Abstract
The Akt and Pim kinases are cytoplasmic serine/threonine kinases that control programmed cell death by phosphorylating substrates that regulate both apoptosis and cellular metabolism. The PI3K-dependent activation of the Akt kinases and the JAK/STAT–dependent induction of the Pim kinases are examples of partially overlapping survival kinase pathways. Pharmacological manipulation of such kinases could have a major impact on the treatment of a wide variety of human diseases including cancer, inflammatory disorders, and ischemic diseases.
Introduction
There is increasing evidence that serine/threonine kinases exist that directly regulate cell survival. Therapeutics that directly target these survival kinases have not yet been developed for clinical use. Activated survival kinases contribute to the pathogenesis of a wide variety of malignancies. In addition, reduced survival kinase signaling may contribute to organ damage following ischemic insults. Selective therapies such as imatinib (1) and gefitinib (2) elicit tumor cell death by indirect inactivation of survival kinases. Would direct inhibition of survival kinases result in better therapeutic efficacy? Alternatively, could therapies that activate survival kinases lead to better organ preservation in ischemic diseases? Many drug discovery programs have begun to develop lead compounds to address these questions. This Review will explore the potential risks and benefits of targeting survival kinases by outlining (a) Akt and Pim kinase action in malignancy, immunity, and vascular disease, (b) the common substrates that survival kinases share, (c) recent advances in the understanding of survival kinase regulation, and (d) investigational agents that target survival kinases.
Kinases that promote cell survival and control cell metabolism
For this Review survival kinases will be defined as cytoplasmic serine/threonine kinases that phosphorylate substrates that collectively contribute to the control of the programmed cell death machinery and cellular metabolism (Figure 1). This coordinated control ensures the maintenance of mitochondrial membrane potential and prevents the mitochondrial release of cytochrome c and other proapoptotic mediators. This coordinated control also maintains cellular ATP production, preventing cells from dying by necrosis (3) or autophagy (4). The best-characterized survival kinases were identified in screens to find suppressors of myc-induced apoptosis. myc is a protooncogene whose overexpression leads to increased proliferation as well as increased apoptosis in nonmalignant cells. Defects in pathways that control apoptosis prevent myc-induced apoptosis and allow myc to act as an oncogene, leading to a malignant phenotype. While deficiency in the tumor suppressor gene p53 and constitutive activation of the antiapoptosis gene bcl-2 are well characterized events that block myc-induced apoptosis, screens using retroviral mutagenesis have uncovered several serine/threonine kinases, including the Akt (5) and Pim (6) kinases, as potent suppressors of myc-induced apoptosis. As described below, these kinases coordinately regulate both apoptosis and cellular metabolism. The ability to reproducibly suppress the strong apoptotic stimulus of myc expression might serve as a criterion to identify other survival kinases.
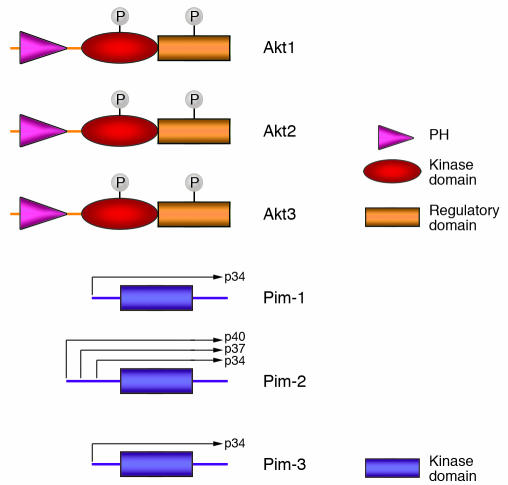
Figure 1.
Domain structure of the Akt and Pim kinases. The structures of human Akt1, Akt2, and Akt3 consist of a pleckstrin homology domain (PH) that binds to PIP3 at membrane surfaces, the kinase domain, and the regulatory domain. The 2 phosphorylation sites necessary for Akt activation are shown. The structures of human Pim-1, Pim-2, and Pim-3 demonstrate a conserved kinase domain and no regulatory domain. There are no required phosphorylation sites for Pim activation. Alternate start codons are depicted in Pim-2 leading to multiple Pim-2 isoforms that retain kinase activity.
