Abstract
Vertebrates exhibit a range of regenerative capacities following spinal cord injury. At one end of the spectrum are chief regenerators, including teleost fish and urodele amphibians. At the other end, most mammalian species exhibit limited repair and multicellular complications following spinal cord injury. Pro-regenerative immune, glial and neuronal injury responses underlie innate spinal cord repair in highly regenerative vertebrates. In many instances, fundamental mechanisms of spinal cord repair represent ancestral neuroprotection mechanisms that are conserved but become overwhelmed by anti-regenerative effects in mammals. Reflecting recent advances in the field, we review how fine-tuned immune responses, pro-regenerative glial cell reactivity and multimodal neuronal repair direct innate spinal cord repair.
Keywords: Spinal cord injury, Regeneration, Zebrafish, Immune cells, Astrocyte reactivity, Neuronal repair
Summary:
This Review gives an overview of how pro-regenerative immune, glial and neuronal strategies are deployed for recovery from spinal cord injury, which is limited to select vertebrate species.
Introduction
The basic form and functions of the spinal cord are conserved across all vertebrates. Yet while common developmental principles guide intricate spinal cord morphogenesis during vertebrate embryogenesis, adult vertebrate species elicit widely varied responses following spinal cord injury (SCI). In most mammalian species, including humans, SCI causes permanent loss of sensory, motor and systemic functions, posing a global medical and socioeconomic burden (Ahuja et al., 2017). Curiously, vertebrates such as teleost fish and urodele amphibians – and even some mammalian species – display a remarkable ability to tolerate damaging injuries and regain lost functions (Zottoli et al., 1994; Becker et al., 1997; Hanslik et al., 2019; Thygesen et al., 2019; Streeter et al., 2020).
The unique ability of some vertebrates to mend a lesioned spinal cord have fascinated scientists for centuries. As neurons are the ultimate effectors of spinal cord function, earlier regeneration studies were largely focused on neuronal repair, mirroring neuron-centered approaches in the neuroscience field. Fueled by considerable understanding of developmental axon growth and neurogenesis, the molecular and cellular mechanisms of axon regrowth and neuronal regeneration in regenerative species were the first to be dissected (Bastmeyer et al., 1991; Becker et al., 1998; Hui et al., 2013). Over the past decade, renewed appreciation for the roles of immunoglial cells, including astrocytes and microglia, has expanded SCI research into new directions (Mokalled et al., 2016; Cavone et al., 2021; de Sena-Tomás et al., 2024; Shaw et al., 2024 preprint). In addition to stimulating immune and glial regeneration research, recent technological advances, including single-cell transcriptomics and imaging tools, have also invigorated studies of neuronal repair (Huang et al., 2021; Pedroni et al., 2024; Saraswathy et al., 2024).
Differential immune, glial and neuronal injury responses underlie disparate injury outcomes between highly and poorly regenerative vertebrates (Fig. 1). In non-regenerative mammals, additional cell types, such as fibroblasts and pericytes, exhibit exaggerated injury responses that impact extracellular matrix deposition and scarring outcomes (Dias et al., 2018; Saxena et al., 2019; Kolb et al., 2023). Mechanisms that differentially promote or inhibit repair have been extensively reviewed and will still be covered in this Review. However, one underappreciated feat of tissue regeneration is the ability of regenerative vertebrates to deploy mechanisms that are often conserved but overwhelmed by anti-regenerative complications in mammals. This Review aims to highlight how fine-tuned immune responses, pro-regenerative glial cell reactivity and multimodal neuronal repair can support spinal cord regeneration in the absence of intrinsic and extrinsic inhibition. Rather than portraying regenerative vertebrates as peculiar exceptions with superior regenerative competence, we present multiple lines of evidence that fundamental mechanisms of spinal cord regeneration are conserved and could be deployed to promote neural repair in mammals. This Review focuses on recent advances that have been made in zebrafish over the past decade and directs readers to reviews covering other regenerative species or prior zebrafish literature.
Fig. 1. Mechanisms underlying differential regenerative competence after SCI in vertebrates.
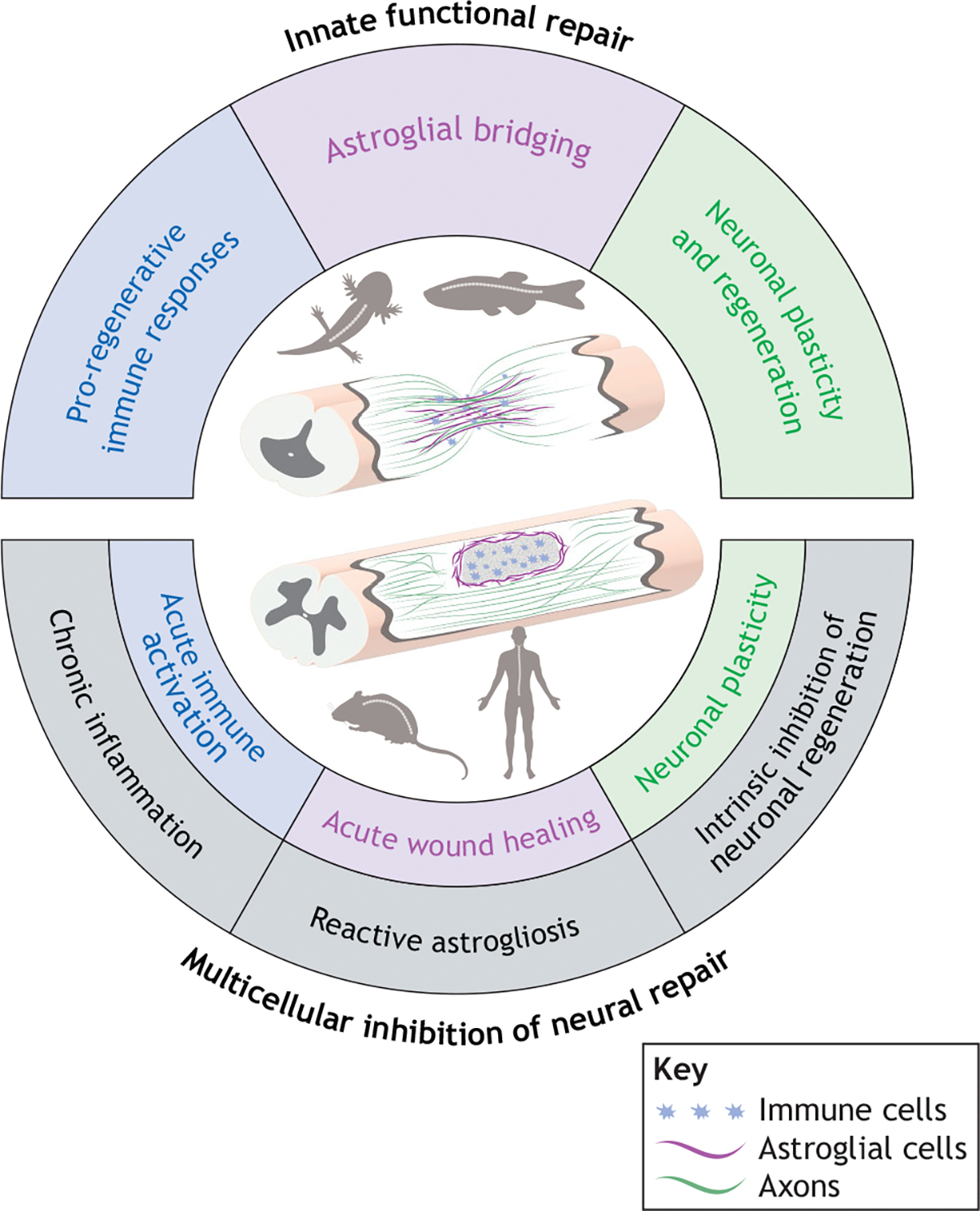
Top: Regenerative species, such as zebrafish and axolotls, exhibit transient immune responses, astroglial bridging and multiple modes of neuronal repair. Bottom: Mammals exhibit some pro-regenerative responses, such as acute immune cell infiltration, astrocytic wound healing and residual neuronal plasticity (inner circle), but these cellular processes are overshadowed by multicellular complications (highlighted in gray) that impede regeneration. Schematics depict lesioned spinal cord tissues in highly regenerative and less-regenerative vertebrates, with immune cells (blue), astroglial cells (magenta) and axons (green).
Regenerative capacity is unevenly distributed among vertebrates
Tissue regeneration is manifested in different mechanisms and efficiencies among vertebrates, including mammals. Relative to other tissues, the central nervous system (CNS) is particularly resistant to undergoing repair following injury or disease (Zheng and Tuszynski, 2023). While the general assumption is that an animal’s regenerative competence inversely correlates with its position and complexity in the phylogenetic tree, gains and losses of regenerative capacity do not adhere to a clear principle and are not confined to specific phylogenetic nodes in evolution (Fig. 2). In fact, nature presents only a few exceptions for which tissue regeneration bestows an unambiguous evolutionary advantage. For instance, adult lizards retain an evolved defense mechanism that allows them to self-amputate their tails and regenerate the entire structure, including spinal cord, following caudal autonomy (Alibardi, 2022). Beyond such rare examples of adaptive regeneration, not all fishes are capable of spinal cord repair, whereas some mammalian species can achieve scarless healing following SCI.
Fig. 2. Distribution of regenerative capacity among vertebrate models of SCI.

For each species, elevated (green) or limited (magenta) regenerative competence is shown throughout the lifespan. Regenerative capacity is generally elevated at younger life stages across the phylogenetic tree. However, unlike less-regenerative vertebrates, regenerative species, such as zebrafish, retain the ability to mend spinal cord lesions in adult stages. Frogs benefit from a temporally restricted regenerative period, although this ability is lost post-metamorphosis. Spiny mice represent an exceptional case among mammals, maintaining life-long scar-free tissue repair.
Among the closest living group to jawed vertebrates are the jawless lamprey fish, which undergo spontaneous repair following repeated spinal cord injuries (Hanslik et al., 2019). Benefiting rom a strategic phylogenetic position between vertebrates and invertebrates, lampreys have greatly advanced our understanding of intrinsic and extrinsic regeneration mechanisms (reviewed by Maxson Jones and Morgan, 2023). Noting that functional recovery is achieved despite imperfect cellular regeneration, lamprey research pioneered the notion of compensatory plasticity after SCI (Hibbard, 1963). Below, we highlight vertebrates such as zebrafish and axolotls as champions of spinal cord repair, the usefulness of studying the regenerative and non-regenerative stages in frogs, and the curious example of the spiny mouse as a mammalian model of successful spinal cord repair. Questions related to the evolution of spinal cord regenerative capacity are fascinating but not amenable to empirical studies. If and why regenerative competence was lost in some species will continue to captivate scientists for years to come.
Elevated regenerative competence in teleost fish and urodele amphibians
Teleosts deployed for SCI research included goldfish before zebrafish became a primary model for teleost regeneration (Zottoli et al., 1994; Becker et al., 1997; Rasmussen and Sagasti, 2017). Zebrafish (Danio rerio) are tropical freshwater fish and an excellent model of successful CNS regeneration. In addition to their elevated regenerative capacity, zebrafish SCI research is rapidly evolving due to their amenability to genetic manipulations. Fish SCI models include complete transection or crush injuries in adults, and mechanical transection or laser ablation in larvae (Becker et al., 1997; Hu et al., 2018; Bremer et al., 2019; Burris et al., 2021; Alper and Dorsky, 2022; Jensen et al., 2023). Benefiting from shorter experimental timelines, advanced imaging tools and amenability to electrophysiological studies, larval zebrafish research is widespread. However, limitations of the larval SCI models include an underdeveloped immune system, delayed microglial maturation and baseline expression of growth factors and regeneration-permissive molecules (Davidson and Zon, 2004; Mokalled et al., 2016; Hui et al., 2017). Although technically more challenging, adult zebrafish have a fully developed innate and adaptive immune system (Davidson and Zon, 2004; Stachura and Traver, 2011), exhibit pro-regenerative glial cell reactivity in the absence of developmental guidance cues (Reyes and Mokalled, 2024), and activate regenerative programs despite eliciting limited growth in adulthood compared to other teleosts (Biga and Goetz, 2006).
