Abstract
Introduction:
As of March 30, 2022, the Food and Drug Administration recommended thyroid function testing for children less than 4 years of age within 3 weeks of receiving intravenous iodinated contrast due to an increased risk of thyroid dysfunction. Multifaceted solutions are needed to identify patients at risk of hypothyroidism, ensure timely testing, interpret results, and communicate information to primary care providers and caregivers. Our objective was to increase compliance with thyroid function test (TFT) completion or communication of the need for TFTs to 20% within 3 months and to 75% within 12 months.
Methods:
We identified patients using the following inclusion criteria: age less than 4 years and receipt of intravenous iodinated contrast. Electronic medical record tools, including conditional and automated SmartText in radiology reports, discharge summaries, and after-visit summaries, were used to identify patients and improve cross-encounter communication.
Results:
We identified 446 children who met the inclusion criteria. Of these, 42% (n = 189) had high-risk comorbidities. Compliance with communicating the need for TFTs to primary care providers through discharge summaries increased from a mean of 5% to 60% and with caregivers through after-visit summaries from a mean of 5% to 81% in 13 months. The percentage of TFTs completed increased from a mean of 10% to 22%, of which 22% (n = 22) were abnormal. Four patients received thyroid hormone supplementation.
Conclusions:
This project has successfully achieved its aim. Although this recommendation is unique to a small cohort of patients, similar strategies could be used to identify patients and coordinate follow-up testing across encounters.
INTRODUCTION
On March 30, 2022, the US Food and Drug Administration (FDA) recommended thyroid monitoring in all children less than 4 years of age within 3 weeks following exposure to iodine-containing intravenous (IV) contrast due to an increased risk of thyroid dysfunction, which has the potential to result in developmental delays.1 The FDA first issued an alert about this risk in 2015 based on a review of 5 studies.2–7 Six additional studies have since identified that patients at higher risk for thyroid dysfunction include children less than 4 years of age, particularly neonates, and those with underlying cardiac conditions, with most cases occurring within 3 weeks of exposure.8–13 Reported rates ranged from 1% to 15%.1 Although most cases of hypothyroidism were transient and did not require long-term treatment, based on the risk and potential for significant lifelong morbidity, the FDA recommended routine monitoring “to avoid future cognitive and other developmental disabilities.”2 In 2023, this recommendation was updated to restrict monitoring based on individualized risk factors (prematurity, very low birth weight [VLBW], and underlying medical conditions affecting thyroid function) rather than age alone.1
Enacting this recommendation provides numerous logistical challenges, particularly given the need for delayed testing. For patients who receive IV contrast in the emergency department (ED) or those discharged before the recommended testing interval, this responsibility was delegated to a separate outpatient provider to ensure completion and/or follow-up on results. Some institutions have achieved success in developing transition clinics, arranging home visits or phone calls for close follow-up with patients at high risk of readmission or specific populations requiring close monitoring.14 However, ensuring follow-up testing in a small cohort of patients who are not easily identified by a single diagnosis or procedure, such as those at risk of thyroid dysfunction, has not been well studied. Additionally, transitions of care have been identified as times of increased risk.15,16 Communication with primary care providers (PCPs), including follow-up needs, relies on accurate, effective, and timely discharge (DC) summaries. Yet, there is evidence that the timeliness and content of DC summaries are often lacking.16–18 As a result, multifaceted solutions are needed to help cross-encounter providers to identify these patients, recall the necessary timing and tests, interpret results, and communicate information to PCPs and caregivers.