Another characteristic of survival kinases is that they are activated by extracellular survival signals through cell surface receptors. Most receptors that can promote cell survival engage multiple signal transduction pathways. Many signaling pathways associated with activated receptor tyrosine kinases — including Src, phospholipase Cγ (PLCγ), and Ras/Raf/MEK/MAPK signaling — appear to promote cell survival. However, the central role of PI3K and Akt in receptor-mediated regulation of cell survival has been demonstrated in a variety of cell types. For example, in VSMCs expressing a number of PDGFR genes that are mutant for 1 or multiple binding sites necessary to activate the Src, Ras, PLCγ, or PI3K signaling pathways, growth factor–induced activation of PI3K/Akt signaling is the only kinase pathway that can prevent cell death induced by diverse stimuli when other kinase pathways are inactivated (7). These findings suggest that many kinase signaling pathways impact cell survival by direct or indirect contributions to PI3K/Akt signaling.
Another family of kinases that satisfies the criteria for survival kinases, and whose function does not appear to be dependent on PI3K/Akt signaling, is the Pim kinase family. The Pim kinases were originally implicated in cell survival by their ability to suppress myc-induced apoptosis in a mouse model of lymphoma (6, 8). Unlike the other serine/threonine kinases mentioned thus far, these kinases are not regulated by membrane recruitment or phosphorylation. The Pim kinases are unusual in that they are regulated primarily by transcription. Activated cytokine receptors recruit JAKs to induce STAT-dependent transcription of the Pim genes. While the role of Akt in promoting the survival of both normal and malignant cells is well established, the role of Pim signaling for cell survival in nontransformed cells has only recently been identified (9).
Although there are numerous pharmacological agents in preclinical and clinical development that can induce cell death by targeting other serine/threonine kinases, discussion of these agents and their targets is beyond the scope of this Review. We will focus on the structure, activation, and pharmacological manipulation of kinases that promote cell survival through the PI3K/Akt and JAK/STAT/Pim pathways.
Structure and regulation of survival kinase activation
There are 8 mammalian isoforms of PI3K, separated into class IA, class IB, class II, and class III. Class I PI3Ks are the only kinases that generate phosphatidylinositide 3,4,5-triphosphate (PIP3). The most common form of class I PI3K associated with receptor tyrosine kinases is a heterodimer consisting of a p110 catalytic subunit and a p85 regulatory subunit. There are 3 p110 isoforms, p110α, p110β, and p110δ, the first 2 of which are widely expressed and the third of which is restricted to lymphocytes (10).
There are 3 isoforms of both Akt and Pim kinases, each encoded by distinct genes (Figure 1). The 3 Akt isoforms, Akt1, Akt2, and Akt3, share a highly conserved pleckstrin homology domain that binds these kinases to membrane surfaces through PIP3. They have highly conserved catalytic and regulatory domains that must undergo phosphorylation for complete kinase activation. When overexpressed and constitutively membrane-localized through myristoylation, all 3 isoforms have the capacity to transform cells in vitro and in vivo (11). Most tissues express all 3 isoforms but at variable levels.
The regulation of Akt is primarily posttranslational. Once translated, the Akt kinases bind to heat shock protein 90 (Hsp90), protecting the inactive Akt proteins from proteosomal degradation (12). Akt is recruited to the cell membrane through PIP3 produced by the lipid kinase activity of PI3K (for review see ref. 13). PI3K is directly associated with many cell surface growth factor and cytokine receptors, and upon ligand binding, PI3K activation generates PIP3. In addition to Akt, PIP3 recruits phosphoinositide-dependent kinase-1 (PDK1) and integrin-linked kinase (ILK) to the cell membrane. Generation of PIP3 is negatively regulated by the activity of phosphatase and tensin homolog (PTEN). PTEN deletion is the most common mechanism of inappropriate Akt activation in human malignancy (14). Akt activation requires 2 phosphorylation events: (a) PDK1 phosphorylation of Akt, and (b) phosphorylation of Akt by a kinase activity referred to as PDK2. Candidate kinases whose activities have been associated with PDK2 activity include ILK (15), DNA-dependent protein kinase (16), and PKCα (17, 18). Recently, the rictor–mammalian target of rapamycin (rictor-mTOR) complex has been suggested as the major contributor to PDK2 kinase activity (19). While ILK activity may contribute to Akt-dependent cell survival, ILK has Akt-independent survival functions as well. Activation of ILK by the cytoplasmic kinase domains of integrin and growth factor receptors (20) maintains cell structure through its cytoskeletal binding partners.
