Abstract
Caspases, a family of cysteine proteases, play a central role in apoptosis. During the last decade, major progress has been made to further understand caspase structure and function, providing a unique basis for drug design. This Review gives an overview of caspases and their classification, structure, and substrate specificity. We also describe the current knowledge of how interference with caspase signaling can be used to pharmacologically manipulate cell death.
Introduction
Apoptosis, or programmed cell death, is a common property of all multicellular organisms (1, 2). It can be triggered by a number of factors, including ultraviolet or γ-irradiation, growth factor withdrawal, chemotherapeutic drugs, or signaling by death receptors (DRs) (3, 4). The central role in the regulation and the execution of apoptotic cell death belongs to caspases (5–7). Caspases, a family of cysteinyl aspartate–specific proteases, are synthesized as zymogens with a prodomain of variable length followed by a large subunit (p20) and a small subunit (p10). The caspases are activated through proteolysis at specific asparagine residues that are located within the prodomain, the p20 and p10 subunits (8). This results in the generation of mature active caspases that consist of the heterotetramer p202–p102. Subsequently, active caspases specifically process various substrates that are implicated in apoptosis and inflammation. Their important function in these processes makes caspases potential targets for drug development. In this Review, we discuss the structures and functions of caspases as well as their role in novel approaches for treating cancer, autoimmune diseases, degenerative disorders, and stroke.
Structure of caspases
General overview.
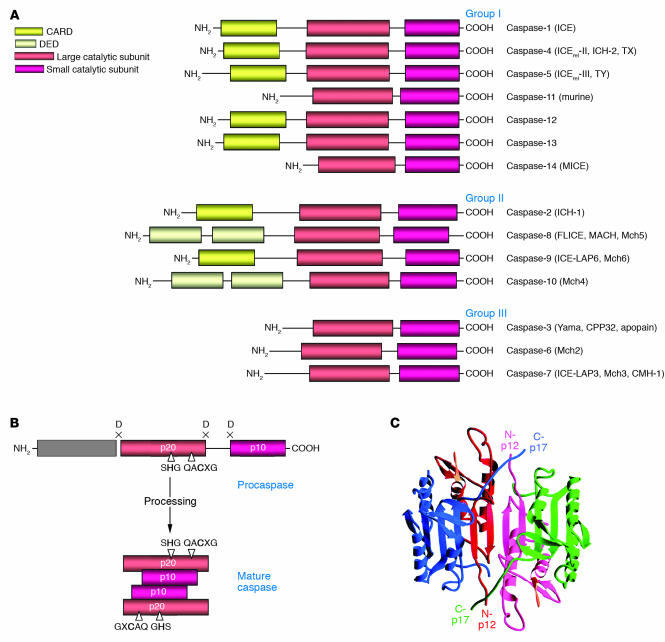
Caspases are zymogens (inactive enzyme precursors, which require a biochemical change to become an active enzyme) that consist of an N-terminal prodomain followed by a large subunit of about 20 kDa, p20, and a small subunit of about 10 kDa, p10 (Figure 1A) (5). In a number of procaspases, the p20 and p10 subunits are separated by a small linker sequence. Depending on the structure of the prodomain and their function, caspases are typically divided into 3 major groups (Figure 1A). The caspases with large prodomains are referred to as inflammatory caspases (group I) and initiator of apoptosis caspases (group II), while caspases with a short prodomain of 20–30 amino acids are named effector caspases (group III).
Figure 1.
Caspase structure. (A) The caspase family. Three major groups of caspases are presented. Group I: inflammatory caspases; group II: apoptosis initiator caspases; group III: apoptosis effector caspases. The CARD, the DED, and the large (p20) and small (p10) catalytic subunits are indicated. (B) Scheme of procaspase activation. Cleavage of the procaspase at the specific Asp-X bonds leads to the formation of the mature caspase, which comprises the heterotetramer p202–p102, and the release of the prodomain. The residues involved in the formation of the active center are shown. (C) The 3D structure of caspase-3 heterotetramer. Each heterodimer is formed by hydrophobic interactions resulting in the formation of mostly parallel β-sheets, composed of 6 antiparallel β-strands. Two heterodimers fit together with formation of a 12-stranded β-sheet that is sandwiched by α-helices. N and C termini of the small and large protease subunits are indicated.
Caspase prodomains.
The large prodomains of procaspases contain structural motifs that belong to the so-called death domain superfamily (9, 10). Death domains are 80- to 100-residue-long motifs involved in the transduction of the apoptotic signal. This superfamily consists of the death domain (DD), the death effector domain (DED), and the caspase recruitment domain (CARD) (11). Each of these motifs interacts with other proteins by homotypic interactions. All members of the death domain superfamily are characterized by similar structures that comprise 6 or 7 antiparallel amphipathic α-helices. Structural similarity suggests a common evolutionary origin for all recruitment domains (12). However, the nature of the homotypic interactions differs within the superfamily. DD and CARD contacts are based on electrostatic interactions, while DED contacts use hydrophobic interactions (13).
Procaspase-8 and -10 possess 2 tandem DEDs in their prodomain (14, 15). The CARD is found in procaspase-1, -2, -4, -5, -9, -11, and -12 (16, 17). DEDs and CARDs are responsible for the recruitment of initiator caspases into death- or inflammation-inducing signaling complexes, resulting in proteolytic autoactivation of caspases that subsequently initiates inflammation or apoptosis.
