Abstract
The aerobic electron transport chain in Mycobacterium smegmatis can terminate in one of three possible terminal oxidase complexes. The structure and function of the electron transport pathway leading from the menaquinol-menaquinone pool to the cytochrome bc1 complex and terminating in the aa3-type cytochrome c oxidase was characterized. M. smegmatis strains with mutations in the bc1 complex and in subunit II of cyctochome c oxidase were found to be profoundly growth impaired, confirming the importance of this respiratory pathway for mycobacterial growth under aerobic conditions. Disruption of this pathway resulted in an adaptation of the respiratory network that is characterized by a marked up-regulation of cydAB, which encodes the bioenergetically less efficient and microaerobically induced cytochrome bd-type menaquinol oxidase that is required for the growth of M. smegmatis under O2-limiting conditions. Further insights into the adaptation of this organism to rerouting of the electron flux through the branch terminating in the bd-type oxidase were revealed by expression profiling of the bc1-deficient mutant strain using a partial-genome microarray of M. smegmatis that is enriched in essential genes. Although the expression profile was indicative of an increase in the reduced state of the respiratory chain, blockage of the bc1-aa3 pathway did not induce the sentinel genes of M. smegmatis that are induced by oxygen starvation and are regulated by the DosR two-component regulator.
The ability of Mycobacterium tuberculosis to grow and persist in its human host and to establish and maintain a state of latent tuberculosis infection from which it can reactivate to cause disease is critical to its extraordinary success as a pathogen. This remarkable competence is attributable to M. tuberculosis's ability to adapt physiologically to the environmental conditions encountered during the course of an infection. These conditions are thought to include restricted nutrient availability and oxidative and nitrosative stress imposed by the host immune response (10, 37, 39, 41).
Of these, the mechanism of physiological adaptation to O2 restriction has been most intensively investigated. These studies have revealed that gradual depletion of O2 from cultures of M. tuberculosis leads to progression of this organism through two stages of nonreplicating persistence, confirming that its replication requires the availability of O2 (41). Of particular importance was the observation that a rapid shift from an aerobic environment to an oxygen-deficient one resulted in bacterial death, indicating that ordered metabolic shutdown is necessary for adaptation to hypoxia or anoxia. Recent studies that have utilized transcriptional profiling to explore the metabolic changes that occur in M. tuberculosis in response to inhibiting aerobic electron flow by O2 depletion (5, 23, 31, 39) and by other means (5, 39) have revealed intriguing insights into the respiratory network of M. tuberculosis that underscore the need to investigate its function and regulation at a molecular level (6).
Mycobacteria possess a branched aerobic respiratory chain in which electrons flow from NADH dehydrogenase and succinate dehydrogenase complexes into the menaquinone-menaquinol pool, from where they are transferred either to an aa3-type cytochrome c oxidase (CcO) via the cytochrome bc1 complex or directly to the cytochrome bd-type menaquinol oxidase (6, 16, 42). The branch terminating in the bd-type oxidase was shown to be important for microaerobic respiration in M. smegmatis (16) and as such is functionally analogous to bd-type oxidase-terminating branches in other organisms (25). In contrast, little is currently known about the CcO-terminating branch in mycobacteria. Genome comparisons suggest that its structure resembles those of other nocardioform actinomycetes, such as Corynebacterium glutamicum (7, 18, 19, 29, 35) and Rhodococcus rhodochrous (36), which are distinguished by the fact that the cytochrome c1 (QcrC) in these organisms is a distinct, diheme c-type cytochrome (18, 35).
A resulting feature of note inferred from studies in C. glutamicum (7, 19) is that the mycobacterial bc1 complex and the cytochrome aa3 oxidase would be expected to form a supercomplex. We have adopted a genetic approach to investigate the function of the bc1-aa3 branch in mycobacteria and, in this paper, report the construction of mutations in M. smegmatis genes encoding the bc1 complex and the aa3-type CcO. The results of this study demonstrate the importance of the cytochrome bc1-aa3 branch for mycobacterial growth and suggest that the disruption of this pathway is accompanied by an adaptation of the respiratory network that is characterized by constitutive up-regulation of the menaquinol oxidase, cytochrome bd-type menaquinol oxidase, and some genes previously shown to be induced by hypoxia, such as uspL (5, 20, 23, 31) and Rv1592c (5, 23, 31), but not of sentinel, DosR-regulated genes (20).
MATERIALS AND METHODS
Bacterial strains and plasmids.
