Abstract
Purpose:
177Lu-DOTATATE is a somatostatin receptor (SSTR)-targeting radiopharmaceutical that shows promise for treating metastatic pheochromocytomas/paragangliomas (PPGLs), a rare SSTR-expressing tumor.
Methods:
In the first stage of this two-stage Simon phase 2 trial, 36 PPGL patients with RECIST 1.1 progression within 12 months were prospectively recruited into two genetic cohorts (SDHx-mutated vs. apparent sporadic, 18 per cohort) and treated with four cycles of 177Lu-DOTATATE. Primary endpoint was progression-free survival (PFS) rate at six months (from initiation of treatment). Secondary endpoints included safety, overall survival (OS), response rate, imaging/serum biomarkers, and anti-hypertensive medication reduction. CT/MRIs and PET/CTs (68Ga-DOTATATE and 18F-FDG) were obtained after 2 and 4 cycles, then every 3 (CT/MRIs) to 6 months (PET/CTs). Patients with systolic blood pressure (SBP) > 200 mmHg despite medical management were treated in the Intensive Care Unit.
Results:
6 months PFS rate for all patients was 0.861 (95% CI: 0.755–0.982), which was significantly lower (P=0.009) for SDHx at 0.72 (95% CI: 0.542–0.962) vs. sporadic at 1.00 (95% CI: 1.0–1.0). Median PFS was 19.9 months (12.9 months SDHx vs. 24.3 months sporadic) and median OS was 51.7 months (31.2 months SDHx vs. not reached in sporadic). Best response was achieved on average 11.0 months after completing 177Lu-DOTATATE. A 17% incidence of grade 3+ catecholamine release syndrome (CRS) was noted which may benefit from preemptive ICU admission. Plasma chromogranin A and normetanephrine were the best tumor-marker surrogates and correlated well with changes in RECIST sum and total tumor lesion uptake on serial 68Ga-DOTATATE PET/CT scans.
Conclusion:
177Lu-DOTATATE demonstrated effectiveness and acceptable safety profile for progressive, metastatic PPGL. CRS may occur but can be mitigated through pre-treatment with antihypertensives, and, when appropriate, intensified monitoring in the ICU with intravenous antihypertensives.
Funding:
NIH grant ZIABC011789
Keywords: pheochromocytoma, paraganglioma, radionuclide therapy, peptide receptor radionuclide therapy, somatostatin receptor
Background
Metastatic pheochromocytoma/paraganglioma (PPGL), defined as the presence of chromaffin cell tumors at locations where chromaffin cells are physiologically absent1, occurs in up to 50% of PPGL patients2,3. It remains an incurable and fatal disease with a median survival of 6.7 years4 and a 5-year survival rate of 37%2. Until very recently, the only FDA-approved therapy for metastatic PPGL had been high-specific-activity 131I-MIBG which unfortunately became commercially unavailable as of early 2024. In May 2025, belzutifan, a hypoxia-inducible factor 2 alpha (HIF-2α) inhibitor, was approved by the FDA for treating metastatic PPGL via priority review, which highlights the fact that data supporting the regulatory approval of additional treatment options are timely and critically important.
Agents targeting somatostatin receptor (SSTR) for Positron Emission Tomography (PET) imaging have demonstrated effective visualization of metastatic PPGL5–7, suggesting that peptide receptor radionuclide therapy (PRRT) using a radiolabeled SSTR agonists, such as 177Lu-DOTA(0)-Tyr(3)-octreotate (177Lu-DOTATATE), may be good treatment alternatives8,9. While some PPGLs may not express SSTR, prior investigations indicated that over 95% of both sporadic and SDHx-associated PPGLs expresses SSTR5,10. Guidelines from organizations such as the National Comprehensive Cancer Network (NCCN)11 and the North American Neuroendocrine Tumor Society (NANETS)12 currently list PRRT as a recommended therapy for PPGL. However, these and other recommendations are based primarily on expert opinions and retrospective data that lack controls for important biological factors such as baseline progression rates, which can range from months to decades13 and genotypic differences that can dramatically affect prognosis, especially those with pathogenic variants in subunit B of succinate dehydrogenase (SDHB), a mutation that is known to cause more aggressive disease14,15. These gaps in the literature make it difficult to determine the best therapies for metastatic PPGL, contribute to the lack of approved therapies, and hinder the clinical management of this fatal disease. Although some agents, such as the tyrosine kinase inhibitors (TKI) sunitinib16 and cabozantinib17, have recently been shown to have activity in this setting, metastatic PPGL remains a deadly disease with only a single FDA-approved agent, and there is an urgent need for prospective data that can lead to the regulatory approval of more effective treatments.
Here, we report the results of the first stage of a prospective, investigator-initiated, single-center two-stage Simon phase 2 trial (NCT03206060) conducted at the National Cancer Institute (NCI) of the United States investigating 177Lu-DOTATATE for patients with progressive, SSTR-positive, and metastatic PPGL.
Methods
Patients with histopathologically-confirmed metastatic PPGL with demonstrated RECIST 1.1 progression within 12 months were recruited from October 2017 until July 2022. Additional eligibility criteria included adequate organ function (kidney/liver/marrow), SSTR-positive disease (see supplementary information for details), and no prior systemic radionuclide therapy. All patients had germline genetic testing and were assigned to either the apparent sporadic cohort (no pathogenic mutations found) or the SDHx-mutated cohort, since those with SDHx mutations, especially SDHB, are at risk of having the most aggressive disease. Those with known pathogenic PPGL susceptibility genes other than SDHx (such as RET, NF1, FH, etc.) were excluded, as our sample size and resource limitations would not have allowed for a meaningful, dedicated analysis of these other mutations. The study was approved by the NIH IRB, performed according to the principles of the Declaration of Helsinki, and all patients signed written informed consents.
Statistical Analysis/Sample Size Calculation
A two-stage Simon design stratified by genetic cohort (apparent sporadic vs SDHx-mutated) was used with a one-sided Type 1 error rate of 0.1 and 90% power. For the first stage, 18 participants were recruited per cohort; if 11 of the 18 patients in a cohort were alive and progression-free at six months, 23 additional patients would be accrued to that cohort in the second stage. Only results from participants in the first stage are reported here.
The primary objective was to evaluate the six-month PFS rate for each cohort (i.e. the proportion of patients within a cohort that remained alive and progression-free at six months), measured from the first 177Lu-DOTATATE infusion. The hypothesis was that the PFS rate in patients with PPGL13 treated with DOTATATE was 75%, as opposed to 55% in untreated patients. 90% confidence intervals were obtained using the Greenwood formula to account for right-censoring. Secondary objectives included the assessment of other clinical endpoints such as safety/tolerability, overall survival (OS), response rate, time to progression expressed as the PFS time, change in plasma biochemical markers, quality of life changes, and the ability to decrease anti-hypertensive medications. For the primary objective, data obtained at the conclusion of the first stage for both cohorts are reported. For secondary objectives, data up to December 31, 2023, are reported. Analyses of the secondary objectives are considered exploratory. Additional details of the statistical analysis can be found in the Supplementary material.
