, and K. Feggestad for help with the mesocosm tanks; S. Glaholt for chlorophyll analyses; and M. Zens for statistical counsel. The manuscript benefited greatly from the comments of two anonymous referees. This work was supported by Superfund Basic Research Grant ES07373 (to C.L.F. and C.Y.C.) from the National Institute of Environmental Health Sciences.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Carpenter S R, Caraco N F, Correll D L, Howarth R W, Sharpley A N, Smith V H. Ecol Appl. 1998;8:559–568. [Google Scholar]
- 2.Smith V H, Tilman G D, Nekola J C. Environ Pollut. 1999;100:179–196. doi: 10.1016/s0269-7491(99)00091-3. [DOI] [PubMed] [Google Scholar]
- 3.Moore M V, Pace M L, Mather J R, Murdoch P S, Howarth R W, Folt C L, Chen C Y, Hemond H F, Flebbe P A, Driscoll C T. Hydrol Process. 1997;11:925–947. [Google Scholar]
- 4.Hall B D, Bodaly R A, Fudge R J P, Rudd J W M, Rosenberg D M. Water Air Soil Pollut. 1997;100:13–24. [Google Scholar]
- 5.Westcott K, Kalff J. Can J Fish Aquat Sci. 1996;53:2221–2228. [Google Scholar]
- 6.Lawson N M, Mason R P. Biogeochemistry. 1998;40:235–247. [Google Scholar]
- 7.Stemberger R S, Chen C Y. Can J Fish Aquat Sci. 1998;55:339–352. [Google Scholar]
- 8.Chen C Y, Stemberger R S, Klaue B, Blum J D, Pickhardt P C, Folt C L. Limnol Oceanogr. 2000;45:1525–1536. [Google Scholar]
- 9.Mason R P, Reinfelder J R, Morel F M M. Water Air Soil Pollut. 1995;80:915–921. [Google Scholar]
- 10.Mason R P, Reinfelder J R, Morel F M M. Environ Sci Technol. 1996;30:1835–1845. [Google Scholar]
- 11.Brooks J L, Dodson S I. Science. 1965;150:28–35. doi: 10.1126/science.150.3692.28. [DOI] [PubMed] [Google Scholar]
- 12.Ives A R, Carpenter S R, Dennis B. Ecology. 1999;80:1405–1421. [Google Scholar]
- 13.Mittelbach G G, Turner A M, Hall D J, Rettig J E, Osenberg C W. Ecology. 1995;76:2347–2360. [Google Scholar]
- 14.Sunda W G, Huntsman S A. Sci Total Environ. 1998;219:165–181. [Google Scholar]
- 15.Dachs J, Eisenreich S J, Hoff R M. Environ Sci Technol. 2000;34:1095–1102. [Google Scholar]
- 16.Larsson P, Okla L, Cronberg G. Can J Fish Aquat Sci. 1998;55:1926–1937. [Google Scholar]
- 17.Chen C Y, Folt C L. Environ Sci Technol. 2000;34:3878–3884. [Google Scholar]
- 18.Jeffree R A, Szymczak R. Environ Sci Technol. 2000;34:1966–1969. [Google Scholar]
- 19.Stemberger R S, Miller E K. Environ Monit Assess. 1998;51:29–51. [Google Scholar]
- 20.Imura N, Sukegawa E, Pan S-K, Nagao K, Kim J-Y, Kwan T, Ukita T. Science. 1971;172:1248–1249. doi: 10.1126/science.172.3989.1248. [DOI] [PubMed] [Google Scholar]
- 21.Klaue B, Blum J D. Anal Chem. 1999;71:1408–1414. doi: 10.1021/ac980846+. [DOI] [PubMed] [Google Scholar]
- 22.Klaue B, Kesler S E, Blum J D. In: 11th Annual International Conference on Heavy Metals in the Environment. Nriagu J, editor. Ann Arbor, MI: Univ. of Michigan, School of Public Health; 2000. , Contribution 1101. [Google Scholar]
- 23.Lauretta D, Klaue B, Blum J D, Buseck P R. Geochim Cosmochim Acta. 2001;65:2807–2818. [Google Scholar]
- 24.Hook S E, Fisher N S. Mar Biol. 2001;138:1131–1140. [Google Scholar]
- 25.Diehl S, Feissel M. Am Nat. 2000;155:200–218. doi: 10.1086/303319. [DOI] [PubMed] [Google Scholar]
- 26.Sarnelle O. Limnol Oceanogr. 1999;44:357–370. [Google Scholar]
- 27.Reynolds C S. The Ecology of Freshwater Phytoplankton. Cambridge, U.K.: Cambridge Univ. Press; 1984. pp. 37–39. [Google Scholar]
- 28.Reinfelder J R, Fisher N S. Science. 1991;251:794–796. doi: 10.1126/science.251.4995.794. [DOI] [PubMed] [Google Scholar]
- 29.Plourde Y, Lucotte M, Pichet P. Can J Fish Aquat Sci. 1997;54:821–831. [Google Scholar]
- 30.Montgomery S, Lucotte M, Cournoyer L. Sci Total Environ. 2000;261:33–41. doi: 10.1016/s0048-9697(00)00593-3. [DOI] [PubMed] [Google Scholar]