We sought to create a system to streamline patient identification in the electronic medical record (EMR) and to use information technology (IT) solutions that facilitate reminders and the timely completion of studies. Because EMR reminders have been successful in aiding medical decision-making, such as improving vaccination rates,19 we hypothesized that using IT solutions to identify patients and prompt the ordering and monitoring of thyroid function tests (TFTs) would be equally effective. Given that communication via DC summaries is problematic, we also hypothesized that automation would improve communication regarding the need for TFTs. Our specific, measurable, achievable, relevant, and time-bound aim was to increase compliance with the completion of TFTs or communication of this need for monitoring in patients less than 4 years of age who received iodine-containing IV contrast to 20% within 3 months and 75% within 1 year. This aim accounts for 2 possible clinical scenarios: further communication was not necessary because TFTs had been completed and were normal, or communication was necessary because TFTs were either abnormal or not yet finished. Given that there is no comparable study or benchmark, improvement targets were based on the successes of quality improvement (QI) projects with EMR interventions19 and increased based on our planned automation of select interventions (after accounting for expected lag time for IT builds and integration).
METHODS
Context
Project team members included representatives from pediatric hospital medicine, endocrinology, cardiology, radiology, intensive care, primary care, a QI expert, a physician informaticist, a senior Epic analyst, and the pediatric department chair. We completed this project across an extensive, Mid-Atlantic hospital system that includes 5 hospital-based EDs (2 dedicated pediatric EDs), 6 free-standing EDs, 2 pediatric inpatient units, 1 pediatric intensive care unit (ICU), 1 pediatric cardiac ICU, 4 neonatal intensive care units, multiple pediatric specialty ambulatory offices, and primary care offices. On the main campus, the children’s hospital includes 226 pediatric beds with approximately 7,000 general pediatric ward admissions, 60,000 pediatric ED visits, and more than 10,000 deliveries annually.
Population of Interest
Our study population included patients from birth to, but not including, 4 years of age who received IV iodinated contrast for a study or procedure within our hospital system from January 1, 2022, to March 30, 2022 (baseline) and from April 1, 2022, to September 30, 2023 (postintervention). Initial chart review was done more than 3 weeks from the contrast administration date. Patients with abnormal TFTs were followed until the conclusion of the study.
Although the FDA ultimately posted a revision to this recommendation in April 2023, our study team did not recognize this until after data collection was nearly complete. Thus, the population of interest for the entire study is based on the original recommendation to screen all patients less than 4 years of age. Those with risk factors, as identified initially by the Pediatric Endocrine Society and/or the FDA, include newborns (age < 3 mo), prematurity (<37 wk gestational age), VLBW (<1500 g), congenital heart disease (CHD), and ICU stay.1,2,20 As such, these will be discussed throughout, even though they are no longer all considered risk factors warranting monitoring, according to the updated recommendation.
Interventions
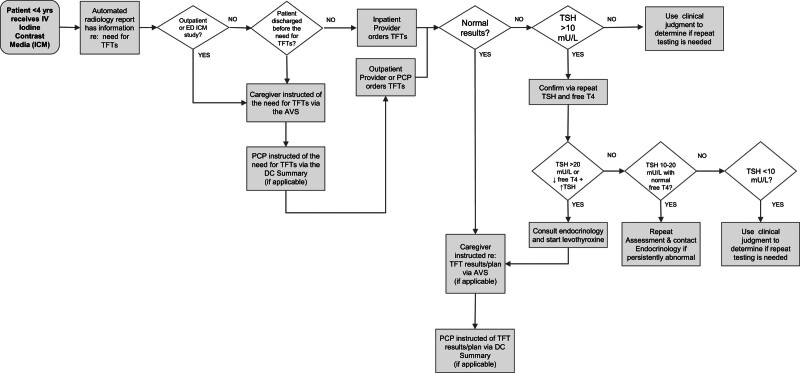
Stakeholders reviewed the existing literature and developed a unified understanding of the project’s goal through the creation of a driver diagram (Fig. 1) and a detailed process map (Fig. 2), which captures encounters over time. Ensuring the completion of thyroid testing requires the sharing of information between the provider ordering the imaging study or procedure, the patient’s caregiver, and the PCP. Interventions focused on 2 areas: identifying patients who meet the criteria for testing and providing reminders with automation.
Fig. 1.
Project key driver diagram.
Fig. 2.
