Abstract
Inactivation of the gene encoding the periplasmic protease DegP confers a high-temperature-sensitive phenotype in Escherichia coli. We have previously demonstrated that a degP mutant of E. coli strain CBM (W3110 pldA1) is not temperature sensitive and showed that this was most likely due to constitutive activation of the sigma E and Cpx extracytoplasmic stress regulons in the parent strain. In this study, further characterization of this strain revealed a previously unknown cryptic mutation that rescued the degP temperature-sensitive phenotype by inducing the extracytoplasmic stress regulons. We identified the cryptic mutation as an 11-bp deletion of nucleotides 1884 to 1894 of the adenylate cyclase-encoding cyaA gene (cyaAΔ11). The mechanism in which cyaAΔ11 induces the sigma E and Cpx regulons involves decreased activity of the mutant adenylate cyclase. Addition of exogenous cyclic AMP (cAMP) to the growth medium of a cyaAΔ11 mutant strain that contains a Cpx- and sigma E-inducible degP-lacZ reporter fusion decreased β-galactosidase expression to levels observed in a cyaA+ strain. We also found that a cyaA null mutant displayed even higher levels of extracytoplasmic stress regulon activation compared to a cyaAΔ11 mutant. Thus, we conclude that the lowered concentration of cAMP in cyaA mutants induces both sigma E and Cpx extracytoplasmic stress regulons and thereby rescues the degP temperature-sensitive phenotype.
Gram-negative bacteria such as Escherichia coli have developed a set of response regulons designed to maintain cell viability under a variety of stressful environmental conditions. Under conditions that increase the amount of misfolded or aggregated proteins in the periplasm, such as those generated under conditions of heat shock or the overproduction of outer membrane porins, a pair of regulons known as sigma E and Cpx are induced. These regulons prevent cell death by inducing the expression of genes that encode chaperones and proteases that function to refold or degrade misfolded proteins in the periplasm (36).
The sigma E extracytoplasmic stress response regulon initially described by Erickson and Gross (16) utilizes a regulated intramembrane proteolytic pathway (5) to transduce a stress signal from the periplasm to the cytoplasm, whereupon genes in the sigma E regulon are induced. A cascade of events initiating with detection of aberrant proteins in the periplasm results in the release of sigma E normally sequestered by the inner-membrane-spanning protein RseA to the cytoplasm. Release allows members of the sigma E regulon to be upregulated, including; rpoE, rseA, rseB, and rseC (14). Other sigma E-controlled genes include degP, which encodes the protease/chaperone DegP (46); fkpA, which encodes the peptidyl/prolyl isomerase FkpA (31); rpoH, which encodes σH of the cytoplasmic heat shock response regulon (16); and many others involved in performing basic cellular functions such as metabolism and phospholipid biosynthesis (37).
The Cpx regulon is a three-component regulatory system composed of an inner-membrane-spanning histidine kinase, CpxA; a periplasmic CpxA repressor, CpxP; and a cytoplasmic response regulator, CpxR. Under conditions that induce the Cpx regulon, such as elevated pH (33), absence of phosphatidylethanolamine in the cell envelope (29), or overexpression of envelope proteins such as outer membrane lipoprotein NlpE (43), inner membrane lipoprotein YafY (32), or pilus subunits (19, 21), induction of the Cpx regulon is initiated by activation of CpxA via removal of the periplasmic inhibitory protein CpxP (35). Once activated, CpxA phosphorylates and thereby activates the cytoplasmic transcription factor CpxR, which in turn directs the transcription of genes in the Cpx regulon. These genes include cpxP, cpxA, and cpxR (10, 15, 35) in addition to degP, ppiA, and ppiD, which encode periplasmic peptidyl-prolyl isomerases (12, 34), and dsbA, which encodes a periplasmic disulfide oxidoreductase (9).
DegP relieves the deleterious effects of misfolded proteins in the periplasm by acting as both a protease (27) and a chaperone, a dual function dependent upon the environmental temperature (44). The importance of DegP in E. coli is confirmed by the finding that degP transcription is induced by both the Cpx and sigma E regulons and that, in the absence of DegP, the cell exhibits a temperature-sensitive (ts) phenotype whereby it cannot grow at a temperature of 42°C or greater (26). Rescue of the ts phenotype can be accomplished by overexpression of the non-heat shock-inducible DegP homologue DegQ (47). This finding suggests that an overlapping function of proteases exists in the E. coli periplasm; however, degQ is not part of either extracytoplasmic stress regulon. The degP ts phenotype has also been shown to be rescued by extragenic expression of the sohA gene (1), which encodes a putative transcriptional regulator protein, by multicopy expression of sohB, which encodes a putative periplasmic serine protease (2), and by overproduction of membrane proteins such as NlpE (43) and certain outer membrane proteins, including OmpX, OmpC, and OmpF (28).
We previously reported that E. coli W3110 degP mutant cells were rescued from the ts phenotype when transduced with the pldA1 allele from strain CBM (7), which encodes inactive outer membrane phospholipase A (OMPLA) (25). We further showed that these transductants were induced for both sigma E and Cpx extracytoplasmic stress regulons. This result suggested that rescue of the ts phenotype was caused by a compensating effect of Cpx and sigma E regulon induction, a mechanism also involved in the rescue of the degP ts phenotype by overexpression of NlpE (11, 43) and YafY (32).
In the studies reported here, we identified a mutation closely linked with pldA1 in strain CBM. The mutation is an 11-bp deletion of the cyaA gene (cyaAΔ11) encoding the enzyme adenylate cyclase. We provide evidence that the lowered concentration of cyclic AMP (cAMP) resulting from the partial activity of the truncated adenylate cyclase encoded by cyaAΔ11 is responsible for induction of the sigma E and Cpx extracytoplasmic regulons and consequent rescue of the degP ts phenotype. Our results indicate that the altered expression of one or more cAMP receptor protein (CRP)-regulated genes likely induces the sigma E and Cpx regulons and serve to explain previous evidence obtained by Delaney (13) that describes the increased thermotolerance of cyaA mutants relative to cyaA+ strains.
MATERIALS AND METHODS
Media, growth conditions, and antibiotics.
All E. coli strains were grown in Luria-Bertani (LB) broth or agar at 30°C, 37°C, 41°C, or 42°C as specified. When required, medium was supplemented with kanamycin (50 μg/ml) and/or tetracycline (10 μg/ml). Strains were also grown on MacConkey agar and on MacConkey agar base supplemented with rhamnose (0.2%).
Bacterial strains.
The bacterial strains used in this study are listed in Table 1.
TABLE 1.
