Abstract
A deletion mutation in the gene rpoZ of Mycobacterium smegmatis causes reduced growth rate and a change in colony morphology. During purification of RNA polymerase from the mutant strain, the β′ subunit undergoes fragmentation but the fragments remain associated with the enzyme and maintain it in an active state until the whole destabilized assembly breaks down in the final step of purification. Complementation of the mutant strain with an integrated copy of the wild-type rpoZ brings back the wild-type colony morphology and improves the growth rate and activity of the enzyme, and the integrity of the β′ subunit remains unaffected.
DNA-dependent RNA polymerase (RNAP) is the central enzyme involved in gene expression and also constitutes a major target for genetic regulation (7, 8, 31). The bacterial RNAP core enzyme consists of four subunits: alpha (α), beta (β), beta′ (β′) and omega (ω) (21, 36). The ω subunit is the least well studied among all the subunits, though the subunit encoded by the rpoZ gene was proposed to be an integral part of the core RNAP several years ago (5, 10). In Escherichia coli ω is not found to be necessary for survival of the bacterium under laboratory conditions (12). At the same time ω homologues are present in the sequenced genomes of free-living bacteria, suggesting an important and conserved role for the protein (23).
It was identified in our laboratory that ω is required for the restoration of denatured core RNAP to its functionally active form (25). Further, we showed that the enzyme purified from an E. coli strain lacking ω recruits large amounts of GroEL (26) and removal of GroEL results in a completely inactive core RNAP which lacks the ability to even associate with σ70 (24). Subsequently, it was demonstrated that ω binds to the β′ subunit and promotes RNAP assembly by facilitating the association of β′ with the previous step of the assembly, α2β (13).
The X-ray crystal structure of Thermus aquaticus RNAP determined at 3.3 Å resolution (36) and subsequent analysis of the ω-β′ interface by Minakhin et al. (23) identified the conserved regions of β′ which ω binds with in a manner that reduces the configurational entropy of β′ and facilitates its interaction with the α2β subassembly. Further experiments in our laboratory showed that the C-terminal tail of the ω subunit is constrained in the presence of β′ (14).
In spite of this well established evidence, a number of recent observations about the ω subunit in different organisms warrant suspecting functional roles for this protein which are not clearly elucidated yet. In Streptomyces kasugaensis, it was observed that a mutation in the gene encoding the ω subunit resulted in characteristic pleiotropic effects (19). The Kranz laboratory recently demonstrated that presence of ω was a prerequisite to obtain an active in vitro assembly of Rhodobacter capsulatus RNAP (30). In a completely different scenario, Periago et al. (29) demonstrated induction of YloH, the ω subunit of Bacillus cereus, by heat stress, suggesting a role for the subunit in stress adaptation of the transcription machinery.
In the present work we have tried to look into the role of ω in Mycobacterium smegmatis, which is used as a model organism to investigate basic mycobacterial biology. The gene rpoZ, encoding the ω subunit in M. smegmatis, was identified by comparing the Mycobacterium tuberculosis rpoZ sequence against the M. smegmatis genomic sequence, which was available as contigs at the TIGR website (http://www.tigr.org/). M. smegmatis ω has 79% and 75% identity with the M. tuberculosis and the Mycobacterium leprae proteins, respectively.
Nucleotide sequence accession number.
The nucleic acid sequence of M. smegmatis rpoZ has been deposited in GenBank with accession number AY973203.
Targeted mutagenesis of rpoZ in M. smegmatis mc2155.
