Abstract
Background:
Masculinizing top surgery (masculinizing mastectomy, thoracoplasty, or mammoplasty) is the most commonly performed gender-affirming surgical procedure for transmasculine individuals. This study presents 3 surgical chest contouring techniques, along with an innovative approach for optimizing chest symmetry, and provides a flowchart to guide the selection of the most appropriate technique for each patient.
Methods:
The medical records of 92 patients who underwent masculinizing top surgery performed by a single surgeon using the free nipple–areolar complex (NAC) graft, NAC flap, or periareolar flap, from 2020 to 2024, were retrospectively reviewed. Technique selection was determined during outpatient evaluations, considering the patient’s body profile, breast volume, degree of ptosis, skin elasticity, and presence of symmastia. Postoperative chest symmetry was also assessed. Statistical significance was defined as a P value of less than 0.05.
Results:
The free NAC graft was the most frequently used (47.2%), followed by the periareolar flap and NAC flap techniques. The overall revision rate was low (14.1%), with 4 patients requiring additional procedures to improve chest symmetry, and no significant differences were observed among the techniques. Dehiscence occurred more frequently after the NAC flap procedure (P < 0.016), whereas other complications showed no significant association with any technique.
Conclusions:
We demonstrated that safe and aesthetically satisfactory outcomes can be achieved by masculinizing top surgery using the approaches presented. By analyzing the preoperative patient clinical characteristics and outcomes, we developed a flowchart that can guide the selection of the most appropriate surgical technique for each patient’s body profile.
Takeaways
Question: How can chest symmetry be ensured during masculinizing top surgery for transmasculine individuals?
Findings: We describe 3 surgical techniques for masculinizing top surgery and present a technical innovation for ensuring chest symmetry. Based on preoperative patient clinical characteristics and outcomes, we developed a flowchart that can guide the selection of the most appropriate surgical technique for each’s patient body profile.
Meaning: Safe and aesthetically satisfactory outcomes can be achieved in masculinizing top surgery using the chest symmetrizing approaches presented, and the flowchart can facilitate appropriate technique choice for each patient.
INTRODUCTION
Transgender individuals have a gender identity that differs from the sex they are assigned at birth, which can lead to discomfort when their ability to express their identity is denied or not respected.1,2 In response, many seek medical interventions to affirm their gender identity. This transition process is highly individualized and may involve various clinical and surgical procedures.3–7
In transmasculine individuals, the transition typically begins with testosterone therapy to induce the development of secondary sexual characteristics. Although the hormone reduces glandular and adipose breast tissue and increases fibrous tissue, it does not significantly alter breast size, skin quantity, or overall appearance. Additionally, testosterone does not reduce the risk of premalignant or malignant breast changes.5–10
Masculinizing top surgery represents a cornerstone of treatment for transmasculine individuals and is the most commonly performed surgical procedure in these individuals.6,11,12 The objective of the surgery is to create a masculine, symmetrical, and aesthetically acceptable chest contour, allowing the patient to assume a new role in his family, social, and sexual life.13,14
In comparison to the female breast, the male chest presents smaller and more laterally positioned areola, smaller nipples, and a less defined inframammary fold. Thus, masculinizing top surgery involves removing the breast tissue, repositioning and reducing the nipple–areolar complex (NAC), releasing the inframammary fold, and excising excess skin.
Several surgical techniques for achieving satisfactory results have been described, but few of these reports address ways to achieve better chest symmetry.6,11,12,15,16 This study proposed an innovative, feasible, and reproducible approach for verifying chest symmetry, developed by the main author, which could be applied to the 3 surgical techniques presented here. Moreover, we presented a flowchart to guide the selection of the most suitable technique for individual patients.
METHODS
Study Design
In this retrospective descriptive study, the medical records of transmasculine patients who underwent top surgery, including the innovative approach for verifying chest symmetry and technical developments developed and conducted by the main author, between May 2020 and September 2024, were reviewed. This study was approved by the research ethics committee of Felicio Rocho Hospital. After excluding 1 patient who declined participation, the final sample comprised 92 patients. All patients provided written informed consent for treatment, data use, and image publication. The patients were followed up by a multidisciplinary team, which included a surgeon, psychologist, and endocrinologist. All patients eligible for surgery underwent bilateral preoperative mammography. Patients were evaluated once or every 2 weeks in the first month after surgery, and then at 6 months following the procedure, when they were evaluated on the necessity of surgical revision procedures.
The collected data included variables such as age, body mass index, and smoking habits. The clinical and surgical features analyzed included testosterone therapy, prior breast surgery, presence of symmastia, degree of ptosis, breast size, skin elasticity, pathological examination (PE), complications, and refinements. The complications included hematoma, dehiscence, infection, seroma, and necrosis. Surgical revisions included improving scar appearance, enhancing chest symmetry, NAC pigmentation, fat grafting, resecting excess skin, revising flaps, and nipple surgery.
