Abstract
Toxic encephalopathy is a central nervous system disorder caused by endogenous or exogenous toxic substances. Granzyme B (GZMB), a key serine protease, plays a crucial role in immune regulation and the progression of various diseases. Abnormal expression of GZMB may contribute to the development and progression of toxic encephalopathy; however, its specific mechanisms remain unclear. This study aims to preliminarily explore the association between granzyme B and toxic encephalopathy through big data. This study retrieved the toxic encephalopathy dataset GSE253309 from the gene expression omnibus database. Differentially expressed genes were identified using the “limma” R package, followed by gene ontology and Kyoto encyclopedia of genes and genomes enrichment analyses. A protein–protein interaction network was constructed using the search tool for the retrieval of interacting genes (STRING) database, key hub genes were identified with Cytoscape software. A total of 994 differentially expressed genes were identified. Among them, GZMB was moderately overexpressed in toxic encephalopathy tissues (log2 fold change = 0.70, P = .00087), suggesting a potential disease association. and was enriched in the TGF-beta signaling pathway. Kyoto encyclopedia of genes and genomes enrichment analysis revealed that GZMB-related genes were involved in several pathways, notably the TGF-beta signaling pathway and apoptosis, both of which are implicated in immune regulation and neuronal injury. Protein–protein interaction network analysis confirmed GZMB as a hub gene potentially contributing to disease progression. Furthermore, β-escin sodium and methylprednisolone may modulate GZMB expression, thereby alleviating neuronal damage and improving outcomes in delayed encephalopathy after carbon monoxide poisoning. Elevated GZMB expression likely contributes to the pathogenesis and progression of toxic encephalopathy through multiple pathways, making it a potential disease biomarker and therapeutic target.
Keywords: bioinformatics, delayed encephalopathy after carbon monoxide poisoning, GZMB, methylprednisolone, toxic encephalopathy, β-escin sodium
1. Introduction
Toxic encephalopathy is a central nervous system disorder caused by various harmful factors, including chemical substances, drugs, heavy metal exposure, and viral infections.[1] Its complex pathogenesis and high disability rate.[2] The prevalence of toxic encephalopathy varies by etiology, population, and exposure settings. For example, the incidence of chronic alcoholic encephalopathy is approximately 10% to 20% among individuals with alcohol dependence, and the global burden is estimated to affect over 200 million people, though this number varies across regions, ethnicities, and lifestyles. The incidence of delayed encephalopathy after acute carbon monoxide poisoning (DEACMP) among hospitalized patients is about 12%, and exposure to hydrogen sulfide is associated with a 23-fold increase in encephalopathy risk compared to the general industrial population.[3]
In developed countries, due to higher occupational safety standards, the incidence of toxic encephalopathy is relatively low. However, a notable proportion remains related to drug abuse. In contrast, developing countries with rapid industrialization but insufficient safety regulation report a higher prevalence, particularly in regions of Africa and Asia where industrial pollution, pesticide misuse, and biomass fuel combustion are widespread. Although accurate global prevalence is difficult to estimate, it is generally believed to range from 0.1% to 1% in the general population, and much higher among occupationally exposed individuals, drug users, and other high-risk groups.[4] In addition to neurological dysfunction, toxic encephalopathy imposes cognitive, emotional, and social burdens on patients and their families, contributing to societal costs and decreased quality of life.[5]
With the advancement of omics technologies such as transcriptomics, proteomics, and metabolomics, researchers are now able to investigate disease mechanisms at the molecular level and identify potential biomarkers or therapeutic targets for toxic encephalopathy.[6–8]
Granzyme B (GZMB), a serine protease secreted by cytotoxic T cells and natural killer cells, plays an essential role in immune regulation by promoting apoptosis of target cells and maintaining immune homeostasis.[9] Recent studies suggest that GZMB may also participate in inflammatory and neurodegenerative processes; however, its role in toxic encephalopathy remains unclear.
Based on public high-throughput datasets and bioinformatics analysis, this study explores whether GZMB is abnormally expressed and functionally relevant in toxic encephalopathy. We aim to identify GZMB-related biological pathways and protein interaction networks, thus providing preliminary evidence for its potential role as a biomarker and foundation for future therapeutic exploration.
1.1. Objectives
This study aims to investigate the potential role of Granzyme B (GZMB) in the pathogenesis of toxic encephalopathy through an integrated bioinformatics and experimental approach. We hypothesize that GZMB is differentially expressed in individuals with toxic encephalopathy and may participate in disease-relevant signaling pathways. To test this hypothesis, we first conducted transcriptomic analyses to identify Differentially expressed genes (DEGs) and assess GZMB expression levels in toxic encephalopathy samples. We then performed pathway enrichment and protein–protein interaction (PPI) network analysis to explore the molecular functions and regulatory networks associated with GZMB. In addition, we employed an animal model of sepsis-associated encephalopathy (SAE) to validate GZMB expression in vivo and to preliminarily evaluate the regulatory effects of β-aescin sodium and methylprednisolone. Through this combined strategy, we aim to provide initial evidence supporting the biomarker potential and functional significance of GZMB in toxic encephalopathy.
