Abstract
The aim of the study was to investigate the causal relationship between chronic obstructive pulmonary disease (COPD) and Barrett’s esophagus (BE) using two-sample Mendelian randomization (MR). We obtained summary-level genome-wide association study data on COPD and BE from the integrative epidemiology unit of the open genome-wide association study project, and selected significant single nucleotide polymorphisms as instrumental variables. Various MR analysis methods were employed to assess the causal effect of COPD on BE and vice versa. The methods selected include inverse variance weighted (IVW) analysis, weighted median analysis, and MR-Egger analysis. The MR resonance imaging revealed a bidirectional causal relationship between COPD and BE. IVW analysis indicated that COPD increased the risk of BE (odds ratio [OR]: 1.24, 95% confidence interval [CI]: 1.12–1.34, P = 3.0 × 10⁻⁵), which was supported using the weighted median method (OR = 1.16, 95% CI: 1.01–1.33, P = .04). These findings were robust in the pleiotropy analysis (MR-Egger intercept = 0.02). Conversely, BE also increases the risk of COPD (IVW OR: 1.080, 95% CI: 1.03–1.13, P = .001), with supporting evidence from the weighted median method (OR = 1.09, 95% CI = 1.03–1.15, P = .005). These findings were robust in the pleiotropy analysis (MR-Egger intercept = 0.001). Our study findings reveal a bidirectional causal link between COPD and BE, highlighting the need for mechanistic research to elucidate the underlying pathways. These insights can improve our knowledge of novel screening and prevention strategies, emphasizing the importance of targeted healthcare measures.
Keywords: Barrett’s esophagus, chronic obstructive pulmonary disease, gastroesophageal reflux disease, genetic variants, Mendelian randomization
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease characterized by airflow obstruction and breathing difficulties.[1–3] It is the 3rd leading cause of death globally,[4] and affects nearly 6% of the US population.[5] The global prevalence of COPD further highlights its significance as a major public health concern. According to the global burden of disease study 2019, approximately 212.3 million people worldwide were affected, with considerable regional variation in both prevalence and disease burden.[6] The disease is a major public health concern worldwide, affecting approximately 10% of individuals aged ≥ 40 years, with significant variations across regions.[7] The global initiative for chronic obstructive lung disease (2023) defined COPD as a variety of chronic respiratory diseases characterized by symptoms (such as dyspnea, cough, sputum production, and/or exacerbations) caused by airway abnormalities (such as bronchitis or small airway disease) and/or emphysema.[8] Based on the new definition from global initiative for chronic obstructive lung disease 2023, in which the recently proposed consensus known as the Rome proposal is endorsed,[9] the definitive diagnosis of COPD primarily relies on pulmonary function tests (spirometry), with a typical criterion of a forced expiratory volume in 1 s/forced vital capacity ratio of < 70%.[8] The main cause of COPD includes tobacco use; however, it can also result from exposure to air pollutants, biomass burning, smoke, and chemical fumes encountered in workplaces.[5]
Barrett’s esophagus (BE) is a well-recognized premalignant condition characterized by the replacement of the normal esophageal squamous epithelium with columnar epithelium. This replacement increases the risk of esophageal adenocarcinoma (EAC). Notably, the progression from intestinal metaplasia to dysplasia, and ultimately to EAC, is well documented and necessitates regular endoscopic surveillance.[10,11] Gastroesophageal reflux disease (GERD) is the primary risk factor for BE;[12] nevertheless, emerging evidence suggests a complex interplay between COPD and BE, which is potentially mediated by GERD and systemic inflammation.[13]
COPD is a common chronic respiratory disease characterized by persistent limited airflow and systemic inflammation with significant extrapulmonary effects.[8] Findings from observational studies reveal a bidirectional association between COPD and GERD, which may contribute to esophageal mucosal damage and BE development.[14] In a large-scale retrospective study, it was demonstrated that patients with COPD have an increased risk of nonerosive esophagitis (odds ratio [OR]: 1.407, P < .01), erosive esophagitis (OR: 1.165, P < .01), esophageal stricture (OR: 1.399, P < .01), and non-dysplastic BE (OR: 1.354, P < .01) compared with individuals without COPD. Moreover, the risk of dysplastic BE and EAC was elevated in patients with COPD, with ORs of 1.327 and 1.235, respectively.[15]
Despite these epidemiological associations, whether COPD causally influences BE remains unclear. Findings from traditional observational studies are influenced by confounding factors, making it challenging to infer causality. Mendelian randomization (MR), which leverages genetic variants as instrumental variables (IVs) is a robust approach for explaining causal relationships by mitigating confounding and reverse causation biases.[16] In previous MR analyses, a bidirectional causal association was established between GERD and COPD, with GERD increasing COPD risk (inverse variance weighted [IVW] OR, 1.376; 95% confidence interval [CI], 1.156–1.637; P < .001), and COPD reciprocally increasing GERD risk (IVW OR: 1.172, 95% CI: 1.061–1.296, P = .002).[17] However, the causal effect of COPD on susceptibility to BE has not been systematically evaluated.
Given the significant clinical burden of BE and its progression to EAC, it is crucial to understand whether COPD is an independent risk factor for BE. Thus, establishing a causal relationship could provide new insights into the pathogenesis of BE and inform the development of targeted screening strategies for high-risk populations. Therefore, in this study, we used MR to assess the causal effect of COPD on BE, offering a novel genetic perspective on the relationship between pulmonary and gastrointestinal diseases.
2. Methods
2.1. Study design
This study was conducted strictly following Mendelian randomization principles, as illustrated in Figure 1. All included data were publicly available from the genome-wide association (GWAS) and Medical Research Council-integrative epidemiology unit (IEU) databases and approved by the relevant ethical review boards. As participants had already provided informed consent, no further ethical review was required. In the inclusion criteria for the GWAS, the data selected were from published, quality-assured datasets for up-to-date, and comprehensive data. Unpublished or outdated data, data with confounding factors, and incomplete datasets were excluded from the analysis. The data on COPD and Barrett’s esophagitis in the MR study were derived from publicly accessible summary-level data provided by the relevant consortia (Table 1).