A recombination cassette was constructed to delete rpoZ from the M. smegmatis mc2155 chromosome (Table 1). It consisted of a 953-bp DNA fragment spanning from the 914th base upstream to rpoZ to the 39th base of rpoZ and a downstream fragment of DNA from the 216th base of rpoZ to the 964th base downstream to it with the EcoRI fragment holding the aph gene from vector pUC4K between them. After the preparative cloning steps this whole recombination cassette was transferred to the suicide vector pPR27 (28) to get the final construct, pOKOI. mc2155 was transformed with pOKOI. The sacB mutant gentamicin-susceptible and kanamycin-resistant colonies were selected for further analysis. Disruption of rpoZ was verified by Southern hybridization as well as PCR in one of the selected colonies (data not shown) and this strain, mcdrz, was used in further studies.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| M. smegmatis | ||
| mc2155 | Parental strain | |
| mcdrz | rpoZ mutant | This work |
| mcdrzco | mcdrz complemented with M. smegmatis rpoZ | This work |
| Plasmids | ||
| pUC4K | Source of aph gene | Pharmacia Biotech |
| pET21b | Cloning vector | Novagen |
| pPR27 | Suicide vector | 28 |
| pMV261 | Cloning vector for hsp60 promoter | 33 |
| pDK20 | Source of integration signal of mycobacteriophage L5 | 9 |
| pSDHy | Cloning vector for hygromycin cassette release | Our laboratory |
| pOKOI | Construct for knocking out M. smegmatis rpoZ | This work |
| pETOsm | Intermediate cloning step for complementation construct | This work |
| pMOsm | Intermediate cloning step for complementation construct | This work |
| phMOsm | Complementation construct for M. smegmatis rpoZ | This work |
mcdrz grows more slowly than mc2155 and possesses a different colony morphology.
The mutant was found to grow at a lower rate than the wild-type strain in Middlebrook 7H9 (MB7H9) broth supplemented with 2% glucose and 0.05% Tween 80 (Fig. 1A). A slow growth phenotype has also been observed for the E. coli rpoZ mutant (26) although it has been suspected to be due to a polar effect on the downstream gene spoT (12). Here it must be mentioned that mycobacteria do not have spoT, as a single gene encodes a bifunctional protein, Rel, which carries out the role of RelA as well as SpoT (1). The appearance of individual colonies grown on MB7H9 agar also varied between the wild type and the mutant, as shown in Fig. 1B. Wild-type bacteria (Fig. 1B, panel 1) formed colonies characteristic of mc2155, with a relatively flat surface and irregular edges. The mutant colonies (Fig. 1B, panel 2) were found to grow to smaller diameters with a drier surface, and viewed from the sides, they appeared as elevated humps. In liquid culture the mutant cells aggregated considerably more than the wild-type cells, even in the presence of 0.05% Tween 80 (data not shown).
FIG. 1.
A. Comparison of growth rates of mc2155, mcdrz, and mcdrzco in Middlebrook 7H9 broth supplemented with 2% glucose and 0.05% Tween 80. B. Effect of ω deletion on the appearance of M. smegmatis colonies. mc2155 (panel 1), mcdrz (panel 2), and mcdrzco (panel 3) colonies are shown. Colonies were grown on MB7H9 agar for 18 days. Bars = 5 mm in all cases.
Complementation of mcdrz with the wild-type rpoZ gene.
To ascertain whether the rpoZ deletion was responsible for the observed phenotypes, the mc2155 rpoZ was amplified from genomic DNA and cloned in pET21b (pETOsm). A transcriptional fusion of the hsp60 promoter of pMV261 (33) and rpoZ along with its translational signals from pET21b was generated (pMOsm). oriM was removed from pMOsm and the backbone was ligated with the integrating signal of mycobacteriophage L5 from pDK20 (9). Since mcdrz carried a kanamycin resistance marker in its genome, the hygromycin resistance cassette from plasmid pSDHy was introduced into pMOsm, resulting in phMOsm. mcdrz was transformed with phMOsm and colonies (mcdrzco) were selected in the presence of hygromycin as well as kanamycin. Adequate expression of ω protein was observed during growth of the culture at 37°C by Western blotting of the cell lysate with anti-M. smegmatis ω antibodies raised in rabbits. The growth rate of the complemented strain improved and the growth curve followed the wild-type one quite closely (Fig. 1A, mcdrzco), and the mcdrzco colonies had an appearance similar to the wild-type colonies (Fig. 1B, panel 3).