Surgical Techniques
The choice of technique is discussed individually in outpatient appointments according to each patient’s body profile, breast volume, degree of ptosis, and skin elasticity. All procedures were conducted under general anesthesia with prophylactic antibiotics. Upon completion, vacuum drains, petrolatum dressings, and chest bindings were placed. The excised breast tissue was sent for PE.
Technique for Evaluating Chest Symmetry
The innovative chest symmetry evaluation was performed in all patients in 3 stages: before the incision, during surgery, and after the surgical site was closed. Initially, with the patient in an upright position, a midline marking was made on the chest based on anatomical landmarks such as the manubrium sterni, sternum, xiphoid process, and umbilicus. The obtained midline was verified by measuring the distance from the line to the coracoid process of the scapula bilaterally. If there was a discrepancy, the process was repeated until the measurements were congruent to establish the true midline in each patient. In the supine position, 2 long silk sutures were placed on the midline: 1 on the manubrium sterni and the other on the xiphoid process. The 2 sutures were brought together, crossed at the most lateral point of the right incision marking, and fixed at this point with a hemostatic clamp. The sutures were then taken to the most lateral point of the left incision marking, where a mark was made to check for symmetry (Fig. 1). This same process was repeated at the midpoint and at the most medial point. Any necessary adjustments to the incision markings were then made to ensure symmetry. Intraoperatively, a second verification was performed to detect and correct any asymmetries. After closure, a final check was performed to confirm the symmetry and appropriate position of the NAC (Fig. 2).
Fig. 1.
Chest symmetry check before skin incision. NAC flap technique.
Fig. 2.
Chest symmetry check after NAC closure using the periareolar technique.
Periareolar Technique
Once symmetry had been marked, the new location of the areola was determined using the intersection of the fifth costal arch and the lateral edge of the pectoralis major (point A) as reference points. Next, an eccentric circle with a radius of 1.5–2 cm was drawn around the NAC, beginning at point A. Alternatively, a double concentric circle was drawn 1–2 cm apart to guide excess skin resection in patients with larger breasts (Fig. 3). The procedure began with an incision and deepithelialization of the previously marked areas. Therefore, it is preferable to use areola markers with diameters smaller than 3 cm. Next, the dermis was incised in the inferolateral region to perform mastectomy, ensuring preservation of skin thickness. Maintaining all the subcutaneous tissue within the skin is crucial; therefore, its thickness varies according to each patient’s body and breast composition and should be kept homogeneous. Another key aspect is the complete release of the inframammary fold; dissection should extend at least 2 cm below the fold. For closure, a pocket was created to accommodate the skin while preserving the diameter of the areolar marker, followed by layered suturing of the skin. A final evaluation of symmetry was conducted, and any necessary adjustments were made.
Fig. 3.
Preoperative marking of excentric and concentric circles for the periareolar technique.
In cases where the patient has a disproportionately large nipple, nipple surgery can be performed during the same surgical session to adjust height. It is advisable to postpone base reduction until revision surgery to minimize the risk of postoperative complications.
NAC Flap Technique
For the NAC flap technique, once symmetry was marked, the inframammary fold was delineated, and the upper part of the incision was positioned above the areola, extending to the inframammary fold. The areola was outlined using markers with diameters smaller than 3 cm, followed by deepithelialization of the flap. An incision was made at the upper marking to perform mastectomy, with attention to preserving uniform skin thickness. A flap measuring 10 cm in width was demarcated from the inframammary fold with a thickness of approximately 2 cm to ensure NAC vascularization (Fig. 4). Excess skin was resected from the medial and lateral regions, followed by skin closure and symmetry evaluation. The areola was positioned 1.5–2 cm above the inframammary fold incision at the level of the fifth costal arch and medial to the lateral edge of the pectoralis major. The skin was incised and resected in the area defined by the areolar marker, and the NAC was exteriorized and closed in layers. A final evaluation of symmetry was conducted, and adjustments were made as needed.
Fig. 4.
Intraoperative view during the NAC flap technique. Both lower pedicles are outlined after resection of lateral and medial excess skin.
Free NAC Graft Technique
In the free NAC graft technique, once symmetry had been marked, the inframammary fold and upper part of the incision were defined as outlined for the NAC flap technique. The areola grafts were removed using areola markers with diameters less than 3 cm and left in vials containing saline solution. An incision was made at the upper mark to perform the mastectomy, with careful attention to skin thickness. Excess skin was resected from the medial and lateral regions, followed by skin closure and symmetry evaluation. The areola was positioned 1.5–2 cm above the inframammary fold incision at the level of the fifth costal arch and medial to the lateral edge of the pectoralis major. The skin was deepithelialized in the area marked, and the NAC graft was positioned. A final evaluation of symmetry was conducted, with any required adjustments, followed by the application of a Brown dressing.