One PI3K-dependent survival kinase that does not phosphorylate Akt directly but augments the activity of Akt is the serum- and glucocorticoid-inducible kinase-1 (SGK1). SGK1 expression is regulated by the transcriptional activity of ligand-bound glucocorticoid receptor (21). In addition, diverse cellular insults such as osmotic stress, ultraviolet radiation, heat, and H2O2 result in induction of SGK1 expression (22). Although SGK1 does not require binding to PIP3, it is PI3K-dependent, because, like Akt, it depends on phosphorylation by PDK1 and PDK2 kinase activities for activation.
In contrast to the Akt kinases, the Pim kinases do not have a regulatory domain and, based on recent crystallography findings, are likely constitutively active when expressed (23). Pim kinase regulation occurs at the level of transcription, translation, and proteosomal degradation. In lymphocytes, upon cytokine engagement of its receptor, JAK phosphorylates and activates STAT proteins. Once phosphorylated, STATs translocate to the nucleus and serve as transcription factors for the Pim genes. In addition to transcriptional control, regulation of pim mRNA stability is also a determinant of Pim activity (24). Adding to the complexity of Pim regulation and activity is the fact that the gene for pim-2 encodes multiple proteins that have the same catalytic activity. Pim proteins are rapidly turned over by proteosomal degradation (25).
Survival kinases regulate common substrates
Once activated, the PI3K-dependent survival kinases and the Pim kinases phosphorylate common substrates that are involved in apoptosis and metabolism (Figure 2). Akt and Pim both directly phosphorylate and inactivate the proapoptotic Bcl-2 protein Bad (26–28). Both Akt and Pim kinases phosphorylate different components of proteins that are critical for maintaining a high rate of protein translation. For example, Akt phosphorylates TSC2, which controls mTOR activity, and mTOR and Pim kinases phosphorylate and inactivate the translational repressor 4EBP1. Akt and SGK1 act in concert to phosphorylate and inactivate FKHRL1, a transcription factor that upregulates proapoptotic Bcl-2 proteins such as Bim and death receptor components such as DR5 (29). Akt (30) and Pim kinases (31) regulate the IκB/NF-κB transcription factor complex by phosphorylating the serine/theronine kinase Cot (32), resulting in the proteasomal degradation of IκB, the activation of NF-κB, and the transcription of an array of antiapoptotic genes. Both Akt and Pim kinases also phosphorylate GSK3B, a regulator of cellular glucose metabolism. Both the Akt and the Pim kinase maintenance of cell survival is dependent on their ability to stimulate glucose uptake and metabolism (33, 34).
Figure 2.
Survival kinases regulate cell death through the phosphorylation of common substrates of the apoptotic machinery and cellular metabolism. PI3K generates PIP3, and PTEN converts PIP3 back to PIP2, negatively regulating PI3K signaling. PIP3 recruits PDK1, ILK, and Akt to the cell membrane. PDK1, ILK, and the rictor-mTOR complex are important for the activation of Akt. Expression of both SGK1 and Pim kinases is inducible. Akt, SGK1, and Pim kinases share common substrates of the apoptosis machinery and cellular metabolism, depicted as color-coded overlapping boxes (see text for full description). PFK2, phosphofructokinase-2.
In addition to these common substrates, Akt has the potential to inactivate 3 pathways of apoptosis initiation: (a) p53-mediated apoptosis, by phosphorylation and activation of MDM2, a protein that binds p53 and facilitates its degradation (35); (b) mitochondrial-dependent apoptosis, by phosphorylation and inactivation of caspase-9 (36) and Bad, and phosphorylation and stabilization of the antiapoptotic protein XIAP (37); and (c) death receptor–mediated apoptosis, by inhibition of the Forkhead family of transcription factors (38). Akt directly controls cellular metabolism by maintaining the association of hexokinase with mitochondria (39) and by phosphorylating and activating ATP-citrate lyase and phosphofructokinase-2 (40). Akt controls the translation of nutrient transporters through the activation of mTOR (41), and the localization of glucose transporters to the plasma membrane (42).
A number of drugs have emerged that target pathways downstream of both Akt and Pim signaling. One example is bortezomib, which inhibits proteasome function, leading to the inhibition of NF-κB, and is now used for multiple myeloma (43). Rapamycin derivatives, which inhibit mTOR, are now being developed for treatment of a broad range of solid tumor malignancies. Strategies such as RNA interference could be used to further validate other survival kinase substrates as pharmacological targets.
