Abstract
Despite considerable current interest in the evolution of intelligence, the intuitively appealing notion that brain volume and “intelligence” are linked remains untested. Here, we use ecologically relevant measures of cognitive ability, the reported incidence of behavioral innovation, social learning, and tool use, to show that brain size and cognitive capacity are indeed correlated. A comparative analysis of 533 instances of innovation, 445 observations of social learning, and 607 episodes of tool use established that social learning, innovation, and tool use frequencies are positively correlated with species' relative and absolute “executive” brain volumes, after controlling for phylogeny and research effort. Moreover, innovation and social learning frequencies covary across species, in conflict with the view that there is an evolutionary tradeoff between reliance on individual experience and social cues. These findings provide an empirical link between behavioral innovation, social learning capacities, and brain size in mammals. The ability to learn from others, invent new behaviors, and use tools may have played pivotal roles in primate brain evolution.
Behavioral innovation and cultural transmission are proposed to be central to the evolution of the human brain (1–3). For example, Wilson's (2) “behavioral drive” hypothesis argues that episodes of innovation and cultural transmission are more frequent in large-brained species, leading these animals to exploit the environment in new ways and so exposing them to novel selection pressures. Such processes may increase the rate of evolution in these taxa. “Social intelligence” hypotheses (4–7) also cite behavioral flexibility as a key factor in the evolution of enhanced brain size. Social intelligence hypotheses posit that complex social interaction was responsible for the selection pressures that favored enhanced primate intelligence. This proposal is currently the focus of considerable attention, with social learning commonly regarded as a core aspect of social intelligence (5–7). Relative neocortex size is positively correlated with social group size in primates, bats, carnivores, and cetaceans (refs. 8 and 9, but see ref. 10 on cetaceans), and Whiten and Byrne (6, 7) have collated evidence that “Machiavellian” strategies of social manipulation and deception have driven simian brain evolution. However, ecological explanations for the evolution of large brains also abound, such as the “extractive foraging” (11) and “cognitive mapping” (12) hypotheses, and the dispute over which variables were of greatest importance in primate brain evolution is far from settled (13). Equally contentious are questions of whether asocial (individual) learning and social learning are part of a domain-general intelligence, or whether “intelligence” is made up of a number of domain-specific, special-purpose modules of cognition (7, 14, 15). If the latter, there is uncertainty over whether social and asocial learning have evolved together, or whether there has been a tradeoff between asocial and social learning abilities (16–18).
Researchers focusing on the links between brain size and various ecological and social variables often make the implicit assumption that brain volume and cognitive capacity are linked. This notion is also embodied in common parlance, with phrases such as “brainy” or “grand cerveau” widespread synonyms for intelligence. In recent years, a number of publications have documented significant advances in our understanding of the evolution of enhanced brain size and intelligence in mammals (e.g., refs. 6–9 and 19–24). However, the widely held (3) assumption that species with larger volumes of the relevant brain structures will show greater behavioral flexibility still remains largely untested (13, 25–29). This is not to say that there has not been important work on the relationships between neural volume and cognitive capacity or behavioral complexity, but these studies have tended to concentrate on perhaps rather specialized behavioral domains or brain regions, such as birdsong repertoire size (focusing on song nuclei; ref. 30), spatial abilities such as food-storing (focusing on the hippocampus; refs. 31–33), and bower-building complexity (34). Notable exceptions are the avian studies of Lefebvre et al. (3, 35–38) mentioned below. To our knowledge, despite ample evidence for links between neural measures and various lifestyles in mammals (8, 9, 19, 22), there is no direct, unequivocal support for a link between brain size and general behavioral flexibility.
There are serious difficulties in making comparative estimates of learning and cognition, because experimental approaches are vulnerable to the criticisms that traditional tests of learning ability are not fair to different species, have little adaptive significance, and do not provide data on large numbers of species (13, 26, 39, 40). An alternative approach, pioneered by Lefebvre et al. (3, 35), is to collate data on the incidence of complex traits associated with behavioral flexibility, on the assumption that incidence is a reflection of that species' intellectual capability. Such an approach circumvents the aforementioned problems with experimental studies, and provides a tractable method of collecting data on tens or even hundreds of species. Hence it is the most appropriate estimate of behavioral flexibility. Here, the tendency to discover novel solutions to environmental or social problems (henceforth “innovation”), to learn skills and acquire information from others (“social learning”), and to use tools were used as ecologically relevant measures of behavioral flexibility.