Structure of active caspase heterotetramers.
Cleavage of a procaspase at the specific Asp-X bonds results in the formation of the mature caspase, which comprises the heterotetramer p202–p102 and causes release of the prodomain (Figure 1B). X-ray structures have been determined for mature caspase-1 (18, 19), caspase-2 (20), caspase-3 (21–23), caspase-7 (24–26), caspase-8 (27), and caspase-9 (28). The overall architecture of all caspases is similar. Each heterodimer (p10–p20) is formed by hydrophobic interactions resulting in the formation of several parallel β-sheets, composed of 6 antiparallel β-strands. Two heterodimers interact via a 12-stranded β-sheet that is surrounded by α-helices (Figure 1C). This so-called caspase fold is a unique quaternary structure among proteases and has been described only for caspases and for gingipain R, the cysteine protease from Porphyromonas gingivalis (29). In the caspase heterotetramer, the 2 heterodimers align in a head-to-tail configuration. Correspondingly, 2 active sites are positioned at opposite ends of the molecule. The architecture of the active center comprises amino acid residues from both subunits. The catalytic machinery involves a diad composed of a cysteine sulfohydryl group (Cys285) and a histidine imidazol ring (His237) (19). Both of them are located in the p20 subunit. The tetrahedral transition state of the cysteine protease is stabilized through hydrogen bonding with the backbone amide protons of Cys285 and Gly238. The asparagine of the substrate seems to be stabilized by 4 residues: Arg179 and Gln283 from the p20 subunit and Arg341 and Ser347 from the p10 subunit.
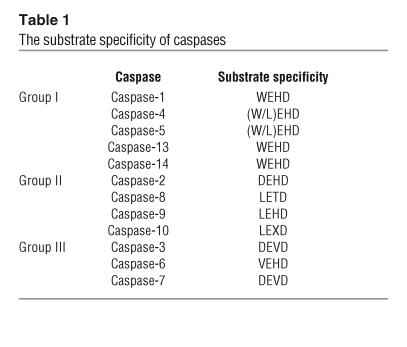
Substrate specificity and synthetic peptide inhibitors of caspases.
Caspases are specific cysteine proteases, recognizing 4 amino acids, named S4-S3-S2-S1. The cleavage takes place typically after the C-terminal residue (S1), which is usually an asparagine (30). A list of substrate specificities of caspases is presented in Table 1. Interestingly, the preferred S3 position is an invariant glutamine for all mammalian caspases. Thus, specificity of caspase cleavage can be described as X-Glu-X-Asp. Caspase-1, -4, and -5 (group I; Figure 1) prefer the tetrapeptide sequence WEHD. Caspase-2, -3, and -7 have a preference for the substrate DEXD, whereas caspase-6, -8, and -9 prefer the sequence (L/V)EXD. Interestingly, the cleavage site between the large and small subunits for initiator caspases carries its own tetrapeptide recognition motif, which is remarkably consistent with the proposed mechanism of autoactivation of initiator caspases (31, 32).
Table 1.
The substrate specificity of caspases
Most of the synthetic peptide caspase inhibitors were developed based on the tetrapeptide caspase recognition motif. Therefore, the selectivity of inhibitors matches the caspase substrate specificities described above (Table 1). The introduction of an aldehyde group at the C terminus of the tetrapeptide results in the generation of reversible inhibitors (33), whereas a fluoromethyl ketone (fmk), a chloromethyl ketone (cmk) (34), or a diazomethyl ketone (dmk) (35) at this position irreversibly inactivates the enzyme.
Caspase signaling
Mechanisms of caspase activation.
All caspases are produced in cells as catalytically inactive zymogens and must undergo proteolytic processing and activation during apoptosis. The effector caspases are activated by initiator or apical caspases. However, one central question of apoptosis is how initiator caspases are activated and how this activation is regulated to prevent spontaneous cell death. It is generally accepted that apical caspase activation takes place in large protein complexes that bring together several caspase zymogens (22–25, 36). All initiator caspases are characterized by the presence of a member of the DD superfamily (DED or CARD), which enables their recruitment into the initiation complex. Several activating complexes for initiator caspases have been reported so far.
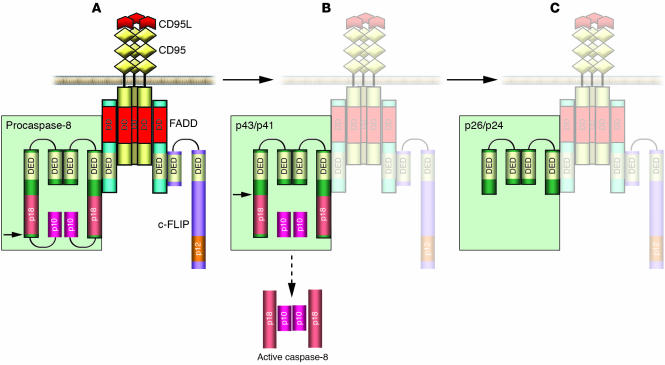
The death-inducing signaling complex as an activating complex for procaspase-8 and -10.