Figure 1.
An overview of the study design. Assumption 1: IVs must be robustly associated with exposure. Assumption 2: IVs must not be associated with any confounding factors of the exposure-outcome relationship. Assumption 3: IVs must affect outcomes only through exposure. *Primary analyses: inverse variance weighted (IVW) method, weighted median, and MR-Egger regression. Sensitivity analyses: MR-PRESSO, Heterogeneity, Cochran Q test, and leave-one-out analysis. BE = Barrett’s esophagus, COPD = chronic obstructive pulmonary disease, IVs = instrumental variables, MR = Mendelian randomization, MR-PRESSO = Mendelian Randomization Pleiotropy RESidual Sum and Outlier, SNP = single-nucleotide polymorphism.
Table 1.
Genetic association study summary data for exposure and outcome.
| Exposure/Outcome | Sample size | Web source | First author | Consortium | Year | Population |
|---|---|---|---|---|---|---|
| COPD | 468,475 | https://gwas.mrcieu.ac.uk/datasets/ | Sakaue S | NA | 2021 | European |
| BE | 56,429 | https://gwas.mrcieu.ac.uk/datasets/ | Ong JS | NA | 2021 | European |
BE = Barrett’s esophagus, COPD = chronic obstructive pulmonary disease.
2.2. Details of GWAS consortium used for exposures and outcome
The European cohort, selected for data availability and practical feasibility, was the largest dataset available for the study team. One of the primary reasons for selecting the European cohort was to minimize potential population stratification biases, which can result from genetic heterogeneity among different ancestries.[18] Using a homogeneous population reduces the confounding effects caused by ancestry-related allele frequency differences, thereby enhancing the validity of causal inference in MR analyses.[19] In addition, the GWAS summary dataset for COPD as an exposure variable was sourced from the IEU Open GWAS project (GWAS ID: ebi-a-GCST90018807) and comprised 468,475 samples and 454,945 controls. Furthermore, BE’s data for this study were obtained from the IEU Open GWAS project (GWAS ID: ebi-a-GCST90000515), which included 56,429 samples and 43,071 controls. From previous studies, it was demonstrated that population stratification can introduce bias into causal estimates if not adequately addressed, further supporting the need for a well-defined ancestry-specific cohort.[20] Detailed information on the data is available at https://gwas.mrcieu.ac.uk/.[21] All the data used in this study were derived from European populations.
2.3. Genetic instrument selection
In this study, when COPD was as an exposure variable, which was used to obtain sufficient candidate single nucleotide polymorphism (SNPs). We set the P-value threshold at 5 × 10−6 and used a tool set for whole-genome association and population-based linkage analyses aggregation to calculate linkage disequilibrium between SNPs for each exposure variable.[22] SNPs with r² < 0.001 were retained and a window size of 10,000 kb was used. Statistical significance was set at P < .05. To avoid weak instrumental bias (F < 10) in the two-sample model, we approximated the F-statistic to estimate the exposure strengths of the IVs, ensuring that the IVs had sufficient validity and strength. The F values and R² were calculated using formulas established in previous studies.[23,24]
2.4. Mendelian randomization analysis
We used the IVW results as our main source of information because these unbiased findings were predicted based on the assumption that there was no horizontal pleiotropy.[25] Moreover, the intercept term in the MR-Egger regression provided valuable insights into whether directional horizontal pleiotropy influenced the MR analysis outcomes.[26] To further corroborate the IVW results, we employed weighted median analyses, which offer more robust estimates across a wide range of scenarios despite their relatively lower efficiency.[27]
Within the context of the Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) analysis, our aim was to minimize variability in the estimated causal effect by excluding SNPs that disproportionately contribute to this variability.[27] In this analysis, heterogeneity was determined using the IVW method and MR-Egger regression. We assessed the presence of heterogeneity using Cochran Q statistic, considering heterogeneity to be significant if the P < .05. Furthermore, to identify potentially influential SNPs, we conducted a “leave-one-out” sensitivity analysis.
2.5. Statistical analysis
All statistical analyses were performed using the two-sample MR package in the R Statistical Software version 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria).[23] Statistical significance for evidence was set P < .05.
3. Results
3.1. MR analysis and sensitivity analysis of COPD on BE
In MR analysis, 31 SNPs were obtained from the COPD dataset as IVs in order to validate the causal effect of COPD on BE. From the MR-PRESSO test, outliers were identified among the ID SNPs (rs72712556, rs10789942, and rs4705862); thus, these SNPs were excluded. Finally, 28 eligible SNPs were selected as IVs for COPD exposure and BE outcomes. All selected SNPs had F-statistics >10 (Table 2; see Table S1, Supplemental Digital Content, https://links.lww.com/MD/P698, for detailed F-statistics).
Table 2.
Details of the IVs used for MR analysis (causal effect of COPD on BE).