As the brain does not evolve as a unitary structure (19–22, 41), rather than total brain volume we collated data on the size of neocortex and striatum of all relevant primates. The neural processing underlying innovation and social learning is widely thought to reside in these brain structures, collectively known as the “executive brain” (41, 42), and it is the neocortex that has received attention from students of the social intelligence hypothesis (9). The neocortex and striatum are genomically and functionally linked, with the maternal genome making a considerable developmental contribution to these brain regions, and there have been evolutionary tradeoffs between the executive and “emotional” (hypothalamus, septum) brain in primates (41). As a consequence of allometric scaling, large-bodied species tend to have large brains (39), so we followed the widely used approach of using brainstem volume as a reference variable on the assumption that such areas are evolutionarily conservative (8, 41). This method of calculating brain volume relative to body size is preferable to using body weight as a reference variable, because body weight, unlike brainstem volume, is a rather inaccurate measure of body size (8). The most appropriate measure of brain size is controversial (8), with each different measure reflecting an often unspoken hypothesis regarding what underlies intelligence, so we used three measures of brain size: (i) “executive brain ratio” (executive brain volume over brainstem), (ii) absolute executive brain volume, and (iii) residual executive brain volume, calculated by including brainstem in a multiple regression. We took independent contrasts (43) to control for phylogeny.
Here, we estimate indices of behavioral flexibility by collating data on the frequency of innovation, social learning and tool use from the published literature to test the following predictions. Social learning, innovation, and tool use have all been proposed as explanations for the evolution of enhanced brain size in primates (1, 4–6, 44, 45), so these authors would predict a positive relationship between relative brain size (specifically, executive brain or neocortex size) and the frequency of these behavior patterns. Species that live a gregarious lifestyle have frequently been predicted to rely more on social learning processes than solitary species (15, 40, 46–49), and proponents of social intelligence hypotheses would also predict that social group size and social learning frequency are positively correlated. The predictions for the relationship between social learning and innovation frequencies are more complex. Those of the opinion that common neural mechanisms and substrates underlie both processes would predict a positive correlation between the two (50). If social learning and innovation processes reside in separate modules of cognition, researchers who assume a tradeoff between asocial learning and social learning processes might predict a negative correlation (16). However, asocial learning and social learning may belong to separate cognitive modules that evolve together (resulting in a positive correlation between social learning and innovation frequencies) or have no evolutionary relationship (resulting in no correlation between social learning and innovation frequencies). Hence, our analysis of the relationship between social and asocial learning is not a test of modularity per se, but rather a test of whether the two processes, if they are two processes, have evolved together.
Materials and Methods
Data Collection.
Approximately 1,000 articles in four primate journals (Primates, American Journal of Primatology, Folia Primatologica, and the International Journal of Primatology), as well as other relevant literature, were searched for examples of innovation, social learning, and tool use. The journal issues examined, further details on data collection methods, and examples of behavior patterns classified as innovations are described by Reader and Laland (51). We read the abstract of each article and the full text of all articles concerning the behavior of nonhuman living primates. We included examples cited in the text of articles, with the final database carefully checked to remove any repeated examples. As in Lefebvre et al.'s bird innovation studies (3, 35), we used keywords such as “novel”, “never seen before,” or “traditional” to classify behavior patterns, so that the judgement of whether a behavior pattern qualified as an instance of (e.g.) innovation was made by the author of the article. This approach aims to avoid any subjective bias on our part imposed during data collation. To maximize the size of the data set, we recorded all episodes across all behavioral contexts, whether they occurred in captivity or in the field, as a result of experimental manipulations, or as a result of human intervention such as provisioning or habitat degradation. However, we describe below results of analyses of a limited data set containing examples from the field where no human intervention was indicated (see Table 1).
Table 1.
Results of reanalysis of a limited data set consisting solely of field reports where no human intervention was indicated
| Independent variable | Dependent variable: reported frequency, corrected for research effort
|
||
|---|---|---|---|
| Innovation | Social learning | Tool use | |
| Executive brain | <0.005 | <0.0001 | <0.001 |
| ratio | >0.1 | <0.01 | >0.1 |
| Innovation | — | <0.0001 (<0.0001) | <0.0001 (<0.0001) |
| frequency | <0.0001 (<0.0001) | <0.0001 (<0.0001) | |
| Tool use | — | <0.0001 (<0.0001) | — |
| frequency | <0.0001 (<0.0001) | ||
Figures indicate P values. Bracketed figures in italics indicate the probability level after executive brain ratio was partialled out by using multiple regression. The top row of figures in each cell indicates across-species results, the bottom row, independent contrast results. The limited data set took only examples from the field, and excluded questionable examples, experimental manipulations, and cases where human intervention was stated or implied. All findings were unaffected by this procedure, apart from the relationships between executive brain ratio and both innovation and tool use frequencies. The tool use result probably reflects the loss of power associated with the relatively small number of species that have been observed using tools in the wild compared with tool use in captivity (45). The fact that the vast majority of the results are robust to the extremely conservative nature of the reanalysis gives us reasonable confidence that the results are not artifacts caused by the inclusion of captive studies.