177Lu-DOTATATE Administration
177Lu-DOTATATE was administered IV every 8 weeks for 4 cycles at a fixed dose of 7.4 GBq (200 mCi) with standard renal-protective amino acids and prophylactic anti-emetics. Patients with high baseline catecholamine symptoms/signs (SBP > 180 mmHg or HR > 100 bpm) in the setting of known high plasma catecholamine levels were treated as inpatients. The highest-risk patients (SBP > 200 mmHg) were electively treated in the ICU, as described previously18. All patients with baseline hypertension received alpha and beta-adrenoceptor blockade medications, as clinically indicated.
Blood and Safety Biomarkers
Blood samples were collected at baseline, at 24 and 48 h after each administration of 177Lu-DOTATATE, and at each clinic visit every 4 weeks. After week 32, clinic visits occurred every 12 weeks for up to 3 years. Blood evaluations included the complete blood count, kidney and liver function, pituitary and other endocrine function, resting plasma catecholamines and metanephrines, and chromogranin A at each of the time points.
Imaging Acquisition and Analysis
Imaging was done at baseline, 4 weeks after cycles 2, 8 weeks after cycle 4, and then every 3 months for up to 3 years. The end-of-treatment (EOT) scans, which were acquired 8 weeks after cycle 4, were used for the analysis of the primary endpoint for all patients, unless progression was detected earlier at the first restaging scan performed after cycle 2. Due to logistical reasons and external factors such as travel restrictions during the COVID pandemic, the EOT scans were obtained with a median of 7.6 months after the start of therapy, with a range of 2.4 (early progressors) to 13.7 (delayed treatment) months. Diagnostic CT/MRI scans were obtained at all restaging time points, whereas 18F-FDG and 68Ga-DOTATATE PET/CT scans were performed at baseline, after cycles 2 and 4, then every 6 months. For each 68Ga-DOTATATE PET/CT scan, a total tumor lesion uptake (TLU) score was generated. This score was determined by summing the volumes of all identified tumor regions on a scan multiplied by the mean standardized uptake value (SUV) for each region (see Supplementary materials for details).
Patient Reported Outcomes
Quality of life evaluations were carried out and scored using the RAND 36-Item Healthy Survey 1.0 at baseline and at each of the imaging re-staging time points. Results are reported descriptively.
Results
Demographics and Tumor Characteristics
40 patients were recruited, 36 of whom (18 sporadic, 18 SDHx pathogenic variants) had evaluable response data (Table 1). Three patients were evaluable only for toxicity (two withdrew consent and one died in an unrelated motor vehicle accident prior to restaging scans), and one withdrew consent before receiving any study drug. In the SDHx cohort, two patients had germline pathogenic variants in SDHA, 15 in SDHB, and one in SDHD. Overall, the most common metastatic sites were the bones (86%), liver (58%), lymph nodes (50%), and lungs (44%). At baseline, 17%, 58%, 42%, 11%, and 72% of the patients had elevated blood or urine levels of epinephrine, norepinephrine, dopamine, metanephrine, or normetanephrine, respectively. Elevated chromogranin A was observed in 92% of the patients. At the time of the first 177Lu-DOTATATE dose, 53% of the patients had no prior history of systemic therapy for PPGL, whereas 33% and 14% had received one or at least two modalities, respectively.
Table 1.
Demographics and disease characteristics of study participants.
| Characteristics | Value Total | % SDHx | % Sporadic |
|---|---|---|---|
| No. of participants | 36 (100) | 18 (100) | 18 (100) |
| No. of females | 16 (44.4) | 7 (38.9) | 9 (50.0) |
| No. of males | 20 (55.6) | 11 (61.1) | 9 (50.0) |
| Race | |||
| Black/African American | 6 (16.7) | 1 (5.5) | 5 (27.8) |
| White | 28 (77.8) | 16 (88.9) | 12 (66.7) |
| Hispanic or multiracial | 2 (5.5) | 1 (5.5) | 1 (5.5) |
| Age at diagnosis (y) | 43.6 ± 14.7 | 39.2 ± 12.7 | 48.0 ± 15.6 |
| Age at metastasis (y) | 49.3 ± 15.0 | 44.3 ± 13.1 | 54.3 ± 15.5 |
| Age at enrollment (y) | 53.2 ± 14.3 | 49.1 ± 13.1 | 57.2 ± 14.6 |
| Time between diagnosis and development of metastases (y) | 5.6 ± 7.2 | 5.1±6.1 | 6.3±8.4 |
| No. of participants who were metastatic at the time of diagnosis | 10 (27.7) | 4 (22.3) | 6 (33.3) |
| Size of the primary tumor (cm) | 8.3 ± 4.8 | 8.3 ± 4.3 | 8.3 ± 5.5 |
| Location of the primary tumor | |||
| Pheochromocytoma only | 9 (25.0) | 0 (0) | 9 (50.0) |
| Paraganglioma (head and neck) only | 3 (8.3) | 1 (5.5) | 2 (11.1) |
| Paraganglioma (extraadrenal) only | 19 (52.8) | 14 (77.8) | 5 (27.8) |
| Both head and neck as well as extraadrenal paraganglioma | 1 (2.8) | 1 (5.5) | 0 (0) |
| Both pheochromocytoma and paraganglioma (all) | 4 (11.1) | 2 (11.1) | 2 (11.1) |
| Biochemical elevations | |||
| Epinephrine/metanephrine | 9 (25) | 4 (22.2) | 5 (27.8) |
| Norepinephrine/normetanephrine | 27 (75) | 12 (66.7) | 15 (83.3) |
| Dopamine | 15 (41.7) | 8 (44.4) | 7 (38.9) |
| Chromogranin A | 33 (91.7) | 17 (94.4) | 16 (88.9) |
| Location of the metastasis | |||
| Lung | 16 (44.4) | 6 (33.3) | 10 (55.5) |
| Liver | 21 (58.3) | 9 (50.0) | 12 (66.7) |
| Bone | 31 (86.1) | 16 (88.9) | 15 (83.3) |
| Lymph nodes | 18 (50.0) | 10 (55.5) | 8 (44.4) |
| Lung or liver or peritoneal/omental/mesenteric deposits | 27 (75.0) | 13 (72.2) | 14 (77.8) |
| Treatment received | |||
| Surgery | 30 (83.3) | 16 (88.9) | 14 (77.8) |
| Chemotherapy and/or TKIs | 11 (30.5) | 6 (33.3) | 5 (27.8) |
| Cold somatostatin analogs (SSAs) | 10 (27.8) | 5 (27.8) | 5 (27.8) |
| External radiation therapy | 17 (47.2) | 12 (66.7) | 5 (27.8) |
| Liver-directed therapies | 6 (16.7) | 6 (33.3) | 0 (0) |
| Bone-directed therapies (bisphosphonates or denosumab) | 6 (16.7) | 5 (27.8) | 1 (5.5) |
| No. of prior systemic treatments | |||
| 0 | 19 (52.8) | 9 (50.0) | 10 (55.5) |
| 1 | 12 (33.3) | 6 (33.3) | 6 (33.3) |
| 2 or more | 5 (13.9) | 3 (16.7) | 2 (11.1) |
TKI = tyrosine kinase inhibitors, SSA = somatostatin analogs.