In cases where excess skin was present in the central region of the chest, between the medial ends of the incisions, such as in cases of symmastia, we use the “fishtail” technique. This technique was performed after closure of the subcutaneous tissue of the primary incisions and involved making a mark on the midline above the incisions (typically 1–2 cm above, referred to as point B) depending on the amount of excess skin. A new incision was made at point B, connecting the initial incisions and enabling excess skin resection (Fig. 5). The same technique can be used in cases where further surgical revision is required to excise any remaining excess skin in the medial region.
Fig. 5.
Intraoperative view during the free NAC graft technique. Connection of the initial incisions along the midline and skin resection using the “fishtail” technique and NAC graft.
Statistical Analysis
Statistical analyses were conducted using RStudio version 2024.04.2 and R version 4.4.0 (https://www.r-project.org/). Statistical significance was set at a P value less than 0.05. Qualitative variables were presented as absolute and relative frequencies, and quantitative variables were presented as the minimum, maximum, mean, SD, median, first quartile, and third quartile. The Shapiro–Wilk normality test was used to evaluate the data distribution of the quantitative variables. The association between 2 qualitative variables was analyzed using the Fisher exact test. Quantitative variables were compared across the 3 surgical techniques using the Kruskal–Wallis test, followed by multiple comparisons using the Dunn test. The Wilcoxon Mann-Whitney test was used to compare quantitative variables between the 2 groups. A 2-factor analysis of variance with the Tukey post hoc test was conducted to assess the duration of testosterone use across techniques and in relation to the PE findings.
RESULTS
The final sample comprised 92 patients with a mean age of 27.7 (±6.9) years. Smoking was reported by 28.6% of participants. A significant difference in body mass index was observed among patients who underwent the 3 surgical techniques, with those undergoing free NAC grafting exhibiting higher values (P < 0.001). This group also had the highest percentage of patients diagnosed with obesity (P = 0.034) (Table 1).
Table 1.
Preoperative Clinical Characteristics of the Entire Sample, Grouped by Surgical Technique
| Variables | Total Sample (n = 92) | Periareolar (n = 27) | NAC Flap (n = 20) | Free NAC Graft (n = 45) | P |
|---|---|---|---|---|---|
| Age, y | 0.200* | ||||
| Min–Max | 18.0–51.0 | 18.0–39.0 | 18.0–45.0 | 18.0–51.0 | |
| Med (Q1; Q3) | 26.5 (23.0; 31.0) | 26.0 (23.0; 28.0) | 27.5 (25.8; 33.0) | 26.0 (22.0; 33.0) | |
| Mean (SD) | 27.7 (6.9) | 26.0 (4.5) | 29.4 (6.3) | 28.0 (8.1) | |
| Valid N | 92 | 27 | 20 | 45 | |
| BMI (kg/m2) | <0.001 * | ||||
| Min–Max | 17.1–50.5 | 17.1–32.4 | 19.5–37.3 | 20.4–50.5 | |
| Med (Q1; Q3) | 25.9 (22.4; 31.0) | 21.3 (20.1; 23.4) | 25.5 (23.3; 27.9) | 29.4 (26.3; 33.1) | |
| Mean (SD) | 27.0 (6.2) | 22.0 (3.0) | 26.0 (4.5) | 30.5 (6.1) | |
| Valid N | 92 | 27 | 20 | 45 | |
| BMI categories, (%) | <0.001 † | ||||
| Underweight | 8 (8.7) | 6 (22.2) | 2 (10.0) | 0 (—) | |
| Healthy weight | 30 (32.6) | 18 (66.7) | 6 (30.0) | 6 (13.3) | |
| Overweight | 29 (31.5) | 2 (7.4) | 10 (50.0) | 17 (37.8) | |
| Obesity | 25 (27.2) | 1 (3.7) | 2 (10.0) | 22 (48.9) | |
| Valid N | 92 | 27 | 20 | 45 | |
| Comorbidities, (%) | |||||
| SAH | 3 (3.3) | 1 (3.7) | 0 (—) | 2 (4.4) | 1.000† |
| DM | 2 (2.2) | 0 (—) | 0 (—) | 2 (4.4) | 0.710† |
| Dyslipidemia | 3 (3.3) | 0 (—) | 0 (—) | 3 (6.7) | 0.310† |
| Obesity | 9 (9.8) | 0 (—) | 1 (5.0) | 8 (17.8) | 0.034 † |
| Anxiety | 13 (14.1) | 2 (7.4) | 2 (10.0) | 9 (20.0) | 0.363† |
| Depression | 6 (6.5) | 1 (3.7) | 1 (5.0) | 4 (8.9) | 0.860† |
| Hypothyroidism | 2 (2.2) | 0 (—) | 0 (—) | 2 (4.4) | 0.710† |
| Asthma | 9 (9.8) | 2 (7.4) | 2 (10.0) | 5 (11.1) | 0.905† |
| Others | 16 (17.4) | 6 (22.2) | 1 (5.0) | 9 (20.0) | 0.252† |
| Valid N | 92 | 27 | 20 | 45 | |
| Smoking, (%) | 26 (28.6) | 8 (29.6) | 6 (31.6) | 12 (26.7) | 0.914† |
| Valid N | 91 | 27 | 19 | 45 | |
| Previous breast surgery, (%) | 0.548† | ||||
| No | 87 (94.6) | 26 (96.3) | 20 (100.0) | 41 (91.1) | |
| Reduction mammoplasty | 3 (3.3) | 0 (—) | 0 (—) | 3 (6.7) | |
| Mastectomy | 2 (2.2) | 1 (3.7) | 0 (—) | 1 (2.2) | |
| Valid N | 92 | 27 | 20 | 45 |
Bold values indicate statistical significance.
