Abstract
NADH:ubiquinone oxidoreductase (complex I) of the electron transport chain is a multimeric mitochondrial enzyme of approximately 1000 kDa consisting of 46 different proteins encoded by both the mitochondrial and nuclear genomes. Little is known about the cellular mechanisms and protein chaperones that guide its assembly. In this issue of the JCI, Ogilvie et al. use genomic sequence data to compare the proteins produced by yeasts with and without complex I in order to generate a list of proteins whose human orthologs might serve as complex I assembly proteins. The gene encoding one of these candidate proteins, B17.2L, was found to harbor a nonsense mutation in one of 28 patients with a deficiency of complex I. B17.2L associated with subcomplexes that are seen when complex I assembly is incomplete. The research described here combines clever model organism genomics and bioinformatics with sophisticated human molecular and biochemical genetics to identify the first mammalian protein required for the normal assembly of complex I.
Complex I of the mitochondrial respiratory chain
Of all the components of the electron transport chain, NADH:ubiquinone oxidoreductase (complex I) is by far the biggest and contains the largest number of structural proteins (1). Complex I contains the mitochondrial NADH-dehydrogenase (EC 1.6.99.3) activity responsible for accepting electrons from NADH and passing them on via ubiquinone to complex III while pumping 4 protons across the inner membrane, thereby contributing to the electrochemical ion gradient across the inner mitochondrial membrane that powers the synthesis of ATP (2) (Figure 1). Based on studies of bovine heart mitochondria, complex I is a very large multisubunit structure, approximately 1000 kDa in size and made up of at least 46 subunits (3), of which 39 are nuclear encoded and 7 are encoded by the mitochondrial genome. According to low-resolution electron micrographs, complex I is an L-shaped heteromultimer (1). A relatively hydrophilic matrix arm extends into the interior of the mitochondria and is connected to a more hydrophobic segment located within the inner mitochondrial membrane (4) (Figure 1). The precise molecular mechanisms that couple electron transfer, ubiquinone reduction, and proton pumping still need to be elucidated (2).
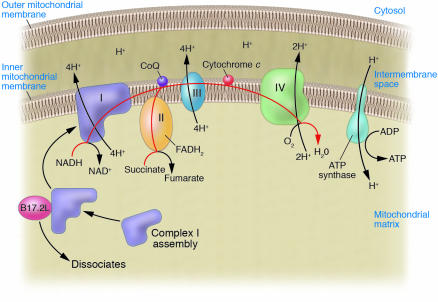
Figure 1.
Schematic diagram of complexes I through IV of the electron transport chain and ATP synthase. The red line traces the path of electrons as they enter and move along the electron transport chain. Complex I is shown at the far left as an L-shaped structure with one portion extending down into the mitochondrial matrix and the other portion embedded in the inner mitochondrial membrane. Ogilvie and colleagues used bioinformatics to perform a virtual whole genome subtraction of yeasts with or without complex I to find candidate complex I assembly factors, identified the human orthologs of these proteins, and showed that one of these orthologs, B17.2L, carried a null mutation in a patient with complex I deficiency (17). The authors also demonstrate that B17.2L — the first identified mammalian protein required for the normal assembly of human complex I — associates with an immature subcomplex of complex I (shown schematically) and facilitates complex I assembly. FADH2, reduced flavin-adenine dinucleotide; CoQ, coenzyme Q (also known as ubiquinone).
As a complicated multisubunit structure of 46 proteins, complex I must be assembled from smaller subcomplexes, which are themselves assembled from smaller intermediates. The existence of subcomplex intermediates in complex I assembly is supported by the observation that 2D blue native PAGE (BN-PAGE) of complex I from muscle mitochondria obtained from 4 patients with complex I deficiency, 1 with a known mutation in the mitochondrial MTND2 gene and the others carrying mutations in undefined nuclear-encoded genes, revealed the existence of several common subcomplexes of varying sizes (5). A different but reproducible subunit expression pattern was seen in an analysis of 2D BN-PAGE of subcomplex assembly occurring after release of a block in mitochondrial protein translation induced by doxycycline (6). The complicated process of assembling such a large multimeric complex most likely needs to be guided by cellular machinery (6). Studies in the aerobic fungus Neurospora crassa have identified 2 of the proteins required to assemble the segment of complex I that is located in the inner mitochondrial membrane from 2 smaller subcomplexes (7).
Researchers have not yet been able to bring the full power of genetic analysis to elucidating the structure and function of complex I because the usual eukaryotic workhorse for such approaches, the yeast Saccharomyces cerevisiae, lacks complex I. Although there has been a lot progress in using prokaryotes or eukaryotic fungi such as N. crassa as alternative model systems (7), the pace of progress seems to now be accelerating through the use of Yarrowia lipolytica, an obligate aerobic yeast and a powerful new model organism for studies of complex I structure and function (1). Complex I from Y. lipolytica appears to be very similar to mammalian complex I in structure and composition, and its identification has facilitated the usual opportunities for genetic manipulation in yeast, such as mutagenesis screens for functional mutants, facile site-directed mutagenesis, and expression of tagged proteins suitable for affinity chromatography and proteomic analysis (8).
