Abstract
Background
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality globally, with bone metastases signifying advanced disease and a poor prognosis. Although bone metastases in HCC are relatively uncommon, they present significant diagnostic and therapeutic challenges. We present a rare case of cervical spine metastasis as a presenting feature of HCC progression, highlighting the need for timely diagnosis and multidisciplinary care.
Case presentation
A 55-year-old North African female with a history of hepatitis C-related cirrhosis, successfully treated with antiviral therapy, was diagnosed with HCC following the detection of hepatic nodules during routine biannual surveillance. After unsuccessful radiofrequency ablation (RFA), she developed intense cervical pain. Imaging revealed osteolytic cervical lesions and a paravertebral mass. Biopsy confirmed metastatic HCC. She received radiotherapy and immunotherapy (atezolizumab-bevacizumab). Despite treatment, her condition deteriorated due to cranial bone metastases, leading to intracranial hypertension and death.
Conclusions
Cervical spine metastasis in HCC is rare and carries a poor prognosis. Early clinical suspicion, advanced imaging, and timely intervention are crucial. Limitations in current treatments emphasize the need for improved therapeutic strategies. A deeper understanding of the molecular mechanisms and oncogenes involved in HCC bone metastases can reveal potential therapeutic pathways for treatment.
Keywords: Hepatocellular carcinoma, cirrhosis, bone metastasis, immunotherapy, radiotherapy, case report
ARTICLE HIGHLIGHTS
HCC accounts for 90% of primary liver cancers and is a leading cause of cancer mortality globally.
Bone metastases occur in up to 37% of metastatic HCC cases, predominantly involving the vertebral column; cervical spine metastases are rare.
We present a case of hepatitis C-related cirrhosis complicated by HCC with cervical spine (C1–C2) metastases confirmed by MRI, bone scintigraphy, and biopsy.
Histopathology revealed macrotrabecular and solid growth patterns associated with aggressive tumor biology and poor prognosis.
Despite locoregional radiofrequency ablation and systemic immunotherapy with atezolizumab-bevacizumab, rapid progression to cranial bone metastases and intracranial hypertension occurred.
Early detection of atypical osseous metastases in HCC is essential for timely multidisciplinary intervention.
External beam radiotherapy remains pivotal for palliation of bone pain and prevention of neurological sequelae in spinal metastases.
Overall prognosis for HCC patients with bone metastases remains dismal, underscoring the need for novel targeted therapeutic strategies.
Comprehensive management requires integration of hepatology, oncology, radiology, and supportive care to optimize outcomes
1. Introduction
HCC accounts for 90% of tumors originating from the liver [1]. The main risk factors for HCC are cirrhosis, and chronic hepatitis B and C [2].
It is the fifth most common malignancy and despite the considerable advancement that has occurred, the overall outcomes of HCC are still far from satisfactory as it represents the second leading cause of cancer-related mortality worldwide [3,4]. The pathogenesis of HCC involves chronic hepatic inflammation, oxidative stress, and repeated cycles of cell injury and regeneration, ultimately leading to genetic and epigenetic alterations [5]. HCC often presents at an advanced stage, the metastatic rate of HCC exceeds 50%, even in small HCC [6] and is a major factor contributing to treatment failure and patient mortality. The lungs are the most common site of extrahepatic metastases (47%), followed by the lymph nodes (45%) and bones (37%) [7]. Bone metastasis often indicates an advanced stage of HCC patients; cervical spine involvement is extremely rare and may cause debilitating pain or neurological compromise [8].
Management of HCC depends on tumor burden, liver function, and performance status, with curative options (resection, transplantation, ablation) reserved for early-stage disease. In advanced cases, systemic therapy—including tyrosine kinase inhibitors and immunotherapy—has become the cornerstone of treatment [9].
We report a unique case of cervical spine metastasis in a patient with hepatitis C-related cirrhosis and HCC, aiming to underscore the importance of early recognition of atypical presentations and the role of a multidisciplinary approach in managing metastatic disease.
2. Case report
A 55-year-old North African female patient, a homemaker with no significant family history of liver disease but a personal history of Hepatitis C-related compensated liver cirrhosis treated with an association of sofosbuvir (400 mg/d) and ledipasvir (90 mg/d) during 24 weeks in 2019 and achieving sustained virological response, was admitted in August 2021 in the Gastroenterology ‘B’ department of La Rabta hospital to investigate suspicious hepatic nodules revealed incidentally on abdominal ultrasound during bi-annual monitoring for HCC screening.