The Kruskal–Wallis test with multiple comparisons by the Dunn test.
The Fisher exact test.
BMI, body mass index; DM, diabetes mellitus; SAH, systemic arterial hypertension.
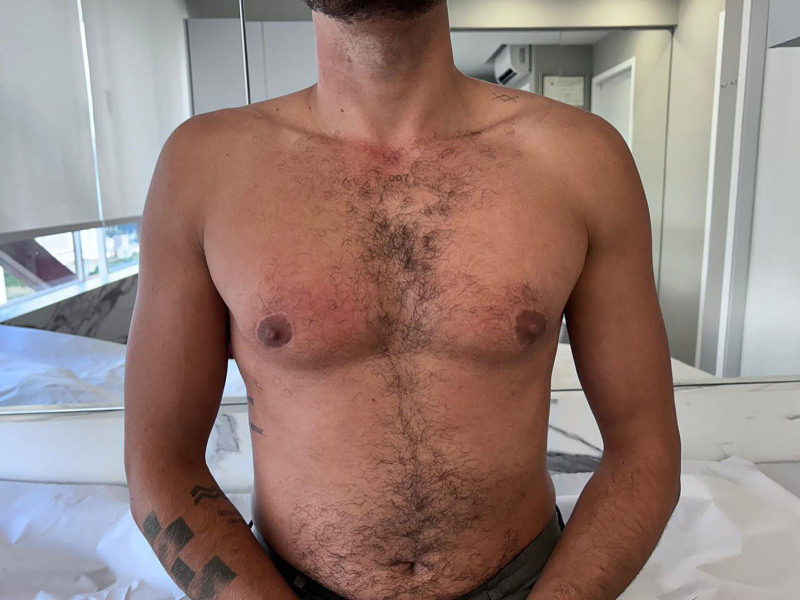
The free NAC graft technique was used in 47.2% of the cases. Within this group, 28.6% of the patients required the “fishtail” technique, whereas 2.4% underwent the procedure without NAC preservation. Preoperative marking and postoperative follow-up results are demonstrated (Figs. 6, 7). The result after areola micropigmentation in this patient is shown in Figure 8.
Fig. 6.
Free NAC graft technique. Anterior view of preoperative marking.
Fig. 7.
Free NAC graft technique. Anterior view of postoperative follow-up.
Fig. 8.
Before and after areola micropigmentation in the patient from Figure 7.
The periareolar approach was used in 30.3% of the cases, with 14.8% of these procedures associated with nipple surgery. Preoperative marking and 6-month postoperative follow-up results are demonstrated in 2 patients (Figs. 9–12).
Fig. 9.
Periareolar technique. Anterior view of the preoperative marking.
Fig. 12.
Periareolar technique. Anterior view at 6-month postoperative follow-up.
Fig. 10.
Periareolar technique. Anterior view at 6-month postoperative follow-up.
Fig. 11.
Periareolar technique. Anterior view of preoperative marking.
Finally, the NAC flap technique was used in 22.5% of the cases. Preoperative marking and 6-month postoperative follow-up results are demonstrated in 2 patients (Figs. 13–16).
Fig. 13.
NAC flap technique. Anterior view of preoperative marking.
Fig. 16.
NAC flap technique. Anterior view at 6-month postoperative follow-up.
Fig. 14.
NAC flap technique. Anterior view at 6-month postoperative follow-up.
Fig. 15.
NAC flap technique. Anterior view of preoperative marking.
Regarding preoperative clinical characteristics, all patients with symmastia (16.3%), grade 3 ptosis (37%), and very large breasts (22%) underwent free NAC grafting (P < 0.001). Moreover, the average breast weight (783.5 g) was higher and skin elasticity was poorer in this group (P < 0.001).
