Abstract
Initiation of eukaryotic DNA replication depends on the function of pre-replication complexes (pre-RC), one of its key component being the six subunits origin recognition complex (ORC). In spite of a significant degree of conservation among ORC proteins from different eukaryotic sources, the regulation of their availability varies considerably in different model systems and cell types. Here, we show that the six ORC genes of Arabidopsis thaliana are regulated at the transcriptional level during cell cycle and development. We found that Arabidopsis ORC genes, except AtORC5, contain binding sites for the E2F family of transcription factors. Expression of AtORC genes containing E2F binding sites peaks at the G1/S-phase. Analysis of AtORC gene expression in plants with reduced E2F activity, obtained by expressing a dominant negative version of DP, the E2F heterodimerization partner, and with increased E2F activity, obtained by inactivation of the retinoblastoma protein, led us to conclude that all AtORC genes, except AtORC5 are E2F targets. Interestingly, Arabidopsis contains two AtORC1 (a and b) genes, highly conserved at the amino acid level but with unrelated promoter sequences. AtORC1b expression is restricted to proliferating cells. However, AtORC1a is preferentially expressed in endoreplicating cells based on our analysis in endoreplicating tissues and in a mutant with altered endocycle pattern. This suggests a differential expression of the two ORC1 genes in Arabidopsis.
INTRODUCTION
Initiation of chromosomal DNA replication is a highly regulated process that depends on the function of a set of initiation factors which act coordinately during the cell cycle. The general strategy for activation of DNA replication origins as well as most of the factors involved seem to be highly conserved throughout evolution in archaea, yeast and higher eukaryotes (1,2). These cellular proteins assemble on the chromatin to form the pre-replication complexes (pre-RC). Upon origin activation, pre-RC facilitate the formation of pre-initiation complexes which finally allow the DNA replication machinery, including DNA polymerase(s) and accessory factors to get access to the activated origin.
One of the key components of pre-RC is the origin recognition complex (ORC), a six subunit complex that can be considered as the initiator complex at eukaryotic origins of DNA replication and a landing pad for the rest of pre-RC components (2). The role of ORC in the process, its DNA-binding properties and cell cycle regulation have been studied in yeast and animal model organisms. A relatively high level of conservation in the type and domain organization of different pre-RC components in eukaryotes has been identified, including those available in plants (3–9).This is in sharp contrast with the highly species-specific strategies that have evolved in different organisms to regulate ORC function (2,10). These include, at least, modulation of gene expression, subcellular localization, chromatin binding, phosphorylation and selective proteolysis. The series of cell cycle-dependent changes in ORC activity and multisubunit organization is referred to as the ‘ORC cycle’ (11). Studies in mammalian cells and subsequently in other eukaryotic systems, revealed specific features, in many cases related to their particular growth characteristics. Human ORC1 is destabilized and released from chromatin, ubiquitinated, and eventually degraded while in other cases, ORC1 is phosphorylated and then either released from chromatin or prevented from rebinding in the same cycle (11). In flies, no direct data are available but current observations strongly suggest cell cycle-dependent changes in ORC activity.
In plants, the situation is far less well understood and largely restricted to the description of ORC genes from several species (4–6,9,12). However, in depth studies on the mechanisms regulating the expression of ORC genes are lacking, e.g. whether E2F transcription factors are involved as it occurs for ORC1 in human cells (13) and Drosophila (14). In addition, plants have very unique growth, developmental and architectural properties. In particular, plant cells have an enormous plasticity in terms of cell proliferation (15), being able to exit and reactivate cell cycle in response to a variety of environmental and developmental cues. Thus, given the species-specific differences in ORC regulation it seems more appropriate to analyze at different levels ORC gene structure and function in plants. Recently, it has been found that disruption of Arabidopsis ORC2 gene causes a zygotic lethal phenotype as well as abnormal endosperm development (6). Furthermore, endoreplication, which is a physiological mode of full-genome re-replication that also occurs in certain animal cell types, is a very frequent event in plants. In these organisms it is frequently associated with specific growth and developmental pathways such as trichome, leaf or endosperm development (3,15–18).
The aim of our study is to understand ORC gene expression in Arabidopsis. Here we show (i) that all AtORC genes, except AtORC5, are regulated by the E2F/DP family of transcription factors both in cultured cells and in planta, and (ii) that the two ORC1 genes present in Arabidopsis seem to be differently regulated in proliferating and endoreplicating cells.
MATERIALS AND METHODS
In silico studies
We used the Patmatch (http://www.arabidopsis.org/cgi-bin/patmatch/nph-patmatch.pl) and the Pattern search (http://mips.gsf.de/proj/thal/db/index.html) tools.
