Abstract
We establish here that iron deficiency causes oxidative stress in the cyanobacterium Anabaena sp. strain PCC 7120. Iron starvation leads to a significant increase in reactive oxygen species, whose effect can be abolished by treatment with the antioxidant tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl). Oxidative stress induced by iron starvation could be a common feature of photosynthetic bacteria.
The availability of iron is crucial for growth, since this element is present in heme and iron sulfur proteins involved in numerous cellular processes, particularly for photosynthetic organisms (3). Many cyanobacteria, such as Synechococcus elongatus PCC 7942, Synechocystis sp. strain PCC 6803, and Anabaena sp. strain PCC 7120, respond to iron limitation by expressing the isiA gene (1, 8, 15).
Transcription of isiA is also induced by oxidative stress in several cyanobacterial species (6, 9, 14, 16), an observation suggesting that multiple signal input pathways control the expression of this gene (12). Alternatively, iron deficiency could lead to oxidative stress, which then triggers isiA expression (10). In this study, we show that iron limitation causes the accumulation of reactive oxygen species (ROS) in Anabaena sp. strain PCC 7120 and provides an explanation of the regulation of isiA by various factors that cause oxidative damage.
Iron starvation and oxidative stress in Anabaena sp. strain PCC 7120 and Synechocystis.
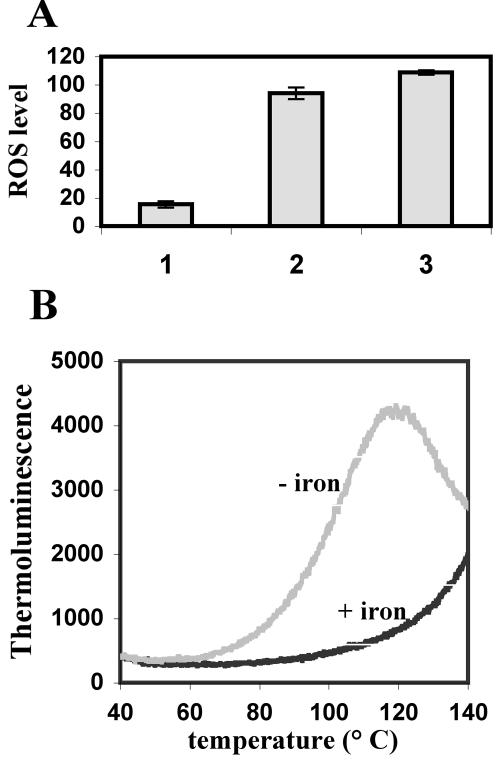
Expression of isiA in Anabaena sp. strain PCC 7120 was induced by methyl viologen treatment or by incubation under high light intensities (data not shown). Anabaena sp. strain PCC 7120 cultures were iron starved by removing ferric ammonium from BG11 medium and adding the iron chelator 2,2′-dipyridyl at a final concentration of 50 μM. We quantified the amount of ROS with a fluorispectrophotometer (SAFAS) by using the fluorescent probe 2,7-dichlorodihydrodihydrofluorescein diacetate (DCFH-DA; Molecular Probes), which detects hydrogen peroxide, hydroxyl radicals, and peroxynitrite anions. Cells were washed twice with 10 mM phosphate buffer, incubated with the probe at a final concentration of 25 μM, and washed again before the fluorescence was measured (5). The results demonstrated a 10-fold increase in ROS levels in iron-starved cells compared to cells grown with the normal amount of iron. ROS production after methyl viologen treatment served as a positive control of oxidative stress (Fig. 1A). Similar results were obtained with Synechocystis sp. strain PCC 6803 under similar conditions (data not shown).
FIG. 1.
Oxidative stress under conditions of iron deficiency in Anabaena sp. strain PCC 7120. (A) ROS generated by Anabaena sp. strain PCC 7120 cells were analyzed after reaction with 2,7-DCFH-DA. The fluorescence intensity was normalized to the optical densities of the samples. Resulting values are presented in arbitrary units. Bars: 1, iron-replete cells; 2, iron-depleted cells; 3, cells treated with methyl viologen. (B) Lipid peroxidation state under oxidative stress induced by iron starvation. Thermoluminescence was measured with cells grown in the presence of iron (black line) or absence of iron (gray line). Experiments were done twice with similar results.