2. Methods
2.1. Toxic encephalopathy dataset
In this study, the toxic encephalopathy dataset GSE253309 profile was downloaded from the gene expression omnibus database (http://www.ncbi.nlm.nih.gov/geo/) generated on the GPL24247 platform. The GSE253309 dataset contains hippocampal RNA-seq data from a total of 6 samples, including 3 control mice and 3 SAE model mice.
The dataset GSE253309 was selected because it is currently the only publicly available transcriptomic dataset specifically related to toxic encephalopathy in the gene expression omnibus database. It includes clearly defined case and control samples, making it suitable for DEG identification and network analysis.
2.2. DEG screening
The R package “limma” was used to preprocess the gene expression matrix from GSE253309, performing probe summarization and background correction. The Benjamini-Hochberg method was applied to adjust the raw p-values. Fold change (FC) was calculated using the false discovery rate (FDR), with the cutoff criteria for DEGs set at P < .05 and FC > 1.5. A volcano plot was generated to visualize the DEGs.
Although an FDR threshold of <0.05 is often used, we adopted a more inclusive cutoff of FDR < 0.25 in the enrichment analysis stage, consistent with common practice in exploratory transcriptomic studies, to avoid missing potentially relevant biological pathways.
2.3. Functional enrichment analysis
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses are computational methods used to evaluate gene functions and biological pathways. In this study, the list of DEGs identified by the limma differential expression analysis was input into the KEGG REST API (https://www.kegg.jp/kegg/rest/keggapi.html) to obtain the latest gene annotations from KEGG Pathway. Using this as a background, genes were mapped to the background set, and enrichment analysis was conducted using the R package “clusterProfiler” (version 3.14.3) to obtain the enriched gene sets. The R package “org.Hs.e.g..db” (version 3.1.0) was also used to annotate the GO terms of the genes, with the minimum gene set size set to 5 and the maximum gene set size to 5000. A P-value of <.05 and an FDR of <0.25 were used as the thresholds for statistical significance.
Additionally, the Metascape database (http://metascape.org/gp/index.html) provides comprehensive gene list annotation and analysis resources with visualization options. We used Metascape to conduct functional enrichment analysis and export visualizations for the DEG list mentioned above.
2.4. Weighted gene co-expression network analysis
First, we calculated the median absolute deviation for each gene in the gene expression matrix of the GSE253309 toxic encephalopathy dataset, excluding the top 50% of genes with the lowest median absolute deviation values. Outliers were removed using the “goodSamplesGenes” function from the R package “WGCNA,” and a scale-free co-expression network was subsequently constructed using WGCNA. Specifically, a Pearson correlation matrix and average linkage method were applied to all paired genes. A power function, Amn=∣Cmn∣βA_{mn} = |C_{mn}|^\betaAmn =∣Cmn ∣β, was used to construct a weighted adjacency matrix, where CmnC_{mn}Cmn is the Pearson correlation between Gene_m and Gene_n, and AmnA_{mn}Amn is the adjacency between Gene m and Gene n. The soft-threshold parameter β\betaβ enhances strong correlations between genes while diminishing the effects of weak correlations and negative correlations. After choosing a power of 10, the adjacency matrix was transformed into a topological overlap matrix (TOM), which measures network connectivity of a gene as the sum of its adjacency with all other genes, and was used to compute the corresponding dissimilarity (1-TOM).
To classify genes with similar expression patterns into gene modules, hierarchical clustering based on TOM dissimilarity measurements was conducted, with a minimum module size (gene group) of 30. Sensitivity was set to 3. For further module analysis, module eigengene dissimilarity was calculated, a cut line was chosen for the module dendrogram, and some modules were merged. Modules with a distance of <0.25 were also combined. Notably, the gray module is considered a set of genes that cannot be assigned to any module. Goodness-of-fit test, by calculating goodness-of-fit indicators under different soft thresholds, such as R² values, selects the soft threshold that makes the network topology most consistent with the scale-free network characteristics (usually R²>0.8 or higher). For stability analysis, the network constructed under different soft thresholds was analyzed several times to observe the stability of the network structure and module composition, and the soft threshold with higher stability was selected. The degree of association between the module and the disease or biological phenotype under different soft thresholds was analyzed, and the soft threshold that can make the module have the most clear biological significance was selected.