The strains and plasmids used in this study are shown in Table 1. The vectors pQCRTBKO and pCTACTBKO were used for knockout of qcrCAB and ctaC, respectively, in M. tuberculosis, whereas pQCRSMKO, pCTADSMKO, pCATD2SMKO, and pCATCSMKO were used for knockout of the qcrCAB, ctaDI, ctaDII, and ctaC genes, respectively, in M. smegmatis. A deletion allele of M. tuberculosis qcrCAB was constructed by ligating the 3,147-bp upstream ScaI/PstI fragment (containing the 5′-terminal 240 bp of qcrC) to the 3,256-bp downstream PstI fragment (containing the 3′-terminal 800 bp of qcrB) in p2NIL (22), thus eliminating an internal, 2,734-bp region from the qcrCAB operon. The hyg-lacZ-sacB marker cassette from pGOAL19 (22) was cloned in the PacI site of the resulting plasmid to produce pQCRTBKO.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristicsa | Source or reference |
|---|---|---|
| M. tuberculosis H37Rv | Laboratory strain ATCC 27294 | Laboratory collection |
| M. smegmatis | ||
| mc2155 | High-frequency transformation mutant of ATCC 607 | 34 |
| ΔqcrCAB::hyg | qcrCAB deletion-replacement mutant of mc2155; Hygr | This work |
| ΔctaC::hyg | ctaC deletion-replacement mutant of mc2155; Hygr | This work |
| ctaDI+/− | Derivative of mc2155 with one wild-type ctaDI and one ΔctaD::hyg allele | This work |
| ΔctaDII::hyg | ctaDII deletion-replacement mutant of mc2155; Hygr | This work |
| mc2155::pBK4 | mc2155 carrying pBK4 integrated at cyd locus; Kmr | 16 |
| ΔqcrCAB::hyg::pBK4 | ΔqcrCAB::hyg carrying pBK4 integrated at cyd locus; Hygr Kmr | This work |
| Plasmids | ||
| pGEM3Z(+)f | E. coli cloning vector; Apr | Promega |
| pGEMTeasy | E. coli cloning vector for cloning PCR products; Apr | Promega |
| pCR2.1-TOPO | E. coli vector for cloning PCR products; Kmr Apr | Invitrogen |
| p2NIL | E. coli vector for cloning homologous recombination substrates; Kmr | 22 |
| pGOAL17 | Vector carrying PAg85-lacZ Phsp60-sacB PacI marker cassette; Apr | 22 |
| pIJ963 | E. coli vector carrying hyg cassette | 2 |
| pBK4 | p2NIL containing the M. smegmatis cydA′::lacZ fusion cassette; Hygr | 16 |
| pQCRTBKO | Knockout vector carrying M. tuberculosis ΔqcrCAB allele; Hygr Kmr | This work |
| pCTACTBKO | Knockout vector carrying M. tuberculosis ΔctaC::hyg allele; Hygr Kmr | This work |
| pQCRSMKO | Knockout vector carrying M. smegmatis ΔqcrCAB::hyg allele; Hygr Kmr | This work |
| pCTADISMKO | Knockout vector carrying M. smegmatis ΔctaDI::hyg allele; Hygr Kmr | This work |
| pCTADIISMKO | Knockout vector carrying M. smegmatis ΔctaDII::hyg allele; Hygr Kmr | This work |
| pCTACSMKO | Knockout vector carrying M. smegmatis ΔctaC::hyg allele; Hygr Kmr | This work |
Apr, ampicillin resistance; Hygr, hygromycin resistance; Kmr, kanamycin resistance.
Deletion alleles of the remaining genes were constructed by PCR amplification of 5′- and 3′-flanking regions using the primer pairs shown in Table 2. In the case of the M. smegmatis genes, the PCR primers were designed based on the open reading frame sequences identified in the preliminary genome sequence of strain mc2155 from the Institute for Genomic Research (http://www.tigr.org/ufmg/). The flanking regions were cloned in pGEMTeasy or pCR2.1-TOPO before simultaneously subcloning both fragments in p2NIL (22). The deletion alleles were marked by the insertion of a hygromycin resistance gene at the junction site before introduction of the lacZ-sacB marker cassette from pGOAL17 (22) in the PacI site of p2NIL (22). The knockout vectors were electroporated into M. tuberculosis or M. smegmatis and mutant selection was carried out as previously described (3, 14, 22).
TABLE 2.
Oligonucleotides and probes used in this study
Restriction sites used for cloning are underlined (HindIII, BamHI, KpnI), and bases changed to introduce the site are given in lowercase letters.
Bacterial culture conditions.
Escherichia coli DH5α used for cloning procedures was grown in Luria broth (LB) or agar (LA) containing 100 μg/ml ampicillin, 50 μg/ml kanamycin, or 200 μg/ml hygromycin where necessary. M. smegmatis strains were grown in LB or MADC-Tw [Middlebrook 7H9 broth (Difco) supplemented with 0.085% NaCl, 0.2% glucose, 0.2% glycerol, and 0.05% Tween 80] or on LA. M. tuberculosis strains were grown in Middlebrook 7H9 medium supplemented with Middlebrook ADC (Difco), 0.2% glycerol, and 0.05% Tween 80 in roller bottles or as stirred cultures. Antibiotic supplements were as follows: kanamycin, 10 μg/ml (solid medium) or 25 μg/ml (liquid medium), and hygromycin, 50 μg/ml.
For growth of cultures of wild-type and mutant strains of M. smegmatis in shaking flasks, starter cultures at an optical density at 600 nm (OD600) of ∼0.013 were prepared by diluting a preculture (OD600 ∼2.0) in 100 ml of MADC-Tw, and incubating at 37°C with shaking (350 rpm). Optical density was monitored at 3-h intervals over a period of 42 to 60 h. Oxystatic growth of M. smegmatis strains was carried out in a New Brunswick Scientific Bioflow 110 fermentor in batch cultures. This system is similar to the Braun BIOSTAT B system employed by Kana et al. (16), with the exception that the rotometer allowed the air supply to be controlled from 0 to 150 ml/min, and agitation speeds ranged from 50 to 1200 rpm.
Oxystatic culturing of reporter strains was carried out as follows. The fermentor was autoclaved with 990 ml of Middlebrook 7H9 and 2 ml of glycerol. Glucose and NaCl were added as the vessel cooled down, to bring the final volume to 1 liter. The vessel was allowed to equilibrate overnight, before the dissolved-O2 probe was calibrated. The 0% air saturation level was set by sparging the medium with 100% nitrogen and the 100% air saturation level was set by sparging with 100% compressed air. The medium was inoculated with 200 ml of a preculture (OD600 = 2.0) to yield a starting culture of ca. 107 CFU/ml. To produce cultures for use in microarray analyses, a total culture volume of 2 liters was used.
cyd expression analysis using a lacZ reporter.
To monitor cyd expression in a cytochrome bc1-deficient background, a reporter strain was constructed by electroporation of plasmid pBK4 into the ΔqcrCAB::hyg mutant, where pBK4 is a suicide plasmid carrying a cydA′::lacZ transcriptional fusion that expresses the lacZ gene under the control of the cyd promoter (16). Single crossovers were selected on media containing kanamycin and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and site specificity of recombination at the cyd locus was confirmed by Southern blot analysis. β-Galactosidase activity assays were performed as described by Kana et al. (16). To determine statistical significance, unpaired t tests were performed using GraphPad Instat version 3.00 (http://www.graphpad.com).