Her initial physical examination revealed a good general health status (ECOG-PS 0), anicteric sclera, and normal blood pressure. She had no clinical ascites or abdominal tenderness. Additionally, no peripheral lymph nodes or organomegaly were observed.
Laboratory investigations revealed inflammatory biologic syndrome with elevated CRP level of 197 mg/L, and thrombopenia of 85000/mm³ but with normal white blood cell count and hemoglobin level. The prothrombin time (PT) was within normal limits at 76%, with an international normalized ratio (INR) of 1.1. Liver enzyme levels were within normal limits. Alpha-fetoprotein level (AFP) was 124 IU/l(4-fold the upper limit of normal (ULN). The CA19-9 level was normal. Screening for esophageal and gastric varices on upper endoscopy was negative.
At the end of this clinical and biological investigation, the cirrhosis was classified as Child-Pugh A6, and the MELD score was 8.
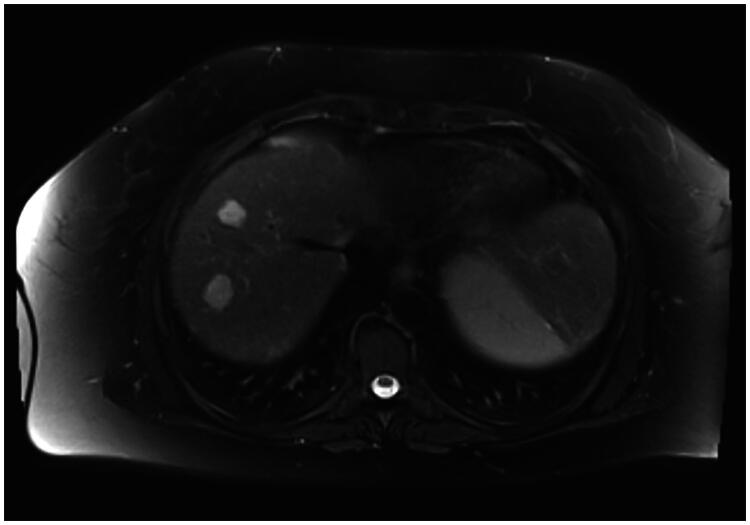
Hepatic MRI revealed two hepatic nodules between segments V and VI. The first nodule measured 12 mm and was classified as LI-RADS V, while the second one measured 8 mm and was classified as LI-RADS IV (Figure 1).
Figure 1.
Hepatic MRI showing two hepatic nodules between segments V and VI classified as LI-RADS V and LI-RADS IV.
A Thoracic CT scan was performed excluding any secondary localization of HCC.
The HCC was classified as BCLC (Barcelona Clinic Liver Cancer) stage A at this stage.
The patient underwent two sessions of radiofrequency ablation for HCC nodules, spaced two months apart—the first during the first week of October and the second during the first week of December. At 6 weeks follow-up appointment, hepatic MRI revealed an increase in tumor size, with the nodules measuring 18 x 12 mm and 25 x 20 mm, respectively. Consequently, the HCC was classified as LR-TR viable.
At the same time, the patient presented with severe, inflammatory-type pain in the cervical spine, with no neurological signs, requiring the use of step 2 analgesics.
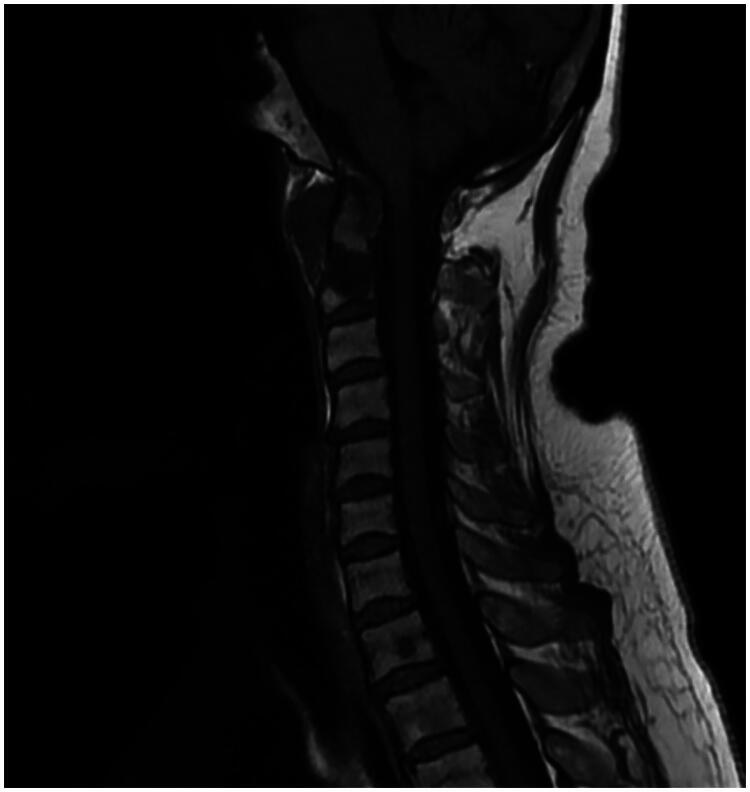
A cervical spine MRI was conducted revealing a lytic lesion of the spine, centered on C1 and C2, the spinous process of D4, and the body of L3. Furthermore, it revealed a soft-tissue mass in the left paravertebral space adjacent to the atlantoaxial joint, extending anteriorly to the retropharyngeal space. The lesion invades the atlas internally (Figure 2).