In total, 533 instances of innovation, 445 observations of social learning, and 607 episodes of tool use were recorded from approximately 2,000 records searched (including the search of the relevant literature as well as the journals mentioned above). Studies covered 116 of the 203 known species of primates (52). The species studied was noted for each journal article examined, regardless of whether that article contained an example of one of the behavior patterns of interest, to estimate the research effort into each species. The frequencies of social learning, innovation, and tool use were corrected for research effort by taking the residual from a natural log–log plot of observation frequency against research effort. The raw data are available from the authors.
The volumes of the relevant brain regions were taken from Stephan et al. (53) and Zilles and Rehkamper (54), which detail the brain sizes of 47 primate species in total. Stephan et al. (53) list data for a species not listed in current phylogenies, Saguinus tamarin, and indicate the genus, but not the species, in two cases. It was possible to identify the species involved as Saguinus midas, Alouatta palliata, and Cebus apella, respectively, by using total brain weight, which matched the figures given by Harvey et al. (55). Executive brain volume was calculated as the sum of neocortex and striatum volumes and brainstem volume as the sum of mesencephalon and medulla oblongata volumes. Stephan et al. (53) correct brain sizes to species means, which reduces the problem of accounting for sex differences in brain size. Body weights were taken from Rowe (56) or Harvey et al. (55), and data on group sizes were taken from Rowe (56), Smuts et al. (57), and Dixson (58).
Phylogenetic Analysis.
Species may show similar characteristics simply because they are closely related rather than because they have evolved independently under similar selection pressures. To treat species as independent data points may overestimate the degrees of freedom and potentially give false positive results, so it is often desirable to make some correction for phylogeny by using approaches such as independent contrasts (59). We used the caic computer program (43) to implement the independent contrasts technique. caic makes fewer type I errors than across-species analyses even when the branch lengths and phylogeny are uncertain or inaccurate (43). New and modified comparative techniques are becoming available (60), but any study of primate brain evolution should note that only a small proportion of primate brains have been measured (53), and conclusions must be necessarily tentative. All brain and body sizes, apart from the executive brain ratio, were natural log-transformed before taking contrasts because caic assumes that different lineages are equally likely to make the same proportional change in size. Across-species analyses were also conducted by using these natural log-transformed values. Independent contrasts were regressed through the origin by using least-squares regression (43). The primate phylogeny was a composite tree derived from 112 previously published phylogenies (52). For the purposes of this analysis, an assumption of equal distances between phylogenetic nodes was made. Recently, questions have been raised over whether independent contrast analyses are always more valid than analyses treating species as independent data points (e.g., ref. 61). Furthermore, any differences between independent contrast and across-species analyses may be biologically informative (62). Hence, we report here the results of both independent contrasts and species-level analyses.
Confounding Variables.
A number of confounding variables could influence our measures of behavioral flexibility. Social learning in particular may be prone to reporting biases as many field reports must, necessarily, infer rather than experimentally establish the presence of social learning. To address this problem we conducted extensive analysis of potential confounding variables and inter-observer reliabilities. A second observer coded 241 previously examined records from the journals Folia Primatologica and Primates, approximately 10% of the total number of records examined. The index of concordance (63) was 0.95 for social learning, 0.83 for innovation, and 0.94 for tool use. In addition to the measures described above to control for common ancestry and differences in research effort, we addressed the effect of field versus captive studies, of experimental manipulations, provisioning, and other human influences (described in Table 1, see also ref. 64). Lefebvre et al. (3, 37, 65) collected avian innovation data from published literature, and demonstrated that their findings were not affected by the following potential confounding variables: mode of juvenile development, population size, journal source, editorial policy, research effort, observer bias (ornithological interest measured as the frequency of photographs in birding publications and by questionnaire), and common ancestry. Furthermore, Lefebvre's innovation measure correlates with laboratory measures of learning performance (38). Combined, these findings suggest that we have a robust operational measure of behavioral flexibility.