177Lu-DOTATATE Demonstrated Clinical Efficacy in PPGL
Five patients in the SDHx cohort had RECIST progression on the first re-staging scan obtained after cycle 2. The remaining 13 out of 18 patients in the SDHx cohort and all 18 patients in the sporadic cohort were alive and progression-free at six months as determined on the EOT scan obtained after cycle 4. Thus, both cohorts met the criteria for continuation into the second stage of the Simon design. The 6 months PFS rate for all patients was 0.861 (95% CI: 0.755–0.982), which was significantly lower (P=0.009) for SDHx at 0.72 (95% CI: 0.542–0.962) vs. sporadic at 1.00 (95% CI: 1.0–1.0). Median PFS (Figure 1a) was 19.9 months (12.9 months in SDHx vs. 24.3 months in sporadic, p=0.162) and median OS (Figure 1b) was 51.7 months (31.2 months in SDHx vs. not reached in sporadic, p=0.110).
Figure 1.

Kaplan–Meier plots of PFS (panel A) and OS (panel B) among patients in the apparent sporadic and SDHx cohorts. The vertical dashed lines indicate the 12-, 24-, 36-, and 48-month marks.
At the EOT scans after 4 cycles of therapy, 31 (86.1%) patients had RECIST stable disease (SD) or better, with five patients (13.8%) achieving an objective partial response (PR) at that time. However, decreases in tumor sizes continued to be observed long after EOT, with 10 patients (27.8%; 4 sporadic, 6 SDHx) eventually reaching PR as their best ORR. Best RECIST sum was achieved on average 11.0 months (range: −4.1 to 30 months) after the last 177Lu-DOTATATE administration. In the sporadic cohort, all 18 patients achieved SD or PR, compared with only 13 patients (72.2%) in the SDHx cohort (P = 0.045). Notably, all five patients who experienced disease progression were in the SDHx cohort (Figure 2).
Figure 2.
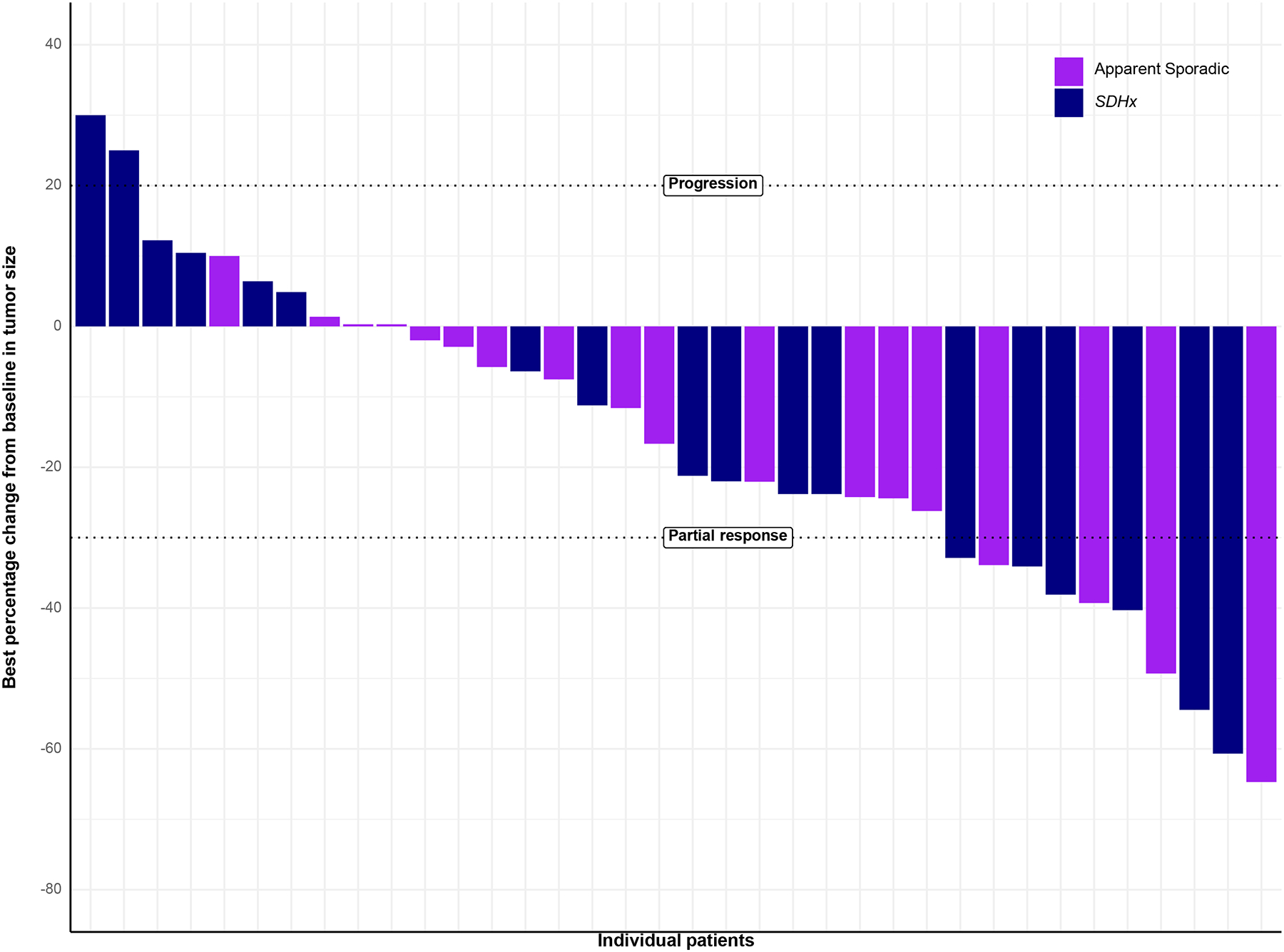
Waterfall plot of the best percentage change in the RECIST sum-of-diameters, according to genetic cohort. Two patients had progression based on > 20% increase in the sum-of-diameters of target lesions, while 3 others had progression based on increases in non-target lesions (or new lesions).