Similar to teleost fish, axolotls are urodele amphibians that stand out for their renowned ability to reverse the anatomical and functional deficits of SCI. Experimental systems include tail amputation, spinal cord transection or crush injuries (Tazaki et al., 2017; Thygesen et al., 2019; Walker et al., 2023). Axolotls remain in neoteny, do not undergo metamorphosis and do not develop adaptive immunity (Menger et al., 2010). Experimentally induced metamorphosis in axolotls reduces the rate and fidelity of regenerative responses but does not abrogate functional recovery, linking metamorphosis to lower regenerative capacity (Monaghan et al., 2014). Recent genetic advances in axolotls have enabled cell fate mapping. For instance, recent lineage tracing showed that Shh+ floor plate cells exclusively contribute to the regenerating floor plate, indicating lineage restriction during regeneration (Arbanas et al., 2024). Molecular studies revealed species-specific roles for the transcription factor Junbb and microRNA miR200a during innate spinal cord repair (Sabin et al., 2019; Walker et al., 2022). Tail and spinal cord regeneration in axolotls have been reviewed by Tazaki et al. (2017).
Regenerative competence is temporally restricted in anuran frogs
Tadpole regenerative capacity is restricted to a limited time window: after tail development is concluded and before the onset of feeding (Filoni and Bosco, 1981). As tadpoles, Xenopus laevis undergo epimorphic regeneration of multiple tissues, including spinal cord, following tail amputation (Cannata et al., 2001). The presence of regeneration-competent and -incompetent phases stimulated intra-species comparisons into the molecular drivers and inhibitors of regeneration. Moreover, inter-species comparative studies between X. laevis and Xenopus tropicalis were prompted by X. tropicalis showing a milder refractory period than X. laevis (Wang and Shi, 2021; Williams et al., 2021). Xenopus studies championed the hypothesis that cold-blooded animals express regenerative genes that are lost in warm-blooded vertebrates. Supporting this hypothesis, genes encoding thioredoxin domain-containing protein Ag1, small GTPases Ras-dva1/2 or cold-blooded animal-specific wound epithelium receptor protein C-answer were lost during the evolution of birds and mammals and are required for tail regeneration in tadpoles (Ivanova et al., 2013, 2018; Tereshina et al., 2014; Korotkova et al., 2019). Despite these drastic examples of complete gene loss, the prevailing model is that injury induces comparable gene sets across species and that nuanced gene regulatory changes alter the timing or specificity of gene expression to impact regeneration. Advances in Xenopus tail and spinal cord regeneration were recently reviewed by Phipps et al. (2020).
The spiny mouse is an exception to limited mammalian regeneration
Mouse species within the genus Acomys, also known as spiny mice, are members of the Muridae family, which includes the widely used experimental Mus and Rattus models. Spiny mice sparked research interests for their unique ability to sustain scar-free healing of the skin and ear (Seifert et al., 2012; Gawriluk et al., 2016; Matias Santos et al., 2016). Acomys were subsequently shown capable of robust regenerative competence after SCI (Streeter et al., 2020; Nogueira-Rodrigues et al., 2022). Whereas Mus axons arrest at the lesion border, Acomys axons are not deterred from entering the lesion after thoracic spinal cord transection. By 12 weeks, corticospinal axons show bilateral sprouting, collateral arborization and partial conduction of compound action potentials across the lesion (Nogueira-Rodrigues et al., 2022). Functionally, Acomys mice regain bladder function and establish some weight bearing and plantar support capacity (Nogueira-Rodrigues et al., 2022). Decreased inflammation, enhanced expression of stem cell and axon guidance genes and pro-regenerative astrocyte reactivity underlie enhanced cellular bridging and functional repair in Acomys compared to Mus (Streeter et al., 2020). Reduced fibrosis is a common characteristic of Acomys responses to various injuries (Saxena et al., 2019). In the spinal cord, Acomys deposit less collagen IV and heparan sulfate proteoglycan and increase deposition of keratin sulfate proteoglycans (KSPGs) (Streeter et al., 2020; Nogueira-Rodrigues et al., 2022). Supporting the hypothesis that increased KSPG biosynthesis provides a pro-regenerative matrix environment, expressing a key KSPG biosynthetic enzyme (β3gnt7) is sufficient to promote neurite outgrowth in vitro (Nogueira-Rodrigues et al., 2022). Why and how Acomys evolved to replace lesion scarring with a scarless tissue bridge is a fascinating observation and open question. Acomys spinal cord regeneration was recently reviewed by Maden and Varholick (2020).
Transient and finely tuned immune responses contribute to spinal cord repair
The first obstacle to regeneration is the requirement to re-enact developmental processes within the hostile environment of adult tissue lesions (Fig. 3). Shortly after mammalian SCI, reactive cell-derived or debris-derived molecules that inhibit regeneration overwhelm the extracellular milieu in and around the lesioned cord (Li et al., 2004; Cafferty et al., 2010; Lee et al., 2010b; Schwab and Strittmatter, 2014; Chio et al., 2022). Attempts to clear cellular debris begin with the phagocytic activity of lesion-adjacent microglia. In addition to their own phagocytic activity, activated microglia secrete pro-inflammatory cytokines that recruit infiltrating immune cells (Kigerl et al., 2009; Hakim et al., 2021). Distinct populations of innate and adaptive immune cells are sequentially recruited to the lesion (Popovich et al., 1996, 1997; Ankeny et al., 2006; Beck et al., 2010).
Fig. 3. Pro-regenerative immune, glial and neuronal responses postSCI in zebrafish.

Schematics represent longitudinal sections of spinal cord tissues in adult zebrafish. (A) In the absence of SCI, neurons and glia are organized around ependymal progenitor cells (gray) lining the central canal (white).
(B) In acute SCI, zebrafish activate a transient, pro-regenerative immune response characterized by the infiltration of immune cells, predominantly microglia and macrophages, into the lesion site. Lesion-adjacent neurons survive the injury and adopt a plasticity signature, while ependymal progenitors proliferate in response to injury.
(C) Chronic SCI is marked by neurogenesis, axonal regrowth and glial regeneration. Glial progenitors (magenta-gray) in the ventral domain undergo epithelial-to-mesenchymal transition (EMT) and contribute to astroglial bridging across the lesion. Neurogenic progenitors (green-gray) generate newborn neurons (green) and axons regrow across the lesion.
Immune cell activation plays dual roles after spinal cord injury
As the first line of defense against neural damage, the immune system dictates the cascade of regenerative events following SCI. After human SCI, immune activation initiates acutely but then persists for years following injury. This has been perceived as inefficient, exaggerated or both (Means and Anderson, 1983; Carlson et al., 1998; Fleming et al., 2006). Consistent with the emergence of overt anti-regenerative inflammation, treatment with anti-inflammatory drugs or depletion of microglia and macrophages improved functional recovery after mouse SCI (Popovich et al., 1999). Sustained, chronic inflammation is a chief contributor to secondary neurotoxicity and the recruitment of scar-depositing fibroblasts to the lesion (Dorrier et al., 2021). Conversely, targeted microglial and macrophage ablation in acute SCI revealed a requirement for early immune activation in mice (Shechter et al., 2009; Greenhalgh et al., 2018; Uderhardt et al., 2019; Brennan et al., 2022). These studies underscore the challenges of discerning specific immune functions in mammals, highlighting the need to improve our understanding of the temporal impact of specific immune populations on regenerative outcomes.
Transient and reversible activation of multiple immune populations in zebrafish
Regenerative vertebrates present a valuable model to untangle pro-regenerative immune functions in the absence of inflammation. The zebrafish immune system comprises innate myeloid cells and adaptive lymphoid cells that are conserved across vertebrates (Davidson and Zon, 2004; Stachura and Traver, 2011). The CNS is also privileged with tissue-resident microglia that elicit a phagocytic response after injury or disease (Wittamer et al., 2011; Nguyen-Chi et al., 2015; Krasemann et al., 2017; Villani et al., 2019). Spinal cord transection in adult zebrafish triggers transient and reversible immune activation, peaking 3 days post-injury (dpi) and significantly cleared by 14 dpi (Shaw et al., 2024 preprint). Neutrophils, blood-derived macrophages and tissue-resident microglia, followed by T cells, serially infiltrate the lesion (Shaw et al., 2024 preprint). Importantly, elevated leukocytes are known to persist in chronic SCI in mouse and humans, whereas immune SCI responses are transient in zebrafish (Gadani et al., 2015; Gillespie and Ruitenberg, 2022; de Sena-Tomás et al., 2024; Shaw et al., 2024 preprint). Establishing an immune requirement for spinal cord repair, blocking immune activation prolongs inflammation and reduces regeneration (Tsarouchas et al., 2018; de Sena-Tomás et al., 2024; Shaw et al., 2024 preprint). Below, we review the identities, activation profiles and roles of major immune subtypes after SCI.
Neutrophils
Neutrophils are the first immune cell population to infiltrate the lesioned spinal cord in regenerative and non-regenerative vertebrates (Taoka et al., 1997; Tsarouchas et al., 2018; de Sena-Tomás et al., 2024; Shaw et al., 2024 preprint). Neutrophils phagocytose cellular debris, recruit microglia and macrophages, and tune inflammatory events in spinal cord lesions (Neirinckx et al., 2014). In zebrafish larvae subjected to SCI, neutrophil infiltration peaks 4–6 h post-injury and is resolved to pre-injury levels by 72 h (de Sena-Tomás et al., 2024). Using transgenic lines in which neutrophils express the photoconvertible protein Kaede, lesion-associated neutrophils were shown to reverse migrate from the lesion to distal anatomical locations without clear organ preference (de Sena-Tomás et al., 2024). The regenerative functions of neutrophils require Cxcr1/2 activation (de Sena-Tomás et al., 2024). Intriguingly, genetic manipulations of rac2, which alters neutrophil mobility and blocks their recruitment to the lesion, had no impact on cellular or functional regeneration, neutrophil clearance or the recruitment of microglia and macrophages (de Sena-Tomás et al., 2024). Thus, spinal cord regeneration can occur in the absence of neutrophils, ostensibly via compensatory immune functions from other cell populations (de Sena-Tomás et al., 2024). Conversely, accelerating neutrophil clearance by administering a Cxcr4 antagonist accelerated progenitor cell proliferation, motor neuron regeneration and cellular bridging across the lesion (de Sena-Tomás et al., 2024). Molecularly, Cxcr4 inhibition induced pro-inflammatory cytokines such as il-1b and tnfa, and activated pro-inflammatory tnfa+ mpeg+ microglia/macrophages, which were shown to promote progenitor cell proliferation and regeneration (Tsarouchas et al., 2018; de Sena-Tomás et al., 2024).