Bacterial strains used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| XL-1 blue | recA (recA1 lac endA1 gyrA96 thi-1 hsdR17 supE44 relA1 [F′ proAB lac1qZΔM15 Tn10]) | 6 |
| W3110 | IN(rrnD-rrnE)1 rph-1 | 20 |
| SP850 | relA1 spoT Δ(cya-1400)::Km thi-1 e14− λ− | 41 |
| GE1050 | MC4100 Δcrp::cam | 39 |
| CBM | W3110 pldA1 cyaAΔ11 | 7, this study |
| GL93 | CBM degP::kan | 25 |
| GL94 | W3110 degP::kan | 25 |
| GL101 | W3110 Δ(argF-lac)U169 | 25 |
| GL102 | CBM Δ(argF-lac)U169 | 25 |
| GL104 | GL101 degP::kan | 25 |
| GL111 | GL101 λRS88 (porfA-dsbA-lacZ) | 25 |
| GL112 | GL101 λRS88 (fkpA-lacZ) | 25 |
| GL113 | GL101 λRS88 (degP-lacZ) | 25 |
| GL123 | GL102 λRS88 (degP-lacZ) | 25 |
| GL123A | GL123 degP::tet | This study |
| GL143 | GL104 λRS88 (degP-lacZ) | 25 |
| GL306 | GL93 Δ(argF-lac)U169 nadA::Tn10 | This study |
| GL308 | GL94 Δ(argF-lac)U169 pldA::kan nadA::Tn10 | This study |
| GL383 | GL93 Δ(argF-lac)U169 pldA::kan (ME) cyaAΔ11 λRS88 (degP-lacZ) | This study |
| GL393 | GL94 Δ(argF-lac)U169 pldA1 (ME) λRS88 (degP-lacZ) | This study |
| GL111A | GL111 degP::tet | This study |
| GL112A | GL112 degP::tet | This study |
| TS2 | W3110 Δ(argF-lac)U169 metE::Tn10 λRS88 (degP-lacZ) | This study |
| TS2-1 | W3110 Δ(argF-lac)U169 λRS88 (degP-lacZ) | This study |
| TS2A | TS2 metE+degP::tet | This study |
| TS2AB | TS2 metE+degP::tet crp::cam | This study |
| TS3 | W3110 Δ(argF-lac)U169 degP::tet λRS88 (degP-lacZ) | This study |
| TS13 | TS2 cyaAΔ11 | This study |
| TS15 | TS2 metE+cyaA::Tn5 | This study |
| TS16 | TS2 metE+cyaA1400::kan | This study |
| TS18 | TS2 metE+cyaAΔ11 (ME) | This study |
| TS14A | TS2 metE+pldA::kan degP::tet | This study |
| TS15A | TS2 metE+cyaA::Tn5 degP::tet | This study |
| TS16A | TS2 metE+cyaA1400::kan degP::tet | This study |
| TS18A | TS2 metE+cyaAΔ11 (ME) degP::tet | This study |
| TS15AB | TS15A crp::cam | This study |
| TS16AB | TS16A crp::cam | This study |
| TS18AB | TS18A crp::cam | This study |
| TS21A | GL111 cyaA::Tn5 degP::tet | This study |
| TS22A | GL111 cyaA1400::kan degP::tet | This study |
| TS23A | GL111 cyaAΔ11 degP::tet | This study |
| TS31A | GL112 cyaA::Tn5 degP::tet | This study |
| TS32A | GL112 cyaA1400::kan degP::tet | This study |
| TS33A | GL112 cyaAΔ11 degP::tet | This study |
| TS40 | GL143 degP::tet | This study |
| TS41 | GL143 pldA1 degP::tet | This study |
| Plasmids | ||
| pBluescript II SK(+) | Cloning vector; Amprlac promoter (lacZα) f1 ColE1 | Stratagene |
| pMRS101 | Suicide vector; Strepr AmprsacBR oriE1 oriR6K mobRK2 | 40 |
β-Galactosidase assays.
Cultures were grown overnight at 30°C in LB broth with the appropriate antibiotic and then subcultured 1:125 into LB broth and grown until an optical density at 600 nm (OD600) between 0.3 and 0.5 was reached. Assays were performed according to Slauch et al. (42) whereby a 1-ml culture volume was permeabilized with 20 μl chloroform and 10 μl 0.1% sodium dodecyl sulfate. The resultant mixture was assayed for β-galactosidase activity (nanomoles per minute per milliliter per OD600 unit) by recording the change in OD420 over time upon addition of 100 μl of a permeabilized cell suspension to a reaction solution containing 10-mg/ml o-nitrophenyl-β-d-galactopyranoside. β-Galactosidase assay results are presented as the average activity of three or four transductants under the growth conditions specified. Error bars represent the standard deviation of these averages.
Measurement of growth under heat shock conditions.
Strains were grown overnight at 30°C in LB broth containing an appropriate antibiotic. They were then subcultured to an OD600 of 0.01 and incubated for 1 h at 30°C. Next, half of the volume of each culture was placed in a second Erlenmeyer flask. The duplicate cultures were incubated at 30°C and 42°C and the OD600 was assessed over time. The effect of exogenous cAMP on growth was determined as described above, except that following 1 h of incubation at 30°C, each culture was subcultured 1:2 into four flasks containing LB broth supplemented with 0, 2, 4, and 8 mM cAMP. Each culture was then incubated at 42°C, and the OD600 was monitored for 6 h.
Marker exchange mutagenesis.
Transfer of alleles by marker exchange was performed using the suicide vector pMRS101 (40). Alleles pldA1, pldA::kan, and cyaAΔ11 were amplified by PCR and cloned into pBluescript II SK(+). Following verification of the correct sequence, the alleles were subcloned into pMRS101 and electroporated into E. coli XL-1 Blue cells. The recombinant plasmids were digested with NotI to remove the pBR322 origin of replication of the plasmid, self-ligated, and transformed into E. coli SM10λpir by selecting for streptomycin resistance. Donor (SM10λpir containing the recombinant pMRS101) and recipient strains for conjugation were grown overnight, subcultured 1:10 in brain heart infusion (BHI) broth without antibiotic, and incubated at 37°C for 1 h prior to conjugation. The conjugation reaction was initiated by mixing a 500-μl volume of donor and recipient cells. Cells in the mixture were collected by centrifugation, and the pellet resuspended in 100 μl was applied to a prewarmed BHI plate and incubated for 3.5 h at 32°C. Half of the cells were scraped off the BHI conjugation pool plate and streaked onto LB agar containing streptomycin and tetracycline to select for potential integrants, which were then streak purified on the same medium. Selection of integrants in which the pMRS101 plasmid has been lost due to a second recombination event was accomplished by growth on LB agar containing 10% sucrose. Verification of allelic exchange was accomplished by PCR.