2.5. Construction and analysis of the protein–protein interaction network
The search tool for the retrieval of interacting genes (STRING) database (http://string-db.org/) aims to collect, score, and integrate all publicly available sources of PPI information and supplements these sources with computational predictions. In this study, we input the list of DEGs into the STRING database to construct a PPI network for predicting core genes (confidence score > 0.4). Cytoscape software provides biologists with tools for biological network analysis and 2-dimensional visualization. Using Cytoscape, we visualized the PPI network generated by the STRING database and identified core genes. First, we imported the PPI network into Cytoscape and used MCODE to identify the most highly correlated modules. We then applied 3 algorithms, MCC, DMNC, EPC, to compute the top 10 genes with the highest correlations and took their intersections to visualize and export the core gene list.
2.6. Gene expression heatmap
Using the R package heatmap, we created a heatmap of the expression levels of the core genes identified in the PPI network within the toxic encephalopathy dataset GSE253309. This allowed us to visualize expression differences of the core genes between toxic encephalopathy and normal tissue samples.
2.7. CTD analysis
Comparative toxicogenomics database (CTD) integrates a large amount of interaction data between chemicals, genes, functional phenotypes, and diseases, providing significant convenience for studying disease-associated environmental exposure factors and potential mechanisms of drug action. We input the core genes into the CTD website to identify diseases most related to the core genes, and we used Excel to create radar charts showing the expression differences of each gene.
2.8. Animal model construction and GZMB expression validation
2.8.1. Experimental grouping
To validate GZMB expression and therapeutic responsiveness in vivo, an animal model of SAE was constructed.
Control group: Normal saline injection served as the negative control.
SAE group: The LPS injection method was used to simulate SAE. In this experiment, C57BL/6 mice were intraperitoneally injected with LPS reagent to induce sepsis.
SAE group + β-aescin sodium combined with methylprednisolone treatment: Mice with SAE were treated with β-aescin sodium combined with methylprednisolone.
2.8.2. Western blotting (WB) was used to detect the expression of GZMB protein in the hippocampal tissue of mice in each group
Total protein in the tissues was extracted using RIPA lysis buffer (with protease inhibitors added within a few minutes before use). The protein concentration was determined via the BCA assay. Proteins were separated by SDS-PAGE electrophoresis under the following conditions: 90V for approximately 30 minutes (stacking gel) and 130V for approximately 60 minutes (separating gel). After electrophoresis, proteins in the gel were transferred to a PVDF membrane. The PVDF membrane was blocked in blocking buffer for 1 hour. Following removal of the blocking buffer, primary antibodies against GZMB (Proteintech, 13588-1-AP, 1:1000, Wuhan) and GAPDH (Proteintech, 10494-1-AP, 1:5000, Wuhan) were added, and the membrane was incubated overnight at 4°C. The primary antibodies were then removed, and the membrane was washed 3 times with TBST (5–10 minutes per wash). The corresponding HRP-conjugated secondary antibody (Solarbio, SE134, 1:1000) was added, and the membrane was incubated at room temperature for 1 hour. After removing the secondary antibody, the membrane was washed 3 times with TBST (5–10 minutes per wash). Finally, chemiluminescence was used for visualization. Grayscale analysis was performed using Image J software, and all data were tested in triplicate for statistical processing.
3. Results
3.1. Differential expression gene analysis
In this study, a total of 994 DEGs were identified from the GSE253309 dataset using the limma package, based on the cutoff criteria of P < .05 and FC > 1.5. Among them, 545 were upregulated and 449 were downregulated in toxic encephalopathy samples compared to controls. The volcano plot (Fig. 1) visually illustrates this distribution, highlighting several significantly upregulated genes including GZMB and Stat3, which may play key roles in disease pathogenesis.
Figure 1.
Volcano plot of DEGs. A total of 994 DEGs were identified in GSE253309 (cutoff: P < .05, FC > 1.5), including 545 upregulated and 449 downregulated genes. The x-axis represents the log2 fold change, and the y-axis represents the–log10 P-value. Red and green dots indicate significantly upregulated and downregulated genes, respectively. GZMB is highlighted as one of the top upregulated genes in toxic encephalopathy tissues. DEGs = differentially expressed genes, GZMB = Granzyme B.
3.2. Functional enrichment analysis
3.2.1. GO and KEGG functional enrichment analysis of DEGs
GO enrichment analysis revealed that the DEGs were significantly associated with biological processes such as organic acid catabolic process, oxidoreductase activity, and chromosomal structural components, suggesting possible mitochondrial and metabolic dysfunction in toxic encephalopathy (Fig. 2A–C). KEGG analysis indicated that DEGs were enriched in several inflammation- and apoptosis-related pathways, including the TGF-beta signaling pathway, apoptosis, and cytokine-cytokine receptor interaction (Fig. 2D). These pathways are known to be involved in neuroinflammatory processes, supporting the pathological basis of toxic encephalopathy.
Figure 2.
Gene ontology and KEGG enrichment analyses of DEGs. (A) Biological process terms significantly enriched among DEGs, including organic acid catabolic process and immune regulation. (B) Cellular component analysis showing enrichment in condensed chromosome and mitochondrial components. (C) Molecular function analysis, with notable enrichment in oxidoreductase and ATPase activity. (D) KEGG pathway analysis revealing top-enriched pathways including the TGF-beta signaling pathway and apoptosis, suggesting potential neuroinflammatory mechanisms. DEGs = differentially expressed genes, KEGG = Kyoto encyclopedia of genes and genomes.