RNA purification and labeling for DNA microarrays.
RNA was extracted as described by Betts et al. (1). RNA from the control experiment (2 μg) was labeled with Cy3-dCTP (Amersham Pharmacia Biotech), whereas that from the subject (4 μg) was labeled with Cy5-dCTP using previously described methods (4).
Construction of a partial-genome amplicon array of M. smegmatis mc2155.
A partial M. smegmatis array of 1,822 genes was constructed to analyze the transcriptional profile of the two samples. Selection was on the basis of homology with the functional genes of Mycobacterium leprae (9), as established via tblastn analysis. A total of 1,327 M. leprae genes demonstrated homology to one or more genes in M. smegmatis. Preliminary sequence data were obtained from the Institute for Genomic Research website at http://www.tigr.org. In addition, 99 homologs of selected M. tuberculosis genes were added to the array (8). Included in this list were homologs of respiratory pathway genes (including cydA, cydB, ctaDII, and MSMEG5584) and of those that are hypoxically inducible (31).
PCR primers with equivalent melting temperatures were designed using PRIMER3 software to amplify internal segments of each gene ranging in size from 100 to 1,000 bp (28) (source code available at http://fokker.wi.mit.edu/primer3/). Primers were synthesized by PROLIGO Australia Pty Ltd. PCR products were produced by a MWG Biotech Roboamp 4200 thermocycler in 96-well plates using the amplification protocol of 94°C for 2 min, 30 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min, followed by 72°C for 5 min. Amplicons were evaluated by agarose gel electrophoresis with only six primer pairs not amplifying single products of the right size. Products were purified in Millipore multiscreen 96-well filtration plates and resuspended in 50% dimethyl sulfoxide (Sigma). The microarrays were printed in triplicate on Corning Gaps II slides using a Genetic Microsystems Inc. GMS 417 Arrayer. Slides were hydrated at 100°C for 5 s, cross-linked using a Stratagene UV Stratalinker 1800, baked at 80°C for 2 h, and boiled for 2 min prior to storage. The MIAME (Minimum Information About a Microarray Experiment) compliance file can be downloaded at http://vbc.med.monash.edu.au/∼powell/M.smegmatis.
Microarray hybridization and data analysis.
The amplicon array slides were washed in 95°C deionized water (2 min), rinsed in 95% ethanol, and quick-dried by centrifugation (500 rpm, 5 min) prior to hybridization. The slides were prehybridized for 1 h at 42°C in a buffer containing 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 1% bovine serum albumin, and 0.1% sodium dodecyl sulfate (SDS). Labeled probes were mixed with blocking reagents (4 μg yeast tRNA, 1.9 μg herring sperm DNA) and made up to a total of 24 μl in hybridization buffer (5× SSC, 25% formamide, 0.1% SDS). Samples were denatured at 98°C for 2 min, snap-cooled on ice, and applied to the array. Hybridization was carried out under a glass coverslip in a humidified slide chamber (Corning) submerged in a 42°C water bath for approximately 16 h. Coverslips were removed in wash buffer I (1× SSC, 0.05% SDS) and slides were washed once in buffer I and twice in buffer II (0.1× SSC) for 2 min each at room temperature before being dried by centrifugation (500 rpm, 5 min). Slides were scanned using a GenePix 4000B instrument (Axon Instruments) and the resulting images were analyzed using GenePix Pro 4.0 software (Axon Instruments). Each experiment was performed using three biological replicates. Since each amplicon was spotted in triplicate on the array, a total of nine data points were obtained for each open reading frame.
Image analysis and quantification were performed using Imagene 6.0 (BioDiscovery). Spots found to be poor were flagged and removed from the analysis. Subsequent analysis was done using Bioconductor (11) and Limma (32). Spot intensities were background corrected by subtracting the background median from the foreground mean. Any resulting nonpositive values were replaced with half the minimum of all positive corrected intensities for that array. The normalization to remove various biases involved two parts. First, each array was normalized independently using print-tip loess normalization (43). Second, diagnostic plots suggested a variation in scale between arrays, so the log-ratios were scaled in such a way that each array had the same median-absolute-deviation (MAD). The normalized data were then used to fit a linear model (43) for each gene using generalized least-squares, which takes into account the correlation between duplicate spots (33). The coefficient of the fitted model for each gene describes the inferred difference in RNA expression between wild-type and mutant cells. Empirical Bayes was then used to calculate the moderated t statistics and associated P values. The P values were adjusted for multiple testing using false-discovery-rate. At a cutoff of 1% false-discovery-rate, 78 genes were found to be differentially expressed; this corresponds to less than one expected false-positive.
RESULTS
Targeted knockout of genes in the cytochrome bc1-aa3 respiratory pathway in M. tuberculosis.
To investigate the role of the cytochrome bc1-aa3 branch in aerobic respiration in M. tuberculosis, the qcrCAB-encoded menaquinol-cytochrome c oxidoreductase was targeted for disruption by allelic exchange mutagenesis using an unmarked ΔqcrCAB allele delivered on a suicide plasmid (22). Both up- and downstream single crossovers were obtained. However, no double crossovers were recovered following counterselection against an upstream single crossover; all Kms clones analyzed (20 of 20) were wild-type revertants generated by second crossover events on the same side of the deletion mutation (data not shown).
To increase the likelihood of identifying potentially growth-impairing mutations in this pathway, allelic exchange mutagenesis was attempted using a hyg-marked deletion-replacement allele of the M. tuberculosis ctaC gene, which encodes subunit II of CcO. The counterselection plates containing sucrose and hygromycin were incubated at 37°C for more than twice the normal length of time (54 versus 21 days) before colonies were picked for screening. However, no double crossovers were recovered; all clones obtained from upstream (25 of 25) and downstream single crossovers (40 of 40) were Kmr, suggesting that they were all spontaneous sacB mutants.
Targeted knockout of genes in the cytochrome bc1-aa3 pathway in M. smegmatis.