Transduction protocol.
Transductions were performed according to Miller (30).
RESULTS
Induction of the extracytoplasmic stress regulons in E. coli strain CBM is caused by a mutation near pldA.
In a previous paper by Langen et al. (25), extracytoplasmic stress regulons sigma E and Cpx were shown to be induced in E. coli strain CBM, which contains the pldA1 allele encoding a mutated form of OMPLA. In order to further examine the extracytoplasmic stress regulon-inducing effect of pldA1, the pldA null allele pldA20::kan (4) was used. We expected that the pldA null allele would induce the sigma E and Cpx extracytoplasmic stress regulons in a manner similar to that of nonfunctional OMPLA encoded by pldA1.
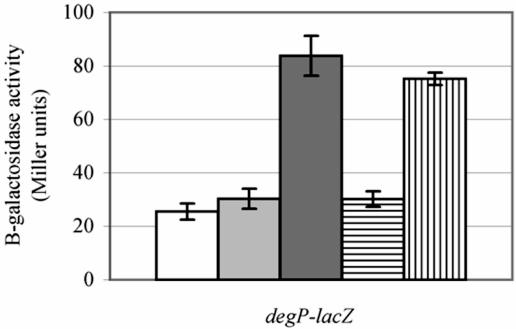
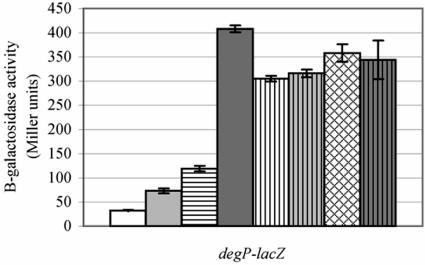
Induction of the sigma E and Cpx extracytoplasmic stress regulons was assessed by lacZ reporter fusion analysis whereby the amount of β-galactosidase activity generated from a chromosomal copy of the lacZ gene fused to the sigma E- and Cpx-inducible degP promoter was quantified. All strains were degP mutants and were grown under conditions of heat shock (41°C) because induction of the extracytoplasmic regulons had previously been shown to be the greatest at this temperature (25). The degP pldA20::kan mutant strain (TS14A) failed to induce the extracytoplasmic regulons to the same level as the degP pldA1 mutant strain (GL123A) (Fig. 1). Instead, the level of β-galactosidase activity generated by the pldA20::kan mutant strain was comparable to that of the pldA+ strain (GL143), suggesting the presence of a second previously unknown mutation in strain CBM. The existence of a cryptic mutation was verified by construction of two strains by marker exchange. In the first mutant, GL383, pldA20::kan replaced pldA1. In the second mutant, GL393, pldA1 replaced pldA20::kan. degP-lacZ reporter gene analysis showed that pldA1 in a non-CBM background (GL393) failed to induce the extracytoplasmic stress regulons, whereas the level of regulon activity in the CBM pldA20::kan mutant strain (GL383) remained high (Fig. 1).
FIG. 1.
An uncharacterized mutation is responsible for induction of the sigma E and/or Cpx regulons in strain CBM. degP mutant strains GL143 (pldA+; white bar), TS14A (pldA::kan; light gray bar), GL383 (pldA::kan mutant strain GL306 by marker exchange; dark gray bar), GL393 (pldA1 mutant strain GL308; horizontally striped bar), and GL123A (pldA1; vertically striped bar) were grown in LB broth at 41°C, and the amount of β-galactosidase activity generated from a degP-lacZ chromosomal fusion was assayed.
A second mutation in strain CBM is located in the adenylate cyclase gene cyaA.
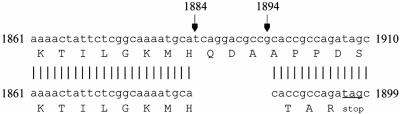
Using a three-factor cross with metE::Tn10 and pldA20::kan and the expression level of the degP-lacZ fusion, the unknown gene was mapped to a position upstream of pldA, distal to metE. The cryptic allele was then identified as a mutant cyaA gene by P1vir transduction mapping employing a set of strains containing Tn5 and Tn10 insertions in genes close to pldA. During this analysis, it was noted that both the cryptic allele and a cyaA::Tn5 allele conferred a white colony phenotype when the strains were grown on MacConkey agar containing various non-phosphotransferase system sugars, including rhamnose (data not shown). The mutant cyaA gene (cyaAΔ11) encoding the enzyme adenylate cyclase contains an 11-bp frameshift deletion of nucleotides 1884 to 1894 (Fig. 2). The protein encoded by cyaAΔ11 is truncated due to the introduction of a stop codon 4 codons downstream of the deletion in the open reading frame (Fig. 2). The resultant adenylate cyclase protein is 631 amino acids in length, 217 amino acids shorter than wild-type adenylate cyclase.
FIG. 2.
The cryptic allele in E. coli strain CBM is a frameshift mutation of cyaA. Comparative nucleotide sequence alignment of nucleotides 1861 to 1910 and the corresponding amino acids encoded by the cyaA+ and cyaAΔ11 ORFs. The cyaAΔ11 ORF has an 11-bp deletion of nucleotides 1884 to 1894. The resultant frameshift mutation generates a stop codon that truncates the adenylate cyclase by 217 amino acids.
The cyaAΔ11 mutation induces expression of the sigma E and Cpx extracytoplasmic stress regulons.
According to degP-lacZ reporter gene fusion analysis, the level of induction of degP in cyaAΔ11 mutant strains was greater than twofold higher than in cyaA+ strains, indicating that the cyaAΔ11 mutation induces the sigma E and/or Cpx extracytoplasmic stress regulons (Fig. 3A). To verify the sigma E and Cpx regulon-inducing effect of mutant adenylate cyclases, we utilized two additional cyaA mutant alleles, cyaA::Tn5 (23) and the cyaA null allele cyaA1400::kan (41) (Fig. 3A). The cyaA::Tn5 strain exhibited induction levels similar to that of cyaAΔ11, whereas the cyaA1400::kan null strain generated induction levels greater than five times that of cyaAΔ11.
FIG. 3.