Intermediate steps of purification of RNA polymerase from mcdrz show reduced transcription activity.
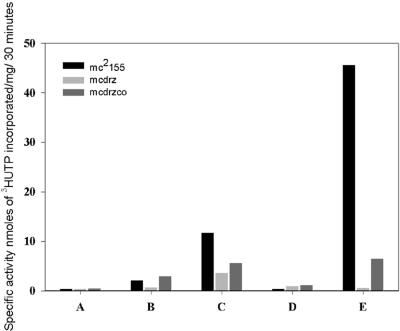
RNAP was purified from M. smegmatis strains by a modification of the protocol described by Kumar and Chatterji (20) and transcription activities of the various steps of purification were checked by the nonspecific transcription assay as described by Lowe et al. (22). In brief, the enzyme purification involved precipitating the lysate (Fig. 2, lanes A) with polymin P, following which the proteins were extracted from the pellet by salt and loaded onto Bio-Gel A-1.5m (Fig. 2, lanes B). The active fractions from Bio-Gel were loaded onto heparin-Sepharose (Fig. 2, lanes C), the unbound fraction of proteins was collected (Fig. 2, lanes D) and the bound proteins were eluted with high salt (Fig. 2, lanes E). It was observed that the various steps of purification of the mutant polymerase except the final chromatography eluate were active, but there was a complete breakdown of transcription activity over the final chromatography with heparin-Sepharose. However, the activities of the intermediate steps were considerably less than the corresponding wild-type fractions in all cases except the lysate. The actual specific activity values showed a variation of nearly 30% between preparations, but the values of the intermediary steps of the knockout preparation always remained within 30 to 50% of the corresponding wild-type values (Fig. 2).
FIG. 2.
Comparison of specific activities of steps of purification of RNA polymerase from mc2155, mcdrz, and mcdrzco. Lanes: A, the cell lysate; B, Biogel A-1.5 load; C, heparin-Sepharose load; D, heparin-Sepharose unbound fraction; and E, heparin-Sepharose eluate. The figure compares one representative RNAP preparation from each strain.
Purification steps of RNA polymerase from mcdrz show lower amounts of associated full-length β′ and increased susceptibility of β′ subunit to proteolytic cleavage.
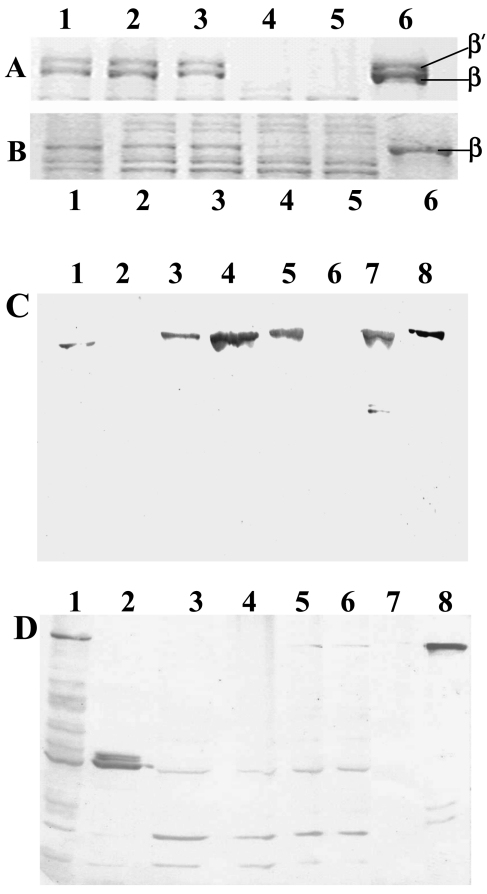
While the RNAP from the wild type behaved as expected with accumulation of β′ subunit in the course of purification (Fig. 3A), the amount of β′ associated with the knockout polymerase was found to be considerably low from the step of Polymin P pellet extraction (Fig. 3B).