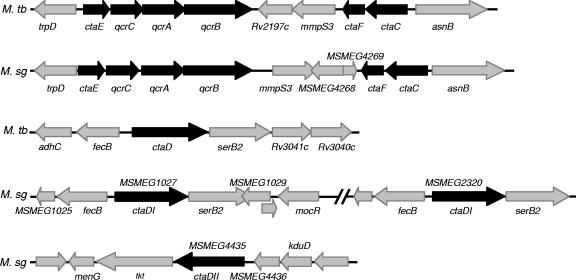
Genes in the corresponding pathway of M. smegmatis were identified by BLAST searches of the unfinished genome sequence of strain mc2155 (http://www.tigr.org/ufmg/) using the corresponding M. tuberculosis genes as query sequences. This analysis revealed the presence of three ctaD homologues encoding subunit I of cytochrome c oxidase in M. smegmatis, two of which are identical but differ from the third. The two identical homologues are located within a region of the genome of mc2155 that is duplicated (12) and was recently shown by genome sequence analysis to be 52 kb in length and flanked by IS1096 elements (http://www.tigr.org/tigr-scripts/CMR2/CMRHomePage.spl). This duplicated ctaD homologue, which is designated herein as ctaDI, shows 86% identity with M. tuberculosis ctaD at the amino acid level and is located in the same chromosomal context as M. tuberculosis ctaD (Fig. 1; MSMEG1027 and MSMEG2320). The second homologue, ctaDII, shares 85% identity with M. tuberculosis ctaD at the amino acid level but is located in a different chromosomal context (Fig. 1; MSMEG4435).
FIG. 1.
Genomic organization of bc1-aa3 respiratory pathway genes in mycobacteria. The arrows denote the respiratory pathway (black) and neighboring genes (gray). The gene annotation for M. tuberculosis H37Rv is taken from Tuberculist (http://genolist.pasteur.fr/TubercuList/), and the annotation for M. smegmatis mc2155 is from the TIGR Comprehensive Microbial Resource (http://www.tigr.org/tigr-scripts/CMR2/CMRHomePage.spl).
The duplication of ctaDI was confirmed by allelic exchange mutagenesis using a knockout vector carrying a ΔctaDI::hyg allele (pCTADISMKO). Southern blot analysis of double crossovers revealed the presence of two cross-hybridizing bands of 4.6 and 2.6 kb, which correspond to the wild-type (ctaDI) and ΔctaDI::hyg alleles, respectively (Fig. 2; ctaDI+/− strain). However, attempts to create a null ctaDI mutant of M. smegmatis by inactivation of the second copy of ctaDI in the ctaDI+/− strain failed to produce double crossovers, suggesting that this gene may be essential (data not shown). In contrast, a ΔctaDII::hyg mutant of mc2155 was readily obtained (data not shown). This strain displayed no discernible growth phenotype under aerobic conditions (data not shown), suggesting that the ctaDII gene is dispensable for the growth of M. smegmatis.
FIG. 2.
Targeted knockout of bc1-aa3 respiratory pathway genes in M. smegmatis mc2155. Each panel shows a schematic representation of the mutant allele. The gene(s) in which internal deletions were made is denoted by a black arrow, the hyg gene is denoted by a white block, and neighboring genes are shown as gray arrows. The positions of the restriction enzymes used for Southern blot analysis shown on the right of each panel are indicated by vertical arrows, and the hybridization probes used in the analysis are shown as hatched boxes above each map. Chromosomal DNA from up- and downstream single-crossover (sco) recombinants, double crossovers (dco), and the parental wild type (mc2155) was digested with ApaI (qcrCAB), BamHI (ctaDI), or SalI (ctaC) and hybridized with the corresponding probe shown above the restriction map (hatched box).
In contrast to the results obtained in M. tuberculosis, viable ΔqcrCAB::hyg and ΔctaC::hyg mutants of M. smegmatis were obtained by allelic exchange mutagenesis (Fig. 2). However, both strains exhibited a marked reduction in growth rate in aerated liquid cultures compared to their parental wild type even though they achieved comparable cell densities in stationary phase (Fig. 3A). The growth defect of the mutant strains was even more pronounced on solid medium (LA), with pinprick colonies taking a minimum of 6 days to appear (Fig. 3B). Both strains displayed visibly altered colony morphology, were highly variable in size, and continued to appear up to 2 weeks after plating.
FIG. 3.
Growth of the ΔqcrCAB::hyg and ΔctaC::hyg mutants of M. smegmatis under aerobic conditions. (A) Growth in liquid medium. Strains were grown at 37°C in shaking flasks (350 rpm) in MADC-Tw medium, and growth was monitored by the absorbance at 600 nm (OD600). ▴, ΔqcrCAB::hyg; ▪, ΔctaC::hyg; ⧫, wild-type mc2155. (B) Growth on solid medium. Strains were grown oxystatically (21% air saturation) in MADC-Tw in a bioreactor, and aliquots were withdrawn and plated on LA. Plates were incubated at 37°C for 3 days (wild type) or 6 to 8 days (mutant).
Overexpression of cytochrome bd oxidase compensates for loss of the bc1 complex.
In prior work, we showed that M. smegmatis possesses a cydAB-encoded cytochrome bd-type menaquinol oxidase, which is induced under microaerobic conditions (16). To investigate the effect of loss of the bc1 complex on expression of cytochrome bd-type menaquinol oxidase, we constructed a reporter strain that carried a cydA′::lacZ transcriptional fusion (16) site-specifically integrated at the cyd locus of the ΔqcrCAB::hyg mutant. Levels of β-galactosidase in the resulting ΔqcrCAB::hyg::pBK4 strain were analyzed under aerobic and microaerobic conditions and were compared with those observed in the control, mc2155::pBK4, in which the same transcriptional fusion was integrated at the cyd locus of the parental wild type. In both strains, the lacZ gene is under control of the cyd promoter.