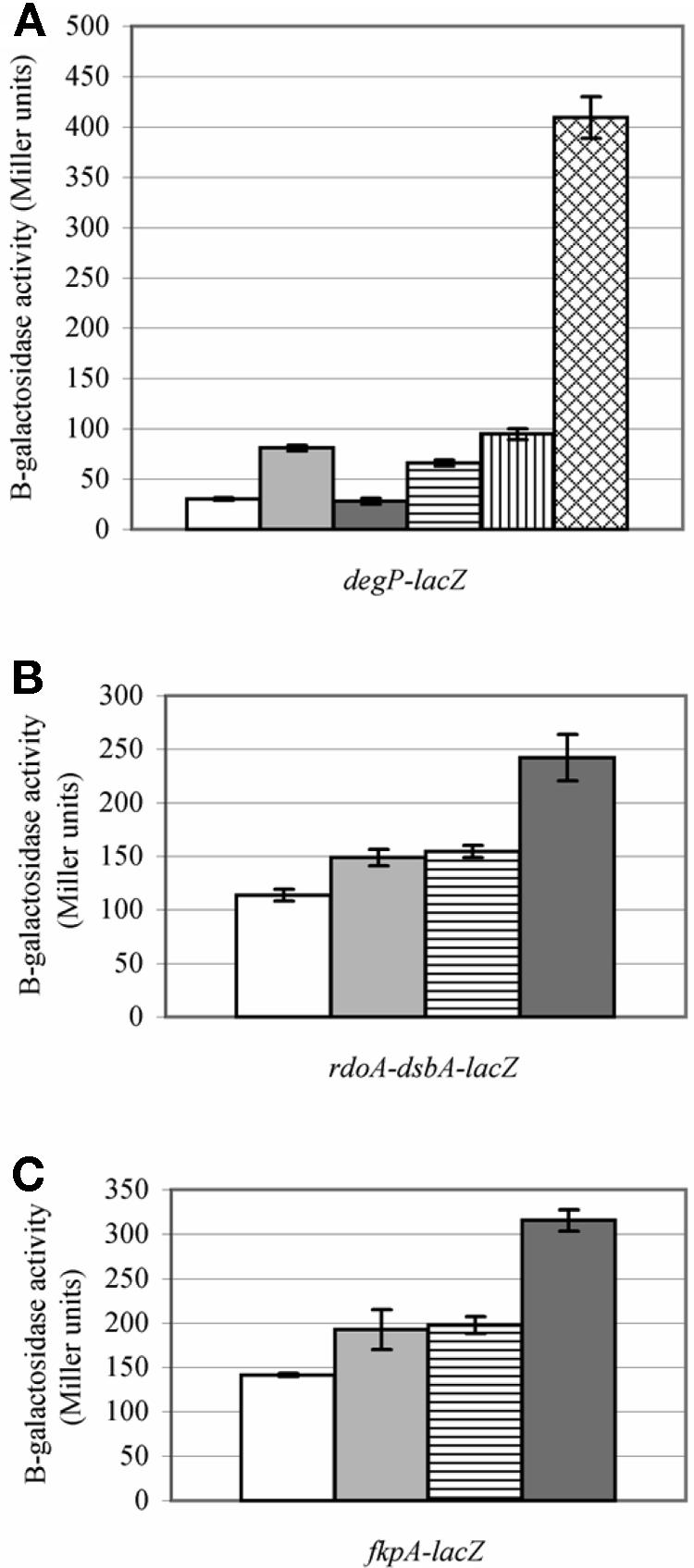
The cyaAΔ11 mutation induces both the sigma E and Cpx regulons. degP mutant strains that contained (A) a degP-lacZ gene reporter fusion, i.e., TS40 (pldA+; white bar), GL123A (pldA1 cyaAΔ11; light gray bar), TS41 (pldA1; dark gray bar), TS18A (cyaAΔ11; horizontally striped bar), TS15A (cyaA::Tn5; vertically striped bar), and TS16A (cyaA1400::kan; cross-hatched bar); (B) an rdoA-dsbA-lacZ gene reporter fusion, i.e., GL111A (cyaA+; white bar), TS23A (cyaAΔ11; light gray bar), TS23A (cyaA::Tn5; horizontally striped bar), and TS22A (cyaA1400::kan; dark gray bar); or (C) an fkpA-lacZ reporter fusion, i.e., GL112A (cyaA+; white bar), TS33A (cyaAΔ11; light gray bar), TS31A (cyaA::Tn5; horizontally striped bar), and TS32A (cyaA1400::kan; dark gray bar), were grown at 41°C, and the amount of β-galactosidase activity was assayed.
The mutant adenylate cyclases cause induction of both the sigma E and Cpx regulons, as shown by the increased β-galactosidase activity in cyaA mutant strains that contain lacZ reporter gene fusions under the control of either Cpx (rdoA-dsbA-lacZ) (3)- or sigma E (fkpA-lacZ) (9)-inducible promoters (Fig. 3B and C). As for the degP-lacZ fusion, the highest sigma E and Cpx regulon activity was generated in the cyaA1400::kan null strain.
The sigma E and Cpx regulon-inducing effect of cyaA mutations is not only apparent under conditions of high temperature (41°C) in a degP background. As shown in Fig. 4, cyaA mutations also induce the sigma E and Cpx regulons when cells are grown at 37°C in a degP+ background.
FIG. 4.
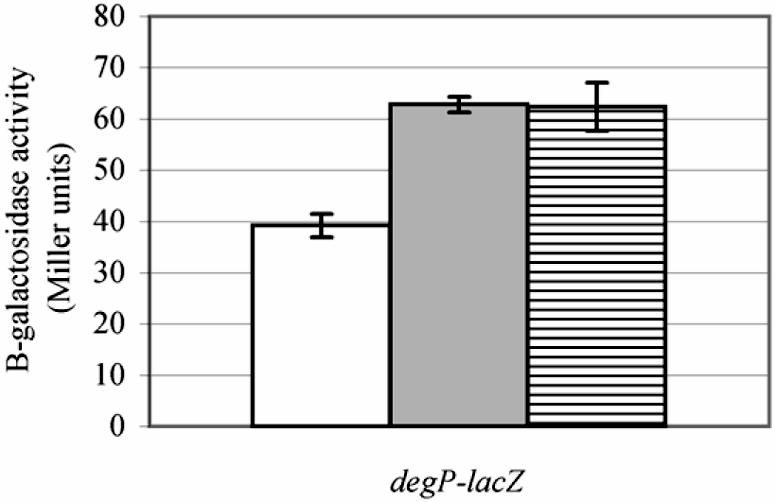
cyaA alleles induce the extracytoplasmic stress regulons in degP+ strains. The amount of β-galactosidase produced when bacteria are grown at 37°C was assayed in degP-lacZ reporter gene fusion-containing strains TS2-1 (cyaA+; white bar), TS18 (cyaAΔ11; light gray bar), and TS15 (cyaA::Tn5; horizontally striped bar).
Lowered intracellular concentrations of cAMP induce the sigma E and Cpx regulons by altered gene regulation involving CRP.