Results and Discussion
We found significant positive correlations between innovation frequency and executive brain ratio (Fig. 1), both before and after taking independent contrasts. A similar relationship was found with absolute executive brain volume (across-species: r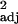 = 0.24, F1,30 = 10.95, P < 0.005, independent contrasts: r
= 0.24, F1,30 = 10.95, P < 0.005, independent contrasts: r = 0.14, F1,29 = 5.92, P < 0.05), but multiple regressions with executive brain volume and brainstem volume as independent variables produced no significant correlation with innovation frequency (across-species: partial r controlling for brainstem = 0.14, t29 = 0.73, P > 0.1, independent contrasts: partial r = 0.14, t28 = 1.13, P > 0.1). Similar results were found with social learning and tool use frequencies (Fig. 1; absolute executive brain volume: social learning: across-species: r
= 0.14, F1,29 = 5.92, P < 0.05), but multiple regressions with executive brain volume and brainstem volume as independent variables produced no significant correlation with innovation frequency (across-species: partial r controlling for brainstem = 0.14, t29 = 0.73, P > 0.1, independent contrasts: partial r = 0.14, t28 = 1.13, P > 0.1). Similar results were found with social learning and tool use frequencies (Fig. 1; absolute executive brain volume: social learning: across-species: r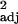 = 0.34, F1,30 = 17.00, P < 0.0005, independent contrasts: r
= 0.34, F1,30 = 17.00, P < 0.0005, independent contrasts: r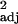 = 0.13, F1,29 = 5.41, P < 0.05; tool use: across-species: r
= 0.13, F1,29 = 5.41, P < 0.05; tool use: across-species: r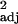 = 0.27, F1,30 = 12.17, P < 0.005, contrasts: r
= 0.27, F1,30 = 12.17, P < 0.005, contrasts: r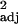 = 0.14, F1,29 = 5.99, P < 0.05; multiple regressions controlling for brainstem: social learning: across-species: partial r = 0.24, t29 = 1.32, P > 0.1, contrasts: partial r = 0.06, t28 = 0.37, P > 0.1; tool use: across-species: partial r = 0.19, t29 = 1.03, P > 0.1, contrasts: partial r = 0.13, t28 = 0.88, P > 0.1). The disparities between brain measures suggest that either the three measures gauge different things, or some measures are more susceptible to type I or type II errors (13). The method of using executive brain volume residuals is perhaps vulnerable to type II errors, because it is the most conservative method of accounting for body size. Residual estimates will also be biased by the data chosen or available to be included in the analysis, which may be a problem with limited sample sizes (66, 67). The correlations between absolute executive brain volume and all three measures of behavioral flexibility support the hypothesized relationship between absolute brain size and learning capacities (26), but could be the result of a confound with body size. Executive brain ratio (and the closely related neocortex ratio) has received theoretical support as an appropriate measure of brain size (39, 68). Combined with the finding that similar correlations between executive brain ratio and innovation and tool use frequencies result when body weight is partialled out (across species: P < 0.05; independent contrasts: P = 0.06 in both cases), we can have reasonable confidence that executive brain ratio is a reliable index of brain size and place greatest emphasis on these results.
= 0.14, F1,29 = 5.99, P < 0.05; multiple regressions controlling for brainstem: social learning: across-species: partial r = 0.24, t29 = 1.32, P > 0.1, contrasts: partial r = 0.06, t28 = 0.37, P > 0.1; tool use: across-species: partial r = 0.19, t29 = 1.03, P > 0.1, contrasts: partial r = 0.13, t28 = 0.88, P > 0.1). The disparities between brain measures suggest that either the three measures gauge different things, or some measures are more susceptible to type I or type II errors (13). The method of using executive brain volume residuals is perhaps vulnerable to type II errors, because it is the most conservative method of accounting for body size. Residual estimates will also be biased by the data chosen or available to be included in the analysis, which may be a problem with limited sample sizes (66, 67). The correlations between absolute executive brain volume and all three measures of behavioral flexibility support the hypothesized relationship between absolute brain size and learning capacities (26), but could be the result of a confound with body size. Executive brain ratio (and the closely related neocortex ratio) has received theoretical support as an appropriate measure of brain size (39, 68). Combined with the finding that similar correlations between executive brain ratio and innovation and tool use frequencies result when body weight is partialled out (across species: P < 0.05; independent contrasts: P = 0.06 in both cases), we can have reasonable confidence that executive brain ratio is a reliable index of brain size and place greatest emphasis on these results.
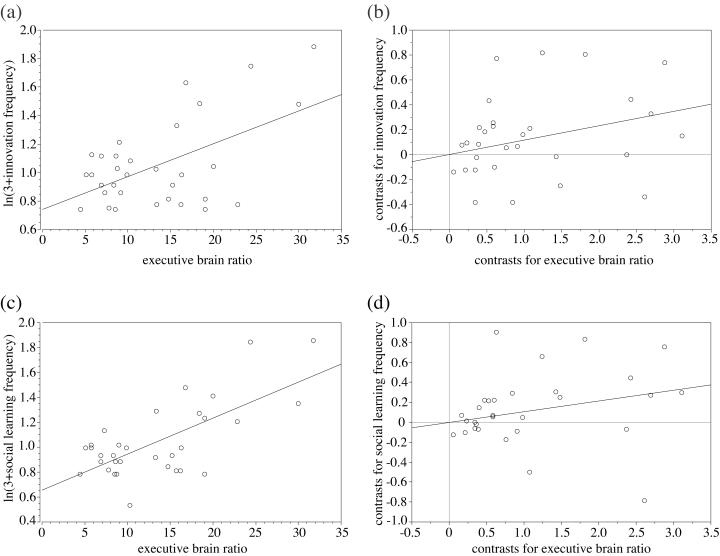
Figure 1.