Microglia and macrophages
Supporting a central regenerative role for macrophages after SCI, irf8 mutant zebrafish larvae lack macrophages and exhibit regeneration defects reminiscent of broad immune inhibition by dexamethasone (Tsarouchas et al., 2018). A subset of lesion-adjacent macrophages express tnfa, promoting progenitor cell proliferation and neuronal differentiation via AP1/Hdac1 signaling (Cavone et al., 2021). By contrast, colony-stimulating factor 1 receptor (csf1ra/b) mutant zebrafish larvae, in which microglial differentiation is impaired, did not exhibit altered axon regeneration (Tsarouchas et al., 2018). These results established a requirement for macrophages after SCI, and suggested microglia are dispensable for larval spinal cord regeneration. However, csf1ra/b mutants show increased macrophage numbers (Tsarouchas et al., 2018), which could compensate for the loss of microglia in this model. Moreover, developmental migration of microglia into the CNS and lymphocyte maturation extend for weeks after zebrafish larval development (Willett et al., 1997; Davidson and Zon, 2004; Xu et al., 2015; Hui et al., 2017). This timeline of immune cell development underscores the need to validate immune cell requirement and functions in adult animals. In adult zebrafish, depletion of mpeg1.1+ microglia and macrophages between 1 and 14 dpi diminished cellular bridging and the recovery of swim function (Shaw et al., 2024 preprint). Notably, allowing mpeg1.1+ cells to repopulate the spinal cord at 14 dpi was not sufficient to direct regeneration between 14 and 28 dpi. These results indicated that mpeg1.1+ microglia and macrophages are acutely required for adult spinal cord repair.
Regulatory T cells
Regulatory T cells (Tregs) comprise a subset of CD4+ T cells, mediate antigen recognition and play important roles in immune suppression (Workman et al., 2009; Josefowicz et al., 2012). After injury, Tregs exert regenerative functions by repressing proinflammatory chemokine expression and dampening innate and adaptive inflammation (reviewed by Gupta et al., 2021). Using a forkhead box P3a (foxp3a)-driven zebrafish reporter line, Tregs were shown to emerge in the juvenile zebrafish thymus (Hui et al., 2017). In adult zebrafish, peripheral and lesion-associated foxp3a+ Tregs peak at 7 dpi in close association with proliferating progenitors and newly born neurons (Hui et al., 2017). Molecularly, spinal Tregs express general Treg activation genes such as nr4a1 and amphiregulin, in addition to CNS-specific growth factors, including neurotrophin 3 (ntf3) (Hui et al., 2017). Conditional ablation of foxp3a+ Tregs results in impaired cellular bridging, disorganized rostral and caudal axon sprouting and diminished swim recovery (Hui et al., 2017). Establishing Treg-derived Ntf3 as a crucial regulator of progenitor cell proliferation, administration of recombinant human NTF3 was sufficient to rescue the number of Sox2+ Pcna+ cells in Treg-depleted animals at 7 dpi (Hui et al., 2017). These findings show the pivotal role played by adaptive immune cells in progenitor cell activation and spinal cord regeneration.
Efficient phagocytosis and lack of chronic inflammation
Adult zebrafish achieve efficient debris clearance and innate repair in the absence of chronic inflammation (Shaw et al., 2024 preprint). Cross-species comparisons between zebrafish and mammals support a model in which zebrafish immune cells elicit superior phagocytic capacity, and identified transcription and immune response regulator (tcim) as a macrophage-enriched zebrafish gene (Shaw et al., 2024 preprint). Genetic deletion of zebrafish tcima and tcimb impedes phagocytic capacity and regeneration, reprogramming leukocytes into a pro-inflammatory transcriptional state similar to that of mice. Conversely, genetic overexpression of human TCIM accelerates debris clearance and regeneration by reprogramming myeloid precursors into activated phagocytes (Shaw et al., 2024 preprint). Manipulations that impact phagocytosis, such as tcim mutagenesis, often leads to excessive accumulation of lipid droplets (Shaw et al., 2024 preprint). These zebrafish observations link lipid processing to phagocytic and regenerative capacities. Similar to zebrafish mutations that impair phagocytosis, murine lipid-laden myeloid cells that fail to process and recycle lipids transition into a hyper-inflammatory, foamy phenotype (Wang et al., 2015). Conversely, lipid breakdown and fatty acid oxidation enhance macrophage differentiation, phagocytosis and microglial neuroprotection in mice (Jacquel et al., 2012; Zhang et al., 2012; Leng et al., 2022). These studies and others emphasize the value of deploying highly regenerative vertebrates to dissect and promote neuroprotective immune mechanisms after SCI. We propose that regenerative vertebrates will inspire approaches to accelerate debris clearance in acute SCI, and to promote immune quiescence in sub-acute to chronic SCI in humans.
Pro-regenerative glial cell reactivity directs scarless spinal cord repair
As the largest and one of the most abundant cell types in the CNS, astrocytes play crucial roles in the development and maintenance of neuronal circuits (Takano et al., 2006; Attwell et al., 2010; Paixão and Klein, 2010; Freeman and Rowitch, 2013; Blanco-Suárez et al., 2017). Astrocytes are highly sensitive to CNS perturbations, exhibiting a spectrum of reactivity states and functional changes, referred to as ‘reactive astrogliosis’ or ‘astrocyte reactivity’ (Zamanian et al., 2012). Although glial cell injury responses have been extensively studied in mammals, the complexities of these responses in mammals and our understanding of how astrocytes respond to SCI in highly regenerative vertebrates are relatively limited.
Reactive astrocytes play dual roles after mammalian spinal cord injury
Mammalian SCI is marked by scarring. The ‘glial scar’ is a historically coined term that depicts the detrimental effects of reactive astrocytes on neuronal survival and repair (Ramón y Cajal et al., 1991; Sofroniew, 2018; Yang et al., 2020). However, as spinal lesions attract multiple cell types in addition to glial cells, the SCI scar has been recently categorized into a fibrotic core bordered by a glial compartment. The fibrotic scar consists of infiltrating immune cells, fibroblasts and pericytes (Göritz et al., 2011; Soderblom et al., 2013; Zhu et al., 2015), whereas the surrounding glial scar comprises astrocytes, microglia and oligodendrocyte progenitor cells (Anderson et al., 2016; Hara et al., 2017; Wanner et al., 2013). Concomitantly, the concept that the glial scar is both detrimental and beneficial for SCI repair has emerged (Pekny et al., 2014; Sofroniew, 2018; Tran et al., 2022; Shafqat et al., 2023). Highly regenerative vertebrates exhibit pro-regenerative glial cell reactivity with minimal detrimental effects, which presents a unique opportunity to dissect the transcriptional, morphological and functional features of pro-regenerative astrocyte reactivity.
Astrocyte reactivity is a conserved defense mechanism across vertebrates
Under physiological conditions, astrocytes provide trophic and metabolic support for neurons, regulate the development and maintenance of synapses, and control spinal blood flow, fluid availability and ion homeostasis in the CNS (Takano et al., 2006; Attwell et al., 2010; Paixão and Klein, 2010; Blanco-Suárez et al., 2017). A conserved, central role for astrocytes is to protect the CNS against external injuries. In acute SCI, astrocytes accumulate around the lesion in zebrafish and mammals (Auguste et al., 2007; Dawley et al., 2012; Goldshmit et al., 2012). In mammals, reactive astrocytes border the fibrotic scar, limit the expansion of inflammation and protect spared neural tissues (Yuan and He, 2013; Sofroniew, 2018). In addition to their wound-healing functions, astrocytes help restore tight junctions and repair the blood–spinal cord barrier (Pitter et al., 2014). Astrocytes clear excess glutamate and were reported to phagocytose dead cells in vitro and in vivo (Lööv et al., 2012; Morizawa et al., 2017). Consequently, depleting reactive astrocytes in mice leads to enlarged lesions, increased inflammation, barrier failure and worsened injury outcomes (Faulkner et al., 2004; Myer et al., 2006; Okada et al., 2006; Sofroniew, 2009).
Reactive astrocytes have detrimental effects in mammals
Despite their acute beneficial roles, mammalian astrocytes form a physical and molecular barrier that obstructs neural repair at later stages (Tran et al., 2022). They increase the expression of anti-regenerative molecules, including intermediate filaments, cytokines, toxic amino acids and extracellular matrix molecules (Silver and Miller, 2004; Massey et al., 2006, 2008; Galtrey and Fawcett, 2007; Andrews et al., 2012; Hara et al., 2017). These molecules trigger neurotoxicity in addition to amplifying cyclical reactivity in surrounding astrocytes (Li et al., 2020). One of the most prominent astrocyte-derived inhibitory molecules is chondroitin sulfate proteoglycan (CSPG), which inhibits axon regrowth, neurogenesis, oligodendrocyte progenitor cell migration, and remyelination (Smith-Thomas et al., 1995; Bradbury et al., 2002; Barkho et al., 2006; Lee et al., 2010a; Siebert and Osterhout, 2011; Siebert et al., 2011; Wang et al., 2011; Hammond et al., 2014). Thus, chronic astrogliosis generates a microenvironment that impedes neuronal repair, hinders remyelination and curbs regeneration.
Bipolar astrocyte morphology correlates with pro-regenerative effects
Bipolar radial glial morphology correlates with pro-regenerative effects across vertebrates. In mammals, astrocytes with bipolar features support neuronal migration and axonal growth in the developing CNS (Joosten and Gribnau, 1989; Vaccarino et al., 2007). After SCI, elongated astrocytes are associated with regeneration-permissive functions in regenerative and non-regenerative species (White et al., 2008; Filous et al., 2010; Goldshmit et al., 2012; Zukor et al., 2013; Mokalled et al., 2016; Shen et al., 2025). Early observations of glial bridging in regenerative species were made in lampreys in 1959. At the time, K. Maron noted the formation of a glial-derived bridge of ependymal cells at the lesion site starting at 5 dpi (Maron, 1959). Subsequent zebrafish studies established glial cells as a central component of cellular regrowth across the lesion (Goldshmit et al., 2012; Mokalled et al., 2016). In 2012, Goldshmit et al. characterized bipolar glial cells that express the general astrocyte marker Gfap in the regenerating tissue at the lesion (Goldshmit et al., 2012). These Gfap+ bridging glia were later identified as astroglial cells that connect the transected spinal cord (Klatt Shaw et al., 2021) (Fig. 3). Genetic lineage tracing and cell ablation showed that bridging astroglia emerge from a confined niche of glial progenitors and are required for functional recovery (Zhou et al., 2023). Intriguingly, glial bridging is required for axon regrowth in adult zebrafish and older larvae but not in younger larvae at 3 days post-fertilization (Wehner et al., 2017; Walker et al., 2025), suggesting the existence of developmental axon guidance cues that can substitute for bridging in larval zebrafish.
Manipulations that either induce or correlate with the emergence of bipolar astrocytes have been shown to improve axon regrowth and regeneration across species (White et al., 2008; Goldshmit et al., 2012; Zukor et al., 2013; Mokalled et al., 2016; Shen et al., 2025). Mouse studies have implicated Pten, TGFα and Wnt signaling in pro-regenerative astrocyte reactivity (White et al., 2008; Zukor et al., 2013; Shen et al., 2025). Advances in transcriptional and mutagenesis tools in zebrafish revealed a gamut of additional factors, including fgf, ctgf, yap1/taz (wwtr1), bach1, egr1 and junb (Goldshmit et al., 2012; Mokalled et al., 2016; Klatt Shaw and Mokalled, 2021; Klatt Shaw et al., 2021). Here, we discuss the roles of some of these pathways.