FIG. 3.
A and B, Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purification profile of RNA polymerase from mc2155 (A) and mcdrz (B). The β-β′ region of the gel is expanded and shown. Lanes: 1, 1 M salt extraction from Polymin P precipitate; 2, the Bio-Gel A-1.5m load; 3, heparin-Sepharose load; 4, heparin-Sepharose unbound fraction; 5, heparin-Sepharose wash; and 6, elution from heparin-Sepharose. The same amount of total protein was loaded for each fraction. C and D, Immunoblot of the RNAP purification profile of mc2155 (C) and mcdrz (D) with anti-M. tuberculosis β′ antibodies. Lanes: 1, cell lysate; 2, 0.4 M salt wash of the Polymin P pellet; 3, 1 M salt extraction of the pellet; 4, Bio-Gel A-1.5m load; 5, heparin-Sepharose load; 6, heparin-Sepharose unbound fraction; 7, the final heparin-Sepharose eluate; and 8, purified β′. The same amount of total protein was loaded for each step of purification.
Western blotting of the steps of purification of polymerase from the wild type as well as mutant mycobacteria was carried out using antibodies raised against the M. tuberculosis β′ subunit in rabbit (Fig. 3C and D, respectively). Purified M. tuberculosis β′ protein was immobilized on normal human serum-Sepharose matrix (Pharmacia Biotech) which was used for isolating mono-specific antibodies against the β′ subunit from the polyclonal rabbit serum. The Western blots showed an increased propensity of the β′ subunit to get fragmented in the case of the mutant RNAP. It can be seen that the full-length β′ subunit is visible only in the lysate stage, with some lower bands. The full-length band is not apparent in the subsequent steps. Interestingly the fragments are of discrete size, and remain the same throughout the purification until the final step. The higher-molecular-mass fragment ran between 60 kDa and 70 kDa in an 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the two fragments of lower molecular masses had mobilities between 40 and 50 kDa. The M. smegmatis β′ subunit being a protein of 146.5 kDa, we do not expect any major cleavage product to be missed in our experiments. These fragments were absent in the final heparin-Sepharose eluate with the bands appearing to come out completely with the unbound fraction of proteins (Fig. 3, panel D, lane 6). On the other hand, the full-length β′ band remains intact in the wild type.
Following this observation we tried reconstituting the inactive mcdrz heparin-Sepharose eluate (Fig. 2, lanes E, mcdrz, and Fig. 3D, lane 7) with the unbound fraction of proteins from the same column (Fig. 2, lanes D, mcdrz, and Fig. 3D, lane 6) according to the protocol described by Igarashi and Ishihama for reconstitution of the RNAP (16). Briefly, 500 μg of mcdrz heparin-Sepharose eluate was denatured by dialysis against denaturation buffer. An aliquot of 25 μg was removed and the rest of the enzyme was divided into aliquots of 25 μg each. To these portions, different quantities of the unbound fraction were added. After being kept on ice for 30 min, dialysis was carried out at 4°C against reconstitution buffer. After 150 min of dialysis, multiple-round transcription assays using calf thymus DNA as template were carried out as described by Lowe et al. (22). As controls the 25-μg aliquot of the eluate as well as the unbound fraction in quantities identical to those used in the assay were separately given the same treatments.
It was seen that the heparin-Sepharose unbound fraction, when mixed with the inactive heparin-Sepharose eluate, could bring about an increase in transcription activity in the reconstituted mixture that was considerably more than the sum of the activities of the individual components (Table 2). The values shown here are representative of a set of experiments done with 25 μg of the eluate and 60 μg of the unbound fraction from the same RNAP preparation. Similar reconstitution of the mcdrz eluate was attempted with the unbound fraction from the heparin-Sepharose step of the wild-type RNAP preparation as well, but it did not result in any increase in activity (data not shown).