| SNP | Exposure | Outcome | MR-PRESSO | |||||
|---|---|---|---|---|---|---|---|---|
| Beta | EAF | SE | Beta | EAF | SE | RSSobs | P value | |
| rs10059100 | 0.055 | 0.354 | 0.012 | 0.031 | 0.388 | 0.015 | 3.47E−04 | 1 |
| rs10151372 | 0.081 | 0.118 | 0.018 | −0.004 | 0.142 | 0.022 | 5.76E−04 | 1 |
| rs10236197 | 0.056 | 0.404 | 0.012 | 0.05 | 0.367 | 0.016 | 1.38E−03 | .465 |
| rs10789942 | −0.058 | 0.399 | 0.013 | −0.055 | 0.538 | 0.015 | 1.73E−03 | .093 |
| rs11065774 | 0.203 | 0.115 | 0.029 | 0.003 | 0.085 | 0.028 | 2.54E−03 | 1 |
| rs112369231 | 0.066 | 0.294 | 0.013 | 0.046 | 0.262 | 0.017 | 9.86E−04 | 1 |
| rs12336219 | 0.059 | 0.692 | 0.012 | 0.033 | 0.685 | 0.016 | 3.76E−04 | 1 |
| rs1246642 | 0.075 | 0.715 | 0.013 | 0.033 | 0.787 | 0.018 | 2.57E−04 | 1 |
| rs12588709 | 0.082 | 0.167 | 0.018 | 0.077 | 0.055 | 0.034 | 3.35E−03 | 1 |
| rs1286753 | −0.085 | 0.171 | 0.016 | 0.004 | 0.108 | 0.024 | 6.39E−04 | 1 |
| rs140750546 | −0.091 | 0.09 | 0.02 | −0.036 | 0.123 | 0.023 | 2.13E−04 | 1 |
| rs1512281 | −0.077 | 0.414 | 0.012 | 0.007 | 0.406 | 0.015 | 7.44E−04 | 1 |
| rs17228058 | 0.069 | 0.192 | 0.015 | 0.0134 | 0.237 | 0.018 | 9.04E−06 | 1 |
| rs17667265 | −0.139 | 0.049 | 0.03 | −0.034 | 0.052 | 0.034 | 4.42E−07 | 1 |
| rs2101195 | 0.06 | 0.411 | 0.012 | −0.023 | 0.342 | 0.016 | 1.49E−03 | .837 |
| rs34946515 | −0.068 | 0.172 | 0.015 | 0.003 | 0.17 | 0.02 | 3.77E−04 | 1 |
| rs4488938 | 0.105 | 0.175 | 0.018 | −0.024 | 0.038 | 0.039 | 2.51E−03 | 1 |
| rs4594972 | −0.067 | 0.431 | 0.013 | −0.013 | 0.285 | 0.017 | 8.23E−06 | 1 |
| rs4705862 | 0.055 | 0.515 | 0.012 | −0.03 | 0.567 | 0.015 | 1.95E−03 | .062 |
| rs4846483 | −0.06 | 0.39 | 0.013 | −0.005 | 0.28 | 0.017 | 9.01E−05 | 1 |
| rs4889606 | −0.061 | 0.516 | 0.013 | −0.023 | 0.376 | 0.016 | 7.85E−05 | 1 |
| rs7124355 | 0.058 | 0.64 | 0.012 | 0.027 | 0.673 | 0.016 | 1.80E−04 | 1 |
| rs72678864 | −0.091 | 0.164 | 0.017 | −0.051 | 0.17 | 0.021 | 9.15E−04 | 1 |
| rs72712556 | −0.057 | 0.315 | 0.012 | −0.076 | 0.344 | 0.016 | 4.11E−03 | <.031 |
| rs72849439 | −0.065 | 0.234 | 0.014 | −0.005 | 0.14 | 0.021 | 1.23E−04 | 1 |
| rs73089398 | 0.182 | 0.037 | 0.031 | 0.009 | 0.025 | 0.048 | 1.29E−03 | 1 |
| rs73157884 | 0.073 | 0.151 | 0.016 | 0.007 | 0.141 | 0.021 | 1.05E−04 | 1 |
| rs76455766 | −0.161 | 0.038 | 0.034 | −0.045 | 0.057 | 0.034 | 4.95E−05 | 1 |
| rs78913805 | −0.115 | 0.118 | 0.024 | −0.014 | 0.119 | 0.023 | 1.99E−04 | 1 |
| rs8073925 | 0.058 | 0.323 | 0.012 | 0.031 | 0.295 | 0.017 | 2.92E−04 | 1 |
| rs9788721 | −0.155 | 0.66 | 0.012 | −0.023 | 0.658 | 0.016 | 2.79E−04 | 1 |
BE = Barrett’s esophagus, COPD = chronic obstructive pulmonary disease, IVs = instrumental variables, MR = Mendelian randomization, MR-PRESSO = Mendelian Randomization Pleiotropy RESidual Sum and Outlier, SNP = single nucleotide polymorphism.
A significant causal relationship between COPD and BE was identified from the MR analysis. Evidence supporting this association was obtained through various MR methods, including the IVW analysis (OR: 1.24, 95% CI: 1.12–1.34, P = 3.0 × 10⁻⁵), the weighted median method (OR = 1.16, 95% CI = 1.01–1.33, P = .04), and the MR-Egger method (OR = 0.98, 95% CI = 0.76–1.26, P = .87; Figs. 2–4). (A significant positive causal association was also observed in the MR analysis, Figs. S1 and S2, Supplemental Digital Content, https://links.lww.com/MD/P699.)
Figure 2.
Forrest plot of the causal effects of COPD-associated SNPs on BE. BE = Barrett’s esophagus, COPD = chronic obstructive pulmonary disease, MR = Mendelian randomization, SNP = single-nucleotide polymorphism.
Figure 4.
MR results for positive control outcomes. BE = Barrett’s esophagus, MR = Mendelian randomization, IVW = inverse variance weighted.
Figure 3.