Induction of cyd gene expression was observed in wild-type M. smegmatis under microaerobic conditions (Fig. 4), in agreement with previous results (16). Importantly, a marked increase in expression of the cyd operon was observed in the ΔqcrCAB::hyg mutant over the air saturation range tested (1 to 21%). The difference in the level of cyd expression between the wild-type and mutant strain was significant under all conditions tested (P < 0.0001). However, comparison of cyd expression levels in the mutant strain under conditions of varying O2 availability revealed no significant induction under microaerobic conditions above the basal expression level observed under full aeration. These results suggest that the loss of cytochrome bc1 resulted in constitutive overproduction of the bd-type oxidase.
FIG. 4.
Effect of cytochrome bc1 disruption on expression of the cyd operon in M. smegmatis. Expression of the cyd operon was assessed using a previously described lacZ reporter under control of the cyd promoter (16) that was integrated at the cyd locus in the wild-type (open bars) and ΔqcrCAB::hyg (black bars) strains. The specific activity of the reporter gene product (β-galactosidase) was assessed at 21, 5, and 1% air saturation, as described under Materials and Methods. All assays were performed in duplicate and the data shown represent the averages and standard deviation of at least two independent experiments.
Expression profiling of the bc1 (ΔqcrCAB::hyg) mutant.
To gain further insight into the effects of rerouting aerobic electron flow through the cytochrome bd-type menaquinol oxidase-terminating branch, expression profiling of aerobically grown cultures of the ΔqcrCAB::hyg mutant was carried out using a partial-genome amplicon array of M. smegmatis. In this experiment, Cy5-labeled cDNA from cultures of the mutant strain grown oxystatically at full aeration (21% pO2) was hybridized against Cy3-labeled cDNA from the wild-type control cultured under identical conditions. A total of 78 genes were found to be differentially expressed, of which 42 were up- and 36 were down-regulated. The top-ranked differentially expressed genes are listed in Table 3, and the complete list is provided in the supplemental material (Table S1).
TABLE 3.
Differentially expressed genes in aerobically grown ΔqcrCAB::hyg mutant of M. smegmatis
| Genes and IDa | Gene | TIGR annotationb | Functionc | Md | P | Classe |
|---|---|---|---|---|---|---|
| Upregulated genes | ||||||
| ribA | ribA2 | MSMEG3082 | Probable riboflavin biosynthesis protein | −2.301 | 0.0020 | 7 |
| ML2274 | MSMEG3498 | Probable conserved secreted protein | −1.914 | 0.0072 | 3 | |
| mihF | mihF | MSMEG3063 | Putative integration host factor | −1.764 | 0.0003 | 2 |
| glgC | glgC | MSMEG5067 | Glucose-1-phosphate adenyl-transferase | −1.753 | 0.0072 | 7 |
| ML1835 | MSMEG6527 | CHP | −1.556 | 0.0030 | 10 | |
| ML0510 | MSMEG3035 | CHP | −1.444 | 0.0004 | 10 | |
| ML0886 | MSMEG4257 | Possible glycosyltransferase | −1.430 | 0.0024 | 7 | |
| ML1312 | MSMEG4598 | CHP | −1.229 | 0.0001 | 10 | |
| argD-g | argD | MSMEG2446 | Probable acetylornithine aminotransferase | −1.027 | 0.0033 | 7 |
| gabD-q | gabD | MSMEG2554 | Probable aldehyde dehydrogenase (NAD dependent) | −1.005 | 0.0052 | 7 |
| Rv3134c | uspL | MSMEG5230 | Universal stress protein | −0.990 | 0.0098 | 10 |
| Rv1623c | cydA | MSMEG3243 | Cytochrome bd oxidase subunit I | −0.931 | 0.0069 | 7 |
| ML1926 | MSMEG0828 | Putative tuberculin-related protein | −0.915 | 0.0058 | 3 | |
| Rv1592c | MSMEG3205 | CHP | −0.878 | 0.0016 | 10 | |
| Downregulated genes | ||||||
| lytB2 | lytB | MSMEG5208 | Probable LytB-related protein, LytB2 | 3.316 | 0.0002 | 3 |
| xseA | xseA | MSMEG5210 | Exo-deoxyribonuclease VII, large subunit | 2.737 | 0.0003 | 2 |
| ML2088-a | MSMEG4807 | Putative cytochrome P450 | 2.039 | 0.0004 | 7 | |
| ctpC-a | MSMEG5384 | Cadmium-translocating P-type ATPase | 1.789 | 0.0081 | 3 | |
| rpoT | sigA | MSMEG2759 | RNA polymerase sigma factor, SigA | 1.513 | 0.0000 | 2 |
| rplE | rplE | MSMEG1464 | Ribosomal protein L5 | 1.198 | 0.0002 | 2 |
| ML2661-e | fadD7 | MSMEG3702 | Fatty acid coenzyme A ligase, FadD7 | 1.197 | 0.0051 | 1 |
| fpg | Fpg | MSMEG2417 | Formamidopyrimidine-DNA glycosylase | 0.961 | 0.0078 | 7 |
| ML1750 | MSMEG2202 | CHP | 0.922 | 0.0098 | 10 |
Gene identifier as defined in the MIAME compliance file (http://vbc.med.monash.edu.au/≈powell/M.smegmatis).
TIGR annotation (http://www.tigr.org/tigr-scripts/CMR2/CMRHomePage.spl).
Function as per Tuberculist and Leproma (http://genolist.pasteur.fr). CHP, conserved hypothetical protein.
Log2 of the fold ratio.
Functional classification as per Tuberculist and Leproma: 1, lipid metabolism; 2, information pathways; 3, cell wall and cell processes; 6, PE/PPE; 7, intermediary metabolism and respiration; 9, regulatory proteins; 10, conserved hypotheticals.