177Lu-DOTATATE is Safe for PPGL Patients but can Induce Catecholamine Release
A total of 39 patients were evaluable for toxicity, with no grade 5 adverse events attributable to 177Lu-DOTATATE. Thirty patients (76.9%) completed all 4 cycles of 177Lu-DOTATATE. One patient discontinued after 3 cycles due to an unrelated pre-existing pulmonary condition and subsequently passed away from it. Eight patients received 2 or fewer cycles due to disease progression (n=5), unrelated mortality from a motor-vehicle accident (n=1), or withdrawal of consent (n=2).
The most common AEs were hematologic (Table 2), with grade 3+ rates of lymphopenia, leukopenia, thrombocytopenia, and anemia reported at 61.5%, 7.7%, 0%, and 15.4%, respectively. All grade 4 hematologic events were from lymphopenia, except one patient who had grade 4 anemia associated with a lower gastrointestinal bleed. One patient with grade 3 anemia required chronic periodic blood transfusions, although a bone marrow biopsy showed no evidence of myelodysplasia. Liver- and kidney-function abnormalities were mostly grade 1–2 and reversible. Temporary pituitary and endocrine AEs were observed within 24 h of each 177Lu-DOTATATE administration, as previously reported19.
Table 2.
Treatment-related adverse events (the number of events is shown with number of affected patients in brackets). A total of 39 patients were evaluable for toxicity (3 of whom were not evaluable for efficacy).
| Treatment-related Adverse Events | All Grades (N = 39) | Grade ≥ 3 (N=39) |
|---|---|---|
| Any adverse event | 678 [38, 97.4%] | 127 [27, 69.2%] |
| General Disorders | ||
| Fatigue | 22 [17, 43.6%] | 3 [3, 7.7%] |
| Metabolism and Nutrition Disorders | ||
| Hypokalemia | 5 [4, 10.3%] | 0 [0, 0%] |
| Hyperkalemia | 2 [2, 5.1%] | 1 [1, 2.6%] |
| Hyponatremia | 4 [3, 7.7%] | 0 [0, 0%] |
| Gastrointestinal Disorder | ||
| Nausea | 38 [21, 53.8%] | 2 [1, 2.6%] |
| Abdominal pain | 7 [5, 12.8%] | 0 [0, 0%] |
| Vomiting | 12 [6, 15.4%] | 0 [0, 0%] |
| Diarrhea | 8 [8, 20.5%] | 0 [0, 0%] |
| Constipation | 2 [1, 2.6%] | 0 [0, 0%] |
| GERD | 2 [2, 5.1%] | 0 [0, 0%] |
| GI bleeding | 1 [1, 2.6%] | 1 [1, 2.6%] |
| ALT increased | 2 [1, 2.6%] | 0 [0, 0%] |
| Nervous system Disorder | ||
| Dizziness | 3 [3, 7.7%] | 0 [0, 0%] |
| Headache | 4 [4, 10.3%] | 0 [0, 0%] |
| Tinnitus | 2 [2, 5.1%] | 0 [0, 0%] |
| Respiratory System Disorder | ||
| Dyspnea | 4 [4, 10.3%] | 1 [1, 2.6%] |
| Pleural effusion | 2 [2, 5.1%] | 0 [0, 0%] |
| Pleural hemorrhage | 2 [1, 2.6%] | 0 [0, 0%] |
| Pneumonitis | 1 [1, 2.6%] | 0 [0, 0%] |
| Cardiovascular Disorders | ||
| Hypotension | 1 [1, 2.6%] | 0 [0, 0%] |
| Hypertension | 8 [6, 15.4%] | 4 [3, 7.7%] |
| Flushing | 3 [3, 7.7%] | 0 [0, 0%] |
| Cardiac troponin I increase | 1 [1, 2.6%] | 1 [1, 2.6%] |
| LV ejection fraction decrease | 1 [1, 2.6%] | 1 [1, 2.6%] |
| Chest pain | 1 [1, 2.6%] | 0 [0, 0%] |
| Palpitations | 2 [2, 5.1%] | 0 [0, 0%] |
| Sinus tachycardia | 1 [1, 2.6%] | 0 [0, 0%] |
| Atrial fibrillation | 2 [2, 5.1%] | 1 [1, 2.6%] |
| Atrial flutter | 1 [1, 2.6%] | 0 [0, 0%] |
| Ventricular arrhythmia | 1 [1, 2.6%] | 1 [1, 2.6%] |
| Hematologic Disorders | ||
| Lymphopenia | 185 [35, 89.7%] | 86 [24, 61.5%] |
| Leukopenia | 103 [23, 59.0%] | 5 [3, 7.7%] |
| Neutropenia | 24 [7, 17.9%] | 1 [1, 2.6%] |
| Thrombocytopenia | 52 [14, 35.9%] | 0 [0, 0%] |
| Anemia | 100 [29, 74.4%] | 11 [6, 15.4%] |
| Renal Disorder | ||
| Creatinine increase | 18 [8, 20.5%] | 0 [0, 0%] |
| Skin Disorders | ||
| Alopecia | 6 [5, 12.8%] | 0 [0, 0%] |
| Rash | 2 [2, 5.1%] | 0 [0, 0%] |
| Endocrine Disorders | ||
| Adrenal insufficiency | 8 [6, 15.4%] | 0 [0, 0%] |
| Hypothyroidism | 3 [3, 7.7%] | 0 [0, 0%] |
| Hypopituitarism | 1 [1, 2.6%] | 0 [0, 0%] |
Considering all grades, relatively high percentages of treatment-related hypertension (15.4%) and arrhythmia (10.3%) adverse events were noted. Grade 3+ catecholamine release syndrome (CRS), defined as the presence of any of the known catecholamine symptoms and signs (hypertension, arrhythmias, palpitations, heart failure, or gastrointestinal paresis including functional bowel obstruction) with a severity of grade 3+, was observed in seven patients (17.8%). In these 7 patients, baseline grade 3, 2, and 1 hypertension was noted in 3, 3, and 1 patient, respectively, which were managed with alpha-adrenoceptor blockade (n=7) such as doxazosin or phenoxybenzamine, beta-adrenoceptor blockade (n=3), and metyrosine (n=1). Three cases of CRS manifested as worsening hypertension with systolic blood pressures reaching as high as the 240’s, although this number may be underestimated due to our practice of treating the highest risk patients in the ICU with prophylactic IV anti-hypertensives (5/39 patients, 12.8%). Two cases of CRS presented as arrhythmias, one as new onset atrial fibrillation subsequently requiring the use of chronic oral anticoagulation agents, and another as torsades-de-pointes requiring eventual implantation of an automatic cardioverter defibrillator device. One case of CRS presented as a temporary cardiac ejection fraction decrease to 35% thought secondary to treatment-related tachycardia, and another case presented as elevated cardiac troponins (max 40 ng/L) thought to be due to demand ischemia. Per investigator discretion, one patient was given a reduced dose of 3.7 GBq (100 mCi) for all 4 cycles due to baseline frail status and perceived high risk of CRS.