Figure 2.
Cervical spine MRI revealing a lytic lesion of the spine, centered on C1 and C2 with a soft-tissue mass in the left paravertebral space.
Bone scintigraphy confirmed the same findings (Figure 3).
Figure 3.
Bone scintigraphy showing hyperfixation at the level of the posterior arch of the 9th rib, the L3 vertebral body, the trochanteric region of the right femur, and the right temporal bone suggestive of secondary nature.
Given the atypical location of the metastasis, a CT-guided biopsy of the paravertebral mass was conducted, revealing an hepatoid tumor proliferation with a macrotrabecular and solid growth pattern confirming the diagnosis of metastasis of HCC (Figure 4(a–c)).
Figure 4.
(a) Hepatoid tumor proliferation(H&Ex40). (b) Tumor proliferation with a macrotrabecular and solid growth pattern (H&Ex100). (c) The tumor cells are large, featuring eosinophilic cytoplasm and irregular, hyperchromatic nuclei containing nucleoli (H&Ex400).
The patient was referred to the oncology department for radiotherapy sessions targeting the cervical spine osseous metastases. Additionally, immunotherapy with atezolizumab and bevacizumab was initiated.
Unfortunately, 8 weeks after the diagnosis of metastasis, the patient’s condition worsened, and she developed intracranial hypertension syndrome due to osseous metastases in the cranial vault, including intense headache, nausea, sudden-onset bilateral deafness and swallowing difficulties. Given the rapid clinical decline and the extent of metastatic disease, further curative options were no longer feasible. The patient’s performance status worsened to ECOG 4, and despite best supportive care, she passed away shortly thereafter due to complications of intracranial hypertension.
3. Discussion
All over the world, HCC is the fifth most common malignancy, and despite significant advancements, its overall outcomes remain unsatisfactory, making it the second leading cause of cancer-related mortality worldwide [3,4]. The metastatic rate of HCC is still high and represents over 50% of the cases [6].
According to a recent Chinese study, between 16.1% and 38.5% of HCC patients are diagnosed with bone metastasis at the time of their initial diagnosis, and 11.7% of those who undergo curative resections subsequently develop bone metastasis [10]. The spine is the most common site of bone metastasis in HCC, occurring in approximately 50% to 75% of patients with bone metastases [11,12].
Clinically, vertebral metastases of HCC are manifested by bone pain and in other cases by neurological signs [13].
Through our case report, we emphasize the importance of a careful clinical examination in search of clinical signs suggestive of vertebral metastases.
Radiographically, typical bone metastases from HCC appear as expansive, destructive lesions with large soft-tissue masses as was observed in our patient. Most bone metastases are osteolytic and detectable using a computed tomography scan. However, osteoblastic lesions are rare in HCC patients. Soft-tissue masses, specific to HCC bone metastasis, are observed in 39%-85.4% of cases [7,8, 14,15]. These masses often replace the normal bone matrix and grow expansively. They can compress peripheric nerves, the spinal cord, or cranial nerves. Spinal metastases can cause spinal cord compression, resulting in paralysis, while skull base metastases can lead to neurological symptoms due to cranial nerve involvement [13].
The pathogenic mechanism of spinal metastasis, as in the case of our patient, may involve several pathways: 1/Presence of pulmonary and brain metastases suggesting arterial dissemination; 2/metastasis through the vertebral venous plexus, facilitating retrograde transport to the spinal cord from the pelvis to cranial sinuses; 3/direct invasion from nearby structures; and 4/intraspinal spread [16]. Our patient subsequently developed brain metastases suggesting hematogenous extension.
