Abstract
The patent introduces a class of agents that target amyloid aggregation, and subsequently, reduce amyloid β-oligomer neurotoxicity. The class encompasses sulfopropanoic acid derivatives and is represented by tramiprosate and its prodrug ALZ-801 (valiltramiprosate). Clinical trials showed that ALZ-801 oral tablet is well tolerated and showed superior pharmacokinetic properties in healthy volunteers and Alzheimer's disease (AD) patients. Results from phase II & III trials of ALZ-801 in early AD have provided evidence of efficacy and to adjudicate the role of amyloid β-oligomers in AD pathogenesis. The confirmatory APOLLOE4 phase III trial of ALZ-801 in APOE4/4 homozygotes with early AD has been initiated. If ALZ-801 is approved, it will be among the first oral disease-modifying drugs for AD.
Keywords: : Alzheimer's disease, Aβ-oligomerization, tramiprosate, valiltramiprosate ALZ-801
Plain language summary
Article highlights.
Background
Anti-amyloid antibodies show promise as disease-modifying treatments. Yet, they are associated with a risk of ARIA-E, requiring MRI monitoring, which is burdensome in the elderly population and could limit their utility in clinical practice.
Aβ42 oligomers are the key drivers of AD pathogenesis and increased concentration of Aβ42 oligomers correlates closely with the onset and progression of clinical symptoms.
An inverse correlation between cognitive impairment severity and the concentration of 3-SPA was identified in subjects having mild to moderate AD.
The potential new treatments
3-SPA and tramiprosate inhibit the aggregation of Aβ42 oligomers.
Discussion
Increasing 3-SPA CSF levels to above those found in AD subjects is expected to protect those subjects from further cognitive decline or reduce the rate of cognitive decline.
Conclusion
Compounds (formula I) that are metabolized to 3-SPA, and therefore increase 3-SPA CSF levels, are claimed to help subjects suffering from Alzheimer's disease, dementia, or cognitive decline.
1. Background
Alzheimer's disease (AD) is a progressive neurodegenerative condition associated primarily with aging. The escalating healthcare expenses linked to AD underscore the significant impact on society, evident in the growing number of affected individuals globally, surpassing 5.7 million in the USA [1] and reaching 43.8 million worldwide [2]. The gradual deterioration of memory, cognition, reasoning, judgment and orientation marks the clinical manifestation of AD. As the disease advances, it also impairs motor, sensory and linguistic abilities, ultimately resulting in a comprehensive decline of various cognitive functions. Although these cognitive losses develop gradually, they typically culminate in severe impairment, often leading to death within a span of 4–12 years.
Currently, approved medications for AD encompass cholinesterase inhibitors (donepezil, rivastigmine and galantamine) and the N-methyl-D-aspartate receptor antagonist (NMDA) memantine [3,4]. Both classes are symptomatic agents designed to address secondary neurotransmitter deficiencies observed in AD. However, neither class demonstrates efficacy beyond a 6-month treatment period in clinical trials, and there is no evidence indicating their impact on the underlying disease pathology. Emerging anti-amyloid antibodies, such as aducanumab and lecanemab, show promise as potential disease-modifying treatments when administered during the early stages of the disease [5,6]. Nevertheless, certain amyloid immunotherapies have been linked to a dose-dependent risk of amyloid-related imaging abnormalities with edema (ARIA-E), with a heightened risk reported in APOE4 carriers [5,6]. This poses a significant developmental challenge, as the two highest doses of aducanumab exhibit amyloid clearance and clinical benefit but are associated with an approximately 40% incidence of ARIA-E [6]. Even with a dose titration regimen, aducanumab still shows an approximately 35% incidence of ARIA-E in APOE4 carriers [7]. While ARIA-E may be asymptomatic or mildly symptomatic in most patients, some individuals experienced seizures or other serious adverse events. The risk of ARIA-E in AD patients necessitates magnetic resonance imaging (MRI) monitoring, which proves burdensome in the elderly population and could potentially limit the practical utility of these drugs in clinical settings.