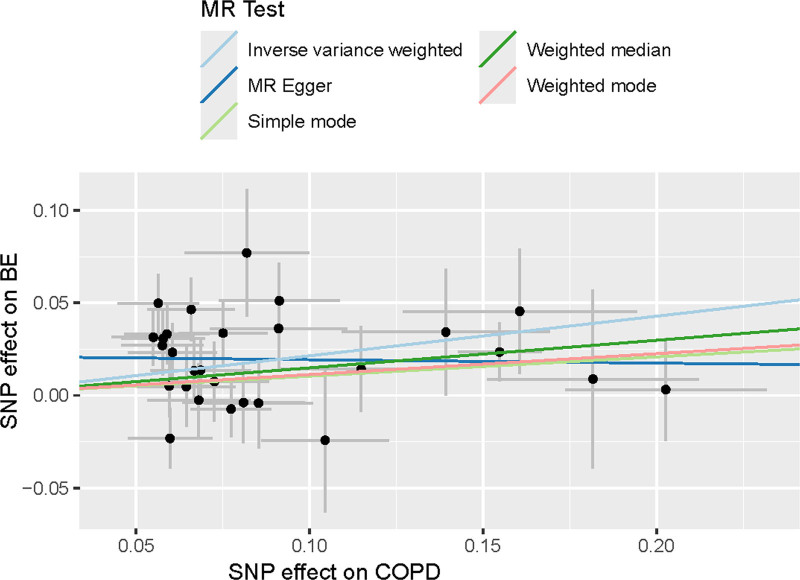
Scatter plots of the Mendelian randomization analyses for the association of COPD with the risk of BE. The red, green, and blue lines represent the inverse variance weighted, weighted median, and MR-Egger estimates, respectively. BE = Barrett’s esophagus, COPD = chronic obstructive pulmonary disease, MR = Mendelian randomization, SNP = single-nucleotide polymorphisms.
Furthermore, the Cochrane Q-test analysis revealed no significant heterogeneity issues following the exclusion of SNPs for both the IVW (Q = 37.01, df = 27, P = .09) and MR-Egger methods (Q = 32.23, df = 26, P = .19). An intercept (0.02) with a P-value exceeding .05 was detected in the MR-Egger intercept analysis, indicating non-significance and potential pleiotropy, but without statistical significance. The MR-PRESSO analysis corroborated this by yielding results (RSSobs = 11.95, P = .6) indicating no pleiotropy. Additionally, all the SNPs showed F values exceeding 10, indicating strong intravenous characteristics. Collectively, these findings support a positive causal relationship between COPD (exposure factors) and BE (outcome factors; Table 3).
Table 3.
Results of heterogeneity by the Cochran Q test and the MR-PRESSO global test.
| Exposure/Outcome | Pleio P value | Intercept | Cochran Q | df | Heterogeneity P value | MR-PRESSO | |
|---|---|---|---|---|---|---|---|
| RSSobs | P value | ||||||
| COPD/BE | .06 | 0.021 | 11.948 | .6 | |||
| MR Egger | 32.228 | 26 | .186 | ||||
| IVW | 37.013 | 27 | .095 | ||||
| BE/COPD | .952 | 0.0007 | 9.96 | .685 | |||
| MR Egger | 63.653 | 49 | .078 | ||||
| IVW | 63.658 | 50 | .093 | ||||
BE = Barrett’s esophagus, COPD = chronic obstructive pulmonary disease, IVW = inverse variance weighted, MR = Mendelian randomization, MR-PRESSO = Mendelian Randomization Pleiotropy RESidual Sum and Outlier.
Statistical significance is expressed directly by the P-value (P < .05).
3.2. MR analysis and sensitivity analysis of BE on COPD
In the MR analysis, 57 SNPs were extracted from the BE dataset as IVs to investigate the causal effects of BE on COPD. Outliers among the ID SNPs (rs10104032, rs10986761, rs13195040, rs205262, and rs769657) were identified and excluded using MR-PRESSO testing. Eventually, 52 eligible SNPs with F-statistics exceeding 10 were selected as IVs for BE exposure and COPD outcomes (Table 4; see Table S2, Supplemental Digital Content, https://links.lww.com/MD/P698, for detailed F-statistics).
Table 4.
Details of the IVs used for MR analysis (causal effect of BE on COPD).
| SNP | Exposure | Outcome | MRPRESSO | |||||
|---|---|---|---|---|---|---|---|---|
| Beta | EAF | SE | Beta | EAF | SE | RSSobs | P value | |
| rs10031416 | 0.315 | 0.012 | 0.068 | 0.057 | 0.010 | 0.058 | 8.40E−03 | 1 |
| rs10039754 | 0.084 | 0.550 | 0.015 | 0.019 | 0.426 | 0.013 | 7.97E−04 | .969 |
| rs10104032 | 0.093 | 0.375 | 0.016 | 0.043 | 0.426 | 0.012 | 1.16E−03 | .171 |
| rs10207635 | 0.137 | 0.135 | 0.022 | 0.032 | 0.089 | 0.020 | 3.28E−04 | 1 |
| rs10224888 | 0.073 | 0.638 | 0.016 | 0.010 | 0.598 | 0.012 | 6.61E−06 | 1 |
| rs10484245 | 0.132 | 0.088 | 0.027 | 0.035 | 0.072 | 0.022 | 4.54E−04 | 1 |
| rs10982622 | 0.085 | 0.462 | 0.015 | 0.009 | 0.535 | 0.012 | 3.51E−04 | 1 |
| rs10986761 | 0.085 | 0.289 | 0.017 | 0.048 | 0.269 | 0.013 | 1.57E−03 | .228 |
| rs11040802 | 0.084 | 0.236 | 0.018 | 0.002 | 0.197 | 0.014 | 4.80E−05 | 1 |
| rs11761457 | 0.099 | 0.208 | 0.019 | 0.009 | 0.257 | 0.013 | 1.94E−06 | 1 |
| rs11766872 | 0.079 | 0.283 | 0.017 | 0.006 | 0.293 | 0.013 | 4.78E−06 | 1 |
| rs11792928 | 0.098 | 0.295 | 0.017 | 0.012 | 0.269 | 0.013 | 5.36E−04 | 1 |