(a and b) Innovation frequencies and executive brain ratio. Frequencies are corrected for research effort by taking residuals from a natural log–log plot through the origin of innovation frequency against research effort. (a) The raw data, with each point representing one species (r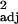 = 0.34, F1,30 = 16.70, P < 0.0005). Stephan et al. (53) generally chose a single representative from each genus for brain volume measurement, so this species-level analysis is effectively similar to a genus-level analysis. (b) Independent contrast data (r
= 0.34, F1,30 = 16.70, P < 0.0005). Stephan et al. (53) generally chose a single representative from each genus for brain volume measurement, so this species-level analysis is effectively similar to a genus-level analysis. (b) Independent contrast data (r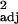 = 0.18, F1,29 = 7.66, P < 0.01). (c and d) Social learning frequencies, corrected for research effort, and executive brain ratio. (c) The raw data, with each point representing one species (r
= 0.18, F1,29 = 7.66, P < 0.01). (c and d) Social learning frequencies, corrected for research effort, and executive brain ratio. (c) The raw data, with each point representing one species (r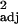 = 0.48, F1,30 = 29.49, P < 0.0001). (d) Independent contrast data (r
= 0.48, F1,30 = 29.49, P < 0.0001). (d) Independent contrast data (r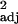 = 0.13, F1,29 = 5.55, P < 0.05). Similar relationships were found between corrected tool use frequency and executive brain ratio (across-species: r
= 0.13, F1,29 = 5.55, P < 0.05). Similar relationships were found between corrected tool use frequency and executive brain ratio (across-species: r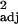 = 0.40, F1,30 = 21.46, P < 0.0001; independent contrasts: r
= 0.40, F1,30 = 21.46, P < 0.0001; independent contrasts: r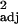 = 0.17, F1,29 = 7.28, P < 0.05).
= 0.17, F1,29 = 7.28, P < 0.05).
Members of large-brained nonhuman primate species innovate, learn from others, and use tools more frequently than members of small-brained primate species. Our findings provide support for Wilson's concept of behavioral drive (2), which could have accelerated evolutionary rates in primates. Macphail (25, 29) has argued that evidence that brain-size measures predict intellectual capacity is lacking. This study provides evidence to the contrary, to the extent that the reported incidence of innovation, social learning, and tool use correlate with ability. Furthermore, the association between large brains and learning capacity supports the argument that one cost of a reliance on learning is an increase in brain size and complexity (69).
Our results suggest an alternative social intelligence hypothesis to those stressing the Machiavellian characteristics of mind-reading, manipulation, and deception (6, 7), especially when we consider that social group size and social learning frequency are not correlated (independent contrasts calculated for 105 species, r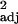 = 0.00, P > 0.1; see ref. 49). There may have been direct selection for an increase in executive brain ratio in primates, because these brain regions facilitate adaptive complex variable strategies, such as inventing new behavior, social learning, or using tools. Individuals capable of inventing new solutions to ecological challenges, or exploiting the discoveries and inventions of others, may have had a selective advantage over less able conspecifics, which generated selection for those brain regions that facilitate complex technical and social behavior. An alternative account is that primates are making opportunistic use of information processing capabilities afforded by a large executive brain that has evolved for some other reason to cope with challenges in new flexible ways. However, as these two hypotheses are not mutually exclusive (3), our findings support the view that social learning and innovation may have been important processes behind the evolution of large brains in primates.
= 0.00, P > 0.1; see ref. 49). There may have been direct selection for an increase in executive brain ratio in primates, because these brain regions facilitate adaptive complex variable strategies, such as inventing new behavior, social learning, or using tools. Individuals capable of inventing new solutions to ecological challenges, or exploiting the discoveries and inventions of others, may have had a selective advantage over less able conspecifics, which generated selection for those brain regions that facilitate complex technical and social behavior. An alternative account is that primates are making opportunistic use of information processing capabilities afforded by a large executive brain that has evolved for some other reason to cope with challenges in new flexible ways. However, as these two hypotheses are not mutually exclusive (3), our findings support the view that social learning and innovation may have been important processes behind the evolution of large brains in primates.