Different forms of amyloid-beta (Aβ) can undergo oligomerization. Primarily, amyloid precursor protein (APP) is cleaved, producing different lengths of Aβ, mainly yielding 40 amino acid long amyloid-beta peptide (Aβ40). Because of brain injury or stress, the upregulated production of APP will result in elevated amounts of all forms of Aβ that could oligomerize and aggregate, if the Aβ clearance system is not adequately functioning. Longer forms of Aβ such as Aβ42/Aβ43 are more prone to oligomerization and aggregation. Importantly, soluble low molecular weight Aβ42 oligomers are now acknowledged as pivotal contributors to AD pathogenesis, and an elevated concentration of Aβ oligomers closely correlates with the initiation and progression of clinical symptoms [7]. These soluble Aβ oligomers have been demonstrated to induce synaptic damage, neuronal death, facilitate tau phosphorylation and contribute to tau pathology [8–12]. Significantly, individuals with APOE 4/4 genotype in AD have been found to bear a higher load of soluble amyloid oligomers [13], likely contributing to the earlier onset of the disease in this specific population. Compounds designed to target Aβ oligomers, such as aducanumab and tramiprosate, have exhibited clinical benefits in AD patients with positive amyloid status.
2. New potential treatments
Tramiprosate, also known as 3-amino-1-propanesulfonic acid (3-APS), is an oral agent designed to counteract amyloid aggregation and mitigate the neurotoxicity of Aβ-oligomers. phase III trials of tramiprosate in individuals with mild-to-moderate AD demonstrated a favorable drug profile. Notably, the trials revealed the drug's ability to impede the reduction of brain hippocampal volume and to enhance cognitive function, as well as overall brain function, in subset analyses [14–16].
Crucially, ALZ-801 (valiltramiprosate) is currently undergoing clinical development as an oral, small-molecule inhibitor targeting the formation of Aβ oligomers for the treatment of AD. ALZ-801 represents a valine conjugate of tramiprosate, characterized by improved pharmacokinetic properties and gastrointestinal tolerability [17]. The active component of ALZ-801, tramiprosate, demonstrates inhibition of Aβ oligomer formation both in vitro and in vivo [18]. Oral tramiprosate underwent evaluation in two phase III studies, encompassing 2,015 patients with mild to moderate AD, treated with either 100 mg BID of tramiprosate, 150 mg BID of tramiprosate, or a placebo. Safety data from these phase III trials, along with findings from the safety extension study, indicate a favorable safety profile for tramiprosate overexposures of up to 2.5 years [19]. In a subgroup analysis focusing on subjects carrying the ε4 allele of apolipoprotein E (APOE4), there was a positive and clinically meaningful cognitive benefit. The biotransformation of ALZ-801 to tramiprosate is illustrated in Figure 1. Notably, a metabolite of tramiprosate, 3-sulfopropanoic acid (3-SPA), has been identified in the cerebrospinal fluid (CSF) and plasma of drug-naive individuals. This metabolite has been shown to effectively inhibit the aggregation of Aβ42, demonstrating efficacy comparable to that of tramiprosate itself.
Figure 1.
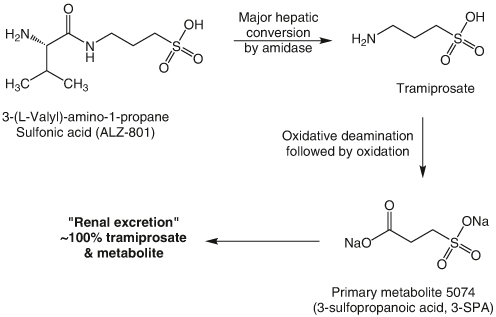
The metabolism of ALZ-801 to 3-sulfopropanoic acid (3-SPA) and their renal excretion.
Notably, a noteworthy finding emerged as an inverse correlation between the severity of cognitive impairment and the concentration of 3-sulfopropanoic acid (3-SPA) was observed in individuals with mild to moderate AD. This suggests that the level of 3-SPA decreases as cognitive impairment becomes more severe, and maintaining higher levels of 3-SPA may have a role in preventing or mitigating the cognitive decline associated with AD. The assessment of cognitive decline was measured through a subject's Mini-Mental State Examination (MMSE) score, a well-established method for determining the severity of AD in the individual [20].
Indeed, individuals with AD who exhibited higher MMSE scores, indicative of less cognitive impairment, displayed elevated levels of 3-SPA in the CSF compared with those with lower MMSE scores. Consequently, a correlation was established between MMSE scores and 3-SPA concentrations in the CSF among subjects within the test population experiencing mild to moderate AD. Based on these findings, the hypothesis emerged that raising CSF levels of 3-SPA above those found in AD subjects with minimal cognitive impairment and maintaining such heightened levels could serve to protect individuals from further cognitive decline or reduce the rate of cognitive decline compared with placebo treatment. The patent (WO 2020/028348) asserts the claim for compounds designed to metabolize into 3-SPA, thereby increasing CSF levels of 3-SPA for individuals in need of protection, such as those with AD, dementia, or cognitive decline. The described compounds encompass those with formula I, as depicted in Figure 2, or a pharmaceutically acceptable salt thereof [21].