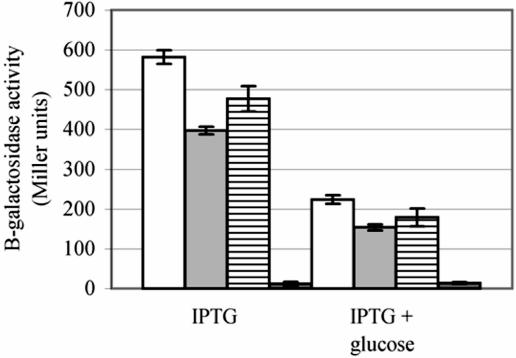
Reporter gene fusion analysis demonstrated that induction of the extracytoplasmic regulons was greatest in the cyaA null mutant. Since the cyaA1400::kan allele is a null mutation (41), this suggested that induction is inversely related to the concentration of cAMP in the cell. Therefore, we measured the relative levels of β-galactosidase activity encoded by the lacZ gene in cyaAΔ11, cyaA::Tn5, and cyaA1400::kan mutant strains compared to that in a cyaA+ strain when grown in the presence of high concentrations of the gratuitous inducer isopropyl-β-d-thiogalactopyranoside (IPTG). In the presence of IPTG, lacZ expression would be solely dependent upon the amount of CRP-cAMP activator. The β-galactosidase activity in cyaA::Tn5 and cyaAΔ11 mutant strains was less than that of the cyaA+ strain (Fig. 5), suggesting that the adenylate cyclases encoded by cyaA mutant alleles have a decreased capacity to produce cAMP compared to wild-type adenylate cyclase. As expected, the cyaA null strain exhibited an extremely low level of β-galactosidase activity.
FIG. 5.
Induced β-galactosidase levels are lower in strains containing cyaA mutant alleles but remain responsive to catabolite repression. W3110 derivative strains W3110 cyaA+ (white bars), W3110 cyaAΔ11 (light gray bars), W3110 cyaA::Tn5 (horizontally striped bars), and W3110 cyaA1400::kan (dark gray bars) were grown in LB broth containing 1 mM IPTG with or without 0.4% glucose at 37°C, and the amount of β-galactosidase was assayed.
It should be noted that although the results described above indicate that a reduction in the cAMP concentration resulting from the mutant adenylate cyclases induces the extracytoplasmic stress regulons, growth under catabolite repression conditions did not induce the regulons. In fact, growth at 37°C in LB medium containing 0.4% glucose did not alter stress levels in cyaA+ strains and decreased the induction of the extracytoplasmic stress regulons in cyaAΔ11 and cyaA::Tn5 mutant strains by approximately 25% (data not shown).
Even though they are apparently partially inactivated, the adenylate cyclases encoded by the cyaAΔ11 and cyaA::Tn5 alleles remain controlled by catabolite repression, as evidenced by lower levels of β-galactosidase in cyaA mutant strains grown in the presence of 1 mM IPTG and 0.4% glucose compared to IPTG alone (Fig. 5). The effect of glucose in the cyaAΔ11 and cyaA::Tn5 mutant strains was similar to the effect in a cyaA+ strain, such that the amount of β-galactosidase activity decreased approximately 64% in each strain when it was grown in the presence of 0.4% glucose.
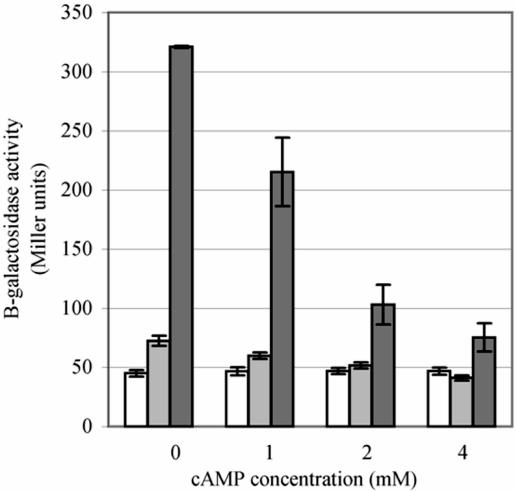
The inverse relationship between the level of cAMP in the cell and the induction level of the sigma E and Cpx regulons was also indicated by the finding that induction of the regulons can be suppressed by addition of cAMP to the growth medium. As shown in Fig. 6, the level of extracytoplasmic regulon activity as measured by the amount of β-galactosidase originating from the degP-lacZ reporter fusion was inversely related to the concentration of exogenous cAMP provided in the growth medium of the degP cyaAΔ11 and cyaA1400::kan mutant strains.
FIG. 6.
Extracytoplasmic stress regulon expression is lowered by addition of exogenous cAMP to the growth medium of cyaA mutant strains. Strains TS2-1 (cyaA+; white bars), TS13 (cyaAΔ11; light gray bars), and TS16 (cyaA1400::kan; dark gray bars) were grown in LB broth supplemented with 0, 1, 2, or 4 mM cAMP as indicated. Cultures were grown at 41°C, and the amount of β-galactosidase activity generated from a degP-lacZ chromosomal fusion was assayed.
If induction of extracytoplasmic stress by the mutant cyaA alleles takes place via the CRP-cAMP mechanism of transcriptional regulation, the absence of CRP should also affect the expression of the stress regulons. We found that the absence of CRP uniformly increased the expression of the degP-lacZ fusion to a level similar to that displayed by the cyaA null strain (Fig. 7). The involvement of CRP-cAMP, not cAMP alone, in induction of the regulons was verified by the finding that induction in a crp::cam null mutant could not be lowered by the addition of exogenous cAMP (data not shown).
FIG. 7.
The extracytoplasmic stress response regulons are strongly induced in the absence of CRP. degP cyaA crp mutant derivative strains were grown in LB broth at 41°C, and the amount of β-galactosidase activity was assayed. The levels of β-galactosidase generated in strains TS2A (cyaA+; white bar), TS18A (cyaAΔ11; light gray bar), TS15A (cyaA::Tn5; horizontally striped bar), TS16A (cyaA1400::kan; dark gray bar), TS2AB (cyaA+ crp::cam; white vertically striped bar), TS18AB (cyaAΔ11 crp::cam; light gray vertically striped bar), TS15AB (cyaA::Tn5 crp::cam; cross-hatched bar), and TS16AB (cyaA1400::kan crp::cam; dark gray vertically striped bar) are shown.
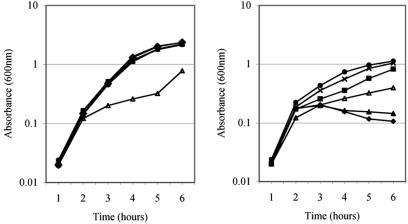
cyaA mutations rescue the degP ts phenotype but are prevented from doing so by the addition of exogenous cAMP.