Blood Biomarker Analysis
The median baseline chromogranin A levels were significantly correlated with OS (P = 0.002, c = 0.736), but not with PFS (P = 0.071, c = 0.61) or best RECIST response (P = 0.060, τ = 0.248) – see Table 1 of Supplementary materials for full details. During treatment and throughout the 3-year follow-up period, changes in chromogranin A and normetanephrine levels were strongly correlated with changes in the RECIST sum-of-diameters value of the tumors (P < 0.001) and changes in the TLU on the DOTATATE PET scan (P < 0.001), as shown in Figure 3.
Figure 3.
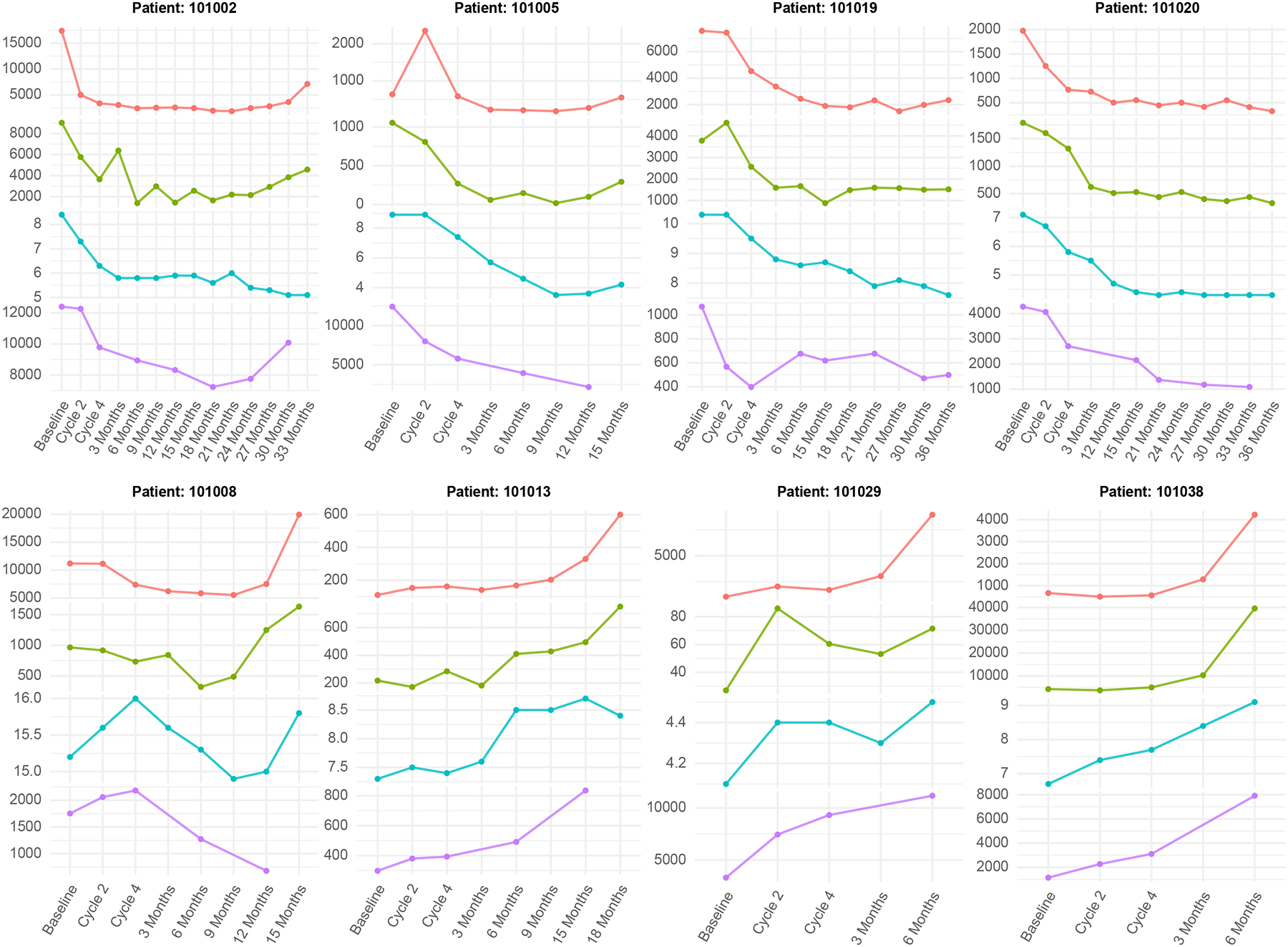
Representative graphical correlation of the blood serum parameters (chromogranin A and free plasma metanephrines) with clinical imaging outcomes, which includes the RECIST sum-of-diameters obtained from anatomic CT/MRI scans, and the total tumor lesion uptake (TLU) on the 68Ga-DOTATATE PET/CT scans, acquired at various time points throughout the treatment and follow-up period.
Use of anti-hypertensive medication
Of the 36 evaluable patients, 30 (83.3%) were on at least one hypertensive medication at baseline. Of these 30 patients, 12 (40.0%) were able to achieve a reduction of 50% or more in at least one anti-hypertensive medication within 12 months after completion of 177Lu-DOTATATE therapy.
Rand-36 Quality of Life Surveys
The median baseline scores in the eight QOL scales ranged from 25 for role limitation caused by physical issues to 100 for role limitation caused by emotional issues. After 24 months of follow-up, improvements were observed in physical functioning, energy levels, emotional well-being, and pain scores (Figure 1 and Table 2, Supplementary materials).
Discussion
Existing retrospective data in the literature suggests that 177Lu-DOTATATE can be successfully used to treat metastatic PPGL. A metanalysis which included 9 studies of 177Lu-DOTATATE in PPGL showed a PFS of 29.6 months20. While promising, these results are heterogeneous in terms of patient population, treatment regimen, and baseline tumor biology which makes interpretation and incorporation into treatment guidelines difficult. For instance, a study of 30 patients in 2019 demonstrated an response rate of 23% and a median PFS of 13 months in patients with sympathetic paraganglioma21, while a case series of 34 PPGL patients treated with 177Lu-DOTATATE showed a response rate of 8.9% and a median PFS of 27.5 months in the sympathetic PPGL group22.