Procaspase-8 and -10 are apical caspases in apoptotic pathways triggered by engagement of death receptors. Several members of the TNF receptor (TNFR) superfamily (TNFR1, CD95 [Fas/APO-1], TRAIL-R1, TRAIL-R2, DR3, DR6) comprise DD in their intracellular domain and are, therefore, termed death receptors (3, 37). Triggering of CD95 and TRAIL-R1/R2 by corresponding ligands results in the formation of a death-inducing signaling complex (DISC) (38–42). CD95 and TRAIL-R1/R2 DISCs consist of oligomerized, probably trimerized, receptors, the DD-containing adaptor molecule FADD/MORT1 (Fas-associated death domain), 2 isoforms of procaspase-8 (procaspase-8/a [FLICE, MACHα1, Mch5] and procaspase-8/b [Machα2]), procaspase-10, and the cellular FLICE-inhibitory proteins (c-FLIPL/S/R) (Figure 2A) (43, 44). The interactions between the molecules at the DISC are based on homotypic contacts. The DD of the receptor interacts with the DD of FADD, while the DED of FADD interacts with the N-terminal tandem DEDs of procaspase-8 and -10 and FLIPL/S/R. Thus, DISC formation results in assembly of procaspase-8 and -10 molecules in close proximity to each other.
Figure 2.
Scheme of procaspase-8 processing at the CD95 DISC. CD95 DISC formation is triggered by extracellular cross-linking with CD95L (depicted in red), which is followed by oligomerization of the receptor. FADD/MORT1 is recruited to the DISC by DD interactions (shown in red); procaspase-8 and -10 as well as c-FLIP proteins are recruited to the DISC by homophilic DED interactions (yellow). Upon recruitment to the DISC, procaspase-8 undergoes processing by forming dimers (depicted in green). (A) The first step of procaspase-8 cleavage occurs between 2 protease subunits. The site of cleavage is shown by a black arrow. As a result of the first cleavage step the p10 subunit is formed, which is not released into the cytosol but remains bound to the DISC as it is involved in the interactions with the large protease subunits. (B) The second cleavage step takes place between the prodomain and the large protease subunit at Asp216. As a result of this cleavage step the active caspase-8 heterotetramer is formed, which is then released into the cytosol. (C) Prodomain p26/p24 remains bound to the DISC.
Activation of procaspase-8 is believed to follow an “induced proximity” model in which high local concentrations and favorable mutual orientation of procaspase-8 molecules at the DISC lead to their autoproteolytic activation (31, 45–47). There is strong evidence from a number of in vitro studies that autoproteolytic activation of procaspase-8 occurs upon oligomerization at the receptor complex (45–47). Furthermore, it has been demonstrated that dimers formed by procaspase-8 molecules possess proteolytic activity, and proteolytic processing of procaspase-8 occurs between precursor dimers (45). Interestingly, it has been shown that procaspase-8 and mature caspase-8 possess different substrate specificities (45). It is likely that conformational changes in the active center of caspase-8 occur upon processing to mature caspase-8.
The processing of procaspase-8/a/b at the DISC is depicted in detail in Figure 2. According to the 2-step model, the processing of procaspase-8 includes 2 cleavage events (39, 45). The first cleavage step occurs between the protease domains, and the second cleavage step takes place between the prodomain and the large protease subunit. Correspondingly, after the first cleavage step, p43/p41 and p10 subunits are formed (Figure 2, A and B). Both cleavage products remain bound to the DISC, p43/p41 by DED interactions and p10 by interactions with the large protease domain (48). As a result of the second cleavage step, p43/41 is processed to the prodomain p26/p24 and p18 (Figure 2C). Thus, the active caspase-8 heterotetramer is formed at the DISC. Subsequently, the mature caspase-8 heterotetramer is released to the cytosol to trigger apoptotic processes.
Procaspase-10 is also activated at the DISC, forming an active heterotetramer (15, 49). However, whether caspase-10 can trigger cell death in the absence of caspase-8 in response to CD95 or TRAIL-R1/R2 stimulation is controversial. Thus, the exact role of caspase-10 remains elusive.
The apoptosome as activating complex for procaspase-9.
A number of apoptotic stimuli, such as cytotoxic stress, heat shock, oxidative stress, and DNA damage, lead to the release of cytochrome c from mitochondria. The release of cytochrome c is followed by the formation of a high–molecular mass cytoplasmic complex referred to as the apoptosome (50). In mammals the central scaffold protein of the apoptosome is a 140-kDa protein known as Apaf-1 (apoptotic protease–activating factor-1), which is a homologue of CED-4, a key protein involved in programmed cell death in the nematode Caenorhabditis elegans (51, 52). In the presence of cytochrome c and dATP, Apaf-1 oligomerizes to form a very large (700–1,400 kDa) apoptosome complex. Procaspase-9 is recruited to the complex by CARD interactions, which results in its activation (53). It has been biochemically demonstrated that activation of procaspase-9 occurs by dimerization (31). Moreover, it has been shown that proteolytic activation of procaspase-9 takes place upon dimerization and subsequent cleavage within an interdomain linker, which itself is important for stabilization of caspase-9 dimers.
The inflammosome as activating complex for caspase-1 and -5.
The activation of the initiator caspase-1 and -5 takes place in a complex that was named the inflammosome (54). The inflammosome comprises procaspase-1 and -5 as well as the CARD-containing protein NALP-1. The formation of this complex results in the processing and activation of the cytokines IL-1β and IL-18, which play a central role in the immune response to microbial pathogens.