Due to the limited sample size of the GSE253309 dataset (n = 6), standard ranked-based gene set enrichment analysis was not applicable in this study. Instead, we employed over-representation analysis using the `clusterProfiler` and Metascape platforms, based on the list of DEGs. over-representation analysis is widely accepted for DEG-based enrichment when sample size or statistical power is limited. The enriched pathways were ranked by adjusted p-values (FDR) and gene ratios, with FDR < 0.25 used as the statistical cutoff for exploratory significance.
3.2.2. Metascape enrichment analysis
Metascape enrichment further supported the involvement of immune and stress response pathways, including the G protein-coupled dopamine receptor signaling, MAPK cascade regulation, and toll-like receptor 2 signaling (Fig. 3A). These findings suggest a convergence of inflammatory and neuronal regulatory mechanisms. The enrichment network maps (Figs. 3B, C, and 4) revealed tight clustering among immune regulation and apoptotic processes, highlighting their potential relevance in the molecular pathology of toxic encephalopathy.
Figure 3.
Metascape-based functional enrichment analysis. (A) Bar plot of top GO and pathway terms enriched in DEGs, color-coded by P-value. (B) Network visualization of enriched terms, grouped by cluster ID. Nodes with the same cluster ID are topologically close and functionally related. (C) Network colored by significance: deeper color indicates stronger statistical enrichment. Immune signaling and cell death–related terms dominated the enrichment profile. DEGs = differentially expressed genes, GO = gene ontology.
Figure 4.
Protein–protein interaction network and MCODE module analysis. PPI network constructed using STRING for DEGs, visualized in Cytoscape. Core submodules were identified using MCODE, highlighting dense connectivity regions related to immune regulation and apoptosis. PPI = protein–protein interaction, STRING = search tool for the retrieval of interacting genes.
3.3. WGCNA
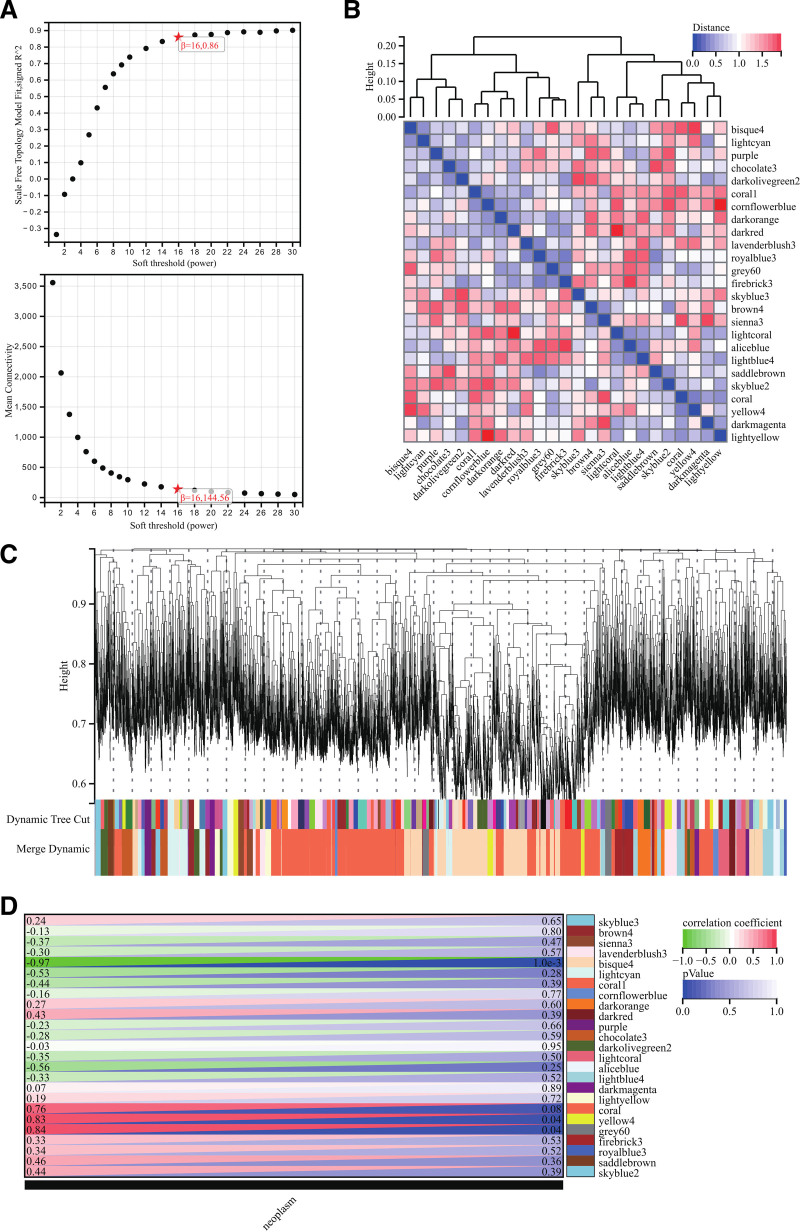
The selection of soft-threshold power is an essential step in WGCNA. To determine the appropriate soft-threshold power, network topology analysis was performed, and the power was set to 16 (Fig. 5A). A hierarchical clustering tree was constructed for all genes, resulting in 25 modules (Fig. 5B). We then analyzed the interactions between key modules (Fig. 5C) and generated a heatmap showing the correlation between modules and phenotypes (Fig. 5D) as well as scatter plots of the gene significance and MM correlations for hub genes in relevant modules (Fig. 6A). To obtain the module membership (MM), we calculated the correlation between module eigengenes and gene expression. According to the cutoff criteria (|MM| > 0.8), 6 highly connected gene modules were identified as hub modules in clinically significant modules, and the genes within these modules were designated as hub genes.