Figure 2.

Representative chemical structures of formula I claimed in the patent.
The patent also asserts claims covering methods for utilizing the disclosed compounds to address AD in individuals with a 3-SPA concentration below a specified baseline threshold level. This threshold is determined as being below the 3-SPA CSF concentration (±10%) value established for an MMSE score of 30 in the best fit of a random population of subjects with AD exhibiting varying degrees of cognitive impairment. Additionally, the patent outlines methods for treating specific AD subjects categorized by different severities of cognitive impairment. Furthermore, the patent encompasses claims related to methods for preventing dementia or halting further cognitive decline in individuals whose 3-SPA concentration falls below a particular baseline threshold level. This threshold is determined as being below the 3-SPA CSF concentration (±10%) value established for an MMSE score of 30 in the best fit of a random population of subjects experiencing cognitive decline.
As examples, the patent describes human CSF samples collection and processing, identification and quantitation of 3-SPA in human CSF by LC-MS/MS, 3-SPA molecular modeling and molecular dynamics simulations, 3-SPA IMS MS binding study, pharmacokinetics, oral absorption and brain exposure of 3-SPA in Sprasue-Dawley rats, and chemical preparation of compounds of formula I, among others.
3. Discussion
The identification of 3-SPA in the CSF of drug-naive individuals marked a significant discovery. The average concentration of 3-SPA from the study was determined to be 11.7 ± 4.3 nM. Demonstrating an anti-Aβ42 oligomeric effect, 3-SPA exhibited both time- and concentration-dependent responses. Furthermore, it was established that 3-SPA boasts a 100% oral bioavailability and a 25% capacity for brain penetration, affirming its effective absorption and ability to traverse the blood-brain barrier. These findings collectively indicate that the heightened CSF concentrations observed in the human brain after the oral administration of ALZ-801 or tramiprosate stem from the penetration of the metabolite 3-SPA into the CNS. An intriguing revelation emerged from an inverse correlation between the concentration of 3-SPA in CSF and the severity of cognitive impairment. Specifically, as the severity of AD decreases, higher concentrations of 3-SPA are detected in CSF. Conversely, as the severity of AD increases, lower concentrations of 3-SPA were observed in CSF. This data strongly suggests that the levels of 3-SPA in the brain play a pivotal role in reducing the likelihood of or delaying the onset of disease progression [21].
Based on these findings, a hypothesis was formulated, suggesting that elevated CSF levels of 3-SPA could offer therapeutic advantages to individuals grappling with AD. The conjecture also proposed that such an increase might confer additional benefits to AD subjects with minimal cognitive impairment and maintaining these heightened levels could shield them from further cognitive decline or mitigate the rate of cognitive deterioration compared with placebo treatment. Achieving an increase in CSF levels of 3-SPA above the established baseline threshold can be accomplished by administering a compound of formula I, as detailed in the patent. This novel therapeutic approach presents avenues for mitigating Aβ42 oligomer neurotoxicity and offers clinical strategies for addressing cognitive disorders such as AD.
In light of the aforementioned results, ALZ-801 was developed as an oral agent with efficient penetration of the blood-brain barrier. This compound selectively interacts with Aβ monomers, inhibiting misfolding and dose-dependently blocking the formation of neurotoxic soluble Aβ42 oligomers. Importantly, this action occurs without affecting insoluble amyloid plaques or fibrils [15–18]. Thus, ALZ-801 represents a prodrug of tramiprosate, exhibiting enhanced gastrointestinal absorption and tolerability. Extensive testing in over 130 elderly volunteers and AD patients demonstrated excellent brain penetration of approximately 40%, with low intersubject variability [22]. Phase 1 trials confirmed that an oral dose of 265 mg ALZ-801, administered twice daily, provides plasma exposure equivalent to oral tramiprosate 150 mg twice daily from the tramiprosate phase III trials [17].
The ongoing phase III trials for ALZ-801 are grounded in a substantial body of clinical data in AD patients, including a favorable safety profile derived from the tramiprosate safety database, encompassing over 2000 AD patients treated for 18 months. The clinical trials also rely on a well-defined target clinical dose established to fully inhibit the formation of Aβ oligomers in the brain, supported by data from the phase III AD trials and an in vitro Aβ oligomer inhibition assay [23]. Employing a precision medicine approach, the trials initially focus on high-risk homozygous APOE4/4 patients with early AD [1]. The oral administration of ALZ-801 offers a convenient at-home dosing option for elderly patients and their caregivers, presenting potential for preventive treatment in presymptomatic individuals at high risk for AD.