Our prospective data show that 177Lu-DOTATATE is active against PPGL, with a median PFS of 19.9 months that is affected by genetic subtype (24.3 months in sporadic vs 12.9 months in SDHx), a median OS of 51.7 months (not reached in sporadic vs 31.2 months in SDHx), and is well-tolerated by most patients. This result is particularly notable because documented progression within 12 months was a requirement for trial enrollment. Differences in the clinical outcome between the two cohorts may be attributed to more aggressive underlying biology of SDHx tumors. As SDHx mutations fall into a category of pseudohypoxic phenotypes, the outcome differences could be due to hypoxia in the tumor microenvironment, with elevations of hypoxia-inducible factor 2 alpha being associated with radio-resistance23. Interestingly, most patients continued to experience tumor shrinkage months after receiving the last cycle of 177Lu-DOTATATE, with the largest reduction observed at 11.0 months after EOT. Therefore, unless a patient exhibits clinical deterioration or conclusive imaging evidence of progression, such as new lesions, a wait-and-watch approach appears to be appropriate for many patients after 177Lu-DOTATATE treatment.
The toxicities detected for 177Lu-DOTATATE in patients with PPGL were similar to those observed in patients with GEP-NET24, with the observed differences likely attributable to differences in metastatic distribution. For instance, as GEP-NET metastasize to the liver at a higher rate, 40–50% transaminase elevation was seen there compared to the 2.5% detected in PPGL. Similarly, because PPGL is much more likely to exhibit extensive bone metastases, the rate of grade 3+ myelosuppression is slightly higher in PPGL compared to GEP-NET. Although there are previous reports of the long-term pituitary effects of 177Lu-DOTATATE therapy25,26, our study demonstrated that pituitary abnormalities can be detected within 24 h of each treatment cycle, but are generally transient, with full recovery observed by 30 days19. Therefore, when these abnormalities are incidentally observed, unnecessary anxiety, additional testing, or interventions can be avoided in most cases.
CRS is a group of adverse events specific to patients with PPGL after 177Lu-DOTATATE and perhaps other systemic treatments. It is characterized by catecholamine-related symptoms or signs that can emerge as early as during infusion and persist for days to weeks after each treatment. Based on our previous limited results18,19 and the current data, CRS is defined as the occurrence of catecholamine-related symptoms and signs in the setting of acute or prolonged catecholamine release induced by systemic therapy, a phenomenon that has been previously reported19. The etiology of CRS may be similar to the serotonin neurohormonal crisis that is observed in patients with carcinoid tumors treated with 177Lu-DOTATATE27. The frequency of Grade 3 CRS in our study was relatively high at 17.8% with potentially dangerous manifestations such as ventricular arrythmias, extreme hypertension and tachycardia, and catecholamine-induced cardiomyopathies. In our experience, patients who had poorly controlled hypertension (frequent episodes of SBP > 200) despite the optimization of oral anti-hypertensive management prior to 177Lu-DOTATATE treatment had the highest risk of developing CRS. Such patients may benefit from preemptive management in an Intensive Care Unit (ICU)18.
In the assessment of treatments for PPGL supported by prospective data, 177Lu-DOTATATE demonstrates a favorable efficacy profile with a reported PFS of 19.9 months and OS of 51.7 months. Results from the FIRSTMAPPP study indicated that sunitinib achieved a PFS of 8.9 months and an OS of 37 months16. Cabozantinib presented a median PFS of 16.6 months and an OS of 24.9 months17. Furthermore, high-specific-activity 131I-MIBG demonstrated a median OS of 37 months. It is noteworthy that sunitinib exhibited a superior overall response rate (ORR) of 36.1%, whereas 177Lu-DOTATATE and 131I-MIBG yielded overall response rates of 27.8% and 23.4%, respectively. Belzutifan, recently approved by the FDA in May 2025 for the treatment of advanced PPGL based on the results of the LITESPARK-015 trial, has preliminary data published in abstract form28, with a reported response rate in PPGL of 26% and a median duration of response of 20.4 months29.
Although there are no direct comparative studies available, 177Lu-DOTATATE exhibited efficacy that aligned with findings from other prospective studies for PPGL, suggesting its potential as a viable option for the management of metastatic PPGL. In terms of toxicity, the adverse-event profiles of these therapies exhibited both similarities and disparities. For example, grade 3+ asthenia, uncommon among patients treated with 177Lu-DOTATATE, was reported in 18% of sunitinib-treated patients. Similarly, cabozantinib was associated with grade 3 hand-and-foot syndrome, rectal fistula, and QT prolongation, all of which are rare for 177Lu-DOTATATE. Conversely, myelosuppression is commonly observed for all radioligand therapies, such as 177Lu-DOTATATE, but is rare in TKI therapy. Worsening hypertension was observed across all agents, although the CRS detected for 177Lu-DOTATATE may be more acute and intense, with some requiring ICU management. Therefore, in patients at high risk for developing CRS, 177Lu-DOTATATE may be best suited for those with access to higher acuity medical care. While no data on the optimal sequencing of treatment is currently available, the choice of therapy could be determined by the baseline profile of the patients or their tolerance of certain agent-specific toxicities30.
Among all measured blood-based biomarkers, chromogranin A and normetanephrine appeared to be the most consistently useful, which confirms and expands upon the results of prior investigations31. Unlike for GEP-NET, baseline chromogranin A levels in PPGL exhibited prognostic value, showing correlations with clinical outcomes. Moreover, both biomarkers were highly and significantly correlated with changes in anatomic (CT/MRI) and functional (68Ga-DOTATATE PET) imaging observed over time, suggesting their possible use for disease monitoring perhaps in lieu of the more costly imaging studies. In fact, a prominent increase in blood chromogranin A or normetanephrine levels (in patients who have abnormally high baseline values of either) was often a harbinger of impending progression subsequently observed on imaging.
Limitations of this report included the fact that the results remain the findings of an interim analysis, with full statistical power only achieved once the study is completed. The patients were enrolled non-consecutively, with some being excluded due to factors such as withdrawal of consent that may have introduced a selection bias. Furthermore, combining the data from both genetic cohorts can inflate the type 1 error, and analyses such as the comparison of PFS and OS between the two cohorts are only exploratory in nature. However, even based on the current results, it is apparent that 177Lu-DOTATATE has activity in metastatic PPGL and that these findings, which has taken 5 years for accrual and 7 years to reach the point of interim analysis, may represent a strong piece of evidence in support of the regulatory approval of 177Lu-DOTATATE for the treatment of this very rare cancer.
Conclusion
177Lu-DOTATATE is generally well-tolerated and shows promising efficacy for the treatment of progressive metastatic PPGLs. Although there is potential for serious toxicities such as CRS, it can be safely managed in most circumstances with proper preparation. An interim analysis of this ongoing study suggests its strong activity, with clinical outcomes that compare favorably against other systemic agents for PPGL. Further data, including those necessary for completion of this study, may further confirm these results.