Disorders of oxidative phosphorylation in humans
Human diseases caused by defects in oxidative phosphorylation are rare (approximately 1 per 10,000 live births) but often take the form of devastating neurological conditions (9). Symptoms can vary from fatal lactic acidosis in the neonate to mental and physical retardation with cardiomyopathy, skeletal myopathy, and hepatic failure in childhood, to acute painless loss of vision (Leber hereditary optic neuropathy) in young adults, to a form of Parkinson disease later in life. One-third of the defects in the electron transport chain that cause genetic oxidative phosphorylation diseases occur in complex I (9, 10). Only a minority of the molecular abnormalities that cause complex I deficiency are known. Laboratories that do extensive molecular diagnostic analysis for complex I deficiency report that only approximately 20–25% of such patients have homoplasmic or heteroplasmic mutations in 1 of 4 mitochondrial-encoded complex I subunit genes; another 20–25% are the result of mutations in 1 of 9 nuclear-encoded complex I structural subunit genes (9, 11–13). The molecular defects in the remaining 50–60% of patients with deficiencies in complex I, but without obvious mutations in genes encoding complex I structural subunits, still remain largely undetermined. It seems a very reasonable supposition that some of these complex I defects without structural subunit mutations are caused by defects in auxiliary proteins required for multimer assembly, as has already been demonstrated in some patients with severe encephalopathy and failure of other organ systems due to mutations in genes such as SURF1 and SCO2 that affect complex IV assembly (14) or mutations in BCS1L affecting complex III assembly (15). Of the 2 assembly proteins that have been shown to be required for N. crassa complex I assembly, 1 has a human ortholog; however, no defects in that gene have been found in patients with complex I deficiency (16). In this issue of the JCI, Ogilvie et al. report the first human protein required for assembly of human complex I (17).
Ogilvie et al. (17) report a female child, born to a normal, nonconsanguineous couple, who developed progressive neurological disease affecting many portions of her central nervous system beginning around 1 year of age and suffered relentless neurological deterioration until her death at 13.5 years of age. Her disease was associated with elevation of cerebral spinal fluid lactate levels and a deficiency of complex I enzyme activity in muscle mitochondria (approximately 38% of control complex I activity) and cultured fibroblasts (less than 20% of control complex I activity). Taking a clever bioinformatics approach in the appropriate model organisms, these researchers carried out a subtraction in silico of genes found in Y. lipolytica and another aerobic yeast with a complex I, Debaryomyces hansenii, but not in other yeasts that lack a complex I, and used the resulting protein sequences to search for human orthologs containing mitochondrial targeting sequences. Their analysis ultimately yielded 14 genes, 1 of which was B17.2L, a paralog of B17.2, which encodes a known structural subunit in the matrix arm of human complex I (18). They sequenced B17.2L in 28 patients with complex I deficiency and found 1 patient, the child described above, who appeared to be homozygous for a nonsense mutation (C182T) in exon 2 of B17.2L that caused premature termination of translation. Her mother was heterozygous for this mutation, but the mutation was not found in her father, suggesting that he was likely to be heterozygous for a deletion allele that the proband inherited from him as her paternal allele. The functional complex I deficiency and defective assembly in this patient, as determined by enzyme assay and 2D BN-PAGE, was corrected by transduction with a vector expressing B17.2L cDNA. Finally, Ogilvie et al. demonstrated that the B17.2L protein associates with a particular 830-kDa subcomplex of complex I that accumulates in a variety of patients with mutations in genes encoding structural components of complex I, but not with the normal intact complex I itself. Based on these data, the authors concluded that B17.2L is a component of the cellular machinery that is involved in the assembly of complex I without being a part of the mature complex I itself and that loss of function of this protein leads to complex I deficiency (Figure 1).
Humans stand at the opposite end of the spectrum from yeast in terms of serving as an easily manipulated genetic system. However, the study of human genetics has much to offer, not only because of the direct involvement with human disease, but also because of the depth of phenotypic richness and the locus and allelic heterogeneity that human genetic disease provides. Indeed, one of the more striking themes of modern molecular genetics has been how progress in understanding fundamental biological processes has come time and again from the marriage of model organism research with careful human genetic studies. The research reported here by Ogilvie and colleagues (17) is an excellent example of just such a successful marriage.
Acknowledgments
The author is supported by the Intramural Research Program of the National Human Genome Research Institute, NIH.
Footnotes
See the related article beginning on page 2784.