Figure 5.
Weighted gene co-expression network analysis. (A) Scale-free topology model fit and soft-thresholding analysis; power β = 16 yielded R2 > 0.85. (B and C) Hierarchical clustering tree generated 25 co-expression modules. (D) Heatmap showing correlation between modules and disease phenotype. Turquoise and brown modules were most associated with toxic encephalopathy.
Figure 6.
Module membership and DEG overlap analysis. (A) Scatter plot of GS vs MM for hub genes. (B) Venn diagram showing overlap between DEGs and WGCNA modules. A total of 880 intersecting genes were used for further analysis. DEGs = differentially expressed genes, GS = gene significance, MM = module membership, WGCNA = Weighted gene co-expression network analysis.
Using the hub genes obtained from WGCNA, we constructed a Venn diagram with DEGs and took the intersection, ultimately identifying 880 intersecting DEGs (Fig. 6B). These intersecting DEGs were subsequently used for the construction of the PPI network.
3.4. Construction and analysis of the PPI network
The PPI network was constructed using the STRING online database and analyzed with Cytoscape software (Fig. 7A). Core gene clusters were identified (Fig. 7B), and the MCC, DMNC, EPC algorithms were applied to determine the core genes of these clusters (Fig. 7C–E). A Venn diagram was generated to obtain the union of the core genes, resulting in 7 core genes: Stat3, Lag3, Mki67, Fcgr2b, Gzmb, Ccr5, Epcam (Fig. 7F).
Figure 7.
Construction and analysis of PPI networks. (A) Construct the PPI network of DEGs using STRING online database and utilize Cytoscape software for analysis. (B) CLUSTER was used to identify the central gene. (C) MCC was used to identify the central gene. (D) DMNC was used to identify the central gene. (E) EPC was used to identify the central gene. (F) Seven core genes (Stat3, Lag3, Mki67, Fcgr2b, Gzmb, Ccr5, Epcam) were obtained by merging using Venn diagrams. DEGs = differentially expressed genes, PPI = protein–protein interaction, STRING = search tool for the retrieval of interacting genes.
3.5. Heatmap of core gene expression
We visualized the expression levels of the 7 core genes identified from the PPI network in the GSE253309 toxic encephalopathy dataset using a heatmap (Fig. 8A). Among these, GZMB exhibited the most distinct differential expression, with consistently higher expression in disease samples compared to controls. This expression pattern was visually more pronounced than that of other core genes, such as Stat3 or Lag3, indicating a uniquely strong disease association.
Figure 8.
Expression heatmap of core genes and CTD disease association analysis. (A) Heatmap of core gene expression in disease (GSM8018656–8658) and control (GSM8018653–8655) samples. GZMB showed the most significant upregulation (P < .01). Red: high expression; green: low expression. (B) CTD analysis linking GZMB to toxicological phenotypes such as inflammation, necrosis, hyperplasia, and clinical symptoms including headache and dizziness, reinforcing its role in toxic encephalopathy. CTD = comparative toxicogenomics database, GZMB = Granzyme B.
These findings support the hypothesis that GZMB may serve as a central regulatory gene in the disease process and justify its selection for further experimental validation. This observation is visually evident in the heatmap, where GZMB displays the most consistent and intense expression shift across all samples.
To statistically compare core gene expression patterns, we calculated mean expression levels in the disease and control groups. As summarized in Table S1, Supplemental Digital Content, https://links.lww.com/MD/P683 GZMB demonstrated the highest disease-specific expression and a statistically significant difference (P = .007), compared to other core genes such as Stat3 or Lag3. This supports its central relevance in the molecular pathology of toxic encephalopathy.