Initial process map of interventions and provider guidance.
EMR analysts identified patients based on their age at the time of the study or procedure, receipt of IV iodinated contrast, and completion of relevant imaging studies or procedures. This allowed for conditional automation of prewritten text regarding the need for TFTs to be included in radiology reports, the after-visit summary (AVS) given to families upon DC, and the DC summary routed to PCPs at DC. This information was conditional, appearing only when specific criteria were met, thereby preventing the risk of incorrect or excessive and redundant note information, as well as the need to delete information for patients who did not meet the criteria. The text in the patient-facing AVS outlines the FDA recommendation and the importance of thyroid monitoring. The DC summary text provides additional information for clinicians. This is achieved through a “vanishing tip” colored text that disappears upon signing the document, along with additional guidance on interpreting TFT results and recommended next steps (Fig. 3). Incorporating information regarding the need for thyroid monitoring within radiology reports served as a reminder to reviewing providers and to caregivers viewing results through the patient portal.
Fig. 3.
Digital recreation of automated, conditional information for PCPs via DC summaries upon DC from the pediatric inpatient units.
We held meetings with physician stakeholders to review interventions and provided education within individual departments. Education was provided to outpatient pediatricians through leadership meetings involving local representatives from outpatient clinics, as well as via information distributed through our pediatric listserv with links to relevant information available on the hospital website. We built reports for tracking and auditing results. Feedback was offered, and results were shared periodically via email and at departmental meetings. We used the Model for Improvement and Plan–Do–Study–Act cycles to evaluate interventions for changes in desired outcomes.
Evolution of Interventions over Time
Isolating the population of interest proved to be difficult. In the first iteration, we identified patients by receipt of iodinated contrast; however, this resulted in the incorrect identification of patients who received these agents by alternative routes (eg, gastrostomy tube evaluation or fluoroscopy). These patients were manually excluded. In the second iteration, we outlined rules to ensure that all the following conditions were met: receipt of a particular subclass of iodinated radiographic contrast media, confirmation of IV administration, and completion of a qualifying imaging study or procedure (eg, computed tomography scan or cardiac catheterization).
IT interventions used to automate information took months to build due to complexity. During this time, we encouraged providers to manually add SmartPhrases, or blocks of text inserted into documentation when a phrase is used in the DC summaries and AVS instructions for appropriate patients. To do so, providers had to individually identify suitable patients and remember to include this information when discharging them.
The AVS information provided upon DC from the ED is done through a separate system within the EMR. In May 2023, we noted that the TFT information had been eliminated for encounters during the prior month due to a system update. Upon identification of this error, the conditional, automated information was reinstated.
Another consideration was for the provider ordering the contrast study to also order TFTs for future completion. However, we were concerned about ensuring follow-up of test completion and result management by providers who may not have further patient contact, as well as barriers to reviewing laboratory tests completed outside of our system, because few PCPs share the same hospital EMR. As a result, we opted to focus on communicating the need for TFTs to PCPs (typically responsible for ordering outpatient tests or follow-up) and caregivers.
A best practice advisory (BPA) was proposed to fire once per provider during the time that TFTs are due to be completed. However, following the updated recommendation, we abandoned our plan to implement a BPA due to the challenges in identifying patients in the EMR based on individual risk factors. Additionally, in response to a statement released by the American College of Radiology supporting the change in monitoring recommendations,21 automated text was removed from radiology reports around August 2023. This change ultimately brought the updated FDA recommendation to our team’s attention. Since then, SmartText and patient-facing information have been adjusted accordingly, and changes have been communicated to each department.