Nonstandard abbreviations used: BN-PAGE, blue native PAGE; complex I, NADH:ubiquinone oxidoreductase.
Conflict of interest: The author has declared that no conflict of interest exists.
References
- 1.Djafarzadeh R, et al. Biophysical and structural characterization of proton-translocating NADH-dehydrogenase (complex I) from the strictly aerobic yeast Yarrowia lipolytica. . Biochim. Biophys. Acta. 2000; 1459:230–238. doi: 10.1016/s0005-2728(00)00154-7. [DOI] [PubMed] [Google Scholar]
- 2.Hirst J. Energy transduction by respiratory complex I–an evaluation of current knowledge. Biochem. Soc. Trans. 2005;33:525–529. doi: 10.1042/BST0330525. [DOI] [PubMed] [Google Scholar]
- 3.Carroll J, Fearnley IM, Shannon RJ, Hirst J, Walker JE. Analysis of the subunit composition of complex I from bovine heart mitochondria. Mol. Cell Proteomics. 2003;2:117–126. doi: 10.1074/mcp.M300014-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Grigorieff N. Three-dimensional structure of bovine NADH:ubiquinone oxidoreductase (complex I) at 22 A in ice. J. Mol. Biol. 1998;277:1033–1046. doi: 10.1006/jmbi.1998.1668. [DOI] [PubMed] [Google Scholar]
- 5.Antonicka H, et al. Identification and characterization of a common set of complex I assembly intermediates in mitochondria from patients with complex I deficiency. J. Biol. Chem. 2003;278:43081–43088. doi: 10.1074/jbc.M304998200. [DOI] [PubMed] [Google Scholar]
- 6.Ugalde C, et al. Human mitochondrial complex I assembles through the combination of evolutionary conserved modules: a framework to interpret complex I deficiencies. Hum. Mol. Genet. 2004;13:2461–2472. doi: 10.1093/hmg/ddh262. [DOI] [PubMed] [Google Scholar]
- 7.Schulte U. Biogenesis of respiratory complex I. J. Bioenerg. Biomembr. 2001;33:205–212. doi: 10.1023/a:1010730919074. [DOI] [PubMed] [Google Scholar]
- 8.Brandt U, et al. Structure-function relationships in mitochondrial complex I of the strictly aerobic yeast Yarrowia lipolytica. Biochem. Soc. Trans. 2005;33:840–844. doi: 10.1042/BST0330840. [DOI] [PubMed] [Google Scholar]
- 9.Triepels RH, Van Den Heuvel LP, Trijbels JM, Smeitink JA. Respiratory chain complex I deficiency. Am. J. Med. Genet. 2001;106:37–45. doi: 10.1002/ajmg.1397. [DOI] [PubMed] [Google Scholar]
- 10.von Kleist-Retzow JC, et al. A high rate (20%-30%) of parental consanguinity in cytochrome-oxidase deficiency. Am. J. Hum. Genet. 1998;63:428–435. doi: 10.1086/301957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.OMIM — Online Mendelian Inheritance in Man. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM.
- 12.Robinson BH. Human complex I deficiency: clinical spectrum and involvement of oxygen free radicals in the pathogenicity of the defect. . Biochim. Biophys. Acta. 1998; 1364:271–286. doi: 10.1016/s0005-2728(98)00033-4. [DOI] [PubMed] [Google Scholar]
- 13.Benit P, et al. Mutant NDUFS3 subunit of mitochondrial complex I causes Leigh syndrome. J. Med. Genet. 2004;41:14–17. doi: 10.1136/jmg.2003.014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rotig A, et al. Molecular diagnostics of mitochondrial disorders. . Biochim. Biophys. Acta. 2004; 1659:129–135. doi: 10.1016/j.bbabio.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 15.de Lonlay P, et al. A mutant mitochondrial respiratory chain assembly protein causes complex III deficiency in patients with tubulopathy, encephalopathy and liver failure. Nat. Genet. 2001;29:57–60. doi: 10.1038/ng706. [DOI] [PubMed] [Google Scholar]
- 16.Janssen R, Smeitink J, Smeets R, van Den Heuvel L. CIA30 complex I assembly factor: a candidate for human complex I deficiency? Hum. Genet. 2002;110:264–270. doi: 10.1007/s00439-001-0673-3. [DOI] [PubMed] [Google Scholar]
- 17.Ogilvie I, Kennaway NG, Shoubridge EA. A molecular chaperone for mitochondrial complex I assembly is mutated in a progressive encephalopathy. J. Clin. Invest. 2005;115:2784–2792. doi:10.1172/JCI26020. doi: 10.1172/JCI26020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smeitink J, et al. Molecular characterization and mutational analysis of the human B17 subunit of the mitochondrial respiratory chain complex I. Hum. Genet. 1998;103:245–250. doi: 10.1007/s004390050813. [DOI] [PubMed] [Google Scholar]