In contrast, the group undergoing surgery using the periareolar technique exhibited a higher incidence of no ptosis (81.5%), whereas 18.5% presented with grade 1 ptosis. Additionally, 76.9% of the breasts were classified as small, and 92.6% had good skin elasticity. This group had an average breast weight (123.6 g) significantly lower than the overall average of 468.2 g (± 447.1 g) (P < 0.001) (Table 2).
Table 2.
Breast Characteristics of the Entire Sample, Grouped by Surgical Technique
| Variables | Total Sample (n = 92) | Periareolar (n = 27) | NAC Flap (n = 20) | Free NAC Graft (n = 45) | P |
|---|---|---|---|---|---|
| Symmastia, (%) | 15 (16.3) | 0 (—) | 0 (—) | 15 (33.3) | <0.001* |
| Valid N | 92 | 27 | 20 | 45 | |
| Degree of ptosis, (%) | <0.001* | ||||
| No ptosis | 24 (26.1) | 22 (81.5) | 1 (5.0) | 1 (2.2) | |
| Grade 1 ptosis | 13 (14.1) | 5 (18.5) | 8 (40.0) | 0 (—) | |
| Grade 2 ptosis | 21 (22.8) | 0 (—) | 11 (55.0) | 10 (22.2) | |
| Grade 3 ptosis | 34 (37.0) | 0 (—) | 0 (—) | 34 (75.6) | |
| Valid N | 92 | 27 | 20 | 45 | |
| Breast size, (%) | <0.001* | ||||
| Small | 22 (24.2) | 20 (76.9) | 1 (5.0) | 1 (2.2) | |
| Medium | 31 (34.1) | 6 (23.1) | 18 (90.0) | 7 (15.6) | |
| Large | 18 (19.8) | 0 (—) | 1 (5.0) | 17 (37.8) | |
| Very large | 20 (22.0) | 0 (—) | 0 (—) | 20 (44.4) | |
| Valid N | 91 | 26 | 20 | 45 | |
| Average breast weight, g | <0.001† | ||||
| Min–Max | 12.0–1956.0 | 12.0–491.0 | 104.0–427.0 | 159.0–1956.0 | |
| Med (Q1; Q3) | 299.0 (143.0; 618.0) | 94.5 (68.2; 141.9) | 233.0 (171.0; 283.6) | 676.5 (476.0; 924.5) | |
| Mean (SD) | 468.2 (447.1) | 123.6 (98.7) | 238.2 (91.9) | 783.5 (456.8) | |
| Valid N | 89 | 26 | 20 | 43 | |
| Skin elasticity, (%) | <0.001* | ||||
| Good | 48 (52.2) | 25 (92.6) | 17 (85.0) | 6 (13.3) | |
| Poor | 44 (47.8) | 2 (7.4) | 3 (15.0) | 39 (86.7) | |
| Valid N | 92 | 27 | 20 | 45 | |
The Fisher exact test.
The Kruskal–Wallis test with multiple comparisons by the Dunn test.
No diagnosis of breast cancer was recorded in PE reports. The most common finding was benign fibrocystic changes, present in 64% of cases, followed by fibroadenomatoid changes in 15.7%. These results were not associated with the surgical technique used.
The overall incidence of hematomas was 14.1%, with no significant difference among the surgical techniques, although a higher percentage was observed in patients who underwent the periareolar technique (25.9%). No significant association was found between the incidence of hematomas and the interval between the last testosterone administration and surgery; nevertheless, patients who underwent the periareolar technique had used testosterone for a longer period.
Other complications included dehiscence (6.5%), wound infection (3.3%), seroma (19.6%), and partial necrosis of the NAC (7.6%) or skin (1.1%), resulting in an overall complication rate of 32.6%. These features showed a significant difference only in the occurrence of dehiscence, which was significantly more prevalent among patients who underwent surgery using the NAC flap technique (P = 0.016). Smoking was not associated with dehiscence or necrosis of the NAC.
Surgical revision was performed in 13 patients. Excess skin resection was exclusively performed in the free NAC graft group (P = 0.002), whereas flap improvement was more frequently required in the periareolar technique (P = 0.040). Most reoperations aimed to enhance scar appearance, without significant difference among the techniques. Other late reoperations were not correlated with the surgical technique. Four patients underwent late symmetry correction (Table 3).
Table 3.