TABLE 2.
Reconstitution of polymerase activitya
| Eluate | Sp act (nmol [3H]UTP incorporated/mg of protein/h) |
|---|---|
| mcdrz heparin-Sepharose | 0.599 ± 0.158 |
| mcdrz heparin-Sepharose unbound fraction | 0.72 ± 0.184 |
| Mixture of bound and unbound mcdrz heparin-Sepharose | 6.88 ± 0.401 |
Reconstitution of polymerase activity by addition of mcdrz heparin-Sepharose unbound fraction to the eluate; 25 μg of the heparin-Sepharose eluate was reconstituted with 60 μg of the unbound fraction. Each value is the mean and standard deviation of three independent reconstitution assays done with the same protein fractions. Specific activity was calculated, taking into account the total protein present in the mixture.
RNAP purified from mcdrzco appears similar to the wild-type enzyme.
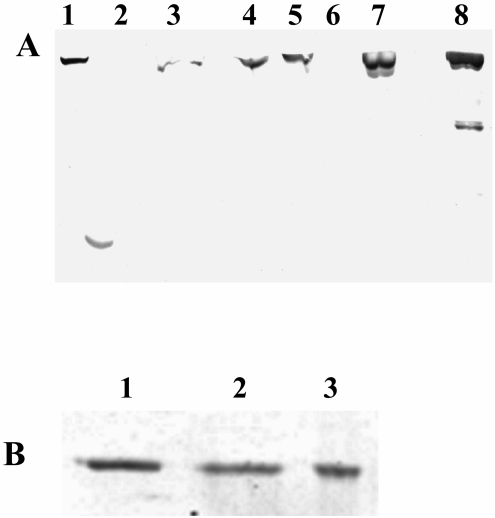
Purification of RNAP from the complemented strain was carried out following the protocol already explained. It can be seen from Fig. 4A that the full-length β′ progressively gets enriched as a function of purification of RNAP from mcdrzco, with no loss of β′ by fragmentation (lanes 1 to 7). Lane 8 contains the control wild-type mc2155 RNAP. We also noted that the α subunit remained intact during the RNAP purification from all three strains, with the final heparin-Sepharose eluates having comparable amounts of the α subunit associated, as can be seen from Fig. 4B.
FIG. 4.
A, Immunoblot of the RNAP purification profile of mcdrzco with anti-M. tuberculosis β′ antibodies. Fractions loaded from left to right: 1, cell lysate; 2, 0.4 M salt wash of the Polymin P pellet; 3, 1 M salt extraction of the pellet; 4, Bio-Gel A-1.5m load; 5, heparin-Sepharose load; 6, heparin-Sepharose unbound fraction; 7, the final heparin-Sepharose eluate; and 8, mc2155 heparin-Sepharose eluate. The same amount of total protein was loaded for each step of purification. B, Immunoblot of the heparin-Sepharose eluates of mc2155 (lane 1), mcdrz (lane 2), and mcdrzco (lane 3) with antibodies raised in rabbits against M. smegmatis RNAP α subunit. Identical quantities of the three proteins were loaded on the gel.
Even though the transcription assay profile (Fig. 2, mcdrzco) showed a recovery of the RNAP activity upon rpoZ integration in strain mcdrzco in comparison to the null strain mcdrz, the RNAP activity remained significantly less than that of the wild type. We reasoned that since the purification of RNAP from the complemented strain was never as clean as that from the wild-type strain (data not shown), the specific activity values, which we have plotted in Fig. 2, go quite off the mark. Taking this into account, we measured the amount of β′ present in the final eluate of mc2155 and mcdrzco (Fig. 3C, lane 7 and Fig. 4A, lane 7, respectively) with quantitative Western blots using enhanced chemiluminescence. The specific activities were normalized for the intensity of the β′ band present in the same amounts of both the proteins and the wild-type value was plotted as 100%. Upon doing this, the mcdrzco enzyme activity value rose to 76.42% of the wild-type value (data not shown).