Innovation and social learning frequencies were found to covary (Fig. 2), even after executive brain ratio was partialled out of the analysis (across-species: partial r controlling for relative executive brain size = 0.67, t29 = 4.87, P < 0.0001; independent contrasts: partial r = 0.69, t28 = 5.07, P < 0.0001). There were similar positive correlations between tool use and innovation frequencies, and tool use and social learning frequencies (Fig. 2, P < 0.0001 in all cases), also unaffected by the inclusion of executive brain ratio as an independent variable. The findings presented above were largely unaffected by limiting the data set to field studies (Table 1). To the extent that innovation is a measure of asocial learning, the correlation between social learning and innovation frequencies suggests that asocial and social learning have evolved together. This pattern suggests that social and asocial learning may be based on the same processes (50), which conflicts with the widely held view that social learning requires distinct psychological abilities from asocial learning (70). However, we cannot rule out the possibility that social and asocial learning are separate, domain-specific capacities (14, 15) that have undergone correlated evolution. Our analysis does not support a third hypothesis, that there has been a tradeoff between asocial learning and social learning capacities over evolutionary time. For the moment, there is little evidence that social learning is an adaptive specialization to particular environmental demands beyond that for a general selection pressure favoring behavioral flexibility (40).
Figure 2.
(a and b) Innovation and social learning frequencies, corrected for research effort, covary. (a) The raw data, with each point representing one species (r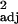 = 0.48, F1,114 = 108.38, P < 0.0001). (b) Independent contrast data (r
= 0.48, F1,114 = 108.38, P < 0.0001). (b) Independent contrast data (r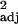 = 0.35, F1,100 = 55.47, P < 0.0001). (c and d) Innovation and tool use (c; r
= 0.35, F1,100 = 55.47, P < 0.0001). (c and d) Innovation and tool use (c; r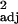 = 0.54, F1,100 = 118.89, P < 0.0001) and social learning and tool use frequencies (d; r
= 0.54, F1,100 = 118.89, P < 0.0001) and social learning and tool use frequencies (d; r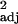 = 0.45, F1,100 = 84.65, P < 0.0001), corrected for research effort, covary. Both c and d show independent contrast data.
= 0.45, F1,100 = 84.65, P < 0.0001), corrected for research effort, covary. Both c and d show independent contrast data.
Our findings are consistent with several hypotheses concerning primate brain evolution that are currently out of favor (6, 7). The reported incidence of tool use correlated with executive brain ratio, supportive of “technical intelligence” hypotheses, which argue that technology or technical skills drove brain evolution (7, 44). Moreover, as the majority of the innovative and socially learned behaviors were recorded in the foraging domain (51), our results can also be interpreted as supporting ecological explanations of the evolution of primate intelligence (6, 11, 12, 19). Our analysis reinforces the findings of other recent studies reporting that ecological hypotheses for brain evolution have been prematurely rejected, that social and ecological intelligence hypotheses should not necessarily be regarded as alternatives, and that multiple sources of selection favored the evolution of a large primate executive brain (7, 8, 13).
Acknowledgments
We thank L. Lefebvre, E. B. Keverne, P. P. G. Bateson, P. Lee, M. Anderson, G. Brown, A. Dixson, R. Boyd, A. Whiten, D. Realé, D. Sol, G. Stirling, P.-O. Cheptou, W. Vickery, and the Université du Québec à Montréal Behavioral Ecology Group for helpful discussion and comments on earlier drafts of this manuscript, R. Day for assistance in calculating interobserver reliabilities, and the Royal Society and Biotechnology and Biological Sciences Research Council for funding.
Footnotes
See commentary on page 4141.
References