3.6. CTD analysis
In this study, we input the core gene list into the CTD website to search for diseases related to the core genes, enhancing our understanding of gene-disease associations. In the CTD disease association analysis (Fig. 8B), GZMB was linked to several clinical phenotypes relevant to toxic encephalopathy, including inflammation, cell death, and headache, further supporting its pathogenic involvement.
3.7. WB results
Results of the Western blotting (WB) assay showed that: GZMB protein exhibited low-level expression in normal mice; in the mouse model of toxic encephalopathy, the expression level of this protein was significantly upregulated (P < .01); furthermore, after mice with toxic encephalopathy received combined treatment with β-aescin sodium and methylprednisolone, the expression level of GZMB protein was significantly downregulated (P < .01).
These findings provided in vivo evidence that GZMB is upregulated in SAE and downregulated upon treatment, supporting its role as a potential therapeutic target.
4. Discussion
Toxic encephalopathy is a central nervous system disorder caused by various harmful factors, including chemical substances, drugs, heavy metals, and viral infections.[10] These factors disrupt the normal functions of neurons through multiple mechanisms, leading to neuronal damage, death, or dysfunction.[11] With its high incidence and disability rates, toxic encephalopathy poses a severe threat to patients’ quality of life and health, imposing significant burdens on families and society. Clinically, it manifests with diverse symptoms, such as headache, nausea, vomiting, consciousness disturbances, motor impairments, and cognitive deficits.[12]
GZMB, as a key cytotoxic protein, plays a crucial role in the immune system. Secreted primarily by cytotoxic T cells and natural killer cells, it regulates immune responses by inducing apoptosis in target cells.[13] Under normal conditions, GZMB activity is tightly regulated to ensure the precision and efficacy of immune responses.[14] In pathological states such as toxic encephalopathy, aberrant expression or hyperactivity of GZMB may lead to excessive neuronal apoptosis, thereby accelerating disease progression. This dysregulation is likely associated with immune imbalances triggered by harmful factors, resulting in the misrecognition and attack of neurons by immune cells.[15]
In recent years, increasing attention has been paid to the potential role of GZMB in toxic encephalopathy. Through big data analysis, this study revealed that GZMB exhibits significant differential expression in various forms of toxic encephalopathy. Notably, in certain autoimmune and infectious encephalopathies, elevated GZMB expression is often closely associated with disease activity and the extent of neuronal damage,[16,17] suggesting a critical role in the disease’s pathogenesis. Further analysis indicates that aberrant GZMB expression may be linked to immune cell activation, neuroinflammatory responses, and oxidative stress in neurons.
Furthermore, although our dataset did not show statistically significant upregulation of other granzyme family members (such as GZMA, GZMK), their involvement in other forms of neuroinflammatory conditions has been suggested in previous literature. Hence, future studies should assess the full granzyme profile to determine potential co-regulation or redundancy in pathogenesis.
This study also explored the potential mechanisms of β-escin sodium combined with methylprednisolone in preventing delayed encephalopathy after carbon monoxide poisoning. As commonly used therapeutic agents,[18] β-escin sodium and methylprednisolone have demonstrated notable efficacy in treating this condition, potentially by modulating the expression and activity of GZMB through multiple mechanisms. β-Escin sodium exerts significant anti-inflammatory and antioxidant effects, alleviating oxidative stress and inflammation induced by harmful factors such as carbon monoxide poisoning.[19] These effects may indirectly suppress the overexpression of GZMB, thereby reducing neuronal apoptosis. Additionally, β-escin sodium can stabilize cell membranes, mitigating direct damage to neurons caused by external toxic factors, and further attenuate apoptosis mediated by GZMB.
Methylprednisolone, as a glucocorticoid, can influence GZMB expression levels by regulating the activity and function of immune cells.[20] It may suppress the hyperactivity of T cells and NK cells,[21,22] thereby reducing the abnormal expression of GZMB. Additionally, methylprednisolone may act directly on neurons, inhibiting GZMB activity and mitigating apoptosis. This effect is likely associated with the enhancement of neuronal survival signaling and inhibition of apoptotic pathways mediated by methylprednisolone.
Notably, this study identified a strong correlation between neuronal apoptosis and GZMB activity following carbon monoxide poisoning. Hypoxia and reperfusion injury induced by carbon monoxide poisoning lead to severe neuronal damage,[23] accompanied by a significant increase in GZMB expression, further exacerbating neuronal apoptosis. However, treatment with β-escin sodium combined with methylprednisolone significantly reduced GZMB expression levels, decreased neuronal apoptosis, and improved patient outcomes.
To support these computational and mechanistic predictions, we further performed in vivo validation using a mouse model of SAE. WB demonstrated that GZMB protein levels were significantly elevated in the hippocampus of SAE mice compared to controls (P < .01), while combined treatment with β-aescin sodium and methylprednisolone significantly suppressed GZMB expression (P < .01). These results, consistent with our bioinformatic predictions, confirm the responsiveness of GZMB to pharmacological modulation in vivo (see Fig. 9).