Pharmacological inhibition of survival kinases
The problem of multiple isoforms: PI3K and Akt.
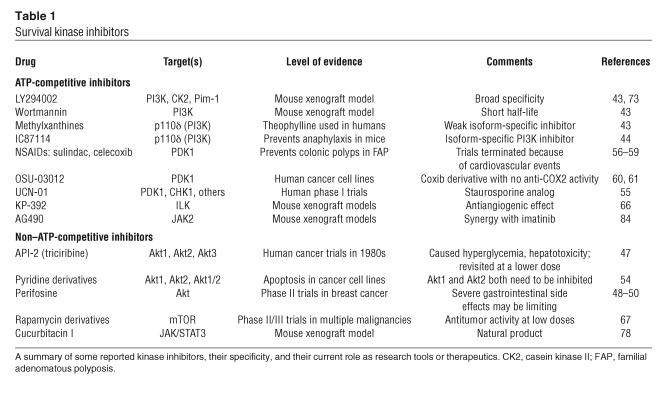
Class I PI3K inhibitors such as wortmannin and LY294002 (Table 1) compete at the ATP binding site of the lipid kinase catalytic domain of all PI3Ks. Although these inhibitors have shown some efficacy in xenograft tumor models, they have not been developed for clinical use because of the broad specificity of kinase inhibition, poor pharmacokinetics, and relatively weak inhibition (44). Moreover, the recent finding that LY294002 binds and inhibits the activity of Pim-1 suggests that LY294002 can no longer be considered a selective tool to study PI3K-dependent biology. Currently a number of initiatives are under way that have identified isoform-selective ATP-competitive inhibitors of the PI3K. A potential validation of PI3K as a targetable survival kinase comes in the form of IC87114, a p110δ-specific PI3K inhibitor, which has been shown to prevent anaphylaxis in a mouse model (45).
Table 1.
Survival kinase inhibitors
Development of a specific inhibitor of Akt has proven difficult because of issues of toxicity. Concerns have been raised about Akt’s role in insulin signaling, and the implications of chronic inhibition of this pathway. There is some evidence that the order of importance in insulin signaling for Akt isoforms is Akt2 > Akt1 > Akt3 (46, 47). Although mild hyperglycemia induced by an Akt inhibitor may be a tolerable side effect for cancer patients, drugs that induce overt diabetes in patients with refractory malignancy might not be seen as worthy of development. An example is the old drug API-2, also known as triciribine, which was tested in human cancer trials in the 1980s as a nucleoside analog. This drug was never fully developed, because it caused hepatotoxicity, hypertriglyceridemia, and hyperglycemia. More recently, this drug was shown to be a non–isoform-specific Akt inhibitor at much lower doses than previously tested (48). Another Akt inhibitor that has now entered phase II trials is the orally bioavailable alkylphospholipid perifosine. Perifosine likely interferes with proper Akt membrane localization, leading to Akt dephosphorylation (49). In phase I trials of perifosine for patients with refractory solid tumors, a few patients benefited from partial responses and disease stabilization with no evidence of hyperglycemia, but gastrointestinal side effects were dose-limiting for the majority of patients (50, 51).
Which Akt isoform would serve as the most effective target in human cancers is also an unanswered question. Gene amplification of AKT2 has been reported in pancreatic (52), breast, and ovarian cancers (53), while gene amplification of AKT3 was found to contribute to the progression of sporadic melanomas (54). Numerous discovery programs are actively engaged in identifying isoform-selective Akt inhibitors. A series of pyridine derivatives were found to selectively inhibit Akt1, or Akt1 and Akt2, by binding to the pleckstrin homology domain (55). Currently, no Akt3-specific inhibitors have been reported.
Single-isoform survival kinases PDK1, ILK, and mTOR.