Plant cell culture
Arabidopsis MM2d suspension cultured cells were used (19). Cell cycle arrest by sucrose starvation was carried out as described (19,20).
Electrophoretic mobility shift assay (EMSA)
EMSA was carried out as described (21), using purified AtE2Fc and AtDPb (22). Oligonucleotides (only the sequence of the sense oligonucleotides is provided for simplicity) containing consensus E2F sites (underlined) were: ORC1a: 5′-GCAAACATTTCCCGCCAAATTTCT; ORC1b: 5′-GCCACCTTTCCCGCCAACTTTCT; ORC2.1: 5′-AAGAAAAACGCGCGGGAAAATTGAGA; ORC2.2: 5′-AAAGTTGTTAACCGGGAAAGACGAAG; ORC3: 5′-CGCTCTTTTTGGCGGGAAAATTCGTG; ORC4: 5′-TGTCAGTTTTCCCGCCAGTCCGATGG; ORC6.1: 5′-AAAAACATTCGCGGCTAAAATTTCAA; ORC6.2: 5′-CGTAAAAAAATCCCGCCAAACGTTGG.
Pull-down assays
The coding regions of all AtORC subunits were cloned into the pDEST15 Gateway vector (Invitrogen) to express AtORC proteins with a GST-tag in bacteria. The pBluescriptII KS± vector was used for in vitro translation of 35S-labeled AtORC subunits using a rabbit reticulocyte kit (Promega), according to the manufaturer's instructions. For the pull-down assays, 5 µg of GST-ORC subunits bound to glutathione-Sepharose beads were incubated with 5–10 µl of 35S-labeled AtORC subunits in phosphate-buffered saline (PBS) for 2 h at 4°C, the beads were washed 5 times with 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100 and then the samples were fractionated by SDS–PAGE.
RNA extraction and real-time RT–PCR
Total RNA was extracted using the Trizol reagent (Invitrogen) and RT–PCRs were carried out with the ThermoScript RT System (Invitrogen). The LightCycler System with the FastStart DNA Master SYBR Green I (Roche) was used for real-time RT–PCR. The concentration of either ubiquitin10 (AtUBQ10) or actin (AtACT2) mRNAs in each sample was determined to normalize for differences of total RNA amount. The data were derived from duplicate experiments, and in the case of the analysis of transgenic plants, at least two independent lines were used. The primer sequences used are available upon request.
Plant material
The promoter region of AtORC1b (from −635 to +15, +1 being the ATG) was fused in frame to the β-galactosidase (GUS) gene in the pBI101 binary vector. To generate transgenic plants A.thaliana (Col-0 ecotype) was transformed with Agrobacterium tumefaciens C58CRifR carrying the pORC1b: GUS construct by the floral dip method (23). Transformed seedlings (T0 generation) were selected on MS agar plates containing 50 µg/ml kanamycin and transferred to soil. T2 homozygous plants were selected for further analysis. Plants expressing a dominant negative version of DP, partially deleted in its DNA-binding domain, have been described (24). Plants expressing the geminivirus RepA, either wild-type or the E198K point mutation that abolish interaction with RBR (25), or transformed with the empty vector are described elsewhere (B. Desvoyes, E. Ramirez-Parra, Q. Xie, N.-H. Chua and C. Gutierrez, manuscript submitted).
In situ hybridization and histochemical analysis
The sense (control) and antisense RNA probes were prepared with the DIG RNA labeling kit (Boehringer Mannheinn), after in vitro transcription from the T7 and T3 promoters of a full length AtORC1 cDNA cloned into the pBluescript vector, as described by the manufacturer. The samples were treated with 0.1 M carbonate buffer (pH 10.2), at 60°C for 1 h. In situ hybridization was carried out essentially as described (26,27). Histochemical detection of GUS activity was done using 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (28), with slight modifications.