Fgf
Fibroblast growth factor (Fgf ) signaling is a pro-regenerative mechanism in the glia of zebrafish and mice (Goldshmit et al., 2012; Kasai et al., 2014). In zebrafish, multiple components of Fgf signaling, including fgf8a, fgf3, spry4, pea3 (etv4) and erm (etv5b), are upregulated in Gfap+ glial progenitors at 3 dpi (Goldshmit et al., 2012). By 14 dpi, fgf3 and spry4 transcripts become restricted to neurons, whereas fgf8a, pea3 and erm maintain their glial expression (Reimer et al., 2009; Goldshmit et al., 2012). The glial bridging functions of Fgf were established using a small molecule inhibitor of the pathway, dominant-negative Fgf receptor manipulations, sprouty mutants and recombinant Fgf8 (Goldshmit et al., 2012). In these experiments, pharmacological and genetic loss-of-function approaches reduced the proliferation and migration of Gfap+ cells into the lesion and compromised axonal bridging. Conversely, Fgf activation using spry4 mutants and intraperitoneal Fgf8 injections increased glial and axonal bridging at the lesion (Goldshmit et al., 2012). Following mouse SCI, exogenous application of Fgf2 lowered the levels of TNFα and CSPG, decreased inflammation and improved motor function (Goldshmit et al., 2014). Importantly, mouse astrocytes treated with Fgf2 adopt a bipolar morphology that is thought to facilitate axonal growth (Goldshmit et al., 2014). These studies correlate the emergence of bipolar astroglia cells with pro-regenerative effects across vertebrates.
Ctgf
Connective tissue growth factor (Ctgf), also known as Ccn2, is a matricellular molecule that elicits a range of cellular responses, including cell proliferation and differentiation (Grotendorst and Duncan, 2005). Zebrafish studies identified ctgfa (ccn2a) as a central glial bridging factor and described the emergence of ctgfa+ gfap+ bridging glia after SCI (Mokalled et al., 2016; Zhou et al., 2023). ctgfa transcripts are upregulated in glial progenitors and the nascent cellular bridge in the regenerating spinal cord (Mokalled et al., 2016). The glial requirements of ctgf during bridging and of glial bridging during spinal cord regeneration were established using genetic mutants and cell-ablation tools (Mokalled et al., 2016; Zhou et al., 2023). ctgfa mutants exhibit reduced swim endurance and fail to extend glial or axonal projections through the lesion (Mokalled et al., 2016). Similar phenotypes were observed following depletion of ctgfa+ cells (Zhou et al., 2023). Conversely, genetic and pharmacological Ctgf gain of function enhance glial bridging and functional repair. Intriguingly, murine CYR61, also known as CCN1, was recently implicated in the reactivity of lesion-remote astrocytes after thoracic hemisection (McCallum et al., 2024 preprint). These studies suggest that CCN family members, including CTGF and CYR61, have conserved pro-regenerative roles in glial cell reactivity.
Epithelial-to-mesenchymal transition
Transcriptional and genetic evidence indicate that epithelial-to-mesenchymal transition (EMT) reprograms glial progenitors into pro-regenerative glia (Klatt Shaw et al., 2021; Zhou et al., 2023). EMT is often linked to stem cell activation and tissue plasticity after injury (Jessen and Arthur-Farraj, 2019; Wilson et al., 2020). gfap+ ctgfa+ bridging glia progenitors displaying hallmarks of EMT, including twist1a expression and Yap1 activation, express mesenchymal markers and downregulate epithelial markers (Klatt Shaw et al., 2021). Phenocopying Fgf and Ctgfa loss of function, CRISPR mutagenesis showed that egr1, junbb and yap1/taz are required for cellular bridging and functional recovery (Klatt Shaw et al., 2021). Furthermore, Twist1 overexpression promotes glial bridging and functional recovery (Klatt Shaw et al., 2021). As EMT gene expression is more pronounced in zebrafish astroglia compared to mammalian astrocytes (Klatt Shaw et al., 2021), whether EMT can confer pro-regenerative functions to mammalian astrocytes remains to be tested.
As disparate as glial cell reactivity may appear between regenerative and non-regenerative vertebrates, the studies reviewed here provide multiple lines of evidence supporting a conserved neuroprotective role for astroglial cells after SCI. We propose that acute glial cell reactivity is an ancestral regenerative function across vertebrates, but that chronic astrogliosis is specific to mammalian SCI. While mammalian research has predominantly focused on dampening the detrimental effects of astrogliosis, regenerative models such as the zebrafish could inform complementary strategies to promote beneficial glial cell reactivity and wound healing.
Intersecting modes of neuronal plasticity and regeneration enable neural repair
Lesioned spinal neurons encounter a multitude of environmental and molecular challenges that are context, injury and species dependent. As discussed above, extrinsic immunoglial effects can be anti- or pro-regenerative. In addition to integrating extrinsic cues, neurons undergo intrinsic molecular changes that impact their ability to regenerate. Recent single-cell transcriptomics identified a small population of injury-surviving spinal neurons that expressed regeneration-associated genes in the mouse spinal cord and were linked to spontaneous plasticity (Matson et al., 2022). Despite this signature of spontaneous plasticity, neurotoxicity and intrinsic inhibition of axon regeneration prevail in less-regenerative vertebrates. These inhibitory effects were recently reviewed by Zheng and Tuszynski (2023). Intriguingly, lesion-adjacent zebrafish neurons that activate regeneration-associated gene expression in acute SCI are associated with increased survival and plasticity (Saraswathy et al., 2024). Innate neuronal repair in regenerative vertebrates is achieved by cooperate survival, plasticity and regeneration mechanisms (Saraswathy et al., 2024) (Fig. 3). Below, we focus our discussion on the multiple modes by which neurons meet the challenges of SCI in zebrafish.
Neuronal plasticity and survival
Plasticity refers to spontaneous reorganization of spared neural circuitry via axon sprouting, not to be confused with regeneration in which a transected axon regrows to its original target site. Neuroplasticity offers a promising therapeutic avenue that does not require de novo cellular or axonal regeneration and is spontaneously detected after mammalian SCI (Tendolkar and Mokalled, 2026). Zebrafish studies have revealed that plasticity precedes cellular regeneration and is required for functional recovery following SCI (Vandestadt et al., 2021; Pedroni et al., 2024; Saraswathy et al., 2024). Similar to lampreys, zebrafish achieve significant functional recovery before axon regrowth and neurogenesis are completed (Hibbard, 1963; Reimer et al., 2008; Kuscha et al., 2012; Pedroni et al., 2024; Saraswathy et al., 2024). In larval zebrafish, caudal calcium propagation and muscle function are restored as early as 2 dpi, when the majority of lesion-associated neurons are not newborn (Vandestadt et al., 2021). Similarly, in adult zebrafish, 5-ethynyl-2’-deoxyuridine (EdU) and 5′-bromo-2′-deoxyuridine incorporation assays showed little neurogenesis in isl1+ or hb9 (mnx1)+ neurons during the first 7 days after injury, despite some recovery in swim function (Reimer et al., 2008; Vandestadt et al., 2021). Imaging of neuronally expressed elavl3: GCaMP6s along with EdU labeling indicated that initial circuit repair relies on the recruitment of pre-existing post-mitotic neurons, rather than neurogenesis (Vandestadt et al., 2021). These findings were supported by recent single-cell transcriptomics revealing a transient population of injury-responsive neurons (iNeurons) that acquire a neuroblast-like transcriptional signature between 3 and 7 dpi (Saraswathy et al., 2024). Similar to the early migrating neurons described by Vandestadt et al. (2021), the majority of iNeurons were morphologically mature and did not incorporate EdU after SCI. Ruling out the notion that mature neurons dedifferentiate after SCI, Vandestadt et al. (2021) showed that early migrating neurons did not express the neural stem cell markers nestin or sox2. However, in-depth molecular characterization by single-cell RNA sequencing supported a model in which mature neurons acquire an immature neuroblast-like cell identity, even if they do not express sox2. By tracking transgenic reporter lines for motor neurons and interneurons, multiple neuronal subtypes were thought to survive SCI and elicited an iNeuron transcriptional signature (Vandestadt et al., 2021; Saraswathy et al., 2024). These studies support a model in which injury-surviving neurons revert to a neuroblast-like stage and are immediate responders to SCI in larval and adult zebrafish.
iNeurons express known regeneration-associated genes during acute to sub-acute SCI (Saraswathy et al., 2024). Using CellChat analysis, cell–cell signaling outgoing from iNeurons and received by other cell types is predicted to account for the majority of all neuronal signaling at 7 dpi. Independent lines of evidence corroborated vesicular trafficking and endocytosis in acute neuronal plasticity after SCI (Vandestadt et al., 2021; Hilton et al., 2022; Saraswathy et al., 2024). Applying inhibitors of clathrin-dependent and -independent endocytosis impaired neuron recruitment to the lesion, establishing a requirement for endocytosis in neuronal plasticity (Vandestadt et al., 2021). Moreover, CRISPR mutagenesis showed that synaptotagmin XI (syt11a/b) and vesicle-associated membrane protein 4 (vamp4) are crucial for functional recovery despite being dispensable for cellular regeneration across the lesion. Syt11 is a non-canonical SNARE that inhibits spontaneous neurotransmission and bulk endocytosis, whereas Vamp4 promotes calcium-dependent neurotransmitter release and bulk endocytosis. These findings are consistent with recent mammalian studies showing that inhibition of the presynaptic release machinery is important for axon regrowth (Hilton et al., 2022). Together, these studies highlight endocytic vesicular trafficking as a putatively essential mechanism that underlies spontaneous plasticity and functional recovery after SCI.
Insights into the mechanisms that activate neuronal survival and plasticity after SCI are only beginning to emerge. Despite experiencing a transient surge in glutamatergic drive, which results in glutamate toxicity in mammals, zebrafish motor neurons employ calcium-binding proteins to buffer excess calcium and tolerate SCI damage (Pedroni et al., 2024). Improved calcium buffering is concomitant with a transient glutamate-mediated upregulation in gap junctional connexins (Cx35 and Cx36) and an increase in bidirectional electrical coupling between motor neurons (Pedroni et al., 2024). A model has been proposed in which double-stranded RNA (dsRNA) molecules secreted by damaged cells activate neuronal toll-like receptors (TLRs), and that dsRNA-TLR activation is an early injury response that stimulates neuronal plasticity (Vandestadt et al., 2021). Supporting this model, treating zebrafish larvae with a global RNase or dsRNA-specific RNaseIII, but not DNases, inhibited the recruitment of isl1+ neurons into the lesion and impaired functional recovery (Vandestadt et al., 2021). Similar phenotypes were obtained upon pharmacological or genetic loss of function of TLR3 or TLR22. Conversely, the application of the dsRNA-mimicking ligand and TLR3 activator Poly(I:C) increased isl1+ neuron migration and functional recovery, without impacting neurogenesis. Importantly, Poly(I:C) treatment was sufficient to rescue RNaseA-induced phenotypes, showing that TLR activation is a major driver of neuronal plasticity and SCI repair (Vandestadt et al., 2021).
Thus, innate spinal cord repair requires more than axon regrowth and de novo neurogenesis to achieve functional recovery. Recent advances in transcriptomic, electrophysiological and imaging tools are finally providing molecular clues regarding why or how regenerative species can regain function prior to cellular regeneration. The discovery of an acute neuroplasticity signature during innate spinal cord repair unlocks new prospects to leverage the zebrafish model for neuronal plasticity research, in addition to regeneration.