Measures
Process measures included the percentage of patient encounters in which communication appropriately identified the need for TFT monitoring in the DC summary and AVS following a hospital encounter. Patients who had TFTs appropriately completed during their hospital stay with normal results were excluded from these measures alone, as no further follow-up was indicated. Outcome measures included the percentage of patients with TFTs completed within our system’s EMR within 3 weeks of contrast administration (among the total number of encounters excluding patients who died before the time that testing was indicated), the percentage of individual patients with abnormal TFTs (any degree of high or low thyroid-stimulating hormone [TSH] or free T4 values outside normal reference ranges as defined by age-specific laboratory manufacturer reference ranges), in addition to those with TSH values greater than 10 μIU/mL (mU/L) warranting confirmatory testing according to the Pediatric Endocrine Society,20 and the number of individual patients who required treatment.
The costs associated with project implementation, the number needed to screen to detect a patient with an abnormal TSH level, and more specifically, the number needed to screen to detect a patient with a TSH level greater than 10 μIU/mL were analyzed as balancing measures. A post hoc analysis was also performed to calculate the number needed to screen based on the revised recommendation.
Analysis
Data were collected through chart review for all patients meeting the inclusion criteria and analyzed on a bimonthly basis. We generated statistical process control charts using Excel’s QI Macros add-in (KnowWare, Denver, CO) with upper and lower control limits defined as more than 3 SDs above or below the mean. Special-cause variation was identified as 8 consecutive points above or below the mean or a single point outside of the upper or lower control limits.22 Chi-square and Fisher’s exact tests were used to determine significant differences between groups. The number needed to screen was generated using 1-sided confidence intervals for binomial proportion P based on Wilson’s method, applied to the parameter 1−(1−p)n, where n is the minimum number of tests required to achieve the desired probabilities, such as 0.95 or 0.99.23
Ethical Considerations
This project was designated as a QI activity and deemed exempt from review by our local institutional review board.
RESULTS
We identified 491 patient encounters, representing 446 unique patients. Patient encounter characteristics are described in Table 1. Nine encounters (representing 8 unique patients) were excluded from further analysis of results as they were deceased at the time TFTs were indicated. Compliance with TFT completion or communication of the need for monitoring across encounters increased from a mean of 11% to 57% within 3 months of intervention implementation, and ultimately to 75% in 1 year (Fig. 4A). Communication regarding the need for TFTs with PCPs through DC summaries increased from a mean of 5% to 47% within 3 months of intervention implementation, and ultimately to 60% following automation (Fig. 4B). Communication to patient caregivers via the AVS increased from a mean of 5% to 57% within 3 months of intervention implementation and to 81% within 13 months (Fig. 4C).
Table 1.
Patient Encounter Characteristics (January 2022–September 2023)
| Total (N = 491 encounters), n (%) | |
|---|---|
| Age at the time of IV contrast study | |
| <1 mo | 58 (11.8) |
| 1 to < 6 mo | 78 (15.9) |
| 6 to < 12 mo | 54 (11.0) |
| 1 to < 2 y | 94 (19.1) |
| 2 to < 3 y | 103 (21.0) |
| 3 to < 4 y | 104 (21.2) |
| Risk factors of interest | |
| <12 wk of age at the time of IV contrast | 89 (18.1) |
| CHD | 161 (32.8) |
| Prematurity (<37 WGA) | 86 (17.5) |
| VLBW | 10 (2.0) |
| ICU stay | 122 (24.8) |
| Any of the above | 217 (44.2) |
| Total (N = 446 patients), n (%) | |
| Unique patients with any risk factor | 189 (42.4) |
| Sex assigned at birth | |
| Male | 246 (55.2) |
| Female | 200 (44.8) |
WGA, weeks gestational age.
Fig. 4.
Control charts of process measures (all are p-charts). A, Compliance with the completion of TFTs or communicating this need for monitoring. B, TFT information included in the DC summaries for PCPs of qualifying patients. C, TFT information included in the AVS for caregivers of qualifying patients. D, Completion of TFTs within our system. CL, center line; LCL, lower control limit; UCL, upper control limit.