The majority of the up-regulated genes (52%) are involved in intermediary metabolism and respiration. Importantly, the presence of cydA in this group of differentially expressed genes was independently validated by the marked induction of cyd expression in the mutant strain, as deduced using the cydA′::lacZ reporter assay (Fig. 4). Other up-regulated genes included uspL and the M. smegmatis homolog of Rv1592c (MSMEG3205), which were previously shown to be induced in response to hypoxia in M. tuberculosis (uspL and Rv1592c) (5, 23, 31) and in M. smegmatis (uspL) (20). The marked up-regulation of mihF in the bc1 mutant was also notable, as this gene is up-regulated in M. smegmatis just prior to stationary phase and may be involved in the expression of genes required for stationary-phase survival (24).
In contrast, many of the down-regulated genes are involved in information pathways (transcription and DNA repair) and in cell wall and cell processes. The most highly down-regulated gene was lytB (MSMEG5208). This gene is involved in the nonmevalonate pathway for the biosynthesis of terpenoids (27) and its down-regulation may thus affect the biosynthesis of menaquinone in the bc1 mutant. The principal σ factor-encoding gene, sigA (13), was also markedly down-regulated in the bc1 mutant, which is significant in light of the down-regulation of sigA that occurs in M. tuberculosis during anaerobiosis (6, 17).
DISCUSSION
Function of the cytochrome bc1-aa3 branch of the respiratory network in mycobacteria.
A genetic approach was employed to investigate the function of the cytochrome bc1-aa3 branch of the respiratory network in mycobacteria. Allelic exchange mutagenesis was used to generate viable mutants of M. smegmatis lacking either subunit II (CtaC) of the aa3-type CcO or the entire bc1 complex (QcrCAB). These mutants were profoundly impaired for growth and in this respect resemble the corresponding bc1-aa3 pathway mutants of C. glutamicum, which displayed similar growth impairment in glucose minimal medium (7, 18). Together, these observations confirm that the bc1-aa3 pathway is the major respiratory route in actinomycetes grown under standard, aerobic culturing conditions.
Two distinct ctaD alleles were identified in the genome of M. smegmatis, ctaDI and ctaDII. CtaD is the only CcO subunit encoded by distinct alleles in M. smegmatis. Inactivation of ctaDII conferred no growth phenotype on M. smegmatis, confirming its dispensability for growth under the conditions tested. In contrast, a null ctaDI mutant lacking both copies of the ctaDI gene could not be recovered. The viability of other bc1-aa3 pathway mutants in M. smegmatis suggests that our failure to recover a null ctaD1 mutant was unlikely to be due to the essentiality of this gene but could be due to polar effects of the ΔctaDI::hyg mutation on the downstream serB2 gene, which may be essential (30).
We could only identify distinct ctaD alleles in the genome of M. smegmatis and not in any other sequenced mycobacterial species (http://www.tigr.org/tigr-scripts/CMR2/CMRHomePage.spl; http://genolist.pasteur.fr). However, two or more ctaD alleles were also identified in the genomes of several other completely sequenced actinobacteria (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi), including Streptomyces, and Nocardia species. Paracoccus denitrificans has similarly been found to contain two distinct ctaD genes (26, 38), suggesting that the presence of multiple ctaD genes is a reasonably common occurrence in bacterial genomes. In P. denitrificans, the ctaDI and ctaDII genes appear to be functionally interchangeable, implying the existence of CcO isoenzymes (26). Importantly, the microarray analysis suggested that ctaDII (MSMEG4435) is expressed in M. smegmatis under aerobic conditions. However, the extent (if any) of functional redundancy and/or interchangeability of the M. smegmatis ctaD alleles has yet to be determined.
The failure to recover allelic exchange mutants in the bc1-aa3 pathway of M. tuberculosis H37Rv suggests that the mutants were either severely attenuated or nonviable under the conditions tested. This conclusion is supported by the underrepresentation of transposon mutations in genes encoding the bc1 complex (qcrCAB) and the CcO (ctaC, ctaD, ctaE) in high-density mutagenized libraries of M. tuberculosis, as determined by transcription site hybridization (30).
Up-regulation of cyd occurs in response to disruption of the bc1-aa3 respiratory pathway.
In M. smegmatis, inactivation of the bc1-aa3 pathway at the level of the bc1 complex or the CcO (data not shown) resulted in pronounced up-regulation of the bd-type terminal oxidase encoded by the cydABDC operon. Analogous observations have been made in P. denitrificans, where changes in electron distribution caused by blockage of a respiratory pathway affected the activities of terminal oxidase promoters (21). The viability of the ctaC and qcrCAB mutants of M. smegmatis attests to the adaptability of the respiratory network in this organism, which allows its energy requirements to be met by increased activity of the bioenergetically less efficient bd-type oxidase. In addition to cydAB, genes encoding a second putative bd-type oxidase have also been identified in M. smegmatis (MSMEG5584 and MSMEG5585) (16). The microarray analysis confirmed that MSMEG5584 is expressed in M. smegmatis grown aerobically, but unlike cydA, this gene was not differentially expressed in response to blockage of the bc1-aa3 pathway.
The apparent essentiality of the bc1-aa3 pathway in M. tuberculosis may be due to an inability of its aerobic respiratory network to adapt in a manner analogous to that of M. smegmatis. However, cyd gene expression in M. tuberculosis has been shown to be highly responsive to interference with the machinery that maintains the proton motive force, as evidenced by its upregulation by inhibitors of CcO and chemicals that affect its maturation (5), by growth on palmitate (5), during adaptation to hypoxia (5, 40), and by protonophores (5). We therefore conclude that respiratory adaptation by cyd up-regulation probably does occur in M. tuberculosis in response to disruption of the bc1-aa3 pathway, but the consequence of single-route electron flow to the bioenergetically less efficient cytochrome bd-type menaquinol oxidase (15) in this slow-growing mycobacterium may be such that mutant colonies might not be detectable under the conditions employed in this study (up to 54 days of incubation).
Other adaptations to rerouting of the electron flux through cytochrome bd oxidase.