One of the consequences of exposing cells to oxidative damage is the peroxidation of lipids producing light-emitting species, which can be estimated by thermoluminescence (4). The signals obtained with iron-depleted cells were significantly higher than those of iron-sufficient cells (Fig. 1B). Our results indicate that when cyanobacterial cells are iron starved, they also undergo an oxidative stress.
isiA transcription in the presence of the antioxidant tempol in Anabaena sp. strain PCC 7120.
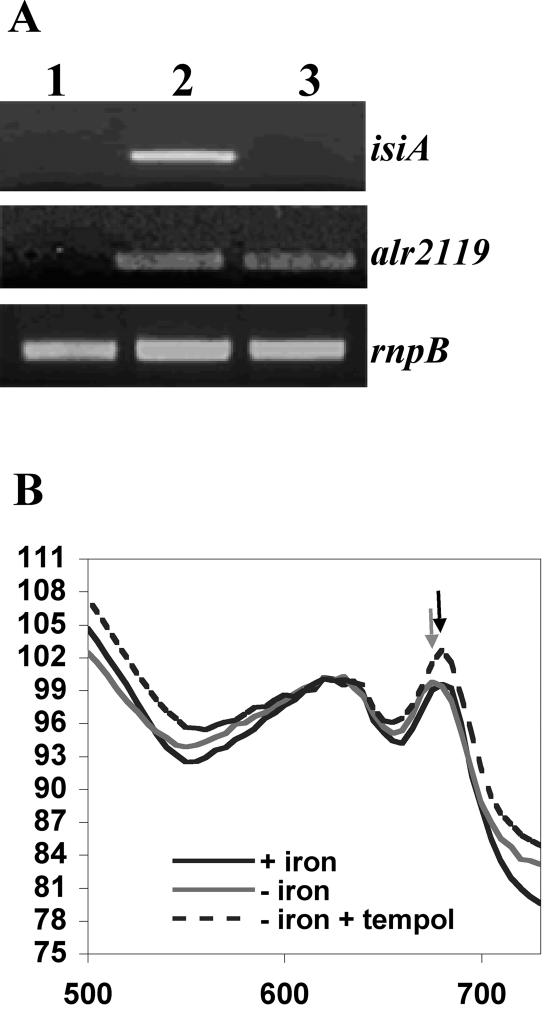
Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl) has been recently described as an antioxidant molecule (2). Tempol is a low-molecular-weight and membrane-permeable antioxidant that protects many biological systems, such as mammalian cells and bacteria, from oxidative damages. Transcription of isiA was analyzed by reverse transcription (RT)-PCR as described previously (15). Expression of the rnpB gene was used as a control (Table 1). RNA preparations were treated with DNase, and the efficiency of this treatment was verified by PCR. When iron-depleted cells were grown in the presence of 10 mM tempol, the expression of isiA was abolished (Fig. 2A). In contrast, the induction of alr2119, whose product is similar to the FeoB protein of Escherichia coli, was not influenced by the presence of tempol. Since FeoB is required for iron transport (7), our data suggest that tempol does not change the iron-starved state of the cells (Fig. 2A).
TABLE 1.
Sequences of the primers used in this study
| Oligonucleotide for indicated gene | Sequence from 5′ to 3′ | Target gene |
|---|---|---|
| isiA | ||
| Top | GCCCGCTTCGCCAATCTCTC | isiA of Anabaena sp. strain PCC 7120 |
| Bottom | CCTGAGTTGTTGCGTCGTAT | |
| rnpB | ||
| Top | AGGGAGAGAGTAGGCGTTGG | rnpB of Anabaena sp. strain PCC 7120 |
| Bottom | GGTTTACCGAGCCAGTACCTCT | |
| feoBEc | ||
| Top | AAGATAACTGGCAGGCAACG | feoB of E. coli |
| Bottom | ACAGGATAACCGCCAGAATG | |
| rpoA | ||
| Top | CAGGGTTCTGTGACAGAGTTTC | rpoA of E. coli |
| Bottom | CTCGTCAGCGATGCTTGCCGGTGG | |
| feoBAna | ||
| Top | AATGTGTTCACCAAAACCT | alr2119 of Anabaena sp. strain PCC 7120 |
| Bottom | GTTCACTCCGAATAATATTAA |
FIG. 2.