It is worth noting that studies have demonstrated that other markers correlate better than amyloid deposition with the cognitive and behavioral manifestations of Alzheimer's patients. Therefore, an argument against the clinical effect of tramiprosate-based therapeutics attributed to their anti-Aβ42 oligomeric effects can be suggested. Tramiprosate appears to be a multimodal agent that acts via different mechanisms including the reduction of amyloid deposition, the improvement of cholinergic transmission and the anti-inflammatory effect. The anti-inflammatory effect can be explained by considering GABAA receptors as plausible molecular targets and mediators for the actions of taurine and homotaurine (and potentially their derivatives) [24–26].
4. Conclusion
Significant clinical and biomarker information from advanced anti-amyloid initiatives indicates a hopeful phase in AD drug development and offers convincing proof of the significant involvement of neurotoxic soluble amyloid oligomers in AD pathogenesis, highlighting them as potential therapeutic targets. In this context, ALZ-801 stands out with its unique mechanism of action, oral delivery method and potential effectiveness in a genetically targeted group, setting it apart from plaque-clearing antibodies. Importantly, treatment with ALZ-801/tramiprosate is not associated with any events of vasogenic brain edema on MRI imaging, consistent with the lack of interaction with insoluble fibrillar and plaque amyloid and clearance of amyloid plaques [27]. In fact, in a completed 2-year phase II biomarker trial, ALZ-801 treatment lowered plasma p-tau181, reduced the rate of hippocampal atrophy, and stabilized clinical AD progression in APOE4 carriers with early AD and high levels of amyloid and tau pathology (Table 1) [28]. Overall, although some of the early studies have some limitations that could interfere with the clinical outcomes and study conclusions, mainly suboptimal study design such as lack or inadequate biomarkers used and small sample size [29], the approval of ALZ-801 could position it as one of the initial oral disease-modifying treatments for AD.
Table 1. Comparison of anti-amyloid therapies.
| Treatment | Status | Plasma P-tau181 (reduction annualized) | Clinical dementia rating- sum of boxes (CDR-SB) % clinical benefit | Ref. |
|---|---|---|---|---|
|
ALZ-801 265 mg oral Twice a day |
Under testing | Approximately 40% | phase III ongoing | |
|
Aducanumab (Aduhelm) 10 mg/kg IV Once a month |
Approved (7 June 2021) treatment of AD in patients with mild cognitive impairment or mild dementia stage of disease | Approximately 10% | Approximately 22% | [30] |
|
Lecanemab (Leqembi) 10 mg/kg IV Twice a month |
Approved (6 January 2023) treatment of AD in patients with mild cognitive impairment or mild dementia stage of disease | Approximately 19% | Approximately 27% | [31] |
|
Gantenerumab 1200 mg SC Every 4 weeks |
Discontinued | Approximately 3% | Approximately 7% |
5. Future perspective
Alzheimer's disease is the most common cause of dementia. Current therapeutics for Alzheimer's disease may help control the symptoms, but they do not prevent or slow cognitive decline. Thus far, no resolution has been established for this problem. Sulfopropanoic acid derivatives such as tramiprosate and its prodrug ALZ-801 provide a novel platform for the development of effective and safe therapeutics to slow cognitive decline in Alzheimer's patients. Molecules in this class appear to inhibit the aggregation of Aβ42 oligomers. These oligomers appear to be the key drivers of AD pathogenesis. Not only that but increased concentration of Aβ42 oligomers also correlates closely with the onset and progression of clinical symptoms. Furthermore, an inverse correlation between cognitive impairment severity and the concentration of 3-SPA was identified in subjects having mild to moderate AD. For better outcomes, more detailed molecular studies are warranted to highlight other potential mechanisms such as the inhibition of neuroinflammation [32–34]. Reliable assays to test sufopropionc acid derivatives for the prevention of Aβ aggregation are also important to develop and use [35]. Last, testing of anti-Aβ42 oligomeric molecules can be performed in combination with one or more of the currently approved drugs, in other words, aducanumab, donepezil, galantamine, memantine, or rivastigmine.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.WWW.Alz.org [internet]. Alzheimer's Association . New Alzheimer's association report reveals sharp increases in Alzheimer's prevalence, deaths, cost of care. [cited 2018 May 30]. Available from: https://www.alz.org/news/2018/new_alzheimer_s_association_report_reveals_sharp_i#:~:text=An%20estimated%205.7%20million%20Americans,and%20older%20affected%20in%202018
- 2.GBD 2016 Dementia Collaborators . Global, regional, and national burden of Alzheimer's disease and other dementias, 1990 – 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106. doi: 10.1016/S1474-4422(18)30403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Horani RA. Steroid sulfatase inhibitors and sulfated C19 steroids for proteotoxicity-related diseases: a patent spotlight. Pharm Pat Anal. 2023;12(5):213–218. doi: 10.4155/ppa-2023-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Horani RA. Riluzole and its prodrugs for the treatment of Alzheimer's disease. Pharm Pat Anal. 2023;12(2):79–85. doi: 10.4155/ppa-2023-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salloway S, Sperling R, Fox NC, et al. Two phase III trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370(4):322–333. doi: 10.1056/NEJMoa1304839 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Important phase III trials of bapineuzumab in mild-to-moderate Alzheimer's disease.