- 1.Wyles J S, Kunkel J G, Wilson A C. Proc Natl Acad Sci USA. 1983;80:4394–4397. doi: 10.1073/pnas.80.14.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson A C. Sci Am. 1985;253(4):148–157. doi: 10.1038/scientificamerican1085-164. (pp. 164–173 in American edition). [DOI] [PubMed] [Google Scholar]
- 3.Lefebvre L, Whittle P, Lascaris E, Finkelstein A. Anim Behav. 1997;53:549–560. [Google Scholar]
- 4.Jolly A. Science. 1966;153:501–506. doi: 10.1126/science.153.3735.501. [DOI] [PubMed] [Google Scholar]
- 5.Humphrey N K. In: Growing Points in Ethology. Bateson P P G, Hinde R A, editors. Cambridge, U.K.: Cambridge Univ. Press; 1976. pp. 303–317. [Google Scholar]
- 6.Byrne R W, Whiten A. Machiavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes and Humans. Oxford: Oxford Univ. Press; 1988. [Google Scholar]
- 7.Whiten A, Byrne R W. Machiavellian Intelligence II. Extensions and Evaluations. Cambridge, U.K.: Cambridge Univ. Press; 1997. [Google Scholar]
- 8.Barton R. In: Comparative Primate Socioecology. Lee P C, editor. Cambridge, U.K.: Cambridge Univ. Press; 1999. pp. 167–194. [Google Scholar]
- 9.Joffe T H, Dunbar R I M. Proc R Soc London Ser B. 1997;264:1303–1307. doi: 10.1098/rspb.1997.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor R C, Mann J, Tyack P L. Trends Ecol Evol. 1998;13:408. doi: 10.1016/s0169-5347(98)01459-1. [DOI] [PubMed] [Google Scholar]
- 11.Parker S T, Gibson K R. J Hum Evol. 1977;6:623–641. [Google Scholar]
- 12.Milton K. In: Machiavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes and Humans. Byrne R W, Whiten A, editors. Oxford: Oxford Univ. Press; 1988. pp. 271–284. [Google Scholar]
- 13.Deaner R O, Nunn C L, van Schaik C P. Brain Behav Evol. 2000;55:44–52. doi: 10.1159/000006641. [DOI] [PubMed] [Google Scholar]
- 14.Tooby J, Cosmides L. Ethol Sociobiol. 1989;10:29–49. [Google Scholar]
- 15.Giraldeau L-A, Caraco T, Valone T J. Behav Ecol. 1994;5:35–43. [Google Scholar]
- 16.Boyd R, Richerson P J. Culture and the Evolutionary Process. Chicago: Univ. of Chicago Press; 1985. [Google Scholar]
- 17.Richerson P J, Boyd R. In: The Evolution of Cognition. Heyes C, Huber L, editors. Cambridge, MA: MIT Press; 2000. pp. 329–346. [Google Scholar]
- 18.Rogers A R. Am Anthropol. 1988;90:819–831. [Google Scholar]
- 19.Harvey P H, Krebs J R. Science. 1990;249:140–146. doi: 10.1126/science.2196673. [DOI] [PubMed] [Google Scholar]
- 20.Barton R A, Harvey P H. Nature (London) 2000;405:1055–1058. doi: 10.1038/35016580. [DOI] [PubMed] [Google Scholar]
- 21.Clark D A, Mitra P P, Wang S S-H. Nature (London) 2001;411:189–193. doi: 10.1038/35075564. [DOI] [PubMed] [Google Scholar]
- 22.De Winter W, Oxnard C E. Nature (London) 2001;409:710–714. doi: 10.1038/35055547. [DOI] [PubMed] [Google Scholar]
- 23.Kaas J H, Collins C E. Nature (London) 2001;411:141–142. doi: 10.1038/35075681. [DOI] [PubMed] [Google Scholar]
- 24.Finlay B L, Darlington R B. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 25.Macphail E M. Brain and Intelligence in Vertebrates. Oxford: Clarendon Press; 1982. [Google Scholar]
- 26.Gibson K R. In: Mammalian Social Learning: Comparative and Ecological Perspectives. Box H O, Gibson K R, editors. Cambridge, U.K.: Cambridge Univ. Press; 1999. pp. 351–366. [Google Scholar]
- 27.Byrne R W, Whiten A. Man. 1992;27:609–627. [Google Scholar]
- 28.Byrne R W. Behav Brain Sci. 1993;16:696–697. [Google Scholar]
- 29.Macphail E M. In: The Evolution of Cognition. Heyes C, Huber L, editors. Cambridge, MA: MIT Press; 2000. pp. 253–271. [Google Scholar]
- 30.DeVoogd T J, Krebs J R, Healy S D, Purvis A. Proc R Soc London Ser B. 1993;254:75–82. doi: 10.1098/rspb.1993.0129. [DOI] [PubMed] [Google Scholar]
- 31.Krebs J R, Sherry D F, Healy S D, Perry H, Vaccerino A L. Proc Natl Acad Sci USA. 1989;86:1388–1392. doi: 10.1073/pnas.86.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hampton R R, Sherry D F, Khurgel M, Ivy G. Brain Behav Evol. 1995;45:54–61. doi: 10.1159/000113385. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs L F, Spencer W D. Brain Behav Evol. 1994;44:125–132. doi: 10.1159/000113584. [DOI] [PubMed] [Google Scholar]