A broader view of the consequences of rerouting of the electron flux through the cytochrome bd-type menaquinol oxidase-terminating branch was obtained from comparative expression profiling of the bc1 mutant and its parental wild type using a partial-genome microarray of M. smegmatis that is enriched for essential genes. In addition to cydA, two other genes known to be induced by hypoxia were also upregulated in the bc1 mutant, uspL (MSMEG5230) and the homolog of Rv1592c (MSMEG3205). Interestingly, UspL is a member of the DosR regulon of M. smegmatis (20).
Although the partial-genome array also contained other genes identified by O'Toole et al. (20) as being hypoxically induced and regulated by DosR, including hspX (MSMEG3937), uspM (MSMEG3957), dosS (MSMEG5226), dosR (MSMEG5229) and acg (MSMEG5231), these genes were not found to be differentially expressed in the bc1 mutant. Therefore, although the results of microarray analysis were suggestive of an overall increase in the reduced state of the respiratory chain, blockage of electron transport via the bc1-aa3 pathway by deletion of the bc1 complex did not induce the DosR regulon of M. smegmatis. This observation suggests that the molecular signal(s) generated by deletion of the bc1 complex is distinct from that generated by O2 starvation, although some genes may respond to both, e.g., cydA (16) and uspL (this study). Current work is aimed at using the respiratory mutant strains as tools for investigating these molecular signals and their transduction pathways.
Supplementary Material
Acknowledgments
This work was supported by grants from the Howard Hughes Medical Institute (International Research Scholar's grants to V.M. and R.L.C.), the Medical Research Council of South Africa, the National Research Foundation of South Africa, the World Health Organization TDR, the Australian National Health and Medical Research Council, the Australian Research Council, and the NIH (#AI43420 to H.R.).
Preliminary sequence data were obtained from the Institute for Genomic Research website at http://www.tigr.org. We thank Marty Voskuil and David Sherman for helpful discussions.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. [DOI] [PubMed] [Google Scholar]
- 2.Blondelet-Rouault, M. H., J. Weiser, A. Lebrihi, P. Branny, and J. L. Pernodet. 1997. Antibiotic resistance gene cassettes derived from the interposon for use in E. coli and Streptomyces. Gene 190:315-317. [DOI] [PubMed] [Google Scholar]
- 3.Boshoff, H. I. M., and V. Mizrahi. 1998. Purification, gene cloning, targeted knockout, overexpression, and biochemical characterization of the major pyrazinamidase from Mycobacterium smegmatis. J. Bacteriol. 180:5809-5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boshoff, H. I. M., M. B. Reed, C. E. Barry III, and V. Mizrahi. 2003. DnaE2 polymerase contributes to in vivo survival and the emergence of drug resistance in Mycobacterium tuberculosis. Cell 113:183-193. [DOI] [PubMed] [Google Scholar]
- 5.Boshoff, H. I. M., T. G. Myers, B. R. Copp, M. R. McNeil, M. A. Wilson, and C. E. Barry III. 2004. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J. Biol. Chem. 279:40174-40184. [DOI] [PubMed] [Google Scholar]
- 6.Boshoff, H. I. M., and C. E. Barry. 2005. Tuberculosis -metabolism and respiration in the absence of growth. Nat. Rev. Microbiol. 3:70-80. [DOI] [PubMed] [Google Scholar]
- 7.Bott, M., and A. Niebisch. 2003. The respiratory chain of Corynebacterium glutamicum. J. Biotech. 104:129-153. [DOI] [PubMed] [Google Scholar]
- 8.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry, III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quali, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, J. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 9.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 10.Dahl, J. L., C. N. Krause, H. I. Boshoff, B. Doan, K. Foley, D. Avarbock, G. Kaplan, V. Mizrahi, H. Rubin, and C. E. Barry III. 2003. The role of RelMTb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc. Natl. Acad. Sci. USA 100:10026-10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudoit, S., and Y. H. Yang. 2003. Bioconductor R packages for exploratory analysis and normalization of cDNA microarray data, p. 45-65. In P. Parmigiani, E. S. Garrett, R. A. Irizarry, and S. L. Zeger, (ed.), The analysis of gene expression data: methods and software. Springer Verlag, New York, NY.
- 12.Galamba, A., K. Soetaert, X.-M. Wang, J. De Bruyn, P. Jacobs, and J. Content. 2001. Disruption of adhC reveals a large duplication in the Mycobacterium smegmatis mc2155 genome. Microbiology 147:3281-3294. [DOI] [PubMed] [Google Scholar]
- 13.Gomez, M., L. Doukhan, G. Nair, and I. Smith. 1998. sigA is an essential gene in Mycobacterium smegmatis. Mol. Microbiol. 29:617-628. [DOI] [PubMed] [Google Scholar]
- 14.Gordhan, B. G., and T. Parish. 2001. Gene replacement using pretreated DNA. Methods Mol. Med. 54:77-92. [DOI] [PubMed] [Google Scholar]
- 15.Hansford, R. 2001. Oxidative phosphorylation, p. 1-8. In Encyclopedia of life sciences. Nature Publishing Group, London, United Kingdom.