Effect of tempol on isiA expression. (A) RT-PCR analysis of isiA, feoB, and rnpB. Total RNAs were isolated from cells grown in the presence of iron (lane 1), in the absence of iron (lane 2), or in absence of iron and supplemented with 10 mM tempol (lane 3). One microgram of RNA was used in each experiment. Samples were collected at the exponential phase of the PCR. All experiments were repeated twice with similar results obtained. (B) Absorption spectra for cell suspensions with iron (+ iron), without iron (− iron), or without iron but in the presence of tempol (− iron + tempol). The gray arrow indicates a shift of the 680-nm chlorophyll a absorption peak.
The presence of CP43′ encoded by isiA results in a shift of the 680-nm chlorophyll a absorption peak (13). Cells starved for iron but incubated with tempol did not exhibit this shift (Fig. 2B). These results are consistent with the suppression of the transcription of isiA by tempol in iron-starved cells.
Iron starvation and oxidative stress in heterotrophic bacteria.
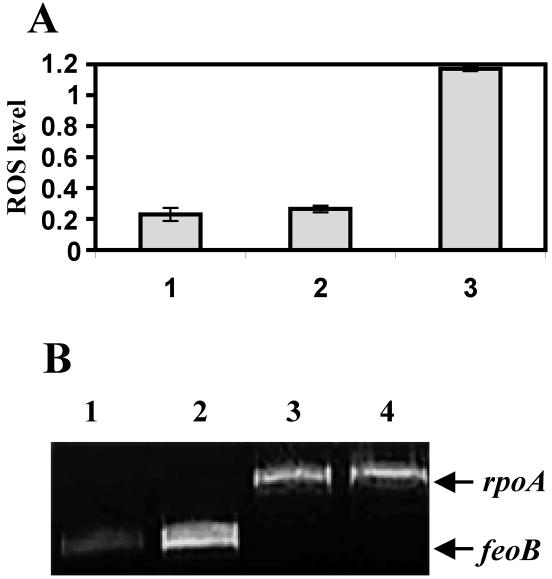
We measured the ROS levels in E. coli cultures grown under normal conditions in MOPS (morpholinepropanesulfonic acid) medium (11) or under conditions of iron deficiency (MOPS medium supplemented with 50 μM of 2,2′-dipyridyl). The ROS production in cells treated with hydrogen peroxide served as a positive control of oxidative stress. Figure 3A shows that when cells of E. coli were iron starved, they did not exhibit a significant increase in the ROS levels. Transcription of the feoB gene was analyzed by RT-PCR. The rpoA gene, encoding the α subunit of the RNA polymerase, was used as a control (Table 1). The transcription of feoB was induced only under conditions of iron deficiency (Fig. 3B), confirming that cells were indeed iron starved under our assay conditions. Similar results were obtained with Bacillus subtilis (data not shown).
FIG. 3.
Oxidative stress under conditions of iron deficiency in E. coli. (A) ROS generated by E. coli DH5α cells were analyzed by using the fluorescent probe 2,7-DCFH-DA. The fluorescence intensity was normalized to the optical densities of the samples. Resulting values are presented in arbitrary units. Bars: 1, iron-replete cells; 2, iron-depleted cells; 3, cells treated with H2O2. (B) RT-PCR analysis of feoB and rpoA mRNAs. Cells were grown normally (lanes 1 and 3) or under conditions of iron limitation (lanes 2 and 4). One microgram of RNA was used in each experiment. Samples were collected at the exponential phase of the PCR. All RT-PCR experiments were repeated twice, with similar results obtained.
Our data indicate that iron deficiency creates an oxidative stress which is the major signal inducing isiA expression in Anabaena sp. strain PCC 7120. Since, under our experimental conditions, oxidative damage induced by iron starvation is not conserved in heterotrophic bacteria, we propose that it could be characteristic of photosynthetic organisms.
Acknowledgments
We thank A. Galinier and the Fondation pour la Recherche Médicale for the helpful use of the fluorispectrophotometer and K. Dudley for English corrections of the manuscript.
This study was supported by “Environnement et santé” grants (AFSSE).