- 6.Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;537(7618):50–56. doi: 10.1038/nature19323 [DOI] [PubMed] [Google Scholar]
- 7.Hampel H, O'Bryant SE, Castrillo JI, et al. PRECISION MEDICINE - the golden gate for detection, treatment and prevention of Alzheimer's disease. J Prev Alzheimers Dis. 2016;3(4):243–259. doi: 10.14283/jpad.2016.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esparza TJ, Zhao H, Cirrito JR, et al. Amyloid-β oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol. 2013;73(1):104–119. doi: 10.1002/ana.23748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto T, Serrano-Pozo A, Hori Y, et al. Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid β peptide. J Neurosci. 2012;32(43):15181–15192. doi: 10.1523/JNEUROSCI.1542-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ono K, Yamada M. Low-n oligomers as therapeutic targets of Alzheimer's disease. J Neurochem. 2011;117(1):19–28. doi: 10.1111/j.1471-4159.2011.07187.x [DOI] [PubMed] [Google Scholar]
- 11.Townsend M, Shankar GM, Mehta T, et al. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572(Pt 2):477–492. doi: 10.1113/jphysiol.2005.103754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95(11):6448–6453. doi: 10.1073/pnas.95.11.6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usui K, Hulleman JD, Paulsson JF, Siegel SJ, Powers ET, Kelly JW. Site-specific modification of Alzheimer's peptides by cholesterol oxidation products enhances aggregation energetics and neurotoxicity. Proc Natl Acad Sci USA. 2009;106(44):18563–18568. doi: 10.1073/pnas.0804758106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauthier S, Aisen PS, Ferris SH, et al. Effect of tramiprosate in patients with mild-to-moderate Alzheimer's disease: exploratory analyses of the MRI sub-group of the Alphase study. J Nutr Health Aging. 2009;13(6):550–557. doi: 10.1007/s12603-009-0106-x [DOI] [PubMed] [Google Scholar]
- 15.Saumier D, Duong A, Haine D, Garceau D, Sampalis J. Domain-specific cognitive effects of tramiprosate in patients with mild to moderate Alzheimer's disease: ADAS-cog subscale results from the Alphase Study. J Nutr Health Aging. 2009;13(9):808–812. doi: 10.1007/s12603-009-0217-4 [DOI] [PubMed] [Google Scholar]
- 16.Aisen PS, Gauthier S, Ferris SH, et al. Tramiprosate in mild-to-moderate Alzheimer's disease - a randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study). Arch Med Sci. 2011;7(1):102–111. doi: 10.5114/aoms.2011.20612 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The Alphase study of tramiprosate in mild-to-moderate Alzheimer's disease.
- 17.Hey JA, Yu JY, Versavel M, et al. Clinical pharmacokinetics and safety of ALZ-801, a novel prodrug of tramiprosate in development for the treatment of Alzheimer's disease. Clin Pharmacokinet. 2018;57(3):315–333. doi: 10.1007/s40262-017-0608-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Important study of clinical pharmacokinetics and safety of ALZ-801.
- 18.Kocis P, Tolar M, Yu J, et al. Elucidating the Aβ42 anti-aggregation mechanism of action of tramiprosate in Alzheimer's disease: integrating molecular analytical methods, pharmacokinetic and clinical data. CNS Drugs. 2017;31(6):495–509. doi: 10.1007/s40263-017-0434-z [DOI] [PMC free article] [PubMed] [Google Scholar]; • An important study for the mechanism of action of tramiprosate in Alzheimer's disease.