Supplementary Material
Appendix Figure 1. Results of the QoL survey on a scale of 0–100, with a score of 100 being the best score in each category. All 8 axis are stable or improved comparing post-therapy to baseline.
Key objective:
Is 177Lu-DOTATATE tolerable and efficacious in pheochromocytomas and paragangliomas (PPGL) associated with either pathogenic variants in succinate dehydrogenase (SDHx) or without any PPGL-associated mutations?
Knowledge generated:
In 36 patients with progressive metastatic PPGL, the 6 months progression free survival (PFS) rate was 0.861, with median PFS and survival of 19.9 and 51.7 months, respectively, and SDHx patients did worse compared to those without mutations. Treatment was well-tolerated but there was a 17% incidence of grade 3+ symptoms related to increased catecholamine release post-therapy which may require higher acuity care in some patients.
Relevance (written by Eileen M. O’Reilly):
Interim analysis of this trial provides prospective non-randomized data supporting the use of radioligand therapy in metastatic paraganglioma and pheochromocytoma; diseases in which there are few regulatory approved therapies and many challenges to conducting clinical trials.
Funding support:
NIH grant ZIABC011789
Contributor Information
Frank I. Lin, National Institutes of Health, National Cancer Institute, Center for Cancer Research, Molecular Imaging Branch, Bethesda, MD, USA.
Jaydira del Rivero, National Institutes of Health, National Cancer Institute, Center for Cancer Research, Developmental Therapeutics Branch, Bethesda, MD, USA.
Jorge A. Carrasquillo, National Institutes of Health, National Cancer Institute, Center for Cancer Research, Molecular Imaging Branch, Bethesda, MD, USA.
Abhishek Jha, National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA.
Joy Zou, National Institutes of Health, National Cancer Institute, Center for Cancer Research, Molecular Imaging Branch, Bethesda, MD, USA.
Inna Shamis, National Institutes of Health, National Cancer Institute, Center for Cancer Research, Molecular Imaging Branch, Bethesda, MD, USA.
Sara Talvacchio, National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA.
Baris Turkbey, National Institutes of Health, National Cancer Institute, Center for Cancer Research, Molecular Imaging Branch, Bethesda, MD, USA.
Erich P. Huang, National Institutes of Health, National Cancer Institute, Division of Cancer Therapy and Diagnosis, Biometric Research Program, Bethesda, MD, USA.
Joanna Shih, National Institutes of Health, National Cancer Institute, Division of Cancer Therapy and Diagnosis, Biometric Research Program, Bethesda, MD, USA.
Joanna Klubo-Gwiezdzinska, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Metabolic Diseases Branch, Bethesda, MD, USA.
Esther Mena, National Institutes of Health, National Cancer Institute, Center for Cancer Research, Molecular Imaging Branch, Bethesda, MD, USA.
Liza Lindenberg, National Institutes of Health, National Cancer Institute, Center for Cancer Research, Molecular Imaging Branch, Bethesda, MD, USA.
Yating Teng, Uniformed Services University of the Health Sciences, Bethesda, MD, USA.
Freddy E. Escorcia, National Institutes of Health, National Cancer Institute, Center for Cancer Research, Molecular Imaging Branch, Bethesda, MD, USA.
Clara Chen, National Institutes of Health, Clinical Center, Division of Nuclear Medicine, Bethesda, MD, USA.
Peter Herscovitch, National Institutes of Health, Clinical Center, PET Department.
Corina Millo, National Institutes of Health, Clinical Center, PET Department.
Peter L. Choyke, National Institutes of Health, National Cancer Institute, Center for Cancer Research, Molecular Imaging Branch, Bethesda, MD, USA.
Karel Pacak, National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA; AKESO, Center for Adrenal Endocrine Tumors, Prague, Czech Republic.
References
- 1.Mete O, Asa SL, Gill AJ, Kimura N, de Krijger RR, Tischler A. Overview of the 2022 WHO Classification of Paragangliomas and Pheochromocytomas. Endocr Pathol 2022;33(1):90–114. (In eng). DOI: 10.1007/s12022-022-09704-6. [DOI] [PubMed] [Google Scholar]
- 2.Hamidi O, Young WF, Iñiguez-Ariza NM Jr., et al. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. J Clin Endocrinol Metab 2017;102(9):3296–3305. (In eng). DOI: 10.1210/jc.2017-00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nölting S, Bechmann N, Taieb D, et al. Personalized Management of Pheochromocytoma and Paraganglioma. Endocr Rev 2022;43(2):199–239. (In eng). DOI: 10.1210/endrev/bnab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hescot S, Curras-Freixes M, Deutschbein T, et al. Prognosis of Malignant Pheochromocytoma and Paraganglioma (MAPP-Prono Study): A European Network for the Study of Adrenal Tumors Retrospective Study. J Clin Endocrinol Metab 2019;104(6):2367–2374. (In eng). DOI: 10.1210/jc.2018-01968. [DOI] [PubMed] [Google Scholar]
- 5.Janssen I, Chen CC, Millo CM, et al. PET/CT comparing (68)Ga-DOTATATE and other radiopharmaceuticals and in comparison with CT/MRI for the localization of sporadic metastatic pheochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging 2016;43(10):1784–91. (In eng). DOI: 10.1007/s00259-016-3357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han S, Suh CH, Woo S, Kim YJ, Lee JJ. Performance of (68)Ga-DOTA-Conjugated Somatostatin Receptor-Targeting Peptide PET in Detection of Pheochromocytoma and Paraganglioma: A Systematic Review and Metaanalysis. J Nucl Med 2019;60(3):369–376. (In eng). DOI: 10.2967/jnumed.118.211706. [DOI] [PubMed] [Google Scholar]
- 7.Kong G, Schenberg T, Yates CJ, et al. The Role of 68Ga-DOTA-Octreotate PET/CT in Follow-Up of SDH-Associated Pheochromocytoma and Paraganglioma. J Clin Endocrinol Metab 2019;104(11):5091–5099. (In eng). DOI: 10.1210/jc.2019-00018. [DOI] [PubMed] [Google Scholar]
- 8.Taïeb D, Garrigue P, Bardiès M, Abdullah AE, Pacak K. Application and Dosimetric Requirements for Gallium-68-labeled Somatostatin Analogues in Targeted Radionuclide Therapy for Gastroenteropancreatic Neuroendocrine Tumors. PET Clin 2015;10(4):477–86. (In eng). DOI: 10.1016/j.cpet.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong G, Grozinsky-Glasberg S, Hofman MS, et al. Efficacy of Peptide Receptor Radionuclide Therapy for Functional Metastatic Paraganglioma and Pheochromocytoma. J Clin Endocrinol Metab 2017;102(9):3278–3287. (In eng). DOI: 10.1210/jc.2017-00816. [DOI] [PubMed] [Google Scholar]
- 10.Janssen I, Blanchet EM, Adams K, et al. Superiority of [68Ga]-DOTATATE PET/CT to Other Functional Imaging Modalities in the Localization of SDHB-Associated Metastatic Pheochromocytoma and Paraganglioma. Clin Cancer Res 2015;21(17):3888–95. (In eng). DOI: 10.1158/1078-0432.Ccr-14-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Neuroendocrine and Adrenal Tumors V2.2024. August/1/2024.