PDK1 is a PI3K-dependent kinase that has been identified as the target of numerous inhibitors. Staurosporine analogs UCN-01 and CGP41251 inhibit PDK1, among other kinases, and have entered phase I cancer trials (56). Another example of an effective multitarget drug class that inhibits PDK1 is NSAIDs. NSAIDs such as sulindac (57) and celecoxib (58), a selective COX-2 inhibitor, were found to have the ability to induce apoptosis in colon cancer cells through inhibition of PDK1. A chemoprevention trial demonstrated that celecoxib reduced the incidence of polyp formation in patients with familial adenomatous polyposis (59). Unfortunately, further clinical evaluation of NSAIDs as chemopreventatives will require caution. An increased incidence of myocardial infarction and stroke in trial participants taking rofecoxib and high-dose celecoxib led to a moratorium on new chemopreventative trials with coxibs (COX-2 inhibitors) (60). Although the mechanism of increased thrombosis induced by COX-2 inhibitors remains unknown, many believe it is a COX-dependent phenomenon, opening the door for coxib-derived molecules that more selectively target PDK1 and not the COX enzymes. Recently, a coxib derivative, OSU-03012, which has no activity against COX enzymes, was found to inhibit PDK1/Akt activity in prostate cancer cells (61) and sensitize imatinib-resistant BCR-ABL clones to imatinib (62).
ILK, another PI3K-dependent kinase implicated in the activation of Akt, has been found to play an important role in malignant pathogenesis. Overexpression of ILK correlates with the clinical stage of many epithelial neoplasms (63–65). The importance of targeting ILK is further supported by the finding that ILK activity is essential for VEGF-dependent tumor angiogenesis (66). Two small-molecule inhibitors of ILK activity have been identified and are currently being developed for clinical trials (67).
While the rictor-mTOR complex responsible for Akt phosphorylation is rapamycin-insensitive, rapamycin-sensitive mTOR activity contributes to Akt-dependent cell survival. Rapamycin and the rapamycin derivatives CCI-779, RAD001, and AP23573 are currently in multiple phase II and phase III clinical trials for both solid tumors and hematological malignancies (68) and have been found to be most effective in tumors with PTEN deletion or Akt activation; this suggests that mTOR inhibitors act to suppress PI3K/Akt–induced cell survival.
Novel targets: Pim kinases.
Pim overexpression has been reported in diffuse B cell lymphoma, chronic lymphocytic leukemia, and prostate cancer, and FLT3-mediated acute myelogenous leukemia (69–72). Besides cancer, Pim kinase activity has been shown to be important in the pathogenesis of vascular smooth muscle proliferation in vessel injury models (73). The only reported inhibitor of Pim function is LY294002, which was originally identified as a specific PI3K inhibitor (74). As knockout of all 3 pim genes leads to a mild phenotype, isoform-specific Pim inhibitors may not be necessary to avoid toxicity (75).
Targeting of the JAK/STAT pathway upstream of Pim expression is an active area of drug discovery. Recently, multiple groups have identified activating JAK2 mutations in a large number of patients with myeloproliferative disorders (76–78). A natural product, cucurbitacin I, was found to specifically inhibit JAK/STAT3 signaling and lead to tumor cell death in a xenograft model (79). Further work is necessary to understand the relative contribution of Pim inhibition to the therapeutic efficacy of JAK/STAT inhibitors.
Activating survival kinases to preserve organ function
A few strategies involving survival kinase activation have emerged to address the problem of postischemic cardiac remodeling leading to heart failure. In rodent models of myocardial infarction, injection of bone marrow–derived mesenchymal stem cells expressing constitutively activated Akt (80) and injection of purified thymosin B4 (81), a naturally secreted peptide that activates ILK, were both found to enhance cardiomyocyte survival and organ function. These data suggest that pharmacological activation of survival kinase activity for postinfarct remodeling could be a promising strategy. The empirical benefit of glucocorticoids, such as prednisone or dexamethasone, seen in a variety of diseases involving epithelial injury may in fact be an unappreciated example of this strategy. In addition to suppression of an exuberant immune response, glucocorticoid activation of SGK1 in epithelial cells may contribute to organ preservation in scenarios such as radiation injury.
In contrast to pharmacological activation of survival kinases, pharmacological inhibition of protein phosphatases could be another fruitful strategy for organ preservation. Inhibition of PTEN or PP2A could be a means of activating Akt pharmacologically. Phosphatase structure and regulation are complex, however. Recently, α4, a noncatalytic subunit of PP2A, was shown to be essential for cell survival, which suggests that certain conformations of phosphatase activity can mimic survival kinase function (82). Further consideration of the regulation of protein phosphatases is required before they can be pursued as therapeutic targets.