REFERENCES
- 1.Burnap, R. L., J. Troyan, and L. A. Sherman. 1993. The highly abundant chlorophyll-protein of iron deficient Synechococcus sp. PCC 7942 (CP43′) is encoded by the isiA gene. Plant Physiol. 103:893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuzzocrea, S., M. C. McDonald, E. Mazzon, D. Siriwardena, G. Costantino, F. Fulia, G. Cucinotta, E. Gitto, S. Cordaro, I. Barberi, A. De Sarro, A. P. Caputi, and C. Thiemermann. 2000. Effects of tempol, a membrane-permeable radical scavenger, in a gerbil model of brain injury. Brain Res. 875:96-106. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira, F., and N. A. Straus. 1994. Iron deprivation. J. Appl. Phycol. 6:199-210. [Google Scholar]
- 4.Havaux, M. 2003. Spontaneous and thermoinduced photon emission: new methods to detect and quantify oxidative stress in plants. Trends Plant Sci. 8:409-413. [DOI] [PubMed] [Google Scholar]
- 5.Jang, H. H., K. O. Lee, Y. H. Chi, B. G. Jung, S. K. Park, J. H. Park, J. R. Lee, S. S. Lee, J. C. Moon, J. W. Yun, Y. O. Choi, W. Y. Kim, J. S. Kang, G. W. Cheong, D. J. Yun, S. G. Rhee, M. J. Cho, and S. Y. Lee. 2004. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117:625-635. [DOI] [PubMed] [Google Scholar]
- 6.Jeanjean, R., E. Zuther, N. Yeremenko, M. Havaux, H. C. P. Matthijs, and M. Hagemann. 2003. A photosystem I psaFJ-null mutant of the cyanobacterium Synechocystis PCC 6803 expresses the isiAB operon under iron replete conditions. FEBS Lett. 549:52-56. [DOI] [PubMed] [Google Scholar]
- 7.Kammler, M., C. Schon, and K. Hantke. 1993. Characterization of the ferrous iron uptake system of Escherichia coli. J. Bacteriol. 19:6212-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonhardt, K., and N. A. Straus. 1994. Photosystem II genes isiA, psbDI and psbC in Anabaena sp. PCC 7120: cloning, sequencing and the transcriptional regulation in iron-stressed and iron-repleted cells. Plant Mol. Biol. 24:63-73. [DOI] [PubMed] [Google Scholar]
- 9.Li, H., A. K. Singh, L. M. McIntyre, and L. A. Sherman. 2004. Differential gene expression in response to hydrogen peroxide and the putative PerR regulon of Synechocystis sp. strain PCC 6803. J. Bacteriol. 186:3331-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michel, K. P., and E. K. Pistorius. 2004. Adaptation of the photosynthetic electron transport chain in cyanobacteria to iron deficiency: the function of IdiA and IsiA. Physiol. Plant. 120:36-50. [DOI] [PubMed] [Google Scholar]
- 11.Neidhardt, F. C., P. L. Bloch, and D. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunert, A., J. Vinnemeier, N. Erdmann, and M. Hagemann. 2003. Repression by Fur is not the main mechanism controlling the iron-inducible isiAB operon in the cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol. Lett. 227:255-262. [DOI] [PubMed] [Google Scholar]
- 13.Singh, A. K., and L. A. Sherman. 2000. Identification of iron-responsive, differential gene expression in the cyanobacterium Synechocystis sp. strain PCC 6803 with a customized amplification library. J. Bacteriol. 182:3536-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh, A. K., H. Li, and L. A. Sherman. 2004. Microarray analysis and redox control of gene expression in the cyanobacterium Synechocystis sp. PCC 6803. Physiol. Plant 120:27-35. [DOI] [PubMed] [Google Scholar]
- 15.Xu, W. L., R. Jeanjean, Y. D. Liu, and C. C. Zhang. 2003. pkn22 (alr2502) encoding a putative Ser/Thr kinase in the cyanobacterium Anabaena sp. PCC 7120 is induced by both iron starvation and oxidative stress and regulates the expression of isiA. FEBS Lett. 553:179-182. [DOI] [PubMed] [Google Scholar]
- 16.Yousef, N., K. Elfriede, E. K. Pistorius, and K. P. Michel. 2003. Comparative analysis of idiA and isiA transcription under iron starvation and oxidative stress in Synechococcus elongatus PCC 7942 wild-type and selected mutants. Arch. Microbiol. 180:471-483. [DOI] [PubMed] [Google Scholar]