Our experiments elucidate the key role played by ω in the assembly and structural stability of the M. smegmatis RNAP. In addition to binding and helping the assembly process, the ω subunit also seems to be physically protecting the β′ subunit in the case we have studied. We believe that in the absence of ω, the newly exposed regions of β′ form preferential sites for proteases present in the system. The mutant RNAP retains activity during intermediate steps of purification, in spite of considerably reduced amounts of associated full-length β′. Core peptides within multisubunit proteins are believed to have mosaic structures with separate functional domains, each being constituted by noncontiguous segments in the primary structure (27). Proteolytic or recombinant fragments of a number of enzymes as well as enzyme subunits are known to reassociate in vitro to reconstitute activity (3, 4, 6, 11, 18, 34, 35). This has been shown clearly in the case of the β subunit of E. coli RNAP (32). The β′ subunit of E. coli RNAP also has been shown to possess such mosaic architecture (17). The β′ equivalents of chloroplasts and some archaebacteria are split into two polypeptides (2). Sequence analyses show that the rpoC genes encoding the β′ subunit homologues contain long evolutionarily nonconserved regions (15). Generally split sites that allow functional assembly of the enzyme occur in regions with poorly conserved sequence homology among homologues.
We find it tempting to hypothesize that the ω subunit in M. smegmatis, while binding to β′, also protects such a region or regions in the β′ sequence which in the absence of ω get exposed to proteolytic cleavage. ω has been shown to bind to regions of β′ which are spread far apart over its primary sequence (13, 23). The split domains remain bound to the rest of the enzyme and are able to carry out transcription, albeit at a reduced level of activity. But the cleavage probably results in physical separation of different functional domains and hence in structural destabilization of the enzyme. This provokes a breakdown of the enzyme during affinity purification over heparin-Sepharose. The loss of the smaller fragments of β′ with the unbound fraction of proteins from heparin explains the lack of activity of the eluate. These conclusions are corroborated by reconstitution of the transcription activity by mixing and reconstituting the inactive heparin-Sepharose eluate with the unbound fraction from the column. The question that still remains is how an RNAP that is compromised by the absence of ω carries out transcription adequately within the cell.
In conclusion, our results show that the role of ω in folding the β′ subunit not only aids the enzyme assembly, but also protects the larger subunit from degradation. The phenotypes that we observe for the mutant also suggest other possible functional roles for ω in M. smegmatis, a question that we are pursuing further.
Acknowledgments
One of us (R.M.) thanks the Indian Institute of Science for a research fellowship.
The work was funded by the Department of Biotechnology (DBT), Government of India.