Finally, the PPARs are intriguing candidates for inducing Akt activation. PPARβ/δ was recently shown to coordinately upregulate PDK1 and ILK while downregulating expression of PTEN in keratinocytes, which implicates this nuclear receptor as an Akt regulator (83). Currently, selective PPARβ/δ agonists such as GW1505 are being developed for treatment of mucosal injury.
Conclusions
Recent advances in survival kinase structure and regulation have identified many potential targets for novel agents that can pharmacologically manipulate cell death. In turn, the basic understanding of the complexities of PI3K/Akt signaling with relation to cell survival will be enriched by the emergence of a new generation of more specific kinase inhibitors. The role of Pim kinases in cell survival will be better understood with the development of specific inhibitors. Evidence already exists to suggest that inhibitors of survival kinases can contribute to cancer therapy. Therapies designed to activate survival kinases may also be useful in preventing organ damage following injury. As our understanding of programmed cell death evolves, additional kinases that contribute to the regulation of cell survival will undoubtedly emerge.
Acknowledgments
We thank Casey Fox and Peter Hammerman for numerous helpful comments. R. Amaravadi is supported by NIH grant R25-CA87812.
Footnotes
Nonstandard abbreviations used: ILK, integrin-linked kinase; mTOR, mammalian target of rapamycin; PDK, phosphoinositide-dependent kinase; PIP3, phosphatidylinositide 3,4,5-triphosphate; PLCγ, phospholipase Cγ; PTEN, phosphatase and tensin homolog; SGK1, serum- and glucocorticoid-inducible kinase-1.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Kawauchi K, Ogasawara T, Yasuyama M, Ohkawa S. Involvement of Akt kinase in the action of STI571 on chronic myelogenous leukemia cells. Blood Cells Mol. Dis. 2003;31:11–17. doi: 10.1016/s1079-9796(03)00070-6. [DOI] [PubMed] [Google Scholar]
- 2.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 3.Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–1282. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J. Clin. Invest. 2005;115:2679–2688. doi:10.1172/JCI26390. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wendel HG, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 6.van Lohuizen M, et al. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989;56:673–682. doi: 10.1016/0092-8674(89)90589-8. [DOI] [PubMed] [Google Scholar]
- 7.Vantler M, Caglayan E, Zimmermann WH, Baumer AT, Rosenkranz S. Systematic evaluation of anti-apoptotic growth factor signaling in vascular smooth muscle cells: only phosphatidylinositol 3’-kinase is important. J. Biol. Chem. 2005;280:14168–14176. doi: 10.1074/jbc.M413310200. [DOI] [PubMed] [Google Scholar]
- 8.Hammerman PS, Fox CJ, Birnbaum MJ, Thompson CB. The Pim and Akt oncogenes are independent regulators of hematopoietic cell growth and survival. Blood. 2005;105:4477–4483. doi: 10.1182/blood-2004-09-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox CJ, Hammerman PS, Thompson CB. The Pim kinases control rapamycin-resistant T cell survival and activation. J. Exp. Med. 2005;201:259–266. doi: 10.1084/jem.20042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fruman DA. Towards an understanding of isoform specificity in phosphoinositide 3-kinase signalling in lymphocytes. Biochem. Soc. Trans. 2004;32:315–319. doi: 10.1042/bst0320315. [DOI] [PubMed] [Google Scholar]
- 11.Mende I, Malstrom S, Tsichlis PN, Vogt PK, Aoki M. Oncogenic transformation induced by membrane-targeted Akt2 and Akt3. Oncogene. 2001;20:4419–4423. doi: 10.1038/sj.onc.1204486. [DOI] [PubMed] [Google Scholar]
- 12.Munster PN, Marchion DC, Basso AD, Rosen N. Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3′-kinase-AKT-dependent pathway. Cancer Res. 2002;62:3132–3137. [PubMed] [Google Scholar]
- 13.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 14.Ali IU, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J. Natl. Cancer Inst. 1999;91:1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- 15.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat. Rev. Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 2004;279:41189–41196. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 17.Partovian C, Simons M. Regulation of protein kinase B/Akt activity and Ser473 phosphorylation by protein kinase Calpha in endothelial cells. Cell. Signal. 2004;16:951–957. doi: 10.1016/j.cellsig.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda T, Guo L, Shi X, Wu C. CH-ILKBP regulates cell survival by facilitating the membrane translocation of protein kinase B/Akt. J. Cell Biol. 2003;160:1001–1008. doi: 10.1083/jcb.200212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda T, Chen K, Shi X, Wu C. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J. Biol. Chem. 2003;278:51324–51333. doi: 10.1074/jbc.M309122200. [DOI] [PubMed] [Google Scholar]