| rs12207319 | 0.075 | 0.309 | 0.016 | 0.000 | 0.221 | 0.014 | 7.22E−05 | 1 |
| rs12474870 | 0.088 | 0.189 | 0.019 | 0.002 | 0.151 | 0.016 | 5.32E−05 | 1 |
| rs1247942 | 0.096 | 0.405 | 0.015 | 0.003 | 0.358 | 0.012 | 6.19E−05 | 1 |
| rs12595438 | 0.122 | 0.092 | 0.026 | 0.021 | 0.096 | 0.019 | 6.69E−05 | 1 |
| rs12607067 | 0.143 | 0.074 | 0.029 | 0.055 | 0.085 | 0.020 | 1.65E−03 | 1 |
| rs12712508 | 0.075 | 0.356 | 0.016 | 0.016 | 0.430 | 0.012 | 6.82E−05 | 1 |
| rs12987825 | 0.082 | 0.288 | 0.017 | 0.020 | 0.416 | 0.013 | 8.46E−04 | 1 |
| rs13085889 | 0.089 | 0.275 | 0.017 | 0.019 | 0.203 | 0.014 | 8.41E−05 | 1 |
| rs13195040 | 0.162 | 0.115 | 0.025 | 0.036 | 0.095 | 0.022 | 2.99E−03 | .684 |
| rs1346563 | 0.081 | 0.285 | 0.017 | 0.007 | 0.250 | 0.015 | 2.55E−04 | 1 |
| rs143384 | 0.079 | 0.401 | 0.015 | 0.016 | 0.364 | 0.012 | 5.41E−05 | 1 |
| rs16862777 | 0.087 | 0.276 | 0.017 | 0.001 | 0.299 | 0.012 | 7.16E−05 | 1 |
| rs16966166 | 0.130 | 0.100 | 0.025 | 0.006 | 0.217 | 0.015 | 6.23E−05 | 1 |
| rs17114682 | 0.109 | 0.118 | 0.023 | 0.035 | 0.159 | 0.016 | 5.51E−04 | 1 |
| rs17451754 | 0.103 | 0.135 | 0.022 | 0.029 | 0.149 | 0.018 | 1.62E−03 | 1 |
| rs1828682 | 0.072 | 0.479 | 0.015 | 0.013 | 0.365 | 0.013 | 2.49E−05 | 1 |
| rs1868915 | 0.089 | 0.587 | 0.015 | 0.030 | 0.506 | 0.012 | 4.16E−04 | 1 |
| rs1876389 | 0.078 | 0.551 | 0.015 | 0.012 | 0.535 | 0.012 | 1.43E−05 | 1 |
| rs205262 | 0.082 | 0.264 | 0.017 | 0.052 | 0.219 | 0.014 | 1.91E−03 | .057 |
| rs2597301 | 0.114 | 0.689 | 0.016 | 0.007 | 0.664 | 0.012 | 4.11E−04 | 1 |
| rs2861695 | 0.107 | 0.806 | 0.019 | 0.012 | 0.807 | 0.016 | 7.36E−07 | 1 |
| rs2877766 | 0.071 | 0.424 | 0.015 | 0.022 | 0.416 | 0.012 | 2.07E−04 | 1 |
| rs288186 | 0.079 | 0.646 | 0.016 | 0.002 | 0.716 | 0.013 | 1.09E−04 | 1 |
| rs3072 | 0.110 | 0.367 | 0.016 | 0.022 | 0.417 | 0.012 | 1.12E−04 | 1 |
| rs31260 | 0.083 | 0.269 | 0.017 | 0.009 | 0.347 | 0.013 | 1.63E−10 | 1 |
| rs4317189 | 0.083 | 0.687 | 0.016 | 0.008 | 0.667 | 0.012 | 8.50E−07 | 1 |
| rs459078 | 0.085 | 0.281 | 0.017 | 0.029 | 0.235 | 0.013 | 4.01E−04 | 1 |
| rs4646607 | 0.080 | 0.418 | 0.015 | 0.003 | 0.500 | 0.012 | 3.76E−05 | 1 |
| rs4655026 | 0.072 | 0.574 | 0.015 | 0.022 | 0.455 | 0.012 | 2.04E−04 | 1 |
| rs4938330 | 0.089 | 0.205 | 0.018 | 0.001 | 0.236 | 0.013 | 1.03E−04 | 1 |
| rs4947741 | 0.080 | 0.284 | 0.017 | 0.007 | 0.270 | 0.013 | 4.55E−06 | 1 |
| rs6032949 | 0.085 | 0.208 | 0.019 | 0.009 | 0.232 | 0.013 | 2.70E−08 | 1 |
| rs622217 | 0.091 | 0.482 | 0.015 | 0.008 | 0.432 | 0.012 | 3.30E−04 | 1 |
| rs6874700 | 0.071 | 0.364 | 0.016 | 0.022 | 0.330 | 0.012 | 9.18E−04 | 1 |
| rs7187365 | 0.111 | 0.823 | 0.020 | 0.012 | 0.823 | 0.015 | 2.15E−11 | 1 |
| rs739414 | 0.101 | 0.742 | 0.018 | 0.011 | 0.757 | 0.013 | 5.11E−04 | 1 |
| rs7537908 | 0.089 | 0.205 | 0.019 | 0.014 | 0.251 | 0.013 | 2.09E−05 | 1 |
| rs7613818 | 0.073 | 0.517 | 0.015 | 0.026 | 0.590 | 0.012 | 3.56E−04 | 1 |
| rs769657 | 0.085 | 0.331 | 0.016 | 0.053 | 0.316 | 0.012 | 2.02E−03 | <.06 |
| rs7720419 | 0.090 | 0.379 | 0.016 | 0.014 | 0.319 | 0.012 | 2.14E−05 | 1 |
| rs773109 | 0.078 | 0.336 | 0.016 | 0.011 | 0.292 | 0.013 | 3.75E−04 | 1 |
| rs7772622 | 0.072 | 0.559 | 0.015 | 0.019 | 0.593 | 0.012 | 1.24E−04 | 1 |
| rs8044476 | 0.103 | 0.150 | 0.021 | 0.002 | 0.176 | 0.015 | 1.70E−04 | 1 |
| rs8067516 | 0.113 | 0.148 | 0.022 | 0.009 | 0.135 | 0.019 | 1.14E−05 | 1 |
| rs8102046 | 0.092 | 0.556 | 0.015 | 0.010 | 0.627 | 0.012 | 9.63E−08 | 1 |
BE = Barrett’s esophagus, COPD = chronic obstructive pulmonary disease, IVs = instrumental variables, MR = Mendelian randomization, MR-PRESSO = Mendelian Randomization Pleiotropy RESidual Sum and Outlier, SNP = single nucleotide polymorphism.