Effector caspase cascade.
The activation of the effector caspase cascade differs between extrinsic (death receptor–mediated) and intrinsic (mitochondria-mediated) pathways.
In death receptor–mediated apoptosis, 2 types of signaling pathways have been established (55). So-called type I cells are characterized by high levels of DISC formation and increased amounts of active caspase-8 (Figure 3). Activated caspase-8 directly leads to the activation of downstream, effector caspase-3 and -7. In type II cells, there are lower levels of DISC formation and, thus, lower levels of active caspase-8. In this case, signaling requires an additional amplification loop that involves the cleavage of the Bcl-2–family protein Bid by caspase-8 to generate tBid and a subsequent tBid-mediated release of cytochrome c from mitochondria (56). The release of cytochrome c from mitochondria results in apoptosome formation, followed by the activation of procaspase-9, which in turn cleaves downstream, effector caspase-3 and -7. Type II signaling might be blocked by Bcl-2 family members such as Bcl-2 and Bcl-xL.
Figure 3.
Caspase signaling and its modulation. In the extrinsic pathway, DISC formation leads to caspase-8 activation. Two signaling pathways downstream from the receptor were established. In type I cells (shown in light blue) caspase-8 directly cleaves caspase-3, which starts the death cascade. In type II cells (shown in light red) an additional amplification loop is required, which involves tBid-mediated cytochrome c release from mitochondria followed by apoptosome formation. Initiation of the intrinsic pathway results in mitochondria-mediated apoptosome formation, followed by caspase-9 and -3 activation, leading to destruction of the cell. Caspase action can be modulated on several levels. Activation of caspases at the DISC is inhibited by c-FLIP proteins; activation of effector caspases is inhibited by IAPs (see text). Effector caspases are shown in light green; cellular caspase inhibitors are presented in yellow. The targets for pharmacological modulation are shown with an orange arrow.
In the intrinsic pathway, which is triggered by a number of factors, including UV or γ-irradiation, growth factor withdrawal, and chemotherapeutic drugs, the release of cytochrome c from mitochondria leads to apoptosome formation and activation of procaspase-9 (53). Subsequently, procaspase-9 cleaves effector caspase-3 and -7, which, correspondingly, initiate the death cascade. There are reports pointing toward a role of procaspase-2 in genotoxic stress acting upstream of mitochondria; however, this question requires further clarification (57, 58).
Cellular inhibitors of caspases.
The action of caspases is regulated on several levels, including blockade of activation of caspases at the DISC as well as inhibition of enzymatic caspase activity (Figure 3). c-FLIP proteins are well-known inhibitors of death receptor–induced apoptosis (44, 59, 60). c-FLIPs possess 2 tandem DEDs at their N termini that facilitate their recruitment to the DISC. There are 3 c-FLIP isoforms described on the protein level, c-FLIPL, c-FLIPS, and c-FLIPR (61). Under conditions of overexpression, all isoforms inhibit activation of procaspase-8 at the DISC by blocking its processing (62) (Figure 3). At the same time, there is increasing evidence that c-FLIPL, when present at the DISC at low concentrations, facilitates the cleavage of procaspase-8 at the DISC by forming c-FLIPL–procaspase-8 heterodimers (45, 63).
The IAP (inhibitor of apoptosis) family of proteins includes 8 mammalian family members, including XIAP, c-IAP1, c-IAP2, and ML-IAP/livin (64–66). They specifically inhibit the initiator caspase-9 and the effector caspase-3 and -7 (Figure 3). The functional unit in IAP is the baculoviral IAP repeat (BIR), which contains about 80 amino acids folded around a central zinc atom. XIAP, c-IAP-1, and c-IAP2 contain 3 BIR domains each. The third BIR domain (BIR3) is involved in interactions with caspase-9 resulting in the inhibition of its activity (67). The linker region between BIR1 and BIR2 selectively targets caspase-3 and -7. The activity of IAPs is regulated by Smac/DIABLO, a structural homologue of the Drosophila proteins Reaper, Hid, and Grim (68, 69) (Figure 3). Smac/DIABLO is released from mitochondria and inhibits IAPs, which facilitates caspase activation during apoptosis. Omi/HtrA2 has been recently identified as another modulator of IAP function (70). Omi/HtrA2 is a mitochondria-located serine protease, which is released into the cytosol and inhibits IAPs by a mechanism similar to that of Smac.
IAPs are not the only natural inhibitors of caspases. The baculoviral p35 protein is a pan-caspase inhibitor, and it targets most caspases, in contrast to IAPs, which affect only caspase-3, -7, and -9 (71). The mechanism of caspase inhibition by p35 involves the formation of an inhibitory complex that is characterized by a protected thioester link between the caspase and p35 (72). Structural analysis of the inhibitory complex between p35 and caspase-8 reveals a unique active-site conformation that protects the intermediary thioester link from hydrolysis. Another pan-caspase inhibitor, serpin CrmA, is derived from the cowpox virus (73). The mechanism of CrmA inhibition is likely to involve covalent modification of the caspase active center.