RESULTS
Characteristics of Arabidopsis ORC cDNAs
Completion of the Arabidopsis genome sequence has provided a powerful tool to identify novel genes, although direct information derived from gene prediction frequently suffers from problems inherent to the algorithms used. Thus, we used homology searches with protein regions conserved among the corresponding human and yeast genes as a query to clone cDNA encoding each Arabidopsis ORC (AtORC) subunit gene (Figure 1A). Comparison of the amino acid sequence derived from these cDNAs with that of the ORC proteins predicted in the Arabidopsis genome sequence databases revealed that all AtORC coding sequences, except for AtORC2 (12), were not predicted correctly. It is striking that the coding sequence of AtORC1a (2427 bp) contains a large single exon, a situation that differs from the exon/intron map predicted in the Arabidopsis genome. It is worth to note that the two AtORC1 showed the highest homology compared with the corresponding ORC proteins of different eukaryotic sources (Table 1), in particular within their C-terminal moiety. Then, AtORC2, AtORC3 and AtORC5 showed a relatively high homology, while AtORC4 and AtORC6 exhibit a high homology with the corresponding human proteins but very low with both budding and fission yeast counterparts. The low homology of AtORC4 with SpORC4 is due to the presence of the AT-hook in the fission yeast protein, which is lacking in the plant protein, as well as in other eukaryotic ORC4 proteins. As expected, the homology is much higher with the ORC proteins of other plant species (Table 1).
Figure 1.
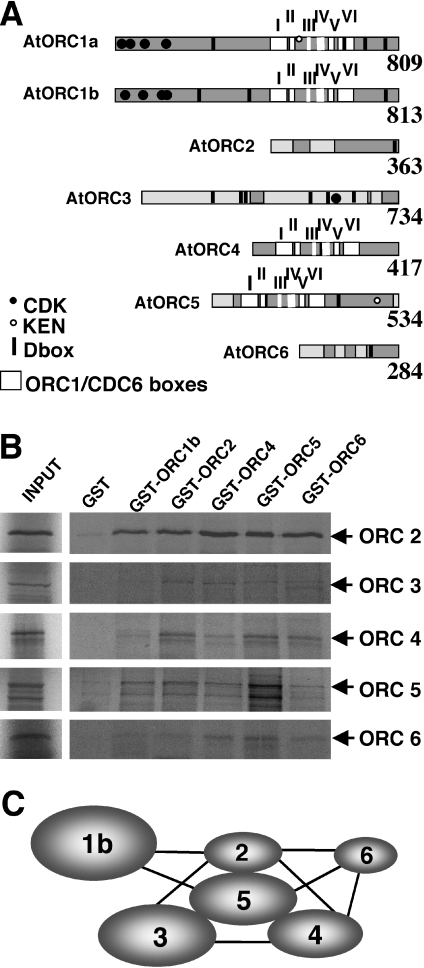
Arabidopsis ORC proteins and subunit interaction map. (A) Summary of domain organization and major landmarks of AtORC proteins deduced from their cDNA sequence. Regions with the highest homology to ORC proteins from other sources appear in grey. Putative CDK phosphorylation sites (closed circles), KEN boxes (empty circles) and D-boxes (bars) are also shown. Note the six domains (hatched) shared among plant and animal ORC1, ORC4 and ORC5 proteins and CDC6 and RFC1. Accession numbers of sequences reported here are: AtORC1a (AJ421410), AtORC1b (AJ426477), AtORC4 (CAE01428), AtORC5 (CAE01429) and AtORC6 (CAE01430). Sequences of AtORC2 and AtORC3 have been reported (U40269 and AY524002, respectively). (B) Pull-down assays of in vitro translated AtORC subunits (ORC2-6) with purified GST-ORC proteins. (C) Schematic representation of the interactions observed among the different AtORC subunits. Lines indicate direct interaction in the pull-down assays.
Table 1.
Amino acid homology of AtORC proteins with the corresponding ORC proteins from different sources
| Saccharomyces cerevisiae | Saccharomyces pombe | Drosophila melanogaster | Homo sapiens | Zea mays | Oryza sativa | |
|---|---|---|---|---|---|---|
| AtORC1a | 19.1 | 23.0 | 24.6 | 24.7 | 57.6 | 56.5 |
| AtORC1b | 19.8 | 24.1 | 25.1 | 25.7 | 58.6 | 57.9 |
| AtORC2 | 16.8 | 20.7 | 18.3 | 21.3 | 59.7 | 57.5 |
| AtORC3 | 14.3 | 16.2 | 19.1 | 18.9 | 42.5 | 39.6 |
| AtORC4 | 17.8 | 13.1 | 27.2 | 27.5 | 58.3 | 58.8 |
| AtORC5 | 16.1 | 19.9 | 18.2 | 23.4 | 31.0a | 42.9 |
| AtORC6 | 10.1 | 11.5 | 18.3 | 26.6 | NAb | 60.7 |
aComparison to a partial clone was described and reported by Witmer et al. (5).
bNot available.