The MR analysis revealed a significant causal effect of BE on COPD. Both the IVW analysis (OR: 1.08, 95% CI: 1.03–1.13, P = .001) and the weighted median method (OR = 1.09, 95% CI = 1.03–1.15, P = .005) confirmed this finding. Although the MR-Egger method provided nonsignificant causal evidence (OR = 1.07, 95% CI = 0.84–1.37, P = .58), the results primarily relied on the IVW analysis and the weighted median method (Figs. 4–6). (A significant positive causal association was also observed in the MR analysis, Figs. S3 and S4, Supplemental Digital Content, https://links.lww.com/MD/P699.)
Figure 6.
Scatter plots of the Mendelian randomization analyses for the association of BE with the risk of COPD. The red, green, and blue lines represent the inverse variance weighted, weighted median, and MR-Egger estimates, respectively. BE = Barrett’s esophagus, COPD = chronic obstructive pulmonary disease, MR = Mendelian randomization, SNP = single-nucleotide polymorphisms.
Figure 5.
Forrest plot of the causal effects of BE-associated SNPs on COPD. BE = Barrett’s esophagus, COPD = chronic obstructive pulmonary disease, MR = Mendelian randomization, SNP = single-nucleotide polymorphism.
The Cochrane Q-test analysis revealed no significant heterogeneity issues after excluding SNPs for either the IVW (Q = 63.66, df = 50, P = .09) or MR-Egger methods (Q = 63.65, df = 49, P = .08). MR-Egger intercept analysis revealed a nonsignificant result (intercept = 0.001, P = .95), indicating no significance or the absence of pleiotropy. Consistent with this finding, MR-PRESSO analysis of the BE results for COPD (RSSobs = 11.95, P = .6) and COPD results for BE (RSSobs = 9.96, P = .69) revealed no pleiotropy. Furthermore, all SNPs exhibited F values exceeding 10, indicating a strong IV. These results show a positive causal relationship between the exposure (BE) and the outcome (COPD) factors (Table 3).
Considering that patients with COPD may have an elevated risk of BE owing to chronic inflammation, oxidative stress, and GERD-related mechanisms, these findings reveal that patients with COPD, particularly those with GERD, may require enhanced BE screening and monitoring. Additionally, BE may influence COPD progression through inflammation and alterations in the airway microenvironment, emphasizing the need to monitor lung health during the long-term management of patients with BE. These results provide a new perspective for early intervention in COPD and BE cases and may guide future clinical screening and prevention strategies.
4. Discussion
In this study, we aimed to investigate the causal relationship between COPD and BE. Our findings, derived from multiple analytical methods, revealed a reciprocal positive causal association between the 2 conditions. These results suggest that COPD may be an independent risk factor for BE, and conversely, BE may also increase the risk of developing COPD. Significant associations were not found in the MR-Egger analysis; however, the IVW and Weighted Median methods provided robust evidence supporting this bidirectional relationship, suggesting that COPD may increase the risk of BE and, conversely, that BE may elevate the risk of COPD. In the pleiotropy analysis, when COPD was the exposure factor and BE was the outcome, the MR-Egger intercept did not show significant bias (P = .303), indicating that horizontal pleiotropy had a minimal influence on causal estimates, thereby strengthening the reliability of our findings.
Moreover, COPD is recognized as a major risk factor for GERD, with previous studies reporting that GERD considerably contributes to BE development, further substantiating the link between COPD and BE.[15,17] Mechanistically, COPD contributes to BE through multiple pathways, including chronic systemic inflammation, oxidative stress, and the frequent coexistence of GERD. Chronic airway inflammation in COPD leads to increased pro-inflammatory cytokine release and oxidative stress, both of which are implicated in esophageal mucosal damage and metaplastic changes characteristic of BE.[28] Furthermore, GERD, which is highly prevalent in patients with COPD owing to increased intrathoracic pressure and impaired esophageal motility, facilitates the progression from esophageal inflammation to BE.[29] Similarly, BE may exacerbate COPD through inflammatory pathways, as esophageal inflammation and acid microaspiration contribute to airway remodeling and lung function decline.[30] These shared pathophysiological mechanisms highlight the complex interplay between COPD and BE, reinforcing the need for integrated management strategies targeting both conditions.