Caspases as targets in drug development
Caspases, being the key effector molecules in apoptosis, are potential targets for pharmacological modulation of cell death (Figure 3). First, increased levels of caspase activity are often observed at sites of cellular damage in a number of diseases, including myocardial infarction, stroke, sepsis, and Alzheimer, Parkinson, and Huntington diseases. Inhibition of caspase activity for these diseases is predicted to be therapeutically beneficial. Second, discovery of drugs that selectively inhibit inflammatory caspases (caspase-1, -4, and -5) may help to control autoimmune diseases like rheumatoid arthritis. Finally, selective activation of caspases would be an approach in the treatment of cancer and chronic viral infections.
The approach of direct caspase inhibition is currently in the center of investigations. Proof-of-concept data have been obtained in several animal models, using suboptimal peptidyl inhibitors of caspases, such as zVAD-fmk, which shows substantial protection in rodent models of stroke, myocardial infarction, hepatic injury, sepsis, amyotrophic lateral sclerosis, and several other diseases (74–76). Furthermore, so-called small-molecule inhibitors of caspases, which have a nonpeptidyl origin, have been developed. One of these caspase inhibitors, IDN6556 (Pfizer), is already in phase II of clinical studies. It is a broad-spectrum caspase inhibitor that forms irreversible adducts with cysteine residues from the active site of caspases (77). IDN6556 is considered to be a candidate for the treatment of acute–tissue injury diseases, which are characterized by excessive apoptosis. This inhibitor is currently being tested in treatment of liver diseases, including HCV and ischemia/reperfusion injury in liver transplantation.
Among the inhibitors of inflammatory caspases, VX-740 (Vertex Pharmaceuticals Inc.) is also in phase II of clinical studies (78). It is a selective and reversible inhibitor of caspase-1 that is developed for treatment of rheumatoid arthritis.
Another promising strategy involves selective activation of caspases in cancer cells, leading to induction of apoptosis. An important contribution to this strategy was achieved by the approach of “forward chemical genetics” (79). This involves screening of small molecules for their ability to perturb cellular pathways and subsequently identifying the specific targets of the active compounds. A number of potential drugs for selective induction of apoptosis were found by high-throughput screening of the compounds activating caspase-3 as a central suicide caspase. Among them are a small molecule, PETCM [α-(trichloromethyl)-4-pyridine ethanol] (80); carbamate and indolone classes of compounds (81); and MX2167, MX116407, MX128504, and MX90745 (82). These compounds engage different pathways; e.g., PETCM accelerates apoptosome formation by interacting with the inhibitor prothymosin-α (80); carbamate and indolone classes of compounds promote Apaf-1 oligomerization and, thereby, apoptosome formation with the subsequent activation of caspase-3 and -9 (81); the action of MX2167 is mediated via the transferrin receptor that is highly overexpressed in a number of tumors; MX116407 is a vascular targeting agent; and MX128504 targets a novel cytoplasmic protein affecting IGF growth signaling pathways. At the same time, all these drugs are characterized by selective induction of apoptosis, and some of them are in preclinical and phase I studies.
Strategies to selectively target cancer cells also involve the application of antibodies coupled to active caspases. There were reports on a chimeric protein, referred to as immunocasp-3, that comprises a single-chain anti–erbB2/HER2 antibody, a translocation domain of Pseudomonas exotoxin A, and constitutively active caspase-3 (83). Immunocasp-3 was shown to selectively bind to erbB2/HER2–overexpressing cancer cells, followed by its internalization and lysosomal cleavage. As a result a COOH-terminal caspase-3–containing fragment is released, which translocates to the cytosol and induces apoptosis. Following this study there was a report on erbB2/HER2–targeted immunocasp-6, which can directly cleave lamin A, leading to nuclear damage, and induce programmed cell death (84). These studies provide the platform for the development of novel therapeutic protocols against tumors that overexpress erbB2/HER2.
In addition to direct targeting of caspases, other strategies involve modulation of cellular caspase inhibitors, such as IAPs, c-FLIPs, and Smac (Figure 3). Some cancers are characterized by overexpression of IAPs that are associated with resistance to apoptosis. The prototypic example is melanoma IAP (MILAP, also known as livin/KIAP), which is found at high levels in melanomas (66). Therefore, strategies of downregulating IAPs play an important role, as this would result in caspase activation and subsequent apoptosis induction in cancer cells. XIAP antisense molecules can directly induce apoptosis as well as sensitize cells to chemotherapy and irradiation (85). Antisense XIAP oligonucleotides are currently in phase I clinical studies.
Another approach to target IAPs is the identification of small molecules that bind to IAPs and prevent their inhibition of caspases. There are 3 main screening strategies to search for IAP antagonists. First, peptides that structurally resemble the N terminus of Smac were shown to bind to the BIR3 pocket of XIAP, subsequently leading to inhibition of XIAP (86). The development of these peptides is promising; however, further work is required to determine whether these peptides can induce apoptosis or sensitize cells to chemotherapy. In a second strategy, phage display was used to identify XIAP-binding peptides. This screen identified peptides unrelated to Smac that bind to the BIR2 domain and can directly induce cell death in leukemia cells (87). In a third approach, biochemically based assays were used to identify small molecules and peptides that inhibit XIAP. The screening was based on the reversed XIAP-mediated repression of caspase-3. Through these screens, both peptidyl and nonpeptidyl XIAP inhibitors were identified (85, 88). The small molecules identified by Wu and colleagues appear to be capable of sensitizing TRAIL-resistant cells toward TRAIL-induced apoptosis.