Neuronal and axonal regeneration
Neuronal regeneration and adult neurogenesis are similar processes involving de novo regeneration of neuronal cells from adult stem cells. In regenerative vertebrates, potent adult progenitors retaining radial glial features replenish lesion-associated neurons (Reimer et al., 2008; Briona et al., 2015; Ribeiro et al., 2017; Donato and Vickaryous, 2022). Newly differentiated neurons populate the regenerating tissue, as pre-existing neurons regrow axons across lesioned tissues (Reimer et al., 2008, 2013; Barreiro-Iglesias et al., 2015). Single-cell transcriptomics has shown that tightly regulated waves of excitatory neuron regeneration precede slower, continuous neurogenesis of inhibitory neurons (Saraswathy et al., 2024). Consequently, excitatory-inhibitory (E/I) ratios are marked by early imbalance towards an excitatory phenotype at 7 dpi, and restoration of baseline E/I activity by 42 dpi (Saraswathy et al., 2024). As altered E/I transmission leads to severe behavioral deficiencies in humans (Isaacson and Scanziani, 2011), how zebrafish progenitors ensure faithful and balanced neurogenesis over the extended timeline of spinal cord regeneration remains unknown.
Axon regeneration refers to axonal regrowth from injured neurons. Mammalian axon regeneration is challenged by intrinsic neuron inhibition (Goldberg et al., 2002; Filbin, 2003; Park et al., 2008) in addition to the extrinsic immune and glial factors discussed above. Conversely, early axon-tracing experiments in adult zebrafish showed that brainstem neurons are capable of long-distance axon regrowth, extending over 3.5 mm beyond the lesion site (Becker et al., 1997). Although brain-derived projections show slight differences in their regrowth capacities, both dopaminergic and serotonergic axon terminals are spontaneously established (Kuscha et al., 2012). Even reticulospinal Mauthner axons, which were thought to represent a unique example of failed regeneration in zebrafish, were subsequently found to be capable of regeneration following two-photon laser-mediated transection (Becker et al., 1997; Hu et al., 2018). A requirement for axon regrowth during innate spinal cord repair was uncovered by creating a physical barrier to axon regrowth at the lesion site and through transection of regenerating axons after complete regeneration (Becker et al., 2004; Kuscha et al., 2012). As neurogenesis and axonal regeneration have been appreciated for decades, we direct readers to recent reviews covering the classic literature related to these topics (Ghosh and Hui, 2018; Tsata and Wehner, 2021).
In more recent studies, transiently overproduced serotonergic neurons were shown to correlate with the regrowth of long spinal projections and functional circuit recovery (Kuscha et al., 2012; Huang et al., 2021). As serotonergic input is both brain and spinal derived, dual spinal cord transections were used to study spinal serotonergic effects independently of brain-derived projections. Depletion of tph2-expressing serotonergic neurons in this two-cut SCI model showed that lesion-associated serotonergic interneurons are required for axon regrowth and functional recovery (Huang et al., 2021). Serotonergic neurons proximal to the lesion have distinct electrophysiological and transcriptional signatures compared to their lesion-remote counterparts (Huang et al., 2021; Saraswathy et al., 2024). Mechanistically, Huang et al. proposed a model in which expression of the serotonergic receptor htr1b in glutamatergic excitatory neurons may facilitate glutamatergic axon regrowth in response to localized serotonergic signaling. In support of this model, glutamatergic axon regrowth and functional recovery are compromised in htr1b mutants (Huang et al., 2021).
Together, these studies uncover an orchestrated series of regenerative events that direct innate neuronal repair following SCI. Crucial to this process is the cooperative regulation of neuronal plasticity, axon regrowth and neurogenesis. We expect future studies will reveal intricate multidimensional regulatory mechanisms that underlie these processes at the cellular, tissue and temporal levels.
Concluding remarks
Despite recent advances highlighted in this Review and beyond, the regeneration field holds significantly more open questions than answers. Reflecting current interests in the field, our Review focuses on acute mechanisms of immune activation, astroglial wound healing and neuronal plasticity. However, our understanding of the signaling mechanisms that guide regenerative patterning, remyelination and functional recovery remain limited. When manifested, SCI repair is largely stem cell derived (Reimer et al., 2008; Donato and Vickaryous, 2022; Maxson Jones and Morgan, 2023; Arbanas et al., 2024). While lineage-tracing experiments in zebrafish and axolotls are beginning to unravel how specific niches of progenitor cells contribute to spontaneous regeneration (Zhou et al., 2023; Arbanas et al., 2024), comprehensive dissection of stem cell niches and the mechanisms that retain stem cell potency in adult animals remain unknown. Finally, the evolutionary principles of tissue regeneration and how observations in regeneration-competent animals would translate to mammals continue to attract regeneration researchers into the field. We propose that a first step to sufficiently address these overarching questions is to acknowledge that regenerative capacity depicts ancestral injury responses that are conserved across species. Rather than picturing regenerative capacity in magic terms that may seem unattainable in mammals, in-depth studies of similarities and differences are more likely to yield a realistic understanding of regenerative mechanisms that are either masked or missing in mammals. We expect this approach, along with continued tool development and cross-species comparisons, will provide the roadmap to understanding and promoting spinal cord repair.
Acknowledgements
We thank V. Cavalli, A. Johnson, P. Williams and members of the Mokalled laboratory for discussion and comments.
Funding details
| S.No. | Funder name | Funder ID | Grant ID |
|---|---|---|---|
|
| |||
| 1 | National Institute of Neurological Disorders and Stroke | http://dx.doi.org/10.13039/100000065 | 2R01-NS113915 |
| 2 | Washington University in St. Louis | http://dx.doi.org/10.13039/100007268 | 1R01-NS123708 |
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (2R01-NS113915 and 1R01-NS123708 to M.H.M.) and funds from the Washington University School of Medicine (Washington University in St. Louis) (to A.T.). Deposited in PMC for release after 12 months.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Special Issue
This article is part of the Special Issue ‘Lifelong Development: the Maintenance, Regeneration and Plasticity of Tissues’, edited by Meritxell Huch and Mansi Srivastava. See related articles at https://journals.biologists.com/dev/issue/152/20.
References
- Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D and Fehlings MG (2017). Traumatic spinal cord injury-repair and regeneration. Neurosurgery 80, S9–S22. doi: 10.1093/neuros/nyw080 [DOI] [PubMed] [Google Scholar]
- Alibardi L (2022). Review. Limb regeneration in lizards under natural and experimental conditions with considerations on the induction of appendages regeneration in amniotes. Ann. Anat. 239, 151844. doi: 10.1016/j.aanat.2021.151844 [DOI] [PubMed] [Google Scholar]
- Alper SR and Dorsky RI (2022). Unique advantages of zebrafish larvae as a model for spinal cord regeneration. Front. Mol. Neurosci. 15, 983336. doi: 10.3389/fnmol.2022.983336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ and Sofroniew MV (2016). Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200. doi: 10.1038/nature17623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews EM, Richards RJ, Yin FQ, Viapiano MS and Jakeman LB (2012). Alterations in chondroitin sulfate proteoglycan expression occur both at and far from the site of spinal contusion injury. Exp. Neurol. 235, 174–187. doi: 10.1016/j.expneurol.2011.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny DP, Lucin KM, Sanders VM, McGaughy VM and Popovich PG (2006). Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J. Neurochem. 99, 1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x [DOI] [PubMed] [Google Scholar]
- Arbanas LI, Cura Costa E, Chara O, Otsuki L and Tanaka EM (2024). Lineage tracing of Shh+ floor plate cells and dynamics of dorsal-ventral gene expression in the regenerating axolotl spinal cord. Dev. Growth Differ. 66, 414–425. doi: 10.1111/dgd.12945 [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA and Newman EA (2010). Glial and neuronal control of brain blood flow. Nature 468, 232–243. doi: 10.1038/nature09613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auguste KI, Jin S, Uchida K, Yan D, Manley GT, Papadopoulos MC and Verkman AS (2007). Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 21, 108–116. doi: 10.1096/fj.06-6848com [DOI] [PubMed] [Google Scholar]
- Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH and Zhao X (2006). Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 15, 407–421. doi: 10.1089/scd.2006.15.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Mysiak KS, Scott AL, Reimer MM, Yang Y, Becker CG and Becker T (2015). Serotonin promotes development and regeneration of spinal motor neurons in zebrafish. Cell Rep. 13, 924–932. doi: 10.1016/j.celrep.2015.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastmeyer M, Beckmann M, Schwab ME and Stuermer CA (1991). Growth of regenerating goldfish axons is inhibited by rat oligodendrocytes and CNS myelin but not but not by goldfish optic nerve tract oligodendrocytelike cells and fish CNS myelin. J. Neurosci. 11, 626–640. doi: 10.1523/JNEUROSCI.11-03-00626.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM and Anderson AJ (2010). Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133, 433–447. doi: 10.1093/brain/awp322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, Wullimann MF, Becker CG, Bernhardt RR and Schachner M (1997). Axonal regrowth after spinal cord transection in adult zebrafish. J. Comp. Neurol. 377, 577–595. doi: [DOI] [PubMed] [Google Scholar]
- Becker T, Bernhardt RR, Reinhard E, Wullimann MF, Tongiorgi E and Schachner M (1998). Readiness of zebrafish brain neurons to regenerate a spinal axon correlates with differential expression of specific cell recognition molecules. J. Neurosci. 18, 5789–5803. doi: 10.1523/JNEUROSCI.18-15-05789.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CG, Lieberoth BC, Morellini F, Feldner J, Becker T and Schachner M (2004). L1.1 is involved in spinal cord regeneration in adult zebrafish. J. Neurosci. 24, 7837–7842. doi: 10.1523/JNEUROSCI.2420-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biga PR and Goetz FW (2006). Zebrafish and giant danio as models for muscle growth: determinate vs. indeterminate growth as determined by morphometric analysis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1327–R1337. doi: 10.1152/ajpregu.00905.2005 [DOI] [PubMed] [Google Scholar]
- Blanco-Suárez E, Caldwell ALM and Allen NJ (2017). Role of astrocyte-synapse interactions in CNS disorders. J. Physiol. 595, 1903–1916. doi: 10.1113/JP270988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LDF, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW and McMahon SB (2002). Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640. doi: 10.1038/416636a [DOI] [PubMed] [Google Scholar]