REFERENCES
- 1.Avarbock, D., J. Salem, L. S. Li, Z. M. Wang, and H. Rubin. 1999. Cloning and characterization of a bifunctional RelA/SpoT homologue from Mycobacterium tuberculosis. Gene 233:261-269. [DOI] [PubMed] [Google Scholar]
- 2.Bergsland, K. J., and R. Haselkorn. 1991. Evolutionary relationships among eubacteria, cyanobacteria, and chloroplasts: evidence from the rpoC1 gene of Anabaena sp. strain PCC 7120. J. Bacteriol. 173:3446-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibi, E., and H. R. Kaback. 1990. In vivo expression of the lacY gene in two segments leads to functional lac permease. Proc. Natl. Acad. Sci. USA 87:4325-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burbaum, J. J., and P. Schimmel. 1991. Assembly of a class I tRNA synthetase from products of an artificially split gene. Biochemistry 30:319-324. [DOI] [PubMed] [Google Scholar]
- 5.Burgess, R. R. 1969. Separation and characterization of the subunits of ribonucleic acid polymerase. J. Biol. Chem. 244:6168-6176. [PubMed] [Google Scholar]
- 6.Corradin, G., and H. A. Harbury. 1971. Reconstitution of horse heart cytochrome c: interaction of the components obtained upon cleavage of the peptide bond following methionine residue 65. Proc. Natl. Acad. Sci. USA 68:3036-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darst, S. A., A. M. Edwards, E. W. Kubalek, and R. D. Kornberg. 1991. Three-dimensional structure of yeast RNA polymerase II at 16 Å resolution. Cell 66:121-128. [DOI] [PubMed] [Google Scholar]
- 8.Darst, S. A., E. W. Kubalek, and R. D. Kornberg. 1989. Three-dimensional structure of Escherichia coli RNA polymerase holoenzyme determined by electron crystallography. Nature 340:730-732. [DOI] [PubMed] [Google Scholar]
- 9.DasGupta, S. K., S. Jain, D. Kaushal, and A. K. Tyagi. 1998. Expression systems for study of mycobacterial gene regulation and development of recombinant BCG vaccines. Biochem. Biophys. Res. Commun. 246:797-804. [DOI] [PubMed] [Google Scholar]
- 10.Dove, S. L., and A. Hochschild. 1998. Conversion of the ω subunit of Escherichia coli RNA polymerase into a transcriptional activator or an activation target. Genes Dev. 12:745-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galakatos, N. G., and C. T. Walsh. 1987. Specific proteolysis of native alanine racemases from Salmonella typhimurium: identification of the cleavage site and characterization of the clipped two-domain proteins. Biochemistry 26:8475-8480. [DOI] [PubMed] [Google Scholar]
- 12.Gentry, D., H. Xiao, R. Burgess, and M. Cashel. 1991. The omega subunit of Escherichia coli K-12 RNA polymerase is not required for stringent RNA control in vivo. J. Bacteriol. 173:3901-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh, P., A. Ishihama, and D. Chatterji. 2001. Escherichia coli RNA polymerase subunit ω and its N-terminal domain bind full-length β′ to facilitate incorporation into the α2β subassembly. Eur. J. Biochem. 268:4621-4627. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh, P., C. Ramakrishnan, and D. Chatterji. 2003. Inter-subunit recognition and manifestation of segmental mobility in Escherichia coli RNA polymerase: a case study with ω-β′ interaction. Biophys. Chem. 103:223-237. [DOI] [PubMed] [Google Scholar]
- 15.Honore, N., S. Bergh, S. Chanteau, F. Doucet-Populaire, K. Eiglmeier, T. Garnier, C. Georges, P. Launois, T. Limpaiboon, S. Newton, et al. 1993. Nucleotide sequence of the first cosmid from the Mycobacterium leprae genome project: structure and function of the Rif-Str regions. Mol. Microbiol. 7:207-214. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi, K., and A. Ishihama. 1991. Bipartite functional map of the E. coli RNA polymerase α subunit: involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell 65:1015-1022. [DOI] [PubMed] [Google Scholar]
- 17.Katayama, A., N. Fujita, and A. Ishihama. 2000. Mapping of subunit-subunit contact surfaces on the beta′ subunit of Escherichia coli RNA polymerase. J. Biol. Chem. 275:3583-3592. [DOI] [PubMed] [Google Scholar]
- 18.Kato, I., and C. B. Anfinsen. 1969. On the stabilization of ribonuclease S-protein by ribonuclease S-peptide. J. Biol. Chem. 244:1004-1007. [PubMed] [Google Scholar]