The percentage of patient encounters with TFTs completed within our hospital system during the appropriate time interval increased from a mean of 10% to 22% during the first 3 months of intervention implementation and remained stable thereafter (Fig. 4D). Table 2 displays the results by department or specialty. At the end of the study period, patients discharged from the pediatric ED and the hospital medicine service were most likely to receive information on the need for TFTs via the AVS (P = 0.003); ICU patients were more likely to have TFTs completed within our system (P < 0.001).
Table 2.
Comparison of Postintervention Patient Encounter Results across Department/Specialty*
| 2022 (Quarter 2–4) | 2023 (Quarter 1–3) | ||
|---|---|---|---|
| n = 214 | n = 226 | P values, Q1–3, 2023 | |
| Encounters,† n (%) | |||
| Outpatient specialties | 50 (23.4) | 41 (18.8) | n/a |
| Inpatient specialties | 12 (5.6) | 19 (8.4) | |
| ED | 42 (19.6) | 40 (17.7) | |
| Hospital medicine | 69 (32.2) | 72 (31.9) | |
| ICU | 41 (19.2) | 54 (23.9) | |
| AVS communication,‡, n (%) | |||
| Outpatient specialties | n/a | n/a | |
| Inpatient specialties | 1 (8.3) | 11 (57.9) | 0.003 |
| ED | 23 (54.8) | 33 (82.5) | |
| Hospital medicine | 18 (27.3) | 57 (85.1) | |
| ICU | 8 (33.3) | 20 (76.9) | |
| DC summary communication, n (%) | |||
| Outpatient specialties | n/a | n/a | |
| Inpatient specialties | 0 (0.0) | 7 (36.8) | 0.5 |
| ED | 19 (45.2) | 21 (52.5) | |
| Hospital medicine | 19 (28.8) | 39 (58.2) | |
| ICU | 13 (54.2) | 20 (76.9) | |
| TFT completion, n (%) | |||
| Outpatient specialties | 6 (12.0) | 8 (19.5) | <0.001 |
| Inpatient specialties | 0 (0.0) | 4 (21.1) | |
| ED | n/a | n/a | |
| Hospital medicine | 3 (4.3) | 9 (12.5) | |
| ICU | 24 (58.5) | 38 (70.4) | |
Data are expressed as number (%); n represents the total number of patient encounters, excluding encounters for patients who were deceased before the time thyroid testing was indicated or as otherwise noted.
Department or specialty caring for the patient at the time thyroid testing was due (if inpatient), at the time of DC (if patient was discharged before the time that testing was indicated), or responsible for ordering of contrast study/procedure done as an outpatient.
Encounters within each respective department/specialty out of the total number of patient encounters, excluding encounters for patients who were deceased before the time thyroid testing was indicated.
For AVS communication, DC summary communication, and TFTs completed, the denominator is the total number of encounters within the respective department/specialty, excluding encounters for patients who had TFTs completed that were normal before the time of DC and those who were deceased before the time that thyroid testing was indicated.
n/a, not applicable.
TFTs were completed across 111 encounters, of which 54% (n = 60) were completed within 2–3 weeks (14–21 d), and 87% (n = 90) within 3 weeks (0–21 d), following contrast administration. Of the 99 individual patients with TFT results within our system, 22% (n = 22) had abnormal results as defined by age-specific laboratory reference range parameters. Of these, 100% (n = 22) had identifiable risk factors; no patients without risk factors had abnormal thyroid screens. Ninety-one percent (n = 20) had an ICU stay, 64% (n = 14) were less than 3 months of age, 32% (n = 7) were premature, 0% (n = 0) had VLBW, and 91% (n = 20) had CHD. Three patients with abnormal results had previously diagnosed hypothyroidism and were receiving levothyroxine. Nine patients with abnormal results received medications with the potential to alter thyroid function or testing (amiodarone, dopamine, beta-blockers, or glucocorticoids)24 24 hours before TFT completion. In total, 5 patients had TSH values greater than 10 μIU/mL. Four patients required treatment, none of whom had been previously treated with levothyroxine.