Complications and Refinements for the Entire Sample, Grouped by Surgical Technique
| Variables | Total Sample (n = 92) | Periareolar (n = 27) | NAC Flap (n = 20) | NAC Graft (n = 45) | P |
|---|---|---|---|---|---|
| Complications | |||||
| Complications requiring immediate reoperation, (%) | 13 (14.1) | 7 (25.9) | 1 (5.0) | 5 (11.1) | 0.134 |
| Valid N | 92 | 27 | 20 | 45 | |
| Hematoma approached (uni- or bilateral), (%) | 12 (13.0) | 6 (22.2) | 1 (5.0) | 5 (11.1) | 0.276 |
| Valid N | 92 | 27 | 20 | 45 | |
| Hematoma not approached, (%) | 2 (2.2) | 2 (7.4) | 0 (—) | 0 (—) | 0.129 |
| Valid N | 92 | 27 | 20 | 45 | |
| Complications managed on an outpatient basis, (%) | 30 (32.6) | 11 (40.7) | 6 (30.0) | 13 (28.9) | 0.543 |
| Dehiscence, (%) | 6 (6.5) | 3 (11.1) | 3 (15.0) | 0 (—) | 0.016 |
| Valid N | 92 | 27 | 20 | 45 | |
| Wound infection, (%) | 3 (3.3) | 0 (—) | 2 (10.0) | 1 (2.2) | 0.197 |
| Valid N | 92 | 27 | 20 | 45 | |
| Seroma, (%) | 18 (19.6) | 8 (29.6) | 1 (5.0) | 9 (20.0) | 0.082 |
| Valid N | 92 | 27 | 20 | 45 | |
| Partial necrosis NAC graft, (%) | 7 (7.6) | 0 (—) | 3 (15.0) | 4 (8.9) | 0.139 |
| Valid N | 92 | 27 | 20 | 45 | |
| Partial skin necrosis, (%) | 1 (1.1) | 0 (—) | 1 (5.0) | 0 (—) | 0.217 |
| Valid N | 92 | 27 | 20 | 45 | |
| Refinements | |||||
| Refinement, (%) | 13 (14.1) | 5 (18.5) | 3 (15.0) | 5 (11.1) | 0.630 |
| Valid N | 92 | 27 | 20 | 45 | |
| Type of reoperation | |||||
| Scar appearance enhancement, (%) | 10 (76.9) | 5 (100.0) | 3 (100.0) | 2 (40.0) | 0.073 |
| Symmetry improvement, (%) | 4 (30.8) | 2 (40.0) | 2 (66.7) | 0 (—) | 0.196 |
| NAC pigmentation correction, (%) | 2 (15.4) | 0 (—) | 1 (33.3) | 1 (20.0) | 0.680 |
| Fat grafting, (%) | 2 (15.4) | 1 (20.0) | 1 (33.3) | 0 (—) | 0.680 |
| Excess skin resection, (%) | 5 (38.5) | 0 (—) | 0 (—) | 5 (100.0) | 0.002 |
| Flap revision, (%) | 5 (38.5) | 4 (80.0) | 1 (33.3) | 0 (—) | 0.040 |
| Nipple surgery, (%) | 1 (7.7) | 1 (20.0) | 0 (—) | 0 (—) | 1.000 |
| Valid N | 13 | 5 | 3 | 5 | |
Bold values indicate statistical significance.
P values by the Fisher exact test.
DISCUSSION
Masculinizing top surgery is one of the main surgical procedures sought by transmasculine individuals as part of their gender reassignment process.6,11,12 This study described and analyzed the results of the periareolar, NAC flap, and free NAC graft techniques for masculinizing top surgery. In addition, we described 2 extended techniques: nipple surgery for adjusting nipple projection, and the “fishtail” technique for resecting excess central skin. In a novel approach, we also described and presented data on a new technique designed to optimize male thoracic symmetry.
Most techniques described in the literature are similar to those used in our practice,15 with variations reflecting surgeon experience.17–20 Some reports have previously described methods for assessing chest symmetry,16 but the technique proposed here is both innovative and efficient.
Our overall surgical revision rate was 14.1%, consistent with the lower range of the incidence rate found in the literature (14.4%21–32%22). Revisions aimed at enhancing chest symmetry were needed in only 4 cases, and 1 of these patients had previously undergone top surgery at another service. This validates the proposed method and demonstrates satisfactory aesthetic results.
Among the 3 surgical techniques described, only the free NAC graft required reoperation for excess skin resection. This technique, often indicated for patients with large breast volumes, symmastia, and poor skin elasticity, presents surgical challenges that justify subsequent refinements and, consequently, more extensive scarring.19,22–24
Our overall complication rate was 32.6%. Wound dehiscence was more prevalent in patients who underwent surgery using the NAC flap technique. This may be explained due to the areolar pedicle. No relationship was found between smoking and dehiscence in our analysis. These findings are supported by the literature, underscoring the relationship between surgical techniques and the occurrence of complications.25
Other complications, such as NAC or skin necrosis, were identified in 8.7% of cases. Among these, 4 had undergone the NAC flap technique and 4 had undergone the NAC graft technique. None of these complications required further revisions such as NAC pigmentation. None of the necrosis occurred in the periareolar group, a technique with greater preservation of NAC irrigation.