Figure 9.
This figure includes Western blotting results and bar graph analysis. The expression levels of GZMB in each group were detected through the WB assay. Group A represents the control group, Group B represents the SAE group, and Group C represents the SAE group + β-aescin sodium combined with methylprednisolone treatment group (** indicates a statistically significant difference with P < .01). GZMB = Granzyme B, SAE = sepsis-associated encephalopathy, WB = Western blotting.
This experimental validation reinforces the central role of GZMB in toxic encephalopathy and supports its potential as a therapeutic target. The ability of β-aescin sodium and methylprednisolone to downregulate GZMB expression in a pathological context provides important translational relevance to our findings. However, further mechanistic studies are warranted to clarify upstream regulatory signals and downstream consequences of GZMB modulation.
Given the pivotal role of GZMB in toxic encephalopathy, therapeutic strategies targeting its expression and activity hold great potential. Regulating GZMB may effectively mitigate neuronal damage and slow disease progression. Future research should focus on elucidating the upstream signaling pathways that regulate GZMB, as well as identifying its downstream apoptotic effectors. The development of specific therapeutic approaches – such as small-molecule inhibitors, receptor antagonists, gene therapy, or cell-based interventions – also represents a promising direction to modulate GZMB expression levels.[24] Moreover, exploring additional neuroprotective targets, including oxidative stress and inflammatory pathways, may help establish a more comprehensive strategy for intervention. Ultimately, combining GZMB-targeted therapies with agents that counteract neuronal injury from multiple angles could offer a more effective and holistic approach to preventing and treating toxic encephalopathy.
4.1. Limitations
This study has several limitations in exploring GZMB as a potential biological target for toxic encephalopathy. First, the big data analysis relies on public datasets and may be affected by sample selection bias, limiting the generalizability of the findings to diverse clinical settings. Second, the complex interplay between GZMB and other genetic or environmental factors was not fully addressed, potentially underestimating the multifactorial etiology. Third, although we now provide preliminary in vivo experimental validation supporting the upregulation of GZMB in SAE and its responsiveness to pharmacological intervention, no large-scale prospective clinical studies have yet verified the efficacy and safety of GZMB-targeted strategies. Further translational research is required to bridge this gap before clinical application.
5. Conclusion
In summary, this study identified GZMB as a key gene associated with toxic encephalopathy through integrated bioinformatics analysis and in vivo validation in a mouse model of SAE. The elevated expression of GZMB in disease states and its responsiveness to β-escin sodium and methylprednisolone suggest its potential functional relevance. However, given the study’s reliance on public transcriptomic data and limited experimental validation, the translational implications remain preliminary. These findings provide an exploratory foundation for future research into the pathophysiological role of GZMB and its therapeutic modulation. Further mechanistic studies and clinical investigations are warranted to validate the potential of GZMB as a biomarker or treatment target.
Author contributions
Conceptualization: Lijie Liu.
Data curation: Lijie Liu, Jiankuo Wang.
Formal analysis: Lijie Liu, Jiankuo Wang.
Methodology: Lijie Liu, Chenqing Liu.
Writing – original draft: Lijie Liu, Jiankuo Wang, Chenqing Liu.
Writing – review & editing: Lijie Liu.
Supplementary Material
Abbreviations:
- CTD
- comparative toxicogenomics database
- DEGs
- differentially expressed genes
- FC
- fold change
- FDR
- false discovery rate
- GEO
- gene expression omnibus
- GO
- gene ontology
- GZMB
- granzyme B
- KEGG
- Kyoto encyclopedia of genes and genomes
- MAD
- median absolute deviation
- MM
- module membership
- PPI
- protein–protein interaction
- STRING
- search tool for the retrieval of interacting genes
- TOM
- topological overlap matrix
This study was approved by the Ethics Committee of Baoding Second Hospital.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Liu L, Wang J, Liu C. Granzyme B as a potential biological target in toxic encephalopathy: A big data-based exploratory analysis. Medicine 2025;104:33(e43879).
Contributor Information
Jiankuo Wang, Email: wangjiankuo@126.com.
Chenqing Liu, Email: yangbiao1981316@163.com.