While this study was in progress, cDNAs encoding AtORC subunits have been reported (6,9). In general, the coincidence is high although some features that were not noticed before are highlighted below. ORC4 was initially considered to be missing in the Arabidopsis genome (5). The similarity of genomic sequences, upstream from the predicted ATG of open reading frame (ORF) At2g01120 with the N-terminal domain of human ORC4, allowed us to isolate a cDNA encoding a 417 amino acid-long AtORC4 gene. The two AtORC1 proteins contain boxes I-VI, highly conserved in the ORC/CDC6 family (12), including the motifs defining the larger ORC/CDC6/RFC superfamily (29). AtORC1 proteins together with AtORC4 and AtORC5 belong to the AAA+ ATPase superfamily and contain the typical Walker A and B motifs of the NTP-binding domain (Figure 1A). However, the Walker A of AtORC4 is atypical (GKA at position 64). The presence of consensus CDK phosphorylation sites (S/TP × K/R) is also characteristic of several AtORC members (Figure 1A). In AtORC1 these are located at positions 14, 18, 45 and 110, in AtORC1b at 12, 42, 100 and 114, and in AtORC3 at position 540. All AtORC subunits contain destruction boxes with the signature R××L at the following positions: AtORC1a at 280, 655, 713 and 768, AtORC1b at 239, 383, 660 and 772, AtORC2 at 354, AtORC3 at 204, 282, 297, 483, 534 and 606, AtORC4 at 200 and 209, AtORC5 at 153 and 362, and AtORC6 at 204. Both AtORC1a and AtORC5 also contain KEN boxes typical of degraded proteins at positions 544 and 470, respectively.
Interactions among Arabidopsis ORC subunits
To gain insight into the possible organization of different AtORC subunits within the complex we analyzed the interactions of all of them with a yeast two-hybrid approach. The ORFs encoding each AtORC subunit were fused to both the GAL4 DNA-binding and activation domains separately, and used in a matrix assay to test yeast growth in all possible combinations. All fusions to the DNA-binding domain were tested for self-transactivation and found to be negative. Except for the interactions of AtORC4 with AtORC2 and AtORC5, other interactions were not very strong (data not shown). It is possible that the AtORC proteins interfere deleteriously with endogenous yeast ORC proteins. Also in several cases, we observed that they did not repeat when the test proteins were exchanged between the two DNA-binding and activation domain plasmids (data not shown), as described previously (9). These problems precluded the generation of a meaningful interaction map of all subunits.
Therefore, we used pull-down assays of in vitro translated AtORC proteins with purified GST-tagged AtORC proteins. We found that AtORC2 and AtORC5 interact strongly with all AtORC subunits while AtORC3 and AtORC4 interact with all, except AtORC1b (Figure 1B). The interaction between AtORC1b and AtORC6 with the rest was significantly weaker (Figure 1B). AtORC1a and b were not included in the assays because the in vitro translated preparations were not sufficiently clean. Also the GST-ORC1a protein could not be expressed properly. The results obtained led us to generate the interaction map shown in Figure 1C.
Expression of AtORC genes occurs in a cell cycle-dependent manner
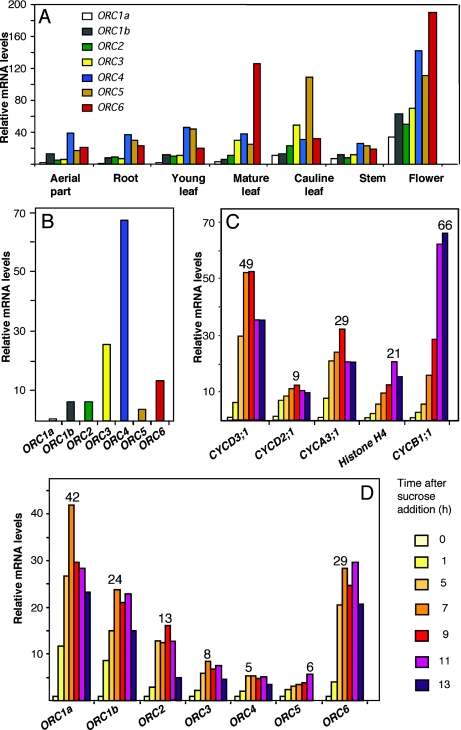
We carried out a detailed analysis of the relative amounts of AtORC mRNAs in different plant tissues by real-time RT–PCR. Samples used included the aerial part and root system of 6 day-old seedlings, young and mature leaves, cauline leaves, stems and flower buds. Buds containing flowers at different developmental stages appeared to be the material that, in general, showed the highest amount of all AtORC transcripts (Figure 2A), most notably AtORC4, AtORC5 and AtORC6. In general, the AtORC1a