- Bremer J, Marsden KC, Miller A and Granato M (2019). The ubiquitin ligase PHR promotes directional regrowth of spinal zebrafish axons. Commun. Biol. 2, 195. doi: 10.1038/s42003-019-0434-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FH, Li Y, Wang C, Ma A, Guo Q, Li Y, Pukos N, Campbell WA, Witcher KG, Guan Z et al. (2022). Microglia coordinate cellular interactions during spinal cord repair in mice. Nat. Commun. 13, 4096. doi: 10.1038/s41467-022-31797-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briona LK, Poulain FE, Mosimann C and Dorsky RI (2015). Wnt/ß-catenin signaling is required for radial glial neurogenesis following spinal cord injury. Dev. Biol. 403, 15–21. doi: 10.1016/j.ydbio.2015.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris B, Jensen N and Mokalled MH (2021). Assessment of swim endurance and swim behavior in adult zebrafish. J. Vis. Exp. 177. doi: 10.3791/63240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WBJ, Duffy P, Huebner E and Strittmatter SM (2010). MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J. Neurosci. 30, 6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannata SM, Bagni C, Bernardini S, Christen B and Filoni S (2001). Nerve-independence of limb regeneration in larval Xenopus laevis is correlated to the level of fgf-2 mRNA expression in limb tissues. Dev. Biol. 231, 436–446. doi: 10.1006/dbio.2001.0161 [DOI] [PubMed] [Google Scholar]
- Carlson SL, Parrish ME, Springer JE, Doty K and Dossett L (1998). Acute inflammatory response in spinal cord following impact injury. Exp. Neurol. 151, 77–88. doi: 10.1006/exnr.1998.6785 [DOI] [PubMed] [Google Scholar]
- Cavone L, McCann T, Drake LK, Aguzzi EA, Oprişoreanu A-M, Pedersen E, Sandi S, Selvarajah J, Tsarouchas TM., Wehner D et al. (2021). A unique macrophage subpopulation signals directly to progenitor cells to promote regenerative neurogenesis in the zebrafish spinal cord. Dev. Cell 56, 1617–1630.e6. doi: 10.1016/j.devcel.2021.04.031 [DOI] [PubMed] [Google Scholar]
- Chio JCT, Punjani N, Hejrati N, Zavvarian M-M, Hong J and Fehlings MG (2022). Extracellular matrix and oxidative stress following traumatic spinal cord injury: physiological and pathophysiological roles and opportunities for therapeutic intervention. Antioxid Redox Signal. 37, 184–207. doi: 10.1089/ars.2021.0120 [DOI] [PubMed] [Google Scholar]
- Davidson AJ and Zon LI (2004). The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 23, 7233–7246. doi: 10.1038/sj.onc.1207943 [DOI] [PubMed] [Google Scholar]
- Dawley EM, Samson SO, Woodard KT and Matthias KA (2012). Spinal cord regeneration in a tail autotomizing urodele. J. Morphol. 273, 211–225. doi: 10.1002/jmor.11019 [DOI] [PubMed] [Google Scholar]
- de Sena-Tomás C, Rebola Lameira L, Rebocho Da Costa M, Naique Taborda P, Laborde A, Orger M, De Oliveira S and Saúde L (2024). Neutrophil immune profile guides spinal cord regeneration in zebrafish. Brain Behav. Immun. 120, 514–531. doi: 10.1016/j.bbi.2024.06.022 [DOI] [PubMed] [Google Scholar]
- Dias DO, Kim H, Holl D, Werne Solnestam B, Lundeberg J, Carlén M, Göritz C and Frisén J (2018). Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell 173, 153–165.e22. doi: 10.1016/j.cell.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato SV and Vickaryous MK (2022). Radial glia and neuronal-like ependymal cells are present within the spinal cord of the trunk (body) in the Leopard Gecko (Eublepharis macularius). J. Dev. Biol. 10, 21. doi: 10.3390/jdb10020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrier CE, Aran D, Haenelt EA, Sheehy RN, Hoi KK, Pintarić L, Chen Y, Lizama CO, Cautivo KM, Weiner GA et al. (2021). CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat. Neurosci. 24, 234–244. doi: 10.1038/s41593-020-00770-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB and Sofroniew MV (2004). Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24, 2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT (2003). Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 4, 703–713. doi: 10.1038/nrn1195 [DOI] [PubMed] [Google Scholar]
- Filoni S and Bosco L (1981). Comparative analysis of the regenerative capacity of caudal spinal cord in larvae of serveral Anuran amphibian species. Acta Embryol. Morphol. Exp. (Halocynthia Assoc) 2, 199–226. [PubMed] [Google Scholar]
- Filous AR, Miller JH, Coulson-Thomas YM, Horn KP, Alilain WJ and Silver J (2010). Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev. Neurobiol. 70, 826–841. doi: 10.1002/dneu.20820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD and Weaver LC (2006). The cellular inflammatory response in human spinal cords after injury. Brain 129, 3249–3269. doi: 10.1093/brain/awl296 [DOI] [PubMed] [Google Scholar]
- Freeman MR and Rowitch DH (2013). Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron 80, 613–623. doi: 10.1016/j.neuron.2013.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Walsh JT, Lukens JR and Kipnis J (2015). Dealing with danger in the CNS: the response of the immune system to injury. Neuron 87, 47–62. doi: 10.1016/j.neuron.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtrey CM and Fawcett JW (2007). The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Rev. 54, 1–18. doi: 10.1016/j.brainresrev.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Gawriluk TR, Simkin J, Thompson KL, Biswas SK, Clare-Salzler Z, Kimani JM, Kiama SG, Smith JJ, Ezenwa VO and Seifert AW (2016). Comparative analysis of ear-hole closure identifies epimorphic regeneration as a discrete trait in mammals. Nat. Commun. 7, 11164. doi: 10.1038/ncomms11164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S and Hui SP (2018). Axonal regeneration in zebrafish spinal cord. Regeneration (Oxf) 5, 43–60. doi: 10.1002/reg2.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie ER and Ruitenberg MJ (2022). Neuroinflammation after SCI: current insights and therapeutic potential of intravenous immunoglobulin. J. Neurotrauma 39, 320–332. doi: 10.1089/neu.2019.6952 [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y and Barres BA (2002). Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296, 1860–1864. doi: 10.1126/science.1068428 [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Sztal TE, Jusuf PR, Hall TE, Nguyen-Chi M and Currie PD (2012). Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. J. Neurosci. 32, 7477–7492. doi: 10.1523/JNEUROSCI.0758-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Frisca F, Pinto AR, Pébay A, Tang JKKY, Siegel AL, Kaslin J and Currie PD (2014). Fgf2 improves functional recovery-decreasing gliosis and increasing radial glia and neural progenitor cells after spinal cord injury. Brain Behav. 4, 187–200. doi: 10.1002/brb3.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göritz C., Dias DO., Tomilin N, Barbacid M, Shupliakov O and Frisen J. (2011). A pericyte origin of spinal cord scar tissue. Science 333, 238–242. doi: 10.1126/science.1203165 [DOI] [PubMed] [Google Scholar]
- Greenhalgh AD, Zarruk JG, Healy LM, Baskar Jesudasan SJ, Jhelum P, Salmon CK, Formanek A, Russo MV, Antel JP, McGavern DB et al. (2018). Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 16, e2005264. doi: 10.1371/journal.pbio.2005264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst GR and Duncan MR (2005). Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 19, 729–738. doi: 10.1096/fj.04-3217com [DOI] [PubMed] [Google Scholar]
- Gupta S, Adhikary S and Hui SP (2021). Decoding the proregenerative competence of regulatory T cells through complex tissue regeneration in zebrafish. Clin. Exp. Immunol. 206, 346–353. doi: 10.1111/cei.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim R, Zachariadis V, Sankavaram SR, Han J, Harris RA, Brundin L, Enge M and Svensson M (2021). Spinal cord injury induces permanent reprogramming of microglia into a disease-associated state which contributes to functional recovery. J. Neurosci. 41, 8441–8459. doi: 10.1523/JNEUROSCI.0860-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TR, Gadea A, Dupree J, Kerninon C, Nait-Oumesmar B, Aguirre A and Gallo V (2014). Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron 81, 588–602. doi: 10.1016/j.neuron.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslik KL, Allen SR, Harkenrider TL, Fogerson SM, Guadarrama E and Morgan JR (2019). Regenerative capacity in the lamprey spinal cord is not altered after a repeated transection. PLoS ONE 14, e0204193. doi: 10.1371/journal.pone.0204193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima Y et al. (2017). Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med. 23, 818–828. doi: 10.1038/nm.4354 [DOI] [PubMed] [Google Scholar]
- Hibbard E (1963). The vascular supply to the central nervous system of the larval lamprey. Am. J. Anat. 113, 93–99. doi: 10.1002/aja.1001130107 [DOI] [PubMed] [Google Scholar]
- Hilton BJ, Husch A, Schaffran B, Lin T-C, Burnside ER, Dupraz S, Schelski M, Kim J, Müller JA, Schoch S. et al. (2022). An active vesicle priming machinery suppresses axon regeneration upon adult CNS injury. Neuron 110, 51–69.e7. doi: 10.1016/j.neuron.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B-B, Chen M, Huang R-C, Huang Y-B, Xu Y, Yin W, Li L and Hu B (2018). In vivo imaging of Mauthner axon regeneration, remyelination and synapses re-establishment after laser axotomy in zebrafish larvae. Exp. Neurol. 300, 67–73. doi: 10.1016/j.expneurol.2017.10.028 [DOI] [PubMed] [Google Scholar]
- Huang C-X, Zhao Y, Mao J, Wang Z, Xu L, Cheng J, Guan NN and Song J (2021). An injury-induced serotonergic neuron subpopulation contributes to axon regrowth and function restoration after spinal cord injury in zebrafish. Nat. Commun. 12, 7093. doi: 10.1038/s41467-021-27419-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui SP, Monaghan JR, Voss SR and Ghosh S (2013). Expression pattern of Nogo-A, MAG, and NgR in regenerating urodele spinal cord. Dev. Dyn. 242, 847–860. doi: 10.1002/dvdy.23976 [DOI] [PubMed] [Google Scholar]
- Hui SP, Sheng DZ, Sugimoto K, Gonzalez-Rajal A, Nakagawa S, Hesselson D and Kikuchi K (2017). Zebrafish regulatory T cells mediate organ-specific regenerative programs. Dev. Cell 43, 659–672.e5. doi: 10.1016/j.devcel.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Isaacson JS and Scanziani M (2011). How inhibition shapes cortical activity. Neuron 72, 231–243. doi: 10.1016/j.neuron.2011.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova AS, Tereshina MB, Ermakova GV, Belousov VV and Zaraisky AG (2013). Agr genes, missing in amniotes, are involved in the body appendages regeneration in frog tadpoles. Sci. Rep. 3, 1279. doi: 10.1038/srep01279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova AS, Korotkova DD, Ermakova GV, Martynova NY, Zaraisky AG and Tereshina MB (2018). Ras-dva small GTPases lost during evolution of amniotes regulate regeneration in anamniotes. Sci. Rep. 8, 13035. doi: 10.1038/s41598-018-30811-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquel A, Obba S, Boyer L, Dufies M, Robert G, Gounon P, Lemichez E, Luciano F, Solary E and Auberger P (2012). Autophagy is required for CSF-1-induced macrophagic differentiation and acquisition of phagocytic functions. Blood 119, 4527–4531. doi: 10.1182/blood-2011-11-392167 [DOI] [PubMed] [Google Scholar]
- Jensen NO, Burris B, Zhou L, Yamada H, Reyes C, Pincus Z and Mokalled MH (2023). Functional trajectories during innate spinal cord repair. Front. Mol. Neurosci. 16, 1155754. doi: 10.3389/fnmol.2023.1155754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR and Arthur-Farraj P (2019). Repair Schwann cell update: adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 67, 421–437. doi: 10.1002/glia.23532 [DOI] [PubMed] [Google Scholar]