In addition to targeting of IAPs in cancer, IAPs might be used in gene therapy strategies to reduce neuronal cell death that is followed by stroke or brain injury. Virus-mediated delivery of IAP into the brain preserved neurons in rodent models of stroke and other types of experimental injury (89). These data indicate that modulation of IAP action might be also used in treating neurological diseases.
Given the current status of research on caspases as targets for pharmacological manipulation, the value of certain strategies is variable. Notably, despite the attraction of using caspase inhibitors, this approach might be ineffective. Blocking caspases often results in triggering of caspase-independent cell death accompanied by the release of cytochrome c, AIF, and EndoG from mitochondria. Thus, how to prevent cross-talk between different pathways involved is not really clear. Strategies of selective activation of caspases in cancer cells by various drugs or site-directed delivery, however, may be more promising and might find their way into the clinic. Moreover, certain compounds might be applied in combination with standard chemotherapy and are likely to be more efficient. Finally, targeting of IAPs by, e.g., IAP antagonists appears to be a potential clinical application and may become a tool in cancer therapy.
Acknowledgments
We would like to thank Lars Weingarten for critical comments, Heidi Sauter for excellent secretarial work, and the Wilhelm Sander Stiftung, the Sonderforschungsbereich 405, and the Tumorzentrum Heidelberg/Mannheim for supporting our work.
Footnotes
Nonstandard abbreviations used: BIR, baculoviral IAP repeat; CARD, caspase recruitment domain; c-FLIP, cellular FLICE-inhibitory protein; DD, death domain; DED, death effector domain; DISC, death-inducing signaling complex; DR, death receptor; FLIP, FLICE-inhibitory protein; fmk, fluoromethyl ketone; IAP, inhibitor of apoptosis.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 2.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 4.Krammer PH. The CD95(APO-1/Fas)/CD95L system [review] Toxicol. Lett. 1998;102–103:131–137. doi: 10.1016/s0378-4274(98)00297-5. [DOI] [PubMed] [Google Scholar]
- 5.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 6.Cohen GM. Caspases: the executioners of apoptosis. Biochem. J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 8.Alnemri ES, et al. Human ICE/CED-3 protease nomenclature [letter] Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 9.Weber CH, Vincenz C. The death domain superfamily: a tale of two interfaces [review]? Trends Biochem. Sci. 2001;26:475–481. doi: 10.1016/s0968-0004(01)01905-3. [DOI] [PubMed] [Google Scholar]
- 10.Martinon F, Hofmann K, Tschopp J. The pyrin domain: a possible member of the death domain-fold family implicated in apoptosis and inflammation. Curr. Biol. 2001;11:R118–R120. doi: 10.1016/s0960-9822(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 11.Fesik SW. Insights into programmed cell death through structural biology. Cell. 2000;103:273–282. doi: 10.1016/s0092-8674(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann K. The modular nature of apoptotic signaling proteins. Cell. Mol. Life Sci. 1999;55:1113–1128. doi: 10.1007/s000180050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberstadt M, et al. NMR structure and mutagenesis of the FADD (Mort1) death-effector domain. Nature. 1998;392:941–945. doi: 10.1038/31972. [DOI] [PubMed] [Google Scholar]
- 14.Muzio M, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death–inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 15.Sprick M, et al. Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8. EMBO J. 2002;21:4520–4530. doi: 10.1093/emboj/cdf441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem. J. 2004;384:201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamkanfi M, et al. A novel caspase-2 complex containing TRAF2 and RIP1. J. Biol. Chem. 2005;280:6923–6932. doi: 10.1074/jbc.M411180200. [DOI] [PubMed] [Google Scholar]
- 18.Walker NP, et al. Crystal structure of the cysteine protease interleukin-1 beta-converting enzyme: a (p20/p10)2 homodimer. Cell. 1994;78:343–352. doi: 10.1016/0092-8674(94)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Wilson KP, et al. Structure and mechanism of interleukin-1 beta converting enzyme. Nature. 1994;370:270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- 20.Schweizer A, Briand C, Grutter MG. Crystal structure of caspase-2, apical initiator of the intrinsic apoptotic pathway. J. Biol. Chem. 2003;278:42441–42447. doi: 10.1074/jbc.M304895200. [DOI] [PubMed] [Google Scholar]
- 21.Rotonda J, et al. The three-dimensional structure of apopain/CPP32, a key mediator of apoptosis. Nat. Struct. Biol. 1996;3:619–625. doi: 10.1038/nsb0796-619. [DOI] [PubMed] [Google Scholar]
- 22.Mittl PR, et al. Structure of recombinant human CPP32 in complex with the tetrapeptide acetyl-Asp-Val-Ala-Asp fluoromethyl ketone. J. Biol. Chem. 1997;272:6539–6547. doi: 10.1074/jbc.272.10.6539. [DOI] [PubMed] [Google Scholar]
- 23.Riedl SJ, et al. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 24.Wei Y, et al. The structures of caspases-1, -3, -7 and -8 reveal the basis for substrate and inhibitor selectivity. Chem. Biol. 2000;7:423–432. doi: 10.1016/s1074-5521(00)00123-x. [DOI] [PubMed] [Google Scholar]