Various studies have identified potential biomarkers in serum, urine, and tumor tissue as well as gene expression profiles and Histological subtypes that strongly predict HCC bone metastasis and determine prognosis in HCC patients [17,18].
For instance, solid and macrotrabecular growth patterns in HCC—as observed in our patient—are associated with poorer prognosis, including reduced overall survival, earlier recurrence, and a higher risk of metastases [19].
Another example is long noncoding RNA H19 which plays an important role in HCC bone metastasis by inhibiting p38 phosphorylation, and reducing osteoprotegerin levels, which normally inhibit osteoclastogenesis [20].
Other pathways were suggested such as overexpression of LGALS3 a lectin galactoside-binding protein secreted by HCC enhances the ability of HCC cells to metastasize to bone [10].
Due to its low incidence, practice patterns and optimal treatment strategies for HCC with bone metastases are not well-established. Patients with bone metastases should be evaluated for systemic therapy or bone-directed therapies, including local radiation, especially for localized disease.
Currently, the combination of atezolizumab with bevacizumab is the preferred first-line treatment for metastatic HCC, due to its demonstrated superior survival benefit over sorafenib. To receive atezolizumab-bevacizumab therapy, patients should have preserved liver function (Child-Pugh A) and no high-risk bleeding signs on upper endoscopy [9].
The benefit of bisphosphonates in bone metastasis of HCC has also been suggested. when embedded in bone structure, bisphosphonates inhibit the formation of osteolytic lesions by promoting apoptosis in osteoclasts and, to a lesser extent, cancer cells. While effective in osteotropic cancers like breast, prostate, and multiple myeloma, their use in HCC bone metastases is limited to a few case reports and retrospective studies [16].
Radiation therapy (RT) is essential for managing bone pain in HCC patients, as well as spinal cord compression. Various RT schedules, such as 30 Gy in 10 fractions, 20 Gy in 5 fractions, and 8 Gy in a single fraction, show similar response rates and late toxicity, though single-fraction treatments often require retreatment. Personalized RT approaches are recommended, with aggressive treatments for patients with higher survival potential and well-controlled liver disease, using a total dose of 48 Gy in 2 Gy fractions for better outcomes and fewer retreatments [16].
Despite the current therapeutic options, the prognosis for HCC patients with bone metastasis remains dire. According to a study by Seong et al., the median survival following the onset of bone metastasis from HCC was 5 months, with a one-year survival rate of 15% [12]. Furthermore, a majority of patients experience skeletal-related events such as pathological fractures and spinal cord compression resulting in severe pain and neurological complications as in the case of our patient. Significant negative prognostic factors for patients with bone metastases include poor performance status, multifocal metastases, tumor stage IV-A, involvement of other organs, elevated tumor markers, uncontrolled intrahepatic tumors, and the presence of ascites at initial presentation.
Several limitations were identified in the management of this case. First, the diagnosis of bone metastases was made at an advanced stage, delaying the initiation of specific local and systemic treatments. This highlights the importance of early and systematic imaging, especially in patients presenting with unexplained bone pain, even in uncommon locations.
Second, the selected treatment—combining radiotherapy for local control and systemic immunotherapy with atezolizumab and bevacizumab—has demonstrated improved survival in advanced HCC, yet it remains palliative in nature. These agents may have limited efficacy once extensive metastases are established, and access to bone-modifying agents like bisphosphonates was lacking, which could have helped reduce skeletal-related events.
Possible causes of death in this patient include the rapid progression of metastatic disease, delayed diagnosis, and the development of intracranial hypertension due to cranial bone metastases.
A deeper understanding of the molecular mechanisms and oncogenes involved in HCC bone metastases can reveal potential therapeutic pathways for future targeted treatments.
4. Conclusion
To summarize, bone metastases in HCC are a rare but significant challenge due to their destructive nature and complex management needs. This case underscores the importance of early recognition of atypical symptoms such as spinal pain, prompt use of advanced imaging, and timely histological confirmation to guide treatment. Delayed diagnosis and limited treatment efficacy likely contributed to the unfavorable outcome.
A deeper understanding of the molecular pathways and oncogenic drivers of HCC bone metastases could open new avenues for targeted therapies and improve outcomes for these patients. A multidisciplinary approach remains essential to optimize both survival and quality of life in such complex cases.
Funding Statement
This paper was not funded.