Based on our initial findings, among a similar population of patients, 18 children less than 4 years of age would need to be tested to yield a 95% chance, or 27 children to yield a 99% chance, of detecting a patient with abnormal TFTs (with 95% confidence). Further, 121 children less than 4 years of age would need to be tested to yield a 95% chance, or 186 children to yield a 99% chance, of detecting a patient with a TSH level greater than 10 μIU/mL. In retrospect, if only patients less than 4 years of age with additional risk factors were screened, 14 children would need to be tested to yield a 95% chance, or 21 children to yield a 99% chance, of detecting a patient with abnormal TFTs (with 95% confidence). Furthermore, 95 children would need to be tested to yield a 95% chance, or 146 children to yield a 99% chance, of detecting a patient with a TSH level greater than 10 μIU/mL.
The primary cost of implementing this project was compensation for approximately 40 hours spent by a senior Epic analyst. Among our study population, 171 patients would meet the current criteria for screening based on the revised recommendation. The total cost of implementation (analyst compensation) and TSH testing for this select population over the same study period would have been $49.89 per patient. This does not account for repeated testing, endocrinology referral visits, or time contributed by QI team members.
DISCUSSION
This QI project successfully improved TFT completion and communicated the need for monitoring to 75% within 12 months. Our greatest success was attributed to the use of automation, though it was not a perfect solution. We learned that significant efforts were required to ensure that information was included for the appropriate patients, particularly with the manual addition of SmartText to templates used across departments and generated by individual providers. Despite these efforts, SmartText could still be manually deleted. For these reasons, we were unable to achieve 100% communication despite automation being a strong and our most impactful intervention. Additional data collection is needed to determine the sustainability of our improvements.
A review of the literature reveals that this project is novel. This study is unique in that it involves a patient population without a unifying diagnosis or procedure. Although there are published projects that improve DC summary timeliness and reliability25 as well as AVS distribution,26 there are few regarding improving testing rates after an exposure that spans multiple encounters.
To our knowledge, this is the first study implementing this recommendation into practice. Even before the recommendation for widespread testing, the number needed to screen had not been studied. Additionally, risk factors extrapolated from prior studies are not well defined, despite the update in recommendations to screen based on these alone. Our study found that all patients with abnormal TFTs had an ICU stay or CHD. Furthermore, each of the 4 patients who were started on levothyroxine was an infant with underlying CHD who had been admitted to the ICU. This suggests that the highest value of monitoring would be for young children, particularly those with CHD, admitted to the ICU. EMR functionalities should be leveraged to track clinical outcomes and process completion. They can further aid in process improvement if outcomes are tracked by department or group, allowing for the targeting of unique workflows. Future considerations could include a BPA linked to the initial order for relevant contrast studies or procedures, or at the time when TFTs are due.
Limitations to our data exist. Because this initiative was conducted within a single system, results may not be generalizable. Risk factor data may be incomplete if a specific risk factor was not identified during the chart review. Other risk factors have been suggested that were not included in our data, namely renal insufficiency, high doses of contrast, and repeated contrast exposure.21 TFTs were completed at varying times after contrast administration, allowing for physiological variability. Another significant limitation was that only TFTs completed within our system and those documented in the EMR could be reviewed. Therefore, the reported completion rates may have been higher than actual. Significant efforts would be needed to follow up with each patient who had testing performed outside of the system. A similar implementation would be best within systems that have hospital-affiliated clinics with a shared EMR. Although we sought input on the process from private pediatricians and our parent advisory committee, we did not formally assess how often PCPs or caregivers read and understood the need for TFT completion.
Although a formal cost analysis was not performed, significant resources were required to develop and implement this process. Once implemented, ongoing costs would include the cost of testing per patient and system upkeep. Ultimately, there was a lack of widespread communication regarding both the initial FDA recommendation and the 2023 update. The delay in recognizing the updated changes, despite our involvement in this initiative, highlights the need for improved communication surrounding such recommendations.