- 21.Wu W, et al. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64:1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 22.Leong ML, Maiyar AC, Kim B, O’Keeffe BA, Firestone GL. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J. Biol. Chem. 2003;278:5871–5882. doi: 10.1074/jbc.M211649200. [DOI] [PubMed] [Google Scholar]
- 23.Qian KC, et al. Structural basis of constitutive activity and a unique nucleotide binding mode of human Pim-1 kinase. J. Biol. Chem. 2004;280:6130–6137. doi: 10.1074/jbc.M409123200. [DOI] [PubMed] [Google Scholar]
- 24.Yip-Schneider MT, Horie M, Broxmeyer HE. Transcriptional induction of pim-1 protein kinase gene expression by interferon gamma and posttranscriptional effects on costimulation with steel factor. Blood. 1995;85:3494–3502. [PubMed] [Google Scholar]
- 25.Wang Z, et al. Pim-1: a serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis. J. Vet. Sci. 2001;2:167–179. [PubMed] [Google Scholar]
- 26.Aho TL, et al. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 2004;571:43–49. doi: 10.1016/j.febslet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 27.Yan B, et al. The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J. Biol. Chem. 2003;278:45358–45367. doi: 10.1074/jbc.M307933200. [DOI] [PubMed] [Google Scholar]
- 28.Datta SR, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 29.Brunet A, et al. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol. Cell. Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr. Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 31.Hammerman PS, et al. Lymphocyte transformation by Pim-2 is dependent on nuclear factor-kappaB activation. Cancer Res. 2004;64:8341–8348. doi: 10.1158/0008-5472.CAN-04-2284. [DOI] [PubMed] [Google Scholar]
- 32.Kane LP, Mollenauer MN, Xu Z, Turck CW, Weiss A. Akt-dependent phosphorylation specifically regulates cot induction of NF-kappaB-dependent transcription. Mol. Cell Biol. 2002;22:5962–5974. doi: 10.1128/MCB.22.16.5962-5974.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- 34.Fox CJ, et al. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 2003;17:1841–1854. doi: 10.1101/gad.1105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardone MH, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 37.Kops GJ, et al. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 38.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 39.Majewski N, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Deprez J, Vertommen D, Alessi DR, Hue L, Rider MH. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J. Biol. Chem. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]
- 41.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Dam EM, Govers R, James DE. Akt activation is required at a late stage of insulin-induced GLUT4 translocation to the plasma membrane. Mol. Endocrinol. 2005;19:1067–1077. doi: 10.1210/me.2004-0413. [DOI] [PubMed] [Google Scholar]
- 43.Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J. Clin. Oncol. 2005;23:630–639. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 44.Workman P. Inhibiting the phosphoinositide 3-kinase pathway for cancer treatment. Biochem. Soc. Trans. 2004;32:393–396. doi: 10.1042/bst0320393. [DOI] [PubMed] [Google Scholar]
- 45.Ali K, et al. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 46.Cho H, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 47.Masure S, et al. Molecular cloning, expression and characterization of the human serine/threonine kinase Akt-3. Eur. J. Biochem. 1999;265:353–360. doi: 10.1046/j.1432-1327.1999.00774.x. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 49.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol. Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 50.Van Ummersen L, et al. A phase I trial of perifosine (NSC 639966) on a loading dose/maintenance dose schedule in patients with advanced cancer. Clin. Cancer Res. 2004;10:7450–7456. doi: 10.1158/1078-0432.CCR-03-0406. [DOI] [PubMed] [Google Scholar]
- 51.Crul M, et al. Phase I and pharmacological study of daily oral administration of perifosine (D-21266) in patients with advanced solid tumours. Eur. J. Cancer. 2002;38:1615–1621. doi: 10.1016/s0959-8049(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 52.Cheng JQ, et al. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc. Natl. Acad. Sci. U. S. A. 1992;89:9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bellacosa A, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int. J. Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 54.Stahl JM, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–7010. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 55.Barnett SF, et al. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem. J. 2005;385:399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senderowicz AM. The cell cycle as a target for cancer therapy: basic and clinical findings with the small molecule inhibitors flavopiridol and UCN-01. Oncologist. 2002;7(Suppl. 3):12–19. doi: 10.1634/theoncologist.7-suppl_3-12. [DOI] [PubMed] [Google Scholar]