- 34.Madden J. Proc R Soc London Ser B. 2001;268:833–838. doi: 10.1098/rspb.2000.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefebvre L, Gaxiola A, Dawson S, Timmermans S, Rosza L, Kabai P. Behavior. 1998;135:1077–1097. [Google Scholar]
- 36.Sol D, Lefebvre L. Oikos. 2000;90:599–605. [Google Scholar]
- 37.Nicolakakis N, Lefebvre L. Behavior. 2000;137:1415–1429. [Google Scholar]
- 38.Timmermans S, Lefebvre L, Boire D, Basu P. Brain Behav Evol. 2000;56:196–203. doi: 10.1159/000047204. [DOI] [PubMed] [Google Scholar]
- 39.Byrne R W. In: Behaviour and Evolution. Slater P J B, Halliday T R, editors. Cambridge, U.K.: Cambridge Univ. Press; 1992. pp. 223–265. [Google Scholar]
- 40.Lefebvre L, Giraldeau L-A. In: Social Learning in Animals: The Roots of Culture. Heyes C M, Galef B G Jr, editors. London: Academic; 1996. pp. 107–128. [Google Scholar]
- 41.Keverne E B, Martel F L, Nevison C M. Proc R Soc London Ser B. 1996;262:689–696. doi: 10.1098/rspb.1996.0103. [DOI] [PubMed] [Google Scholar]
- 42.Jolicoeur P, Pirlot P, Baron G, Stephan H. Syst Zool. 1984;33:14–29. [Google Scholar]
- 43.Purvis A, Rambaut A. Comp Appl Biosci. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- 44.Passingham R E. The Human Primate. Oxford: Freeman; 1982. [Google Scholar]
- 45.Byrne R W. In: Machiavellian Intelligence II. Extensions and Evaluations. Whiten A, Byrne R W, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 289–311. [Google Scholar]
- 46.Klopfer P H. Behavior. 1959;14:282–299. [Google Scholar]
- 47.Lee P. In: Primate Responses to Environmental Change. Box H O, editor. London: Chapman & Hall; 1991. pp. 39–56. [Google Scholar]
- 48.Roper T J. Sci Prog. 1986;70:571–583. [PubMed] [Google Scholar]
- 49.Reader S M, Lefebvre L. Behav Brain Sci. 2001;24:353–355. [Google Scholar]
- 50.Heyes C M. Biol Rev. 1994;69:207–231. doi: 10.1111/j.1469-185x.1994.tb01506.x. [DOI] [PubMed] [Google Scholar]
- 51.Reader S M, Laland K N. Intl J Prim. 2001;22:787–805. [Google Scholar]
- 52.Purvis A. Philos Trans R Soc London Ser B. 1995;348:405–421. doi: 10.1098/rstb.1995.0078. [DOI] [PubMed] [Google Scholar]
- 53.Stephan H, Frahm H, Baron G. Folia Prim. 1981;35:1–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- 54.Zilles K, Rehkamper G. In: Orang-Utan Biology. Schwartz J H, editor. Oxford: Oxford Univ. Press; 1988. pp. 157–176. [Google Scholar]
- 55.Harvey P H, Martin R D, Clutton-Brock T H. In: Primate Societies. Smuts B B, Cheney D L, Seyfarth R M, Wrangham R W, Struhsaker T T, editors. Chicago: Univ. of Chicago Press; 1987. pp. 181–196. [Google Scholar]
- 56.Rowe N. The Pictorial Guide to the Living Primates. New York: Pogonias; 1996. [Google Scholar]
- 57.Smuts B B, Cheney D L, Seyfarth R M, Wrangham R W, Struhsaker T T. Primate Societies. Chicago: Univ. of Chicago Press; 1987. [Google Scholar]
- 58.Dixson A F. Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes and Human Beings. Oxford: Oxford Univ. Press; 1998. [Google Scholar]
- 59.Harvey P H, Pagel M D. The Comparative Method in Evolutionary Biology. Oxford: Oxford Univ. Press; 1991. [Google Scholar]
- 60.Pagel M. Nature (London) 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- 61.Harvey P H, Rambaut A. Philos Trans R Soc London Ser B. 2000;355:1599–1605. doi: 10.1098/rstb.2000.0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Price T. Philos Trans R Soc London Ser B. 1997;352:519–529. doi: 10.1098/rstb.1997.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin P R, Bateson P. Measuring Behaviour: An Introductory Guide. Cambridge, U.K.: Cambridge Univ. Press; 1986. [Google Scholar]
- 64.Reader S M. Ph.D. thesis. Cambridge, U.K.: Univ. of Cambridge; 2000. [Google Scholar]
- 65.Lefebvre L. In: The Evolution of Cognition. Heyes C, Huber L, editors. Cambridge, MA: MIT Press; 2000. pp. 311–328. [Google Scholar]
- 66.Martin R D. Nature (London) 1981;293:57–60. doi: 10.1038/293057a0. [DOI] [PubMed] [Google Scholar]
- 67.Holloway R L, Post D G. In: Primate Brain Evolution: Methods and Concepts. Armstrong E, Falk D, editors. New York: Plenum; 1982. pp. 57–75. [Google Scholar]
- 68.Dunbar R I M. J Hum Evol. 1995;28:287–296. [Google Scholar]
- 69.Johnston T D. Adv Study Behav. 1982;12:65–106. [Google Scholar]
- 70.Tomasello M, Call J. Primate Cognition. New York: Oxford Univ. Press; 1997. [Google Scholar]