We confirmed that cyaAΔ11-mediated induction of the extracytoplasmic stress regulons was responsible for rescue of the degP ts phenotype by a growth assay. In this assay, the ability of degP derivatives to grow at both 30°C and the nonpermissive temperature of 42°C was assessed by determining cell density over a 6-h growth period (Fig. 8). Strains which contained the cyaAΔ11 or cyaA::Tn5 allele grew best at 42°C. In contrast, pldA1 cyaA+, pldA::kan cyaA+, and pldA+ cyaA+ strains that were degP mutants grew very poorly, exhibiting a decrease in cell density after incubation at 42°C for 2 h.
FIG. 8.
Partial or complete inactivation of adenylate cyclase rescues the degP ts phenotype. Cell densities of degP mutant strains TS2A (pldA+ cyaA+; ♦), TS41 (pldA1; ▴), GL123A (pldA1 cyaAΔ11; ▪), TS18A (cyaAΔ11; •), TS15A (cyaA::Tn5; ×), and TS16A (cyaA1400::kan; ▵) grown in LB broth and incubated at (A) 30°C or (B) 42°C were determined.
The degP cyaA1400::kan mutant exhibited a greatly reduced growth rate in comparison with other cyaA mutant derivatives when incubated at both 30°C and 42°C. This result can be attributed to a complete lack of cAMP in the cell due to the absence of adenylate cyclase. The null strain has a “sickly” phenotype identifiable by small colony size when grown on various media, including LB agar and MacConkey agar, and a reduced growth rate in liquid medium. However, although it confers a poor-growth phenotype even under nonstress conditions, cyaA1400::kan rescues the degP ts phenotype. This is evident by a comparison of the growth curve of the cyaA1400::kan mutant strain with that of a cyaA+ strain. The cyaA null strain grew, albeit at a reduced rate, when incubated at both 30°C (Fig. 8A) and 42°C (Fig. 8B), whereas the cyaA+ degP mutant strain could not continue to grow following a 2-h incubation at 42°C.
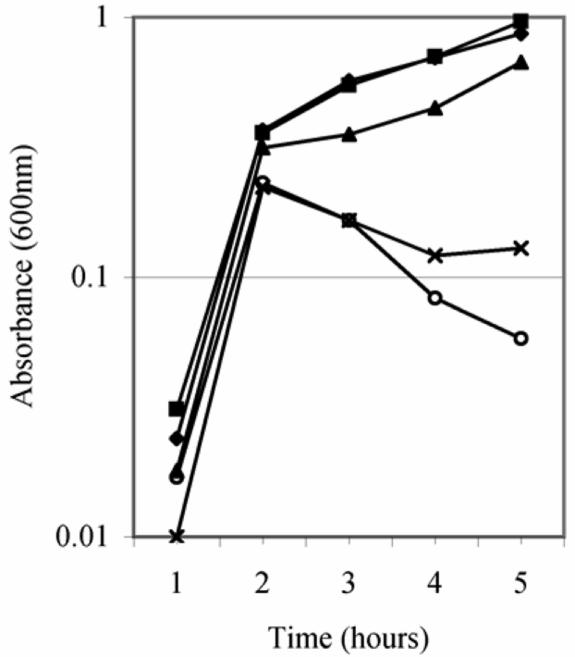
The results shown in Fig. 6 show that addition of exogenous cAMP to the growth medium of cyaA degP mutant strains decreases Cpx and sigma E induction levels in a dose-dependent manner. Since rescue of the degP ts phenotype is attributed to the induction of these regulons, we determined whether addition of exogenous cAMP to the growth medium would also affect rescue in a dose-dependent manner. degP cyaAΔ11 mutant strain TS18A exhibits a specific set of growth patterns when grown at 42°C in LB medium supplemented with increasing concentrations of exogenous cAMP (Fig. 9). The best growth was observed when the cells were grown in LB broth containing no or 1 mM cAMP. A decrease in the growth rate was observed in concentrations of exogenous cAMP greater than 1 mM. In fact, addition of 4 mM cAMP decreased the growth rate to a level near that observed for a degP cyaA+ mutant strain (Fig. 9). In control experiments, addition of exogenous cAMP did not affect the inability of a degP cyaA+ mutant strain to grow at nonpermissive temperature, nor did it alter the growth of a degP+ cyaA+ strain at 42°C (data not shown).
FIG. 9.
Addition of exogenous cAMP changes the growth profile of a degP cyaA mutant strain grown at 42°C. The cell density of a degP cyaAΔ11 mutant strain (TS18A) grown in LB broth supplemented with 0 (♦), 1 (▪), 2 (▴), or 4 (×) mM cAMP and incubated at 42°C was determined. The growth curve of cyaA+ degP mutant strain TS2A grown in the absence of exogenous cAMP (○) is shown for comparison.
DISCUSSION
E. coli responds to conditions that increase the amount of misfolded or aggregated proteins in the periplasm such as high temperature and the overproduction of outer membrane proteins, by inducing the sigma E and Cpx extracytoplasmic stress regulons (36). Members of these regulon families act as proteases, chaperones, and isomerases that function to degrade and refold aberrant periplasmic proteins. DegP is a periplasmic protease/chaperone whose expression is controlled by both the sigma E and Cpx regulons and whose activity is integral to the ability of the cell to remain viable at high temperature, as evidenced by the ts phenotype of a degP mutant (26, 27, 44, 45, 46). Rescue of the degP ts phenotype has been shown to involve the induction of both the Cpx and sigma E extracytoplasmic regulons, which presumably compensate for the lack of DegP by upregulating the expression of proteins with overlapping functions (25, 43). In this work, we show that rescue of the degP ts phenotype in E. coli strain CBM by induction of the extracytoplasmic stress regulons is accomplished by a decrease in the cAMP concentration, resulting from the decreased activity of adenylate cyclase encoded by a mutant cyaAΔ11 allele.
The cyaAΔ11 allele contains an 11-bp deletion of nucleotides 1884 to 1894 of the 2,544-nucleotide cyaA open reading frame (ORF) and generates a stop codon 4 codons from the site of the deletion (Fig. 2). The mutant gene thus encodes a 631-amino-acid protein that contains the entire catalytic domain composed of amino acids 82 to 341 (18) and only 30% of the putative C-terminal regulatory domain normally composed of amino acids 536 to 848 (38).
The adenylate cyclases encoded by cyaAΔ11 and a second mutant cyaA allele, cyaA::Tn5 (encodes amino acids 1 to 507), apparently have a reduced ability to produce cAMP compared to the wild-type enzyme but surprisingly retain the ability to be regulated by catabolite repression (Fig. 5). This suggests that the C-terminal domain is not involved in regulation by catabolite repression but instead is required for maximal adenylate cyclase activity. This result is difficult to reconcile with the proposal that the (C-terminal) regulatory domain is inhibitory to the catalytic domain (8) and that the regulatory domain is essential for catabolite repression by glucose (38).