- 25.Chai J, et al. Structural basis of caspase-7 inhibition by XIAP. Cell. 2001;104:769–780. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, et al. Structural basis of caspase inhibition by XIAP: differential roles of the linker versus the BIR domain. Cell. 2001;104:781–790. [PubMed] [Google Scholar]
- 27.Blanchard H, et al. The three-dimensional structure of caspase-8: an initiator enzyme in apoptosis. Structure Fold. Des. 1999;7:1125–1133. doi: 10.1016/s0969-2126(99)80179-8. [DOI] [PubMed] [Google Scholar]
- 28.Renatus M, Stennicke HR, Scott FL, Liddington RC, Salvesen GS. Dimer formation drives the activation of the cell death protease caspase 9. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14250–14255. doi: 10.1073/pnas.231465798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eichinger A, et al. Crystal structure of gingipain R: an Arg-specific bacterial cysteine proteinase with a caspase-like fold. EMBO J. 1999;18:5453–5462. doi: 10.1093/emboj/18.20.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornberry NA, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 31.Boatright KM, et al. A unified model for apical caspase activation. Mol. Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 33.Graybill TL, Dolle RE, Helaszek CT, Miller RE, Ator MA. Preparation and evaluation of peptidic aspartyl hemiacetals as reversible inhibitors of interleukin-1 beta converting enzyme (ICE) Int. J. Pept. Protein Res. 1994;44:173–182. doi: 10.1111/j.1399-3011.1994.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 34.Estrov Z, et al. Effect of interleukin-1 beta converting enzyme inhibitor on acute myelogenous leukemia progenitor proliferation. Blood. 1995;86:4594–4602. [PubMed] [Google Scholar]
- 35.Thornberry NA, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 36.Chang DW, et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 38.Kischkel FC, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scaffidi C, Medema JP, Krammer PH, Peter ME. FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b. J. Biol. Chem. 1997;272:26953–26958. doi: 10.1074/jbc.272.43.26953. [DOI] [PubMed] [Google Scholar]
- 40.Scaffidi C, et al. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J. Biol. Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- 41.Scaffidi C, Krammer PH, Peter ME. Isolation and analysis of components of CD95 (APO-1/Fas) death-inducing signaling complex. Methods. 1999;17:287–291. doi: 10.1006/meth.1999.0742. [DOI] [PubMed] [Google Scholar]
- 42.Scaffidi C, Kischkel FC, Krammer PH, Peter ME. Analysis of the CD95 (APO-1/Fas) death-inducing signaling complex (DISC) by high resolution two-dimensional gel electrophoresis. Methods Enzymol. 1999;322:363–373. doi: 10.1016/s0076-6879(00)22033-8. [DOI] [PubMed] [Google Scholar]
- 43.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 44.Golks A, Brenner D, Fritsch C, Krammer PH, Lavrik IN. C-FLIPR: a new regulator of death receptor-induced apoptosis. J. Biol. Chem. 2005;280:14507–14513. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- 45.Chang DW, Xing Z, Capacio VL, Peter ME, Yang X. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 2003;22:4132–4142. doi: 10.1093/emboj/cdg414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. An induced proximity model for caspase-8 activation. J. Biol. Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 47.Martin DA, Siegel RM, Zheng L, Lenardo MJ. Membrane oligomerization and cleavage activates the caspase-8 (FLICE/MACHalpha1) death signal. J. Biol. Chem. 1998;273:4345–4349. doi: 10.1074/jbc.273.8.4345. [DOI] [PubMed] [Google Scholar]
- 48.Lavrik I, et al. The active caspase-8 heterotetramer is formed at the CD95 DISC. Cell Death Differ. 2003;10:144–145. doi: 10.1038/sj.cdd.4401156. [DOI] [PubMed] [Google Scholar]
- 49.Kischkel FC, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J. Biol. Chem. 2001;276:46639–46646. doi: 10.1074/jbc.M105102200. [DOI] [PubMed] [Google Scholar]
- 50.Acehan D, et al. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol. Cell. 2002;9:423–432. doi: 10.1016/s1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- 51.Hengartner MO. Programmed cell death in the nematode C. elegans [review] Recent Prog. Horm. Res. 1999;54:213–222; discussion 222–214. [PubMed] [Google Scholar]
- 52.Chinnaiyan AM. The apoptosome: heart and soul of the cell death machine. Neoplasia. 1999;1:5–15. doi: 10.1038/sj.neo.7900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang X, Wang X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J. Biol. Chem. 2000;275:31199–31203. doi: 10.1074/jbc.C000405200. [DOI] [PubMed] [Google Scholar]
- 54.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Scaffidi C, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korsmeyer SJ, et al. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 57.Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S. Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J. Biol. Chem. 2002;277:29803–29809. doi: 10.1074/jbc.M204185200. [DOI] [PubMed] [Google Scholar]
- 58.Lassus P, Opitz-Araya X, Lazebnik Y. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science. 2002;297:1352–1354. doi: 10.1126/science.1074721. [DOI] [PubMed] [Google Scholar]