The potential mechanisms by which COPD affects the BE are multifaceted: (1) studies have reported a high prevalence of GERD in COPD cases.[31] Chronic cough and dyspnea, which are common symptoms in patients with COPD, may lead to increased gastric acid reflux.[32] Gastric acid reflux is a major cause of BE because refluxed acid can cause epithelial cells in the lower esophagus to degenerate, eventually leading to Barrett’s. Furthermore, patients with COPD often require long-term use of respiratory medications such as inhaled bronchodilators, anticholinergics, and corticosteroids, which may increase the risk of developing GERD.[33,34] (2) Patients with COPD experience chronic lung inflammation and recurrent infections, which can interact with inflammatory cells either directly or indirectly. These interactions activate signaling pathways that promote the synthesis and secretion of inflammatory cytokines (such as tumor necrosis factor-α, interleukin-6, and NF-kB).[35] Elevated levels of oxidative stress in the body can stimulate DNA damage in esophageal epithelial cells, thereby increasing the risk of carcinogenesis and promoting the development of BE. Factors such as long-term smoking, activation of inflammatory cells, and impaired respiratory function contribute to significantly elevated levels of reactive oxygen species in patients with COPD.[36] The accumulation of oxidative stress in these patients may lead to GERD onset.[37] Additionally, changes in acidity caused by prolonged inflammation and oxidative stress, along with acid reflux, can induce columnar metaplasia of the esophageal epithelium, resulting in BE.[38]
The potential mechanisms by which BE affects COPD include: (1) gastroesophageal reflux: the association of BE with GERD symptoms has been widely accepted for decades.[39] BE is often caused by GERD, which is an independent risk factor for acute exacerbations of COPD.[40] In a prospective study involving 82 patients with COPD exacerbations, it was found that gastric acid reflux was significantly associated with a 6-fold increased risk of COPD exacerbations.[17] This conclusion was also supported by findings from a systematic review and meta-analysis conducted in Brazil, confirming that the relative risk of COPD exacerbation in patients was 7.6.[41] (2) Inhalation pneumonia is associated with the presence of distal esophageal acid, which stimulates airway irritation and inflammatory responses, releasing potent bronchoconstrictive mediators.[42] Refluxed gastric contents can move into the proximal esophagus and enter the lower pharynx, directly triggering laryngeal or tracheal reactions that may manifest as coughing, wheezing, or dyspnea.[40] Similarly, gastric acid reflux can cause aspiration of gastric contents into the respiratory tract, leading to pulmonary inflammation and infection, thereby triggering or exacerbating COPD.[41] (3) Regarding immune dysfunction, increasing evidence suggests that autoimmunity plays a role in COPD pathogenesis.[43] BE is a chronic inflammatory condition. Studies have shown that during the progression of BE, there is increased expression of the inflammatory cytokines IL-6 and C-X-C motif chemokine ligand 8, and their associated receptors CXCR1/CXCR2, as well as an increase in M2 macrophage expression.[44] A chronic inflammatory state in patients with BE can lead to systemic immune dysfunction. Autoimmune abnormalities may also render the lungs more susceptible to infections and inflammatory attacks, thereby increasing the risk of COPD.[45]
Despite uncovering the potential causal relationships and mechanisms between COPD and BE, this study has some limitations. First, multiple MR methods have been employed to infer causality and each method operates under specific assumptions and may be influenced by unmeasured confounding factors or horizontal pleiotropy. However, MR strengthens the observational findings by reducing confounding and reverse causation, thereby enhancing the robustness of the conclusions. Second, the data used in this study were predominantly derived from European populations, which limits the generalizability of these findings to other ethnic groups with different genetic backgrounds and environmental exposures. Future studies incorporating diverse populations are essential to validate these results and to explore potential ethnic differences in genetic susceptibility. Third, a comprehensive analysis of existing GWAS data was integrated in this study, further experimental and longitudinal clinical research is required to validate these findings and elucidate the underlying biological mechanisms. Finally, the complex interplay between COPD and BE highlights the need to further explore the molecular pathways that mediate this relationship. The focus of translational opportunities should be on identifying genetic predispositions and implementing lifestyle modifications such as GERD management and smoking cessation to mitigate disease risk and progression.
5. Conclusion
Our study findings revealed evidence of a bidirectional causal relationship between COPD and BE. These findings underscore the importance of further mechanistic studies to elucidate the pathways that link COPD and BE, thereby providing avenues for novel screening and preventive strategies. Implementing screening and preventive measures for COPD in individuals with BE and vice versa will be crucial in future healthcare management. Further studies are required to better understand the mechanisms underlying the causal relationship between COPD and BE.
Acknowledgments
We would like to thank Editage (www.editage.cn) for the English language editing.
Author contributions
Data curation: Jun Huang, Zhejun Deng.
Project administration: Nan Yi, Yunxiao Liang.
Writing – original draft: Nan Yi.
Writing – review & editing: Nan Yi.
Supplementary Material
Abbreviations:
- BE
- Barrett’s esophagus
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CXCR
- C-X-C chemokine receptor
- EAC
- esophageal adenocarcinoma
- GERD
- gastroesophageal reflux disease
- GWAS
- genome-wide association study
- IEU
- integrative epidemiology unit
- IVs
- instrumental variables
- IVW
- inverse variance weighted
- MR
- Mendelian randomization
- MR-PRESSO
- Mendelian Randomization Pleiotropy RESidual Sum and Outlier
- OR
- odds ratio
- SNP
- single nucleotide polymorphism
This study was supported by Guangxi Appropriate Technologies for Medical and Health Care (grant no. S S2024016).
An ethical approval statement is not required for this study because the genome-wide association study database is publicly available and contains unidentified patient information. Patient consent was also not required for publication.
The authors have no conflicts of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
Supplemental Digital Content is available for this article.
How to cite this article: Yi N, Huang J, Deng Z, Liang Y. The causal relationship between chronic obstructive pulmonary disease and Barrett’s esophagitis: A two-sample Mendelian randomization study. Medicine 2025;104:33(e43913).
Contributor Information
Jun Huang, Email: huangjun1997@163.com.
Zhejun Deng, Email: 576947169@qq.com.
Yunxiao Liang, Email: yxliang@gxams.org.cn.