Quantification of β-galactosidase activity originating from lacZ reporter gene fusions whose promoters (that do not contain consensus CRP-cAMP binding sites) are controlled by sigma E (fkpA, Fig. 3C), Cpx (rdoA-dsbA, Fig. 3B), or both (degP-lacZ, Fig. 3A) demonstrated the sigma E and Cpx regulon-inducing effect of mutant adenylate cyclase proteins. We propose that an inverse correlation exists between the magnitude of sigma E and Cpx regulon induction and the concentration of cAMP in the cell. That is, a cyaA+ strain should contain the highest concentration of cAMP (Fig. 5) and exhibits the lowest induction level of the extracytoplasmic stress regulons (Fig. 3 and 4). cyaAΔ11 and cyaA::Tn5 mutant strains generate higher levels of regulon induction compared to a cyaA+ strain. The cyaA1400::kan degP mutant strain does not produce any cAMP (17) and exhibits a highly elevated sigma E and Cpx induction level (Fig. 3). Therefore, these results suggested that it is a reduction of the cellular cAMP concentration that is responsible for induction of the extracytoplasmic stress response regulons, which in turn rescues the ts phenotype of the degP mutants. This conclusion is supported by the finding that the activity level of the regulons in cyaA mutant strains was reduced in a dose-dependent manner by addition of exogenous cAMP to the growth medium, while at the same time this addition compromised the ability of the cyaA mutant strains to rescue the degP ts phenotype (Fig. 6 and 9).
The most likely mechanism by which a decreased cAMP concentration induces the sigma E and Cpx regulons would involve an alteration of gene expression mediated by CRP-cAMP. cAMP acts as a second messenger in the cell whereby it is used to regulate transcription when complexed with CRP. In keeping with this hypothesis, we found that the absence of CRP causes high-level induction of the sigma E and Cpx regulons similar to that observed for the cyaA null mutant (Fig. 7).
Because there are a large number of metabolic processes regulated by CRP-cAMP, there are many candidate genes that could be responsible for the induction of the stress regulons in cyaA mutants. We do not yet know whether the decreased CRP-cAMP concentrations induce the sigma E and Cpx regulons by actually generating periplasmic stress; however, all other reported cases of suppression of the degP ts phenotype involve overexpression of either normal or aberrant envelope proteins (1, 2, 28, 43, 47). It is unlikely that direct effects on the expression of the cpx and rpoE genes are involved, since CRP sites have not been detected in the cpxR and rpoE promoters, and in any case increased expression of these regulators would not necessarily increase the expression of their regulons since they are held inactive at the inner membrane in the absence of periplasmic stress.
Changes in the expression of the responsible gene could also be indirectly caused by the decrease in CRP-cAMP concentrations. For example, the transcription levels of the cytoplasmic stress sigma factor genes rpoS and rpoH are repressed by cAMP (22, 24). It has been reported, however, that cytoplasmic stress responses were not elevated in cyaA mutants (13). The same study also found that despite the inability to generate a cytoplasmic heat shock response when grown at 42°C, the cyaA null strain exhibited increased thermoresistance compared to a cyaA+ strain. The author attributed this finding to the existence of an uncharacterized heat shock pathway, but the results presented here suggest it could have been due to induction of the extracytoplasmic stress response regulons.
It is interesting that in control experiments, growth in the presence of glucose did not cause increases in the extracytoplasmic stress regulon expression level, even though a decrease in cAMP levels in glucose-containing medium is a major mechanism of catabolite repression. This suggests that, in addition to CRP-cAMP, the gene whose altered expression generates periplasmic stress could also be regulated by the catabolite repressor/activator protein, which functions as a cAMP-independent mechanism of gene regulation.
In summary, the studies described here indicate that cyaA mutations rescue the ts phenotype of degP cells. Our results further indicate that this rescue results from induction of the Cpx and sigma E stress regulons and that this is caused by the effect of decreased cAMP concentrations on the CRP-cAMP regulon. Further experiments are required to identify the genes involved in induction of the stress response and the mechanisms involved in their homeostasis during cellular growth in various nutrient environments.
Acknowledgments
We thank Hughes Goldie, Jan Tommassen, Frederick R. Blattner, and George M. Weinstock for kindly providing strains used in this study.
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada to S.P.H.
REFERENCES
- 1.Baird, L., and C. Georgopoulos. 1990. Identification, cloning, and characterization of the Escherichia coli sohA gene, a suppressor of the htrA (degP) null phenotype. J. Bacteriol. 172:1587-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird, L., B. Lipinska, S. Raina, and C. Georgopoulos 1991. Identification of the Escherichia coli sohB gene, a multicopy suppressor of the HtrA (DegP) null phenotype. J. Bacteriol. 173:5763-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belin, P., and P. L. Boquet. 1994. The Escherichia coli dsbA gene is partly transcribed from the promoter of a weakly expressed upstream gene. Microbiology 140:3337-3348. [DOI] [PubMed] [Google Scholar]
- 4.Brok, R. G. P. M., E. Brinkman, R. van Boxtel, A. C. A. P. A. Bekkers, H. M. Verheij, and J. Tommassen. 1994. Molecular characterization of enterobacterial pldA genes encoding outer membrane phospholipase A. J. Bacteriol. 176:861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, M. S., R. B. Rawson, and J. L. Goldstein. 2000. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100:391-398. [DOI] [PubMed] [Google Scholar]
- 6.Bullock, W. O., J. M. Fernandez, and J. M. Short. 1987. XL1-Blue: A high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. BioTechniques 5:376-379. [Google Scholar]
- 7.Cavard, D., S. P. Howard, and C. Lazdunski. 1989. Functioning of the colicin A lysis protein is affected by Triton X-100, divalent cations and EDTA. J. Gen. Microbiol. 135:1715-1726. [DOI] [PubMed] [Google Scholar]
- 8.Crasnier, M., V. Dumay, and A. Danchin. 1994. The catalytic domain of Escherichia coli K-12 adenylate cyclase as revealed by deletion analysis of the cya gene. Mol. Gen. Genet. 243:409-416. [DOI] [PubMed] [Google Scholar]
- 9.Danese, P. N., and T. J. Silhavy. 1997. The σE and Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 11:1183-1193. [DOI] [PubMed] [Google Scholar]
- 10.Danese, P. N., and T. J. Silhavy. 1998. CpxP, a stress-combative member of the Cpx regulon. J. Bacteriol. 180:831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danese, P. N., W. B. Snyder, C. L. Cosma, L. J. B. Davis, and T. J. Silhavy. 1995. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 9:387-398. [DOI] [PubMed] [Google Scholar]