- 19.Kojima, I., K. Kasuga, M. Kobayashi, A. Fukasawa, S. Mizuno, A. Arisawa, and H. Akagawa. 2002. The rpoZ gene, encoding the RNA polymerase omega subunit, is required for antibiotic production and morphological differentiation in Streptomyces kasugaensis. J. Bacteriol. 184:6417-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar, K. P., and D. Chatterji. 1988. An improved method for the purification of DNA-dependent RNA polymerase from Escherichia coli. J. Biochem. Biophys. Methods. 15:235-240. [DOI] [PubMed] [Google Scholar]
- 21.Lonetto, M., M. Gribskov, and C. A. Gross. 1992. The σ70 family: sequence conservation and evolutionary relationships. J. Bacteriol. 174:3843-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowe, P. A., D. A. Hager, and R. R. Burgess. 1979. Purification and properties of the sigma subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry 18:1344-1352. [DOI] [PubMed] [Google Scholar]
- 23.Minakhin, L., S. Bhagat, A. Brunning, E. A. Campbell, S. A. Darst, R. H. Ebright, and K. Severinov. 2001. Bacterial RNA polymerase subunit ω and eukaryotic RNA polymerase subunit RPB6 are sequence, structural, and functional homologs and promote RNA polymerase assembly. Proc. Natl. Acad. Sci. USA 98:892-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee, K., and D. Chatterji. 1999. Alteration in template recognition by E. coli RNA polymerase lacking the ω subunit: a mechanistic analysis through gel retardation and foot-printing studies. J. Biosci. 24:453-459. [Google Scholar]
- 25.Mukherjee, K., and D. Chatterji. 1997. Studies on the ω subunit of Escherichia coli RNA polymerase—its role in the recovery of denatured enzyme activity. Eur. J. Biochem. 247:884-889. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee, K., H. Nagai, N. Shimamoto, and D. Chatterji. 1999. GroEL is involved in activation of Escherichia coli RNA polymerase devoid of the ω subunit in vivo. Eur. J. Biochem. 266:228-235. [DOI] [PubMed] [Google Scholar]
- 27.Nomura., T., N. Fujita, and A. Ishihama. 1999. Mapping of subunit-subunit contact surfaces on the β subunit of Escherichia coli RNA polymerase. Biochemistry 38:1346-1355. [DOI] [PubMed] [Google Scholar]
- 28.Pelicic, V., M. Jackson, J. M. Reyrat, W. R. Jacobs, Jr., B. Gicquel, and C. Guilhot. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10955-10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Periago, P. M., W. van Schaik, T. Abee, and J. A. Wouters. 2002. Identification of proteins involved in the heat stress response of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 68:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard, C. L., A. Tandon, N. R. Sloan, and R. G. Kranz. 2003. RNA polymerase subunit requirements for activation by the enhancer-binding protein Rhodobacter capsulatus NtrC. J. Biol. Chem. 278:31701-31708. [DOI] [PubMed] [Google Scholar]
- 31.Schultz, P., H. Celia, M. Riva, A. Sentenac, and P. Oudet. 1993. Three-dimensional model of yeast RNA polymerase I determined by electron microscopy of two-dimensional crystals. EMBO J. 12:2601-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Severinov, K., A. Mustaev, E. Severinova, I. Bass, M. Kashlev, R. Landick, V. Nikiforov, A. Goldfarb, and S. A. Darst. 1995. Assembly of functional Escherichia coli RNA polymerase containing β subunit fragments. Proc. Natl. Acad. Sci. USA 92:4591-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456-460. [DOI] [PubMed] [Google Scholar]
- 34.Strittmatter, P., R. E. Barry, and D. Corcoran. 1972. Tryptic conversion of cytochrome b5 reductase to an active derivative containing two peptide chains. J. Biol. Chem. 247:2768-2775. [PubMed] [Google Scholar]
- 35.Wrubel, W., U. Stochaj, U. Sonnewald, C. Theres, and R. Ehring. 1990. Reconstitution of an active lactose carrier in vivo by simultaneous synthesis of two complementary protein fragments. J. Bacteriol. 172:5374-5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, G., E. A. Campbell, L. Minakhin, C. Richter, K. Severinov, and S. A. Darst. 1999. Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 Å resolution. Cell 98:811-824. [DOI] [PubMed] [Google Scholar]