CONCLUDING SUMMARY
This project successfully increased both the communication of the need for monitoring and the completion of TFTs in patients at risk of hypothyroidism. Although this recommendation applies to a limited cohort of patients, the strategies used could be applied to other aspects of care where the patient population is not easily identifiable or timely follow-up is required, particularly across multiple encounters. The implementation of IT interventions, including conditional automation, contributed to our success, although this required significant effort to accomplish, and room for improvement still exists. Overall, a large number of patients would need to be screened to detect 1 patient who would potentially benefit from treatment; this supports targeted testing while highlighting the importance of data-driven identification of specific risk factors or other variables such as those that have been previously proposed (eg, cumulative doses of contrast and repeated exposure) and would benefit from further study.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Inchi Hu for a portion of the statistical support provided for this project.
Footnotes
Published online August 20, 2025.
Katherine Moyer and Courtney Port contributed equally as co-senior authors.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Presented at the Pediatric Academic Society Conference, May 6, 2024, Toronto, Ontario; Academic Pediatric Association QI Conference, May 3, 2024, Toronto, Ontario; Children's Hospital Association Transforming Quality Conference, March 11, 2024, Chicago, Ill.; American Academy of Pediatrics Conference, October 23, 2023, Washington, D.C.; Pediatric Hospital Medicine Conference, August 4, 2023, Philadelphia, Pa.; Academic Pediatric Association QI Conference, April 28, 2023, Washington, D.C.; Pediatric Academic Society Conference, April 28, 2023, Washington, D.C.; and Society of Hospital Medicine Converge, March 27, 2023, Austin, Tex.
To cite: Klingaman C, Callahan C, Deveau-Rosen S, Gibson C, Lazareva O, Moyer K, Port C. Thyroid Monitoring Following Intravenous Iodinated Contrast Administration in Children: A Quality Improvement Initiative. Pediatr Qual Saf 2025;10:e841.
REFERENCES
- 1.Center for Drug Evaluation and Research. FDA advises thyroid monitoring in kids after iodine contrast dye. U.S. Food and Drug Administration. Available at https://www.fda.gov/drugs/drug-safety-and-availability/fda-recommends-thyroid-monitoring-babies-and-young-children-who-receive-injections-iodine-containing. March 30, 2022. Accessed December 12, 2023. [Google Scholar]
- 2.Center for Drug Evaluation and Research. FDA recommends thyroid monitoring in babies and young children who receive injections of iodine-containing contrast media for medical imaging. U.S. Food and Drug Administration. Available at https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-advises-rare-cases-underactive-thyroid-infants-given-iodine. November 17, 2015. Accessed May 16, 2023. [Google Scholar]
- 3.Dembinski J, Arpe V, Kroll M, et al. Thyroid function in very low birthweight infants after intravenous administration of the iodinated contrast medium iopromide. Arch Dis Child Fetal Neonatal Ed. 2000;82:F215–F217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parravincini E, Fontana C, Paterlini GL, et al. Iodine, thyroid function, and very low birth weight infants. Pediatrics. 1996;98:730–734. [PubMed] [Google Scholar]
- 5.Ares S, Pastor I, Quero J, et al. Thyroid complications, including overt hypothyroidism, related to the use of non-radiopaque silastic catheters for parenteral feedings in prematures requiring injection of small amounts of an iodinated contrast medium. Acta Paediatr. 1995;84:579–581. [DOI] [PubMed] [Google Scholar]
- 6.Bona G, Zaffaroni M, Defilippi C, et al. Effects of iopamidol on neonatal thyroid function. Eur J Radiol. 1992;14:22–25. [DOI] [PubMed] [Google Scholar]