- 12.Fishbein L, Del Rivero J, Else T, et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Management of Metastatic and/or Unresectable Pheochromocytoma and Paraganglioma. Pancreas 2021;50(4):469–493. (In eng). DOI: 10.1097/mpa.0000000000001792. [DOI] [PubMed] [Google Scholar]
- 13.Hescot S, Leboulleux S, Amar L, et al. One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 2013;98(10):4006–12. (In eng). DOI: 10.1210/jc.2013-1907. [DOI] [PubMed] [Google Scholar]
- 14.Taïeb D, Nölting S, Perrier ND, et al. Management of phaeochromocytoma and paraganglioma in patients with germline SDHB pathogenic variants: an international expert Consensus statement. Nat Rev Endocrinol 2024;20(3):168–184. (In eng). DOI: 10.1038/s41574-023-00926-0. [DOI] [PubMed] [Google Scholar]
- 15.Araujo-Castro M, García Sanz I, Mínguez Ojeda C, et al. Local recurrence and metastatic disease in pheochromocytomas and sympathetic paragangliomas. Front Endocrinol (Lausanne) 2023;14:1279828. (In eng). DOI: 10.3389/fendo.2023.1279828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baudin E, Goichot B, Berruti A, et al. Sunitinib for metastatic progressive phaeochromocytomas and paragangliomas: results from FIRSTMAPPP, an academic, multicentre, international, randomised, placebo-controlled, double-blind, phase 2 trial. Lancet 2024;403(10431):1061–1070. (In eng). DOI: 10.1016/s0140-6736(23)02554-0. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez C, Habra MA, Campbell MT, et al. Cabozantinib in patients with unresectable and progressive metastatic phaeochromocytoma or paraganglioma (the Natalie Trial): a single-arm, phase 2 trial. Lancet Oncol 2024;25(5):658–667. (In eng). DOI: 10.1016/s1470-2045(24)00133-5. [DOI] [PubMed] [Google Scholar]
- 18.Phelps TE, Del Rivero J, Chertow DS, Rosing D, Pacak K, Lin FI. Managing Catecholamine Release Syndrome During and Following Lu-177-DOTATATE in High-Risk Pheochromocytoma Patients. JCEM Case Rep 2024;2(4):luae049. (In eng). DOI: 10.1210/jcemcr/luae049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gubbi S, Al-Jundi M, Auh S, et al. Early short-term effects on catecholamine levels and pituitary function in patients with pheochromocytoma or paraganglioma treated with [(177)Lu]Lu-DOTA-TATE therapy. Front Endocrinol (Lausanne) 2023;14:1275813. (In eng). DOI: 10.3389/fendo.2023.1275813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prado-Wohlwend S, Del Olmo-García MI, Bello-Arques P, Merino-Torres JF. Response to targeted radionuclide therapy with [(131)I]MIBG AND [(177)Lu]Lu-DOTA-TATE according to adrenal vs. extra-adrenal primary location in metastatic paragangliomas and pheochromocytomas: A systematic review. Front Endocrinol (Lausanne) 2022;13:957172. (In eng). DOI: 10.3389/fendo.2022.957172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zandee WT, Feelders RA, Smit Duijzentkunst DA, et al. Treatment of inoperable or metastatic paragangliomas and pheochromocytomas with peptide receptor radionuclide therapy using 177Lu-DOTATATE. Eur J Endocrinol 2019;181(1):45–53. (In eng). DOI: 10.1530/eje-18-0901. [DOI] [PubMed] [Google Scholar]
- 22.Severi S, Bongiovanni A, Ferrara M, et al. Peptide receptor radionuclide therapy in patients with metastatic progressive pheochromocytoma and paraganglioma: long-term toxicity, efficacy and prognostic biomarker data of phase II clinical trials. ESMO Open 2021;6(4):100171. (In eng). DOI: 10.1016/j.esmoop.2021.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatt RS, Landis DM, Zimmer M, et al. Hypoxia-inducible factor-2alpha: effect on radiation sensitivity and differential regulation by an mTOR inhibitor. BJU Int 2008;102(3):358–63. (In eng). DOI: 10.1111/j.1464-410X.2008.07558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FDA Label for Lutathera. April/23/2024. (https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/208700s031lbl.pdf).
- 25.Sundlöv A, Sjögreen-Gleisner K, Tennvall J, et al. Pituitary Function after High-Dose 177Lu-DOTATATE Therapy and Long-Term Follow-Up. Neuroendocrinology 2021;111(4):344–353. (In eng). DOI: 10.1159/000507761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teunissen JJ, Krenning EP, de Jong FH, et al. Effects of therapy with [177Lu-DOTA 0,Tyr 3]octreotate on endocrine function. Eur J Nucl Med Mol Imaging 2009;36(11):1758–66. (In eng). DOI: 10.1007/s00259-009-1151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brabander T, van der Zwan WA, Teunissen JJM, et al. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res 2017;23(16):4617–4624. (In eng). DOI: 10.1158/1078-0432.Ccr-16-2743. [DOI] [PubMed] [Google Scholar]
- 28.Qui JZ J; Cai L; Kong W; Xue W; Zhang J; Dong P; Liu J; Li W; Li N; Naik G; Gong K Belzutifan monotherapy in Chinese patients (pts) with von Hippel-Lindau (VHL) disease–associated tumors: Results of LITESPARK-015 study. Journal of Clinical Oncology 2025;43:5_suppl. [Google Scholar]
- 29.FDA approves belzutifan for pheochromocytoma or paraganglioma. May 14th, 2025. (https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-belzutifan-pheochromocytoma-or-paraganglioma).
- 30.Pacak K, Taieb D, Lin FI, Jha A. Approach to the Patient: Concept and Application of Targeted Radiotherapy in the Paraganglioma Patient. J Clin Endocrinol Metab 2024;109(9):2366–2388. (In eng). DOI: 10.1210/clinem/dgae252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jimenez C, Chin BB, Noto RB, et al. Biomarker response to high-specific-activity I-131 meta-iodobenzylguanidine in pheochromocytoma/paraganglioma. Endocr Relat Cancer 2023;30(2) (In eng). DOI: 10.1530/erc-22-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 1. Results of the QoL survey on a scale of 0–100, with a score of 100 being the best score in each category. All 8 axis are stable or improved comparing post-therapy to baseline.