Authors’ contributions
Salma Merhaben: drafting the manuscript. Haythem Yacoub, Hajer Hassine, Habiba Debbabi, Dhouha Cherif, Aida Khadhar, Aziz Abdelghani: critical revision of the manuscript for important intellectual content. Slim Haouet, Hela Kchir, Nadia Maamouri: final approval of the manuscript.
Availability of supporting data
All data supporting the findings of this study are included in this published article.
Consent for publication
Written informed consent could not be obtained as the patient was deceased at the time of writing this case report. Efforts were made to ensure the case was anonymized, and no identifiable information has been included.
Disclosure statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
* of Interest
** of considerable interest (It provides a serious analysis and reliable information. It was very helpful especially in the discussion part)
- 1.Kawasaki M, Shioya A, Takata M, et al. A case of bone metastasis of hepatocellular carcinoma: Mallory hyaline bodies can lead to the correct cytological diagnosis. Diagn Cytopathol. 2023;51(2):E70–E74. doi: 10.1002/dc.25072 [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 4.Wen N, Cai Y, Li F, et al. The clinical management of hepatocellular carcinoma worldwide: a concise review and comparison of current guidelines: 2022 update. Biosci Trends. 2022;16(1):20–30. doi: 10.5582/bst.2022.01020 [DOI] [PubMed] [Google Scholar]
- 5.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 6.Lin YL, Li Y.. Study on the hepatocellular carcinoma model with metastasis. Genes Dis. 2020;7(3):336–350. doi: 10.1016/j.gendis.2020.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uka K, Aikata H, Takaki S, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13(3):414–420. doi: 10.3748/wjg.v13.i3.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Chun M, Wang H, et al. Bone metastasis from primary hepatocellular carcinoma: characteristics of soft tissue formation. Cancer Res Treat. 2007;39(3):104–108. doi: 10.4143/crt.2007.39.3.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S, Xu Y, Xie C, et al. RNF219/α-catenin/LGALS3 axis promotes hepatocellular carcinoma bone metastasis and associated skeletal complications. Adv Sci (Weinh). 2021;8(4):2001961. doi: 10.1002/advs.202001961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaizu T, Karasawa K, Tanaka Y, et al. Radiotherapy for osseous metastases from hepatocellular carcinoma: a retrospective study of 57 patients. Am J Gastroenterol. 1998;93(11):2167–2171. doi: 10.1111/j.1572-0241.1998.00648.x [DOI] [PubMed] [Google Scholar]
- 12.Seong J, Koom WS, Park HC.. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int. 2005;25(2):261–265. doi: 10.1111/j.1478-3231.2005.01039.x [DOI] [PubMed] [Google Scholar]
- 13.Hayashi S, Tanaka H, Hoshi H.. Palliative external-beam radiotherapy for bone metastases from hepatocellular carcinoma. World J Hepatol. 2014;6(12):923–929. doi: 10.4254/wjh.v6.i12.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhlman JE, Fishman EK, Leichner PK, et al. Skeletal metastases from hepatoma: frequency, distribution, and radiographic features. Radiology. 1986;160(1):175–178. doi: 10.1148/radiology.160.1.3726123 [DOI] [PubMed] [Google Scholar]
- 15.Chen HY, Ma XM, Bai YR.. Radiographic characteristics of bone metastases from hepatocellular carcinoma. Contemp Oncol (Pozn). 2012;16(5):424–431. doi: 10.5114/wo.2012.31594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longo V, Brunetti O, D’Oronzo S, et al. Bone metastases in hepatocellular carcinoma: an emerging issue. Cancer Metastasis Rev. 2014;33(1):333–342. doi: 10.1007/s10555-013-9462-4 [DOI] [PubMed] [Google Scholar]
- 17.Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359(19):1995–2004. doi: 10.1056/NEJMoa0804523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10(2):99–111. doi: 10.1016/j.ccr.2006.06.016 [DOI] [PubMed] [Google Scholar]
- 19.Kumar D, Hafez O, Jain D, et al. Can primary hepatocellular carcinoma histomorphology predict extrahepatic metastasis? Hum Pathol. 2021;113:39–46. doi: 10.1016/j.humpath.2021.03.011 [DOI] [PubMed] [Google Scholar]
- 20.Huang Z, Chu L, Liang J, et al. H19 promotes HCC bone metastasis through reducing osteoprotegerin expression in a protein phosphatase 1 catalytic subunit alpha/p38 mitogen-activated protein kinase–dependent manner and sponging microRNA-200b-3p. Hepatology. 2021;74(1):214–232. doi: 10.1002/hep.31679 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the findings of this study are included in this published article.