- 19.Abushakra S, Porsteinsson A, Scheltens P, et al. Clinical effects of tramiprosate in APOE4/4 homozygous patients with mild Alzheimer's disease suggest disease modification potential. J Prev Alzheimers Dis. 2017;4(3):149–156. doi: 10.14283/jpad.2017.26 [DOI] [PubMed] [Google Scholar]; • Important randomized, double-blind, placebo-controlled parallel-arm multi-center studies to determine the optimal stage of AD for future trials in APOE4/4 homozygotes.
- 20.Pangman VC, Sloan J, Guse L. An examination of psychometric properties of the mini-mental state examination and the standardized mini-mental state examination: implications for clinical practice. Appl Nurs Res. 2000;13(4):209–213. doi: 10.1053/apnr.2000.9231 [DOI] [PubMed] [Google Scholar]
- 21.Kocis P, Hey J, Tolar M. Sulfopropanoic acid derivatives for treating neurodegenerative disorders. WO 2020/028348 Al. Published: June 2, 2020. Available from: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020028348 [Google Scholar]
- 22.Tolar M, Hey J, Power A, et al. Neurotoxic soluble amyloid oligomers drive Alzheimer's pathogenesis and represent a clinically validated target for slowing disease progression. Int J Mol Sci. 2021;22(12):6355. doi: 10.3390/ijms22126355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alzheon.com [internet]. Alzheon, Inc.; [cited 2023 Dec 26]. Available from: https://alzheon.com/clinical-data/ [Google Scholar]
- 24.Meera P, Uusi-Oukari M, Lipshutz GS, et al. GABAA receptors as plausible molecular targets and mediators for taurine and homotaurine actions. Front Pharmacol. 2023;14:1271203. doi: 10.3389/fphar.2023.1271203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358–372. doi: 10.1038/nrn3880 [DOI] [PubMed] [Google Scholar]
- 26.Jorfi M, Maaser-Hecker A, Tanzi RE. The neuroimmune axis of Alzheimer's disease. Genome Med. 2023;15(1):6. doi: 10.1186/s13073-023-01155-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gervais F, Paquette J, Morissette C, et al. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging 2007;28(4):537–547. doi: 10.1016/j.neurobiolaging.2006.02.015 [DOI] [PubMed] [Google Scholar]; • Animal studies show that tramiprosate results in a significant reduction in the brain amyloid plaque load and a significant decrease in the cerebral levels of Aβ-40 and Aβ-42.
- 28.Alzheon.com [internet]. Alzheon, Inc.; [cited 2023 Dec 26]. Available from: https://alzheon.com/pipeline/alz-801-development/ [Google Scholar]
- 29.Manzano S, Agüera L, Aguilar M, Olazarán J. A review on tramiprosate (Homotaurine) in Alzheimer's disease and other neurocognitive disorders. Front Neurol. 2020;11:614. doi: 10.3389/fneur.2020.00614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase III studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197–210. doi: 10.14283/jpad.2022.30 [DOI] [PubMed] [Google Scholar]; •• Phase III trial shows results of aducanumab use in early Alzheimer's disease.
- 31.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9–21. doi: 10.1056/NEJMoa2212948 [DOI] [PubMed] [Google Scholar]; •• Phase III trial shows that lecanemab reduced markers of amyloid in early Alzheimer's disease and resulted in moderately less decline in measures of cognition.
- 32.McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126(4):479–497. doi: 10.1007/s00401-013-1177-7 [DOI] [PubMed] [Google Scholar]
- 33.Tian J, Dang H, Wallner M, Olsen R, Kaufman DL. Homotaurine, a safe blood-brain barrier permeable GABAA-R-specific agonist, ameliorates disease in mouse models of multiple sclerosis. Sci Rep. 2018;8(1):16555. doi: 10.1038/s41598-018-32733-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian J, Kaufman DL. The GABA and GABA-receptor system in inflammation, anti-tumor immune responses, and COVID-19. Biomedicines. 2023;11(2):254. doi: 10.3390/biomedicines11020254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang A, Portugal Barron D, Chen EW, Guo Z. A protein aggregation platform that distinguishes oligomers from amyloid fibrils. Analyst. 2023;148(10):2283–2294. doi: 10.1039/d3an00487b [DOI] [PMC free article] [PubMed] [Google Scholar]