Hematomas occurred in 14.1% of our cases, with a higher rate in patients undergoing the periareolar technique, although there was no significant difference among the groups. This may be explained by the reduced direct intraoperative visibility due to the small incision used with this technique.19,22–27 The literature presents conflicting data regarding hematomas, emphasizing the need for preventive measures, such as discontinuing testosterone therapy, implementing intraoperative measures, and using compression bandages.19,22–27 Although some studies report no significant association between the duration of use or last administration of testosterone therapy and the incidence of hematomas, others suggest a potential association.17,19,20 In our practice, discontinuing testosterone therapy 1 month before surgery, along with other prophylactic measures, has helped reduce the incidence of hematomas.
The average breast weight was significantly higher in the free NAC graft group, whereas the periareolar group exhibited the lowest average breast weight, corresponding to smaller breast sizes. These variations underscore the influence of breast size on surgical technique selection. Our results are consistent with those in the literature.15,17,21,22,25
Clinical evaluation of patients undergoing masculinizing top surgery should include breast size (small, medium, large, or very large), degree of ptosis (no ptosis, grade 1, 2, or 3), skin elasticity (good or poor), and the presence of symmastia. These factors help define a suitable surgical technique for the individual patient.15,21–23,25 Some studies have previously proposed flowcharts based on these features, highlighting their importance in guiding surgical decision-making.2,15,23,28
Based on these parameters, we have developed a surgical flowchart to guide the selection of the most appropriate technique for each patient, incorporating chest symmetry evaluation and the potential use of extended techniques. Our flowchart differs from those presented in the literature,29 because it incorporates an innovative technique for checking thoracic symmetry, optimizing surgical decisions, and contributing to better aesthetic results. Our flowchart emphasizes the need for a systematic and visual approach to guide the choice of each of the 3 surgical techniques described, all using verification of thoracic symmetry (Fig. 17).
Fig. 17.
Surgical flowchart for selecting the most appropriate masculinizing top surgery technique and surgical extensions.
Several patients did not attend the recommended follow-up appointments at 6 months postsurgery. This absence impedes the long-term evaluation of surgical techniques and their potential indications for surgical revisions, limiting the assessment of late-stage outcomes. Prospective studies with long follow-up, including revision rates, assessment of satisfaction, and quality-of-life comparisons between techniques must be carried out so that we can improve our indications and results.
CONCLUSIONS
Masculinizing top surgery is a safe and effective gender-affirming procedure for the transmasculine population. Our flowchart for selecting the appropriate surgical technique and corresponding modifications has led to excellent aesthetic outcomes and reproducible results tailored specifically to each patient’s body profile. The integration of strategies aimed at enhancing the appearance of incisions and optimizing NAC positioning contributes to a more harmonious postoperative experience in line with the chest symmetry evaluation proposed in this study. It is essential to clearly present the available surgical options and to manage patient expectations regarding realistic outcomes, considering modifiable and nonmodifiable factors, such as the individual’s physical and anatomical characteristics.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
PATIENT CONSENT
Patients provided written consent for the use of their images.
Footnotes
Published online 20 August 2025.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Ribeiro CR, Ahmad AF, Dantas BS, et al. Masculinidades em construção, corpos em (re)construção: desejos, contradições e ambiguidades de homens trans no processo transexualizador. Ciên Saúde Colet. 2022;27:3901–3911. [DOI] [PubMed] [Google Scholar]
- 2.Sousa D, Iriart J. “Viver dignamente”: necessidades e demandas de saúde de homens trans em Salvador, Bahia, Brasil. Cad Saúde Pública. 2018;34:e00036318. [DOI] [PubMed] [Google Scholar]
- 3.Winter S, Diamond M, Green J, et al. Transgender people: health at the margins of society. Lancet. 2016;388:390–400. [DOI] [PubMed] [Google Scholar]
- 4.Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23:S1–S259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imprensa Nacional. Resolução No 2.265, de 20 de setembro de 2019 - DOU. Imprensa Nacional. Available at https://www.in.gov.br/web/dou/-/resolucao-n-2.265-de-20-de-setembro-de-2019-237203294. Accessed July 9, 2025. [Google Scholar]
- 6.Oles N, Darrach H, Landford W, et al. Gender affirming surgery: a comprehensive, systematic review of all peer-reviewed literature and methods of assessing patient-centered outcomes (part 1: breast/chest, face, and voice). Ann Surg. 2021;275:e52–e66. [DOI] [PubMed] [Google Scholar]