- 7.l’Allemand D, Gruters A, Beyer P, et al. Iodine in contrast agents and skin disinfectants is the major cause for hypothyroidism in premature infants during intensive care. Horm Res. 1987;28:42–49. [DOI] [PubMed] [Google Scholar]
- 8.Kubicki R, Grohmann J, Kunz KG, et al. Frequency of thyroid dysfunction in pediatric patients with congenital heart disease exposed to iodinated contrast media—a long-term observational study. J Pediatr Endocrinol Metab. 2020;33:1409–1415. [DOI] [PubMed] [Google Scholar]
- 9.Jick SS, Hedderson M, Xu F, et al. Iodinated contrast agents and risk of hypothyroidism in young children in the United States. Invest Radiol. 2019;54:296–301. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg V, Michel A, Chodick G, et al. Hypothyroidism in young children following exposure to iodinated contrast media: an observational study and a review of the literature. Pediatr Endocrinol Rev. 2018;16:256–265. [DOI] [PubMed] [Google Scholar]
- 11.Belloni E, Tentoni S, Puci MV, et al. Effect of iodinated contrast medium on thyroid function: a study in children undergoing cardiac computed tomography. Pediatr Radiol. 2018;48:1417–1422. [DOI] [PubMed] [Google Scholar]
- 12.Thaker VV, Galler MF, Marshall AC, et al. Hypothyroidism in infants with congenital heart disease exposed to excess iodine. J Endocr Soc. 2017;1:1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dechant MJ, van der Werf-Grohmann N, Neumann E, et al. Thyroidial response following iodine excess for cardiac catheterisation and intervention in early infancy. Int J Cardiol. 2016;223:1014–1018. [DOI] [PubMed] [Google Scholar]
- 14.Auger KA, Kenyon CC, Feudtner C, et al. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251–260. [DOI] [PubMed] [Google Scholar]
- 15.Forster AJ, Murff HJ, Peterson JF, et al. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167. [DOI] [PubMed] [Google Scholar]
- 16.Kripalani S, LeFevre F, Phillips CO, et al. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831–841. [DOI] [PubMed] [Google Scholar]
- 17.Lakhaney D, Banker SL. An evaluation of the content of pediatric discharge summaries. Hosp Pediatr. 2020;10:949–954. [DOI] [PubMed] [Google Scholar]
- 18.Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan M, Huntman J, Nelson M, et al. Use of peer comparison, provider education, and electronic medical record triggers to increase influenza vaccination rates in hospitalized children. Hosp Pediatr. 2020;10:76–83. [DOI] [PubMed] [Google Scholar]
- 20.Pediatric Endocrine Society. Statement on thyroid monitoring in infants and young children receiving iodine-containing contrast media. Available at https://pedsendo.org/news-announcements/pes-statement-on-thyroid-monitoring-in-infants-and-young-children-receiving-iodine-containing-contrast-media/. May 10, 2022. Accessed May 15, 2022.
- 21.American College of Radiology. ACR-backed FDA action to monitor young at-risk children who receive iodinated contrast media. American College of Radiology. Available at https://www.diagnosticimaging.com/view/ct-update-fda-changes-course-on-post-icm-thyroid-monitoring-in-young-children. April 27, 2023. Accessed November 21, 2023. [Google Scholar]
- 22.Provost LP, Murray S. The Health Care Data Guide: Learning from Data for Improvement. Jossey-Bass; 2011. [Google Scholar]
- 23.Filliben JJ. NIST/SEMATECH e-Handbook of Statistical Methods. December 12, 2023. Available at http://www.itl.nist.gov/div898/handbook/. Accessed October 28, 2023. [Google Scholar]
- 24.Burch HB. Drug effects on the thyroid. N Engl J Med. 2019;381:749–761. [DOI] [PubMed] [Google Scholar]
- 25.Shen MW, Hershey D, Bergert L, et al. Pediatric hospitalists collaborate to improve timeliness of discharge communication. Hosp Pediatr. 2013;3:258–265. [DOI] [PubMed] [Google Scholar]
- 26.Williamson J, Holstine J, Balch Samora J. A quality improvement initiative to improve after-visit summary distribution in orthopedic outpatient clinics. Pediatr Qual Saf. 2022;7:e620. [DOI] [PMC free article] [PubMed] [Google Scholar]