References
- [1].Verma R, Chakraborty R, Giri P. Acute toxic encephalopathy in occupational exposure with polyvinyl chloride (PVC) fumes: a case series. Neurol India. 2023;71:531–5. [DOI] [PubMed] [Google Scholar]
- [2].Dobbs MR. Toxic encephalopathy. Semin Neurol. 2011;31:184–93. [DOI] [PubMed] [Google Scholar]
- [3].Bojsen JA, Lunau L, Nguyen NT, Rasmussen B. Amphetamine-induced toxic encephalopathy. Ugeskr Laeger. 2022;184:V12210924 [pii]. [PubMed] [Google Scholar]
- [4].Biswas S, Pendharkar HS, Murumkar VS. MRI spectrum of toxic encephalopathy-an institutional experience. Neurol India. 2022;70:1525–33. [DOI] [PubMed] [Google Scholar]
- [5].Song IU, Chung SW. Chorea as the first neurological symptom of delayed encephalopathy after carbon monoxide intoxication. Intern Med. 2010;49:1037–9. [DOI] [PubMed] [Google Scholar]
- [6].Zhang P, Dai Y, Xiong J, et al. iTRAQ-based differential proteomic analysis of the brains in a rat model of delayedcarbon monoxide encephalopathy. Brain Res Bull. 2018;137:329–37. [DOI] [PubMed] [Google Scholar]
- [7].Hu H, Pan X, Wan Y, Zhang Q, Liang W. Factors affecting the prognosis of patients with delayed encephalopathy after acute carbon monoxide poisoning. Am J Emerg Med. 2011;29:261–4. [DOI] [PubMed] [Google Scholar]
- [8].Li ZK, Li CH, Yue AC, et al. Therapeutic effect and molecular mechanism of Salvia Miltiorrhiza on rats with acute brain injury after carbon monoxide poisoning based on the strategy of internet pharmacology. Environ Toxicol. 2022;37:413–34. [DOI] [PubMed] [Google Scholar]
- [9].Thompson R, Cao X. Reassessing granzyme B: unveiling perforin-independent versatility in immune responses and therapeutic potentials. Front Immunol. 2024;15:1392535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lerner DP, Tadevosyan A, Burns JD. Toxin-induced subacute encephalopathy. Neurol Clin. 2020;38:799–824. [DOI] [PubMed] [Google Scholar]
- [11].Kelafant GA. Encephalopathy and peripheral neuropathy following carbon monoxide poisoning from a propane-fueled vehicle. Am J Ind Med. 1996;30:765–8. [DOI] [PubMed] [Google Scholar]
- [12].Erkkinen MG, Berkowitz AL. A clinical approach to diagnosing encephalopathy. Am J Med. 2019;132:1142–7. [DOI] [PubMed] [Google Scholar]
- [13].Prager I, Liesche C, van Ooijen H, et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J Exp Med. 2019;216:2113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aliyu M, Zohora FT, Anka AU, et al. Interleukin-6 cytokine: an overview of the immune regulation, immune dysregulation, and therapeutic approach. Int Immunopharmacol. 2022;111:109130. [DOI] [PubMed] [Google Scholar]
- [16].Kaur S, Bansal Y, Kumar R, Bansal G. A panoramic review of IL-6: structure, pathophysiological roles and inhibitors. Bioorg Med Chem. 2020;28:115327. [DOI] [PubMed] [Google Scholar]
- [17].Chen Y, Guo Y, Li S, et al. Tumor-derived IL-6 promotes chordoma invasion by stimulating tumor-associated macrophages M2 polarization and TNFα secretion. Int Immunopharmacol. 2024;143(Pt 1):113315. [DOI] [PubMed] [Google Scholar]
- [18].Ji DB, Xu B, Liu JT, Ran FX, Cui JR. β-Escin sodium inhibits inducible nitric oxide synthase expression via downregulation of the JAK/STAT pathway in A549 cells. Mol Carcinog. 2011;50:945–60. [DOI] [PubMed] [Google Scholar]
- [19].Wang Y, Han X, Wan X, Niu F, Zhou C. β-Escin: an updated review of its analysis, pharmacology, pharmacokinetics, and toxicity. Am J Chin Med. 2023;51:2095–120. [DOI] [PubMed] [Google Scholar]
- [20].Tęsiorowski M, Potaczek T, Jasiewicz B, Sapa J, Zygmunt M. Methylprednisolone- acute spinal cord injury, benefits or risks?. Postepy Hig Med Dosw (Online). 2013;67:601–9. [DOI] [PubMed] [Google Scholar]
- [21].Nakamura T, Ebihara I, Tomino Y, Okumura K, Koide H. Perforin mRNA expression in the inflamed tissues of NZB/W F1 lupus mice decreases with methylprednisolone treatment. Am J Pathol. 1991;139:731–6. [PMC free article] [PubMed] [Google Scholar]
- [22].Langhoff E, Ladefoged J, Dickmeiss E. The immunosuppressive potency of various steroids on peripheral blood lymphocytes, T cells, NK and K cells. Int J Immunopharmacol. 1985;7:483–9. [DOI] [PubMed] [Google Scholar]
- [23].Olson KR. Carbon monoxide poisoning: mechanisms, presentation, and controversies in management. J Emerg Med. 1984;1:233–43. [DOI] [PubMed] [Google Scholar]
- [24].Shi Z, Yan J, Zhao M, Li S, She T, Qian X. Co-encapsulation of granzyme B and perforin in nanocapsules for tumour therapy: biomimicking immune cells. J Control Release. 2024;369:658–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.