References
- [1].Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389:1931–40. [DOI] [PubMed] [Google Scholar]
- [2].Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399:2227–42. [DOI] [PubMed] [Google Scholar]
- [3].Zou M, Zhang W, Shen L, Xu Y, Zhu Y. Major depressive disorder plays a vital role in the pathway from gastroesophageal reflux disease to chronic obstructive pulmonary disease: a Mendelian randomization study. Front Genet. 202319;14:1198476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Upadhyay P, Wu CW, Pham A, et al. Animal models and mechanisms of tobacco smoke-induced chronic obstructive pulmonary disease (COPD). J Toxicol Environ Health B Crit Rev. 2023;26:275–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cagle SD, Jr, Landrum LS, Kennedy AM. Chronic obstructive pulmonary disease: diagnosis and management. Am Fam Physician. 2023;107:604–12. [PubMed] [Google Scholar]
- [6].Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I; NIHR RESPIRE Global Respiratory Health Unit. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10:447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Agustí A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61:2300239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Celli BR, Fabbri LM, Aaron SD, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the rome proposal. Am J Respir Crit Care Med. 2021;204:1251–8. [DOI] [PubMed] [Google Scholar]
- [10].Stawinski PM, Dziadkowiec KN, Kuo LA, et al. Barrett’s esophagus: an updated review. Diagnostics (Basel). 2023;13:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30–50; quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–31. [DOI] [PubMed] [Google Scholar]
- [13].Zou M, Zhang W, Xu Y, Zhu Y. Relationship between COPD and GERD: a bibliometrics analysis. Int J Chron Obstruct Pulmon Dis. 2022;17:3045–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rascon-Aguilar IE, Pamer M, Wludyka P, et al. Role of gastroesophageal reflux symptoms in exacerbations of COPD. Chest. 2006;130:1096–101. [DOI] [PubMed] [Google Scholar]
- [15].Menon S, Nightingale P, Trudgill N. Chronic obstructive pulmonary disease and the risk of esophagitis, barrett’s esophagus, and esophageal adenocarcinoma: a primary care case-control study. J Clin Gastroenterol. 2019;53:e451–5. [DOI] [PubMed] [Google Scholar]
- [16].Davies NM, Holmes MV, Smith GD. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang X, Wright Z, Wang J, Roy S, Fass R, Song G. Elucidating the link: chronic obstructive pulmonary disease and the complex interplay of gastroesophageal reflux disease and reflux-related complications. Medicina (Kaunas). 2023;59:1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–63. [DOI] [PubMed] [Google Scholar]
- [20].Wojcik GL, Graff M, Nishimura KK, et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature. 2019;570:514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sakaue S, Kanai M, Tanigawa Y, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. 2021;53:1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Slatkin M. Linkage disequilibrium: understanding the evolutionary past and mapping the medical future. Nat Rev Genet. 2008;9:477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Palmer TM, Lawlor DA, Harbord RM, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21:223–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li B, Li M, Qi X, Tong T, Zhang G. The causal associations of circulating lipids with Barrett’s esophagus and esophageal cancer: a bi-directional, two sample mendelian randomization analysis. Hum Genomics. 2024;18:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35:1880–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ong JS, MacGregor S. Implementing MR-PRESSO and GCTA-GSMR for pleiotropy assessment in Mendelian randomization studies from a practitioner’s perspective. Genet Epidemiol. 2019;43:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wright JL, Tai H, Churg A. Cigarette smoke induces persisting increases of vasoactive mediators in pulmonary arteries. Am J Respir Cell Mol Biol. 2004;31:501–9. [DOI] [PubMed] [Google Scholar]
- [30].Mokhlesi B, Morris AL, Huang CF, Curcio AJ, Barrett TA, Kamp DW. Increased prevalence of gastroesophageal reflux symptoms in patients with COPD. Chest. 2001;119:1043–8. [DOI] [PubMed] [Google Scholar]
- [31].Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–42. [DOI] [PubMed] [Google Scholar]
- [32].Iliaz S, Iliaz R, Onur ST, et al. Does gastroesophageal reflux increase chronic obstructive pulmonary disease exacerbations? Respir Med. 2016;115:20–5. [DOI] [PubMed] [Google Scholar]
- [33].Lee AS, Lee JS, He Z, Ryu JH. Reflux-aspiration in chronic lung disease. Ann Am Thorac Soc. 2020;17:155–64. [DOI] [PubMed] [Google Scholar]
- [34].Mungan Z, Pinarbaşi Şimşek B. Which drugs are risk factors for the development of gastroesophageal reflux disease? Turk J Gastroenterol. 2017;28(Suppl 1):S38–43. [DOI] [PubMed] [Google Scholar]
- [35].Wight TN, Kang I, Evanko SP, et al. Versican-A critical extracellular matrix regulator of immunity and inflammation. Front Immunol. 2020;11:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yamaguchi T, Yoshida N, Tomatsuri N, et al. Cytokine-induced neutrophil accumulation in the pathogenesis of acute reflux esophagitis in rats. Int J Mol Med. 2005;16:71–7. [PubMed] [Google Scholar]
- [37].Liu B, Chen M, You J, Zheng S, Huang M. The causal relationship between gastroesophageal reflux disease and chronic obstructive pulmonary disease: a bidirectional two-sample mendelian randomization study. Int J Chron Obstruct Pulmon Dis. 2024;19:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rieder F, Biancani P, Harnett K, Yerian L, Falk GW. Inflammatory mediators in gastroesophageal reflux disease: impact on esophageal motility, fibrosis, and carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Taylor JB, Rubenstein JH. Meta-analyses of the effect of symptoms of gastroesophageal reflux on the risk of Barrett’s esophagus. Am J Gastroenterol. 2010;105:1729, 1730–7; quiz 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lee AL, Goldstein RS. Gastroesophageal reflux disease in COPD: links and risks. Int J Chron Obstruct Pulmon Dis. 2015;10:1935–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sakae TM, Pizzichini MM, Teixeira PJ, Silva RM, Trevisol DJ, Pizzichini E. Exacerbations of COPD and symptoms of gastroesophageal reflux: a systematic review and meta-analysis. J Bras Pneumol. 2013;39:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Canning BJ, Mazzone SB. Reflex mechanisms in gastroesophageal reflux disease and asthma. Am J Med. 2003;115(Suppl 3A):45S–8S. [DOI] [PubMed] [Google Scholar]
- [43].Bhat TA, Panzica L, Kalathil SG, Thanavala Y. Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12(Suppl 2):S169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lagisetty KH, McEwen DP, Nancarrow DJ, et al. Immune determinants of Barrett’s progression to esophageal adenocarcinoma. JCI Insight. 2021;6:e143888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Caramori G, Casolari P, Barczyk A, Durham AL, Di Stefano A, Adcock I. COPD immunopathology. Semin Immunopathol. 2016;38:497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.