- 16.Kana, B. D., E. A. Weinstein, D. Avarbock, S. S. Dawes, H. Rubin, and V. Mizrahi. 2001. Characterization of the cydAB-encoded cytochrome bd oxidase from Mycobacterium smegmatis. J. Bacteriol. 183:7076-7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kendall, S. L., F. Movahedzadeh, S. C. G. Rison, L. Wernisch, T. Parish, K. Duncan, J. C. Betts, and N. G. Stoker. 2004. The Mycobacterium tuberculosis dosRS two-component system is induced by multiple stresses. Tuberculosis 84:247-255. [DOI] [PubMed] [Google Scholar]
- 18.Niebisch, A., and M. Bott. 2001. Molecular analysis of the cytochrome bc1-aa3 branch of the Corynebacterium glutamicum respiratory chain containing an unusual diheme cytochrome c1. Arch. Microbiol. 175:282-294. [DOI] [PubMed] [Google Scholar]
- 19.Niebisch, A., and M. Bott. 2003. Purification of a cytochrome bc1-aa3 supercomplex with quinol oxidase activity from Corynebacterium glutamicum. Identification of a fourth subunit of cytochrome aa3 oxidase and mutational analysis of diheme cytochrome c1. J. Biol. Chem. 278:4339-4346. [DOI] [PubMed] [Google Scholar]
- 20.O'Toole, R., M. J. Smeulders, M. C. Blokpoel, E. J. Kay, K. Lougheed, and H. D. Williams. 2003. A two-component regulator of universal stress protein expression and adaptation to oxygen starvation in Mycobacterium smegmatis. J. Bacteriol. 185:1543-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otten, M. F., D. M. Stork, W. N. M. Reijnders, H. V. Westerhoff, and R. J. M. Van Spanning. 2001. Regulation of expression of terminal oxidases in Paracoccus denitrificans. Eur. J. Biochem. 268:2486-2497. [DOI] [PubMed] [Google Scholar]
- 22.Parish, T., and N. G. Stoker. 2000. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 146:1969-1975. [DOI] [PubMed] [Google Scholar]
- 23.Park, H. D., K. M. Guinn, M. I. Harrell, R. Liao, M. I. Voskuil, M. Tompa, G. K. Schoolnik, and D. R. Sherman. 2003. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 48:833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedulla, M. L., and G. F. Hatfull. 1998. Characterization of the mIHF gene of Mycobacterium smegmatis. J. Bacteriol. 180:5473-5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole, R. K., and G. M. Cook. 2000. Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv. Microb. Physiol. 43:165-224. [DOI] [PubMed] [Google Scholar]
- 26.Raitio, M., J. M. Pispa, T. Metso, and M. Saraste. 1990. Are there isoenzymes of cytochrome c oxidase in Paracoccus denitrificans? FEBS Lett. 261:431-435. [DOI] [PubMed] [Google Scholar]
- 27.Rohdich, F., S. Hecht, K. Gärtner, P. Adam, C. Krieger, S. Amslinger, D. Arigoni, A. Bacher, and W. Eisenreich. 2002. Studies on the nonmevalonate terpene biosynthetic pathway: Metabolic role of IspH (LytB) protein. Proc. Natl. Acad. Sci. USA 99:1158-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365-386. [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto, J., T. Shibata, T. Mine, R. Miyahara, T. Torigoe, S. Noguchi, K. Matsushita, and N. Sone. 2001. Cytochrome c oxidase contains an extra charged amino acid cluster in a new type of respiratory chain in the amino-acid-producing Gram-positive bacterium Corynebacterium glutamicum. Microbiology 147:2865-2871. [DOI] [PubMed] [Google Scholar]
- 30.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48:77-84. [DOI] [PubMed] [Google Scholar]
- 31.Sherman, D. R., M. I. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding α-crystallin. Proc. Natl. Acad. Sci. USA 98:7534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyth, G. K. 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3:1-26. [DOI] [PubMed] [Google Scholar]
- 33.Smyth, G. K., J. Michaud, and H. S. Scott. 2005. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics 0:2701 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 34.Snapper, S. B., R. E. Melton, T. Mustafa, T. Keiser, and W. R. Jacobs, Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911-1919. [DOI] [PubMed] [Google Scholar]
- 35.Sone, N., K. Nagata, H. Kojima, J. Tajima, Y. Kodera, T. Kanamaru, S. Noguchi, and J. Sakamoto. 2001. A novel hydrophobic diheme c-type cytochrome: Purification from Corynebacterium glutamicum and analysis of the qcrCBA operon encoding three subunit proteins of a putative cytochrome reductase complex. Biochim. Biophys. Acta 1503:279-290. [DOI] [PubMed] [Google Scholar]
- 36.Sone, N., M. Fukuda, S. Katayama, A. Jyoudai, M. Syugyou, S. Noguchi, and J. Sakamoto. 2003. QcrCAB operon of a nocardia-form actinomycete Rhodococcus rhodochrous encodes cytochrome reductase complex with diheme cytochrome cc subunit. Biochim. Biophys. Acta 1557:125-131. [DOI] [PubMed] [Google Scholar]
- 37.Timm, J., F. A. Post, L. G. Bekker, G. B. Walther, H. C. Wainwright, R. Manganelli, W. T. Chan, L. Tsenova, B. Gold, I. Smith, G. Kaplan, and J. D. McKinney. 2003. Differential expression of iron-, carbon-, and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. Proc. Natl. Acad. Sci. USA 100:14321-14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Oost, J., T. Haltia, M. Raitio, and M. Saratse. 1991. Genes encoding for cytochrome c oxidase in Paracoccus denitrificans. J. Bioenerg. Biomembr. 23:257-267. [DOI] [PubMed] [Google Scholar]
- 39.Voskuil, M. I., D. Schnappinger, K. C. Visconti, M. I. Harrell, G. M. Dolganov, D. R. Sherman, and G. K. Schoolnik. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voskuil, M. I., K. C. Visconti, and G. K. Schoolnik. 2004. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis 84:281-327. [DOI] [PubMed] [Google Scholar]
- 41.Wayne, L. G., and C. D. Sohaskey. 2001. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55:139-163. [DOI] [PubMed] [Google Scholar]
- 42.Weinstein, E. A., T. Yano, L.-S. Li, D. Avarbock, A. Avarbock, D. Helm, A. A. McColm, K. Duncan, J. T. Lonsdale, and H. Rubin. 2005. Inhibitors of type II NADH:menaquinone oxidoreductase represent a class of antitubercular drugs. Proc. Natl. Acad. Sci. USA 102:4548-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, Y. H., S. Dudoit, P. Luu, and T. P. Speed. 2001. Normalisation for cDNA microarray data, p. 141-152. In M. L. Bittner, Y. Chen, A. N. Dorsel, and E. R. Dougherty (ed.), Microarrays: optical technologies and informatics. Proceedings of the International Society for Optical Engineering, vol. 4266. International Society for Optical Engineering, San Jose, California.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.