- 12.Dartigalongue, C., and S. Raina. 1998. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 17:3968-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delaney, J. M. 1990. A cya deletion mutant of Escherichia coli develops thermotolerance but does not exhibit a heat-shock response. Genet. Res. 55:1-6. [DOI] [PubMed] [Google Scholar]
- 14.De Las Penas, A. L., L. Connolly, and C. A. Gross. 1997. The sigma E mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of sigma E. Mol. Microbiol. 24:373-385. [DOI] [PubMed] [Google Scholar]
- 15.De Wulf, P., O. Kwon, and E. C. C. Lin. 1999. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J. Bacteriol. 181:6552-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson, J. W., and C. A. Gross. 1989. Identification of the sigma E subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes Dev. 3:1462-1471. [DOI] [PubMed] [Google Scholar]
- 17.Glaser, P., A. Roy, and A. Danchin. 1989. Molecular characterization of two cya mutations, cya-854 and cyaR1. J. Bacteriol. 171:5176-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holland, M. M., T. K. Leib, and J. A. Gerlt. 1988. Isolation and characterization of a small catalytic domain released from the adenylate cyclase from Escherichia coli by digestion with trypsin. J. Biol. Chem. 263:14661-14668. [PubMed] [Google Scholar]
- 19.Hung, D. L., T. L. Raivio, C. H. Jones, T. J. Silhavy, and S. J. Hultgren. 2001. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 20:1508-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen, K. F. 1993. The Escherichia coli K-12 “wild-types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J. Bacteriol. 175:3401-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, C. H., P. N. Danese, J. S. Pinkner, T. J. Silhavy, and S. J. Hultgren. 1997. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 16:6394-6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kallipolitis, B. H., and P. Valentin-Hansen. 1998. Transcription of rpoH, encoding the Escherichia coli heat-shock regulator σ32, is negatively controlled by the cAMP-CRP/CytR nucleoprotein complex. Mol. Microbiol. 29:1091-1099. [DOI] [PubMed] [Google Scholar]
- 23.Kang, Y., T. Durfee, J. D. Glasner, Y. Qiu, D. Frisch, K. M. Winterberg, and F. R. Blattner. 2004. Systematic mutagenesis of the Escherichia coli genome. J. Bacteriol. 186:4921-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lange, R., and R. Hengge-Aronis. 1994. The cellular concentration of the sigma S subunit of RNA-polymerase in Escherichia coli is controlled at the levels of transcription, translation and protein stability. Genes Dev. 8:1600-1612. [DOI] [PubMed] [Google Scholar]
- 25.Langen, G. R., J. R. Harper, T. J. Silhavy, and S. P. Howard. 2001. Absence of the outer membrane phospholipase A suppresses the temperature-sensitive phenotype of Escherichia coli degP mutants and induces the Cpx and σE extracytoplasmic stress responses. J. Bacteriol. 183:5230-5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipinska, B., O. Fayet, L. Baird, and C. Georgeopoulos. 1989. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for growth only at elevated temperatures. J. Bacteriol. 171:1574-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipinska, B., M. Zylicz, and C. Georgeopoulos. 1990. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J. Bacteriol. 172:1791-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mecsas, J., P. E. Rouviere, J. W. Erickson, T. J. Donohue, and C. A. Gross. 1993. The activity of σE, an Escherichia coli heat-inducible σ-factor, is modulated by expression of outer membrane proteins. Genes Dev. 7:2618-2628. [DOI] [PubMed] [Google Scholar]
- 29.Mileykosvskaya E., and W. Dowhan. 1997. The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J. Bacteriol. 179:1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Missiakas, D., J. M. Betton, and S. Raina. 1996. New components of protein folding in extracytoplasmic compartments of Escherichia coli SurA, FkpA and Skp/OmpH. Mol. Microbiol. 21:871-884. [DOI] [PubMed] [Google Scholar]
- 32.Miyadai, H., K. Tanaka-Matsuda, S. Matsuyama, and H. Tokuda. 2004. Effects of lipoprotein overproduction on the induction of DegP (HtrA) involved in quality control in the Escherichia coli periplasm. J. Biol. Chem. 279:39807-39813. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama S. I., and H. Watanabe. 1995. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J. Bacteriol. 177:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pogliano, J. A., S. Lynch, D. Belin, E. C. C. Lin, and J. Beckwith. 1997. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 11:1169-1182. [DOI] [PubMed] [Google Scholar]
- 35.Raivio, T. L., D. L. Popkin, and T. J. Silhavy. 1999. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J. Bacteriol. 181:5263-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raivio, T. L., and T. J. Silhavy. 2001. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55:591-624. [DOI] [PubMed] [Google Scholar]
- 37.Rezuchova, B., H. Miticka, D. Homerova, M. Roberts, and J. Kormanec. 2003. New members of the Escherichia coli sigma E regulon identified by a two-plasmid system. FEMS Microbiol. Lett. 225:1-7. [DOI] [PubMed] [Google Scholar]
- 38.Roy, A., A. Danchin, E. Joseph, and A. Ullmann. 1983. Two functional domains in adenylate cyclase of Escherichia coli. J. Mol. Biol. 165:197-202. [DOI] [PubMed] [Google Scholar]
- 39.Salles, B., and G. M. Weinstock. 1989. Interaction of the CRP-cAMP complex with the cea regulatory region. Mol. Gen. Genet. 215:537-542. [DOI] [PubMed] [Google Scholar]
- 40.Sarker, M. R., and G. R. Cornelis. 1997. An improved version of suicide vector pKNG101 for gene replacement in gram-negative bacteria. Mol. Microbiol. 23:410-411. [DOI] [PubMed] [Google Scholar]
- 41.Shah, S., and A. Peterkofsky. 1991. Characterization and generation of Escherichia coli adenylate cyclase deletion mutants. J. Bacteriol. 173:3238-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slauch, J. M., S. Garrett, D. E. Jackson, and T. J. Silhavy. 1988. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J. Bacteriol. 170:439-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder, W. B., L. J. Davis, P. N. Danese, C. L. Cosma, and T. J. Silhavy. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol. 177:4216-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spiess, C., A. Biel, and M. Ehrmann. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339-347. [DOI] [PubMed] [Google Scholar]
- 45.Strauch, K. L., and J. Beckwith. 1988. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc. Natl. Acad. Sci. USA 85:1576-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strauch, K. L., K. Johnson, and J. Beckwith. 1989. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J. Bacteriol. 171:2689-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waller, P. R. H., and R. T. Sauer. 1996. Characterization of degQ and degS, Escherichia coli genes encoding homologs of the DegP protease. J. Bacteriol. 178:1146-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]