- 59.Thome M, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 60.Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 1999;274:1541–1548. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 61.Golks A, Brenner D, Fritsch C, Krammer PH, Lavrik IN. C-FLIPR: a new regulator of death receptor-induced apoptosis. J. Biol. Chem. 2005;280:14507–14513. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- 62.Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular flice-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the cd95 death-inducing signaling complex. J. Biol. Chem. 2001;276:20633–20640. doi: 10.1074/jbc.M101780200. [DOI] [PubMed] [Google Scholar]
- 63.Micheau O, et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J. Biol. Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 64.Deveraux QL, Reed JC. IAP family proteins: suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 65.Ashhab Y, Alian A, Polliack A, Panet A, Ben Yehuda D. Two splicing variants of a new inhibitor of apoptosis gene with different biological properties and tissue distribution pattern. FEBS Lett. 2001;495:56–60. doi: 10.1016/s0014-5793(01)02366-3. [DOI] [PubMed] [Google Scholar]
- 66.Vucic D, Stennicke HR, Pisabarro MT, Salvesen GS, Dixit VM. ML-IAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomas. Curr. Biol. 2000;10:1359–1366. doi: 10.1016/s0960-9822(00)00781-8. [DOI] [PubMed] [Google Scholar]
- 67.Srinivasula SM, et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 68.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 69.Ekert PG, Silke J, Hawkins CJ, Verhagen AM, Vaux DL. DIABLO promotes apoptosis by removing MIHA/XIAP from processed caspase 9. J. Cell Biol. 2001;152:483–490. doi: 10.1083/jcb.152.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki Y, et al. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell. 2001;8:613–621. doi: 10.1016/s1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- 71.Miller LK. An exegesis of IAPs: salvation and surprises from BIR motifs. Trends Cell Biol. 1999;9:323–328. doi: 10.1016/s0962-8924(99)01609-8. [DOI] [PubMed] [Google Scholar]
- 72.Xu G, et al. Covalent inhibition revealed by the crystal structure of the caspase-8/p35 complex. Nature. 2001;410:494–497. doi: 10.1038/35068604. [DOI] [PubMed] [Google Scholar]
- 73.Renatus M, et al. Crystal structure of the apoptotic suppressor CrmA in its cleaved form. Structure Fold. Des. 2000;8:789–797. doi: 10.1016/s0969-2126(00)00165-9. [DOI] [PubMed] [Google Scholar]
- 74.Endres M, et al. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J. Cereb. Blood Flow Metab. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Wiessner C, Sauer D, Alaimo D, Allegrini PR. Protective effect of a caspase inhibitor in models for cerebral ischemia in vitro and in vivo. Cell. Mol. Biol. (Noisy-le-grand). 2000;46:53–62. [PubMed] [Google Scholar]
- 76.Rabuffetti M, et al. Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of proinflammatory cytokines. J. Neurosci. 2000;20:4398–4404. doi: 10.1523/JNEUROSCI.20-12-04398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoglen NC, et al. Characterization of IDN-6556 (3-{2-(2-tert-butyl-phenylaminooxalyl)-amino]-propionylamino}-4-oxo-5-(2,3,5,6-tetrafluoro-phenoxy)-pentanoic acid): a liver-targeted caspase inhibitor. J. Pharmacol. Exp. Ther. 2004;309:634–640. doi: 10.1124/jpet.103.062034. [DOI] [PubMed] [Google Scholar]
- 78.Leung-Toung R, Li W, Tam TF, Karimian K. Thiol-dependent enzymes and their inhibitors: a review. Curr. Med. Chem. 2002;9:979–1002. doi: 10.2174/0929867024606704. [DOI] [PubMed] [Google Scholar]
- 79.Stockwell BR. Chemical genetics: ligand-based discovery of gene function. Nat. Rev. Genet. 2000;1:116–125. doi: 10.1038/35038557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang X, et al. Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science. 2003;299:223–226. doi: 10.1126/science.1076807. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen JT, Wells JA. Direct activation of the apoptosis machinery as a mechanism to target cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7533–7538. doi: 10.1073/pnas.1031631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maxim Pharmaceuticals. 2004. Annual Report 2004.
- 83.Jia LT, et al. Specific tumoricidal activity of a secreted proapoptotic protein consisting of HER2 antibody and constitutively active caspase-3. Cancer Res. 2003;63:3257–3262. [PubMed] [Google Scholar]
- 84.Xu YM, et al. A caspase-6 and anti-human epidermal growth factor receptor-2 (HER2) antibody chimeric molecule suppresses the growth of HER2-overexpressing tumors. J. Immunol. 2004;173:61–67. doi: 10.4049/jimmunol.173.1.61. [DOI] [PubMed] [Google Scholar]
- 85.Schimmer AD, et al. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 2004;5:25–35. doi: 10.1016/s1535-6108(03)00332-5. [DOI] [PubMed] [Google Scholar]
- 86.Glover CJ, et al. A high-throughput screen for identification of molecular mimics of Smac/DIABLO utilizing a fluorescence polarization assay. Anal. Biochem. 2003;320:157–169. doi: 10.1016/s0003-2697(03)00389-0. [DOI] [PubMed] [Google Scholar]
- 87.Tamm I, et al. Peptides targeting caspase inhibitors. J. Biol. Chem. 2003;278:14401–14405. doi: 10.1074/jbc.M210133200. [DOI] [PubMed] [Google Scholar]
- 88.Wu TY, Wagner KW, Bursulaya B, Schultz PG, Deveraux QL. Development and characterization of nonpeptidic small molecule inhibitors of the XIAP/caspase-3 interaction. Chem. Biol. 2003;10:759–767. doi: 10.1016/s1074-5521(03)00157-1. [DOI] [PubMed] [Google Scholar]
- 89.Reed J. Apoptosis-based therapies. Nat. Rev. Drug Discov. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]