- 7.Richards C, Barrett J. The case for bilateral mastectomy and male chest contouring for the female-to-male transsexual. Ann R Coll Surg Engl. 2013;95:93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grynberg M, Fanchin R, Dubost G, et al. Histology of genital tract and breast tissue after long-term testosterone administration in a female-to-male transsexual population. Reprod Biomed Online. 2010;20:553–558. [DOI] [PubMed] [Google Scholar]
- 9.Schultz JJ, Naides AI, Bai D, et al. Pathological evaluation of breast specimens in transgender chest masculinization: incidental findings and effect of prior chest binding and androgen therapy in 74 consecutive patients. Transgend Health. 2021;6:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blok CJM de, Wiepjes CM, Nota NM, et al. Breast cancer risk in transgender people receiving hormone treatment: nationwide cohort study in the Netherlands. BMJ. 2019;365:l1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamali A, Sigurjónsson H, Gran I, et al. Improved surgical outcome with double incision and free nipple graft in gender confirmation mastectomy. Plast Reconstr Surg Glob Open. 2021;9:e3628–e3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuccolo NG, Kang CO, Boskey ER, et al. Masculinizing chest reconstruction in transgender and nonbinary individuals: an analysis of epidemiology, surgical technique, and postoperative outcomes. Aesthetic Plast Surg. 2019;43:1575–1585. [DOI] [PubMed] [Google Scholar]
- 13.Ramella V, Papa G, Stocco C, et al. New algorithm for chest-wall surgery and quality of life assessment in female-to-male reassignment patients. Plast Reconstr Surg Glob Open. 2020;8:e3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal CA, Scheefer MF, Wright LN, et al. Quality of life improvement after chest wall masculinization in female-to-male transgender patients: a prospective study using the BREAST-Q and Body Uneasiness Test. J Plast Reconstr Aesthet Surg. 2018;71:651–657. [DOI] [PubMed] [Google Scholar]
- 15.Top H, Balta S. Transsexual mastectomy: selection of appropriate technique according to breast characteristics. Balkan Med J. 2017;34:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen A, Alcon A, Parmeshwar N, et al. A technique for optimizing symmetry in gender-affirming mastectomy. Plast Reconstr Surg Glob Open. 2021;9:e3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frederick MJ, Berhanu AE, Bartlett R. Chest surgery in female to male transgender individuals. Ann Plast Surg. 2017;78:249–253. [DOI] [PubMed] [Google Scholar]
- 18.Kääriäinen M, Salonen K, Helminen M, et al. Chest-wall contouring surgery in female-to-male transgender patients: a one-center retrospective analysis of applied surgical techniques and results. Scand J Surg. 2016;106:74–79. [DOI] [PubMed] [Google Scholar]
- 19.Berry MG, Curtis R, Davies D. Female-to-male transgender chest reconstruction: a large consecutive, single-surgeon experience. J Plast Reconstr Aesthet Surg. 2012;65:711–719. [DOI] [PubMed] [Google Scholar]
- 20.Whitehead DM, Weiss PR, Podolsky D. A single surgeon’s experience with transgender female-to-male chest surgery. Ann Plast Surg. 2018;81:353–359. [DOI] [PubMed] [Google Scholar]
- 21.Startseva OI, Safronov VV, Adamyan RT, et al. Abordagem moderna para masculinizar a mamoplastia. Cirurgia Plástica e Medicina Estética. 2021:13. [Google Scholar]
- 22.Monstrey S, Selvaggi G, Ceulemans P, et al. Chest-wall contouring surgery in female-to-male transsexuals: a new algorithm. Plast Reconstr Surg. 2008;121:849–859. [DOI] [PubMed] [Google Scholar]
- 23.Wolter A, Scholz T, Pluto N, et al. Subcutaneous mastectomy in female-to-male transsexuals: optimizing perioperative and operative management in 8 years of clinical experience. J Plast Reconstr Aesthet Surg. 2018;71:344–352. [DOI] [PubMed] [Google Scholar]
- 24.Naides AI, Schultz JJ, Shulzhenko NO, et al. Chest masculinization technique and outcomes in 72 double-incision chest-contouring procedures with free nipple grafting. Plast Reconstr Surg Glob Open. 2021;9:e3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J, Wang E, Liu S, et al. Impact of surgical technique on outcome measures in chest masculinization: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2023;87:109–116. [DOI] [PubMed] [Google Scholar]
- 26.Wilson SC, Morrison SD, Anzai L, et al. Masculinizing top surgery: a systematic review of techniques and outcomes. Ann Plast Surg. 2018;80:679–683. [DOI] [PubMed] [Google Scholar]
- 27.McEvenue G, Xu FZ, Cai R, et al. Female-to-male gender affirming top surgery: a single surgeon’s 15-year retrospective review and treatment algorithm. Aesthet Surg J. 2017;38:49–57. [DOI] [PubMed] [Google Scholar]
- 28.Van de Grift TC, Elfering L, Bouman MB, et al. Surgical indications and outcomes of mastectomy in transmen: a prospective study of technical and self-reported measures. Plast Reconstr Surg. 2017;140:415e–424e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ammari T, Sluiter EC, Gast K, et al. Female-to-male gender-affirming chest reconstruction surgery. Aesthet Surg J. 2019;39:150–163. [DOI] [PubMed] [Google Scholar]