- 57.Piazza GA, et al. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57:2452–2459. [PubMed] [Google Scholar]
- 58.Arico S, et al. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J. Biol. Chem. 2002;277:27613–27621. doi: 10.1074/jbc.M201119200. [DOI] [PubMed] [Google Scholar]
- 59.Steinbach G, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 60.Solomon SD, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 61.Zhu J, et al. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res. 2004;64:4309–4318. doi: 10.1158/0008-5472.CAN-03-4063. [DOI] [PubMed] [Google Scholar]
- 62.Tseng PH, et al. Synergistic interactions between imatinib and the novel phosphoinositide-dependent kinase-1 inhibitor OSU-03012 in overcoming imatinib resistance. Blood. 2005;105:4021–4027. doi: 10.1182/blood-2004-07-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takanami I. Increased expression of integrin-linked kinase is associated with shorter survival in non-small cell lung cancer. BMC Cancer. 2005;5:1. doi: 10.1186/1471-2407-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito R, et al. Expression of integrin-linked kinase is closely correlated with invasion and metastasis of gastric carcinoma. Virchows Arch. 2003;442:118–123. doi: 10.1007/s00428-002-0718-6. [DOI] [PubMed] [Google Scholar]
- 65.Graff JR, et al. Integrin-linked kinase expression increases with prostate tumor grade. Clin. Cancer Res. 2001;7:1987–1991. [PubMed] [Google Scholar]
- 66.Tan C, et al. Regulation of tumor angiogenesis by integrin-linked kinase (ILK) Cancer Cell. 2004;5:79–90. doi: 10.1016/s1535-6108(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 67.Persad S, et al. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:3207–3212. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives [review] Ann. Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 69.Alizadeh AA, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 70.Valdman A, Fang X, Pang ST, Ekman P, Egevad L. Pim-1 expression in prostatic intraepithelial neoplasia and human prostate cancer. Prostate. 2004;60:367–371. doi: 10.1002/pros.20064. [DOI] [PubMed] [Google Scholar]
- 71.Cohen AM, et al. Increased expression of the hPim-2 gene in human chronic lymphocytic leukemia and non-Hodgkin lymphoma. Leuk. Lymphoma. 2004;45:951–955. doi: 10.1080/10428190310001641251. [DOI] [PubMed] [Google Scholar]
- 72.Mizuki M, et al. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood. 2003;101:3164–3173. doi: 10.1182/blood-2002-06-1677. [DOI] [PubMed] [Google Scholar]
- 73.Katakami N, et al. Role of pim-1 in smooth muscle cell proliferation. J. Biol. Chem. 2004;279:54742–54749. doi: 10.1074/jbc.M409140200. [DOI] [PubMed] [Google Scholar]
- 74.Jacobs MD, et al. PIM-1 ligand-bound structures reveal the mechanism of serine/threonine kinase inhibition by LY294002. J. Biol. Chem. 2005;280:13728–13734. doi: 10.1074/jbc.M413155200. [DOI] [PubMed] [Google Scholar]
- 75.Mikkers H, et al. Mice deficient for all PIM kinases display reduced body size and impaired responses to hematopoietic growth factors. Mol. Cell. Biol. 2004;24:6104–6115. doi: 10.1128/MCB.24.13.6104-6115.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones, A.V., et al. 2005. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. doi:10.1182/blood-2005-03–1320. [DOI] [PubMed]
- 77.Kralovics R, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 78.Levine RL, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 79.Blaskovich MA, et al. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- 80.Mangi AA, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 81.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 82.Kong M, et al. The PP2A-associated protein alpha4 is an essential inhibitor of apoptosis. Science. 2004;306:695–698. doi: 10.1126/science.1100537. [DOI] [PubMed] [Google Scholar]
- 83.Di-Poi N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol. Cell. 2002;10:721–733. doi: 10.1016/s1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 84.Sun X, Layton JE, Elefanty A, Lieschke GJ. Comparison of effects of the tyrosine kinase inhibitors AG957, AG490, and STI571 on BCR-ABL-expressing cells, demonstrating synergy between AG490 and STI571. Blood. 2001;97:2008–2015. doi: 10.1182/blood.v97.7.2008. [DOI] [PubMed] [Google Scholar]