- Joosten EAJ and Gribnau AAM (1989). Astrocytes and guidance of outgrowing corticospinal tract axons in the rat. An immunocytochemical study using anti-vimentin and anti-glial fibrillary acidic protein. Neuroscience 31, 439–452. doi: 10.1016/0306-4522(89)90386-2 [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu L-F and Rudensky AY (2012). Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564. doi: 10.1146/annurev.immunol.25.022106.141623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai M, Jikoh T, Fukumitsu H and Furukawa S (2014). FGF-2-responsive and spinal cord-resident cells improve locomotor function after spinal cord injury. J. Neurotrauma 31, 1584–1598. doi: 10.1089/neu.2009.1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ and Popovich PG (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt Shaw D and Mokalled MH (2021). Efficient CRISPR/Cas9 mutagenesis for neurobehavioral screening in adult zebrafish. G3 (Bethesda) 11, jkab089. doi: 10.1093/g3journal/jkab089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt Shaw D, Saraswathy VM, Zhou L, McAdow AR, Burris B, Butka E, Morris SA, Dietmann S and Mokalled MH (2021). Localized EMT reprograms glial progenitors to promote spinal cord repair. Dev. Cell 56, 613–626.e7. doi: 10.1016/j.devcel.2021.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb J, Tsata V, John N, Kim K, Möckel., Rosso G, Kurbel V, Parmar A, Sharma G, Karandasheva. et al. (2023). Small leucine-rich proteoglycans inhibit CNS regeneration by modifying the structural and mechanical properties of the lesion environment. Nat. Commun. 14, 6814. doi: 10.1038/s41467-023-42339-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova DD, Lyubetsky VA, Ivanova AS, Rubanov LI, Seliverstov AV, Zverkov OA, Martynova NY, Nesterenko AM, Tereshina MB, Peshkin L et al. (2019). Bioinformatics screening of genes specific for well-regenerating vertebrates reveals c-answer, a regulator of brain development and regeneration. Cell Rep. 29, 1027–1040.e6. doi: 10.1016/j.celrep.2019.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z et al. (2017). The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 47, 566–581.e9. doi: 10.1016/j.immuni.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuscha V, Frazer SL, Dias TB, Hibi M, Becker T and Becker CG (2012). Lesion-induced generation of interneuron cell types in specific dorsoventral domains in the spinal cord of adult zebrafish. J. Comp. Neurol. 520, 3604–3616. doi: 10.1002/cne.23115 [DOI] [PubMed] [Google Scholar]
- Lee H, McKeon RJ and Bellamkonda RV (2010a). Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 107, 3340–3345. doi: 10.1073/pnas.0905437106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B and Zheng B (2010b). Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron 66, 663–670. doi: 10.1016/j.neuron.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng L, Yuan Z, Pan R, Su X, Wang H, Xue J, Zhuang K, Gao J, Chen Z, Lin H et al. (2022). Microglial hexokinase 2 deficiency increases ATP generation through lipid metabolism leading to β-amyloid clearance. Nat. Metab. 4, 1287–1305. doi: 10.1038/s42255-022-00643-4 [DOI] [PubMed] [Google Scholar]
- Li S, Liu BP, Budel S, Li M, Ji B, Walus L, Li W, Jirik A, Rabacchi S, Choi E et al. (2004). Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 24, 10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li M, Tian L, Chen J, Liu R and Ning B (2020). Reactive astrogliosis: implications in spinal cord injury progression and therapy. Oxid. Med. Cell Longev. 2020, 9494352. doi: 10.1155/2020/9494352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lööv C, Hillered L, Ebendal T and Erlandsson A (2012). Engulfing astrocytes protect neurons from contact-induced apoptosis following injury. PLoS ONE 7, e33090. doi: 10.1371/journal.pone.0033090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M and Varholick JA (2020). Model systems for regeneration: the spiny mouse, Acomys cahirinus. Development 147, dev167718. doi: 10.1242/dev.167718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marón K (1959). Regeneration capacity of the spinal cord in Lampetra fluviatilis larvae. Folia Biol. 7, 179–189. [Google Scholar]
- Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J and Onifer SM (2006). Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J. Neurosci. 26, 4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey JM, Amps J, Viapiano MS, Matthews RT, Wagoner MR, Whitaker CM, Alilain W, Yonkof AL, Khalyfa A, Cooper NGF et al. (2008). Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp. Neurol. 209, 426–445. doi: 10.1016/j.expneurol.2007.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias Santos D, Rita AM, Casanellas I, Brito Ova A, Araújo IM, Power D and Tiscornia G (2016). Ear wound regeneration in the African spiny mouse Acomys cahirinus. Regeneration (Oxf) 3, 52–61. doi: 10.1002/reg2.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson KJE, Russ DE, Kathe C, Hua I, Maric D, Ding Y, Krynitsky J, Pursley R, Sathyamurthy A, Squair JW et al. (2022). Single cell atlas of spinal cord injury in mice reveals a pro-regenerative signature in spinocerebellar neurons. Nat. Commun. 13, 5628. doi: 10.1038/s41467-022-33184-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson Jones K and Morgan JR (2023). Lampreys and spinal cord regeneration: “a very special claim on the interest of zoologists,” 1830s-present. Front. Cell Dev. Biol. 11, 1113961. doi: 10.3389/fcell.2023.1113961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum S, Suresh KB, Islam T, Saustad AW, Shelest O, Patil A, Lee D, Kwon B, Yenokian I, Kawaguchi R et al. (2024). Lesion-remote astrocytes govern microglia-mediated white matter repair. bioRxiv. doi: 10.1101/2024.03.15.585251 [DOI] [Google Scholar]
- Means ED and Anderson DK (1983). Neuronophagia by leukocytes in experimental spinal cord injury. J. Neuropathol. Exp. Neurol. 42, 707–719. doi: 10.1097/00005072-198311000-00009 [DOI] [PubMed] [Google Scholar]
- Menger B, Reimers K, Kuhbier JW and Vogt PM (2010). Lessons from the Mexican axolotl: amphibian limb regeneration and its impact on plastic surgery. Plast. Reconstr. Surg. 125, 260e–261e. doi: 10.1097/PRS.0b013e3181d45e16 [DOI] [PubMed] [Google Scholar]
- Mokalled MH, Patra C, Dickson AL, Endo T, Stainier DYR and Poss KD (2016). Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science 354, 630–634. doi: 10.1126/science.aaf2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan JR, Stier AC, Michonneau F, Smith MD, Pasch B, Maden M and Seifert AW (2014). Experimentally induced metamorphosis in axolotls reduces regenerative rate and fidelity. Regeneration (Oxf) 1, 2–14. doi: 10.1002/reg2.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizawa YM, Hirayama Y, Ohno N, Shibata S, Shigetomi E, Sui Y, Nabekura J, Sato K, Okajima F, Takebayashi H et al. (2017). Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat. Commun. 8, 28. doi: 10.1038/s41467-017-00037-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA and Sofroniew MV (2006). Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 129, 2761–2772. doi: 10.1093/brain/awl165 [DOI] [PubMed] [Google Scholar]
- Neirinckx V, Coste C, Franzen R, Gothot A, Rogister B and Wislet S (2014). Neutrophil contribution to spinal cord injury and repair. J. Neuroinflammation 11, 150. doi: 10.1186/s12974-014-0150-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Chi M, Laplace-Builhe B, Travnickova J, Luz-Crawford P, Tejedor G, Phan QT, Duroux-Richard I, Levraud J-P, Kissa K, Lutfalla G et al. (2015). Identification of polarized macrophage subsets in zebrafish. eLife 4, e07288. doi: 10.7554/eLife.07288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Rodrigues J, Leite SC, Pinto-Costa R, Sousa SC, Luz LL, Sintra MA, Oliveira R, Monteiro AC, Pinheiro GG, Vitorino M et al. (2022). Rewired glycosylation activity promotes scarless regeneration and functional recovery in spiny mice after complete spinal cord transection. Dev. Cell 57, 440–450.e7. doi: 10.1016/j.devcel.2021.12.008 [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y et al. (2006). Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 12, 829–834. doi: 10.1038/nm1425 [DOI] [PubMed] [Google Scholar]
- Paixaõ S. and Klein R. (2010). Neuron-astrocyte communication and synaptic plasticity. Curr. Opin. Neurobiol. 20, 466–473. doi: 10.1016/j.conb.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M et al. (2008). Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963. doi: 10.1126/science.1161566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroni A, Dai Y-WE, Lafouasse L, Chang W, Srivastava I, Del Vecchio L and Ampatzis K (2024). Neuroprotective gap-junction-mediated bystander transformations in the adult zebrafish spinal cord after injury. Nat. Commun. 15, 4331. doi: 10.1038/s41467-024-48729-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Wilhelmsson U and Pekna M (2014). The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 565, 30–38. doi: 10.1016/j.neulet.2013.12.071 [DOI] [PubMed] [Google Scholar]
- Phipps LS, Marshall L, Dorey K and Amaya E (2020). Model systems for regeneration: Xenopus. Development 147, dev180844. doi: 10.1242/dev.180844 [DOI] [PubMed] [Google Scholar]
- Pitter KL, Tamagno I, Feng X, Ghosal K, Amankulor N, Holland EC and Hambardzumyan D (2014). The SHH/Gli pathway is reactivated in reactive glia and drives proliferation in response to neurodegeneration-induced lesions. Glia 62, 1595–1607. doi: 10.1002/glia.22702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Horner PJ, Mullin BB and Stokes BT (1996). A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp. Neurol. 142, 258–275. doi: 10.1006/exnr.1996.0196 [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P and Stokes BT (1997). Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 377, 443–464. doi: [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, Van Rooijen N and Stokes BT (1999). Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 158, 351–365. doi: 10.1006/exnr.1999.7118 [DOI] [PubMed] [Google Scholar]
- Ramón Y Cajal S, Jones EG, Ramâon Y Cajal S, Defelipe J and Oxford Scholarship Online, Neuroscience. (1991). Cajal’s Degeneration and Regeneration of the Nervous System. New York; Oxford: Oxford University Press. [Google Scholar]
- Rasmussen JP and Sagasti A (2017). Learning to swim, again: Axon regeneration in fish. Exp. Neurol. 287, 318–330. doi: 10.1016/j.expneurol.2016.02.022 [DOI] [PubMed] [Google Scholar]
- Reimer MM, Sörensen I, Kuscha V, Frank RE, Liu C, Becker CG and Becker T (2008). Motor neuron regeneration in adult zebrafish. J. Neurosci. 28, 8510–8516. doi: 10.1523/JNEUROSCI.1189-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer MM, Kuscha V, Wyatt C, Sörensen I, Frank RE, Knüwer, M., Becker, T. and Becker CG. (2009). Sonic hedgehog is a polarized signal for motor neuron regeneration in adult zebrafish. J. Neurosci. 29, 15073–15082. doi: 10.1523/JNEUROSCI.4748-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer MM, Norris A, Ohnmacht J, Patani R, Zhong Z, Dias TB, Kuscha V, Scott AL, Chen Y-C, Rozov S et al. (2013). Dopamine from the brain promotes spinal motor neuron generation during development and adult regeneration. Dev. Cell 25, 478–491. doi: 10.1016/j.devcel.2013.04.012 [DOI] [PubMed] [Google Scholar]
- Reyes C and Mokalled MH (2024). Astrocyte-neuron interactions in spinal cord injury. Adv. Neurobiol. 39, 213–231. doi: 10.1007/978-3-031-64839-7_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro A, Monteiro JF, Certal AC, Cristovaõ AM and Saúde L (2017). Foxj1a is expressed in ependymal precursors, controls central canal position and is activated in new ependymal cells during regeneration in zebrafish. Open Biol. 7, 170139. doi: 10.1098/rsob.170139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin KZ, Jiang P, Gearhart MD, Stewart R and Echeverri K (2019). AP-1 (cFos/JunB)/miR-200a regulate the pro-regenerative glial cell response during axolotl spinal cord regeneration. Commun. Biol. 2, 91. doi: 10.1038/s42003-019-0335-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswathy VM, Zhou L and Mokalled MH (2024). Single-cell analysis of innate spinal cord regeneration identifies intersecting modes of neuronal repair. Nat. Commun. 15, 6808. doi: 10.1038/s41467-024-50628-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Vekaria H, Sullivan PG and Seifert AW (2019). Connective tissue fibroblasts from highly regenerative mammals are refractory to ROS-induced cellular senescence. Nat. Commun. 10, 4400. doi: 10.1038/s41467-019-12398-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME and Strittmatter SM (2014). Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 27, 53–60. doi: 10.1016/j.conb.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM and Maden M (2012). Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 489, 561–565. doi: 10.1038/nature11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafqat A, Albalkhi I, Magableh HM, Saleh T, Alkattan K and Yaqinuddin A (2023). Tackling the glial scar in spinal cord regeneration: new discoveries and future directions. Front. Cell Neurosci. 17, 1180825. doi: 10.3389/fncel.2023.1180825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DK, Saraswathy VM, McAdow AR, Zhou L, Park D, Mote R, Johnson AN and Mokalled MH (2024). Elevated phagocytic capacity directs innate spinal cord repair. bioRxiv. doi: 10.1101/2024.06.11.598515 [DOI] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G et al. (2009). Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 6, e1000113. doi: 10.1371/journal.pmed.1000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Feng B, Lim WL, Woo T, Liu Y, Vicenzi S, Wang J, Kwon BK and Zou Y (2025). Astrocytic Ryk signaling coordinates scarring and wound healing after spinal cord injury. Proc. Natl. Acad. Sci. USA 122, e2417400122. doi: 10.1073/pnas.2417400122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert JR and Osterhout DJ (2011). The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J. Neurochem. 119, 176–188. doi: 10.1111/j.1471-4159.2011.07370.x [DOI] [PubMed] [Google Scholar]
- Siebert JR, Stelzner DJ and Osterhout DJ (2011). Chondroitinase treatment following spinal contusion injury increases migration of oligodendrocyte progenitor cells. Exp. Neurol. 231, 19–29. doi: 10.1016/j.expneurol.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Silver J and Miller JH (2004). Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156. doi: 10.1038/nrn1326 [DOI] [PubMed] [Google Scholar]
- Smith-Thomas LC, Stevens J, Fok-Seang J, Faissner A, Rogers JH and Fawcett JW (1995). Increased axon regeneration in astrocytes grown in the presence of proteoglycan synthesis inhibitors. J. Cell Sci. 108, 1307–1315. doi: 10.1242/jcs.108.3.1307 [DOI] [PubMed] [Google Scholar]
- Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P et al. (2013). Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J. Neurosci. 33, 13882–13887. doi: 10.1523/JNEUROSCI.2524-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647. doi: 10.1016/j.tins.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2018). Dissecting spinal cord regeneration. Nature 557, 343–350. doi: 10.1038/s41586-018-0068-4 [DOI] [PubMed] [Google Scholar]
- Stachura DL and Traver D (2011). Cellular dissection of zebrafish hematopoiesis. Methods Cell Biol. 101, 75–110. doi: 10.1016/B978-0-12-387036-0.00004-9 [DOI] [PubMed] [Google Scholar]
- Streeter KA, Sunshine MD, Brant JO, Sandoval AGW, Maden M and Fuller DD (2020). Molecular and histologic outcomes following spinal cord injury in spiny mice, Acomys cahirinus. J. Comp. Neurol. 528, 1535–1547. doi: 10.1002/cne.24836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Tian G-F, Peng W, Lou N, Libionka W, Han X and Nedergaard M (2006). Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 9, 260–267. doi: 10.1038/nn1623 [DOI] [PubMed] [Google Scholar]
- Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, Naruo M, Okabe H and Takatsuki K (1997). Role of neutrophils in spinal cord injury in the rat. Neuroscience 79, 1177–1182. doi: 10.1016/S0306-4522(97)00011-0 [DOI] [PubMed] [Google Scholar]
- Tazaki A, Tanaka EM and Fei J-F (2017). Salamander spinal cord regeneration: the ultimate positive control in vertebrate spinal cord regeneration. Dev. Biol. 432, 63–71. doi: 10.1016/j.ydbio.2017.09.034 [DOI] [PubMed] [Google Scholar]
- Tendolkar A and Mokalled MH (2026). Plasticity meets regeneration during innate spinal cord repair. Neural Regen. Res. 21, 1136–1137. doi: 10.4103/NRR.NRR-D-24-01197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereshina MB, Ermakova GV, Ivanova AS and Zaraisky AG (2014). Ras-dva1 small GTPase regulates telencephalon development in Xenopus laevis embryos by controlling Fgf8 and Agr signaling at the anterior border of the neural plate. Biol. Open 3, 192–203. doi: 10.1242/bio.20147401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen MM, Lauridsen H, Pedersen M, Orlowski D, Mikkelsen TW and Rasmussen MM (2019). A clinically relevant blunt spinal cord injury model in the regeneration competent axolotl (Ambystoma mexicanum) tail. Exp. Ther. Med. 17, 2322–2328. doi: 10.3892/etm.2019.7193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran AP, Warren PM and Silver J (2022). New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 387, 319–336. doi: 10.1007/s00441-021-03477-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarouchas TM, Wehner D, Cavone L, Munir T, Keatinge M, Lambertus M, Underhill A, Barrett T, Kassapis E, Ogryzko N et al. (2018). Dynamic control of proinflammatory cytokines Il-1beta and Tnf-alpha by macrophages in zebrafish spinal cord regeneration. Nat. Commun. 9, 4670. doi: 10.1038/s41467-018-07036-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsata V and Wehner D (2021). Know how to regrow-axon regeneration in the zebrafish spinal cord. Cells 10, 1404. doi: 10.3390/cells10061404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uderhardt S, Martins AJ, Tsang JS, Lämmermann T. and Germain RN. (2019). Resident macrophages cloak tissue microlesions to prevent neutrophil-driven inflammatory damage. Cell 177, 541–555.e17. doi: 10.1016/j.cell.2019.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino FM, Fagel DM, Ganat Y, Maragnoli ME, Ment LR, Ohkubo Y, Schwartz ML, Silbereis J and Smith KM (2007). Astroglial cells in development, regeneration, and repair. Neuroscientist 13, 173–185. doi: 10.1177/1073858406298336 [DOI] [PubMed] [Google Scholar]
- Vandestadt C, Vanwalleghem GC, Khabooshan MA, Douek AM, Castillo HA, Li M, Schulze K, Don E, Stamatis S-A, Ratnadiwakara M et al. (2021). RNA-induced inflammation and migration of precursor neurons initiates neuronal circuit regeneration in zebrafish. Dev. Cell 56, 2364–2380.e8. doi: 10.1016/j.devcel.2021.07.021 [DOI] [PubMed] [Google Scholar]
- Villani A, Benjaminsen J, Moritz C, Henke K, Hartmann J, Norlin N, Richter K, Schieber NL, Franke T, Schwab Y et al. (2019). Clearance by microglia depends on packaging of phagosomes into a unique cellular compartment. Dev. Cell 49, 77–88.e7. doi: 10.1016/j.devcel.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Walker SE, Sabin KZ, Gearhart MD, Yamamoto K and Echeverri K (2022). Regulation of stem cell identity by miR-200a during spinal cord regeneration. Development 149, dev200033. doi: 10.1242/dev.200033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S, Santos-Ferreira T and Echeverri K (2023). A reproducible spinal cord crush injury in the regeneration-permissive axolotl. Methods Mol. Biol. 2636, 237–246. doi: 10.1007/978-1-0716-3012-9_13 [DOI] [PubMed] [Google Scholar]
- Walker WJ, Underwood KL, Garrett PI, Lorbacher KB, Linch SM, Rynes TP, Sloop C and Mruk K (2025). Effects of age on the response to spinal cord injury: optimizing the larval zebrafish model. Dev. Biol. 526, 111–127. doi: 10.1016/j.ydbio.2025.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S and Shi Y-B (2021). Evolutionary divergence in tail regeneration between Xenopus laevis and Xenopus tropicalis. Cell Biosci. 11, 71. doi: 10.1186/s13578-021-00582-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR and Cao QL (2011). Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J. Neurosci. 31, 6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cao K, Sun X, Chen Y, Duan Z, Sun L, Guo L, Bai P, Sun D, Fan J et al. (2015). Macrophages in spinal cord injury: phenotypic and functional change from exposure to myelin debris. Glia 63, 635–651. doi: 10.1002/glia.22774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y and Sofroniew MV (2013). Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J. Neurosci. 33, 12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner D, Tsarouchas TM, Michael A, Haase C, Weidinger G, Reimer MM, Becker T and Becker CG (2017). Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish. Nat. Commun. 8, 126. doi: 10.1038/s41467-017-00143-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RE, Yin FQ and Jakeman LB (2008). TGF-alpha increases astrocyte invasion and promotes axonal growth into the lesion following spinal cord injury in mice. Exp. Neurol. 214, 10–24. doi: 10.1016/j.expneurol.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett CE, Zapata AG, Hopkins N and Steiner LA (1997). Expression of zebrafish rag genes during early development identifies the thymus. Dev. Biol. 182, 331–341. doi: 10.1006/dbio.1996.8446 [DOI] [PubMed] [Google Scholar]
- Williams MC, Patel JH, Kakebeen AD and Wills AE (2021). Nutrient availability contributes to a graded refractory period for regeneration in Xenopus tropicalis. Dev. Biol. 473, 59–70. doi: 10.1016/j.ydbio.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MM, Weinberg RA, Lees JA and Guen VJ (2020). Emerging mechanisms by which EMT programs control stemness. Trends Cancer 6, 775–780. doi: 10.1016/j.trecan.2020.03.011 [DOI] [PubMed] [Google Scholar]
- Wittamer V, Bertrand JY, Gutschow PW and Traver D (2011). Characterization of the mononuclear phagocyte system in zebrafish. Blood 117, 7126–7135. doi: 10.1182/blood-2010-11-321448 [DOI] [PubMed] [Google Scholar]
- Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR and Vignali DAA (2009). The development and function of regulatory T cells. Cell. Mol. Life Sci. 66, 2603–2622. doi: 10.1007/s00018-009-0026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu L, He S, Wu Y, Jin W, Yu T, Qu JY and Wen Z (2015). Temporal-spatial resolution fate mapping reveals distinct origins for embryonic and adult microglia in zebrafish. Dev. Cell 34, 632–641. doi: 10.1016/j.devcel.2015.08.018 [DOI] [PubMed] [Google Scholar]
- Yang T, Dai YJ, Chen G and Cui SS (2020). Dissecting the dual role of the glial scar and scar-forming astrocytes in spinal cord injury. Front. Cell Neurosci. 14, 78. doi: 10.3389/fncel.2020.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y-M and He C (2013). The glial scar in spinal cord injury and repair. Neurosci. Bull. 29, 421–435. doi: 10.1007/s12264-013-1358-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG and Barres BA (2012). Genomic analysis of reactive astrogliosis. J. Neurosci. 32, 6391–63410. doi: 10.1523/JNEUROSCI.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Morgan MJ, Chen K, Choksi S and Liu Z-G (2012). Induction of autophagy is essential for monocyte-macrophage differentiation. Blood 119, 2895–2905. doi: 10.1182/blood-2011-08-372383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B and Tuszynski MH (2023). Regulation of axonal regeneration after mammalian spinal cord injury. Nat. Rev. Mol. Cell Biol. 24, 396–413. doi: 10.1038/s41580-022-00562-y [DOI] [PubMed] [Google Scholar]
- Zhou L, McAdow AR, Yamada H, Burris B, Klatt Shaw D, Oonk K, Poss KD and Mokalled MH (2023). Progenitor-derived glia are required for spinal cord regeneration in zebrafish. Development 150, dev201162. doi: 10.1242/dev.201162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Soderblom C, Krishnan V, Ashbaugh J, Bethea JR and Lee JK (2015). Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiol. Dis. 74, 114–125. doi: 10.1016/j.nbd.2014.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottoli SJ, Bentley AP, Feiner DG, Hering JR, Prendergast BJ and Rieff HI (1994). Spinal cord regeneration in adult goldfish: implications for functional recovery in vertebrates. Prog. Brain Res. 103, 219–228. doi: 10.1016/S0079-6123(08)61138-3 [DOI] [PubMed] [Google Scholar]
- Zukor K, Belin S, Wang C, Keelan N, Wang X and He Z (2013). Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J. Neurosci. 33, 15350–15361. doi: 10.1523/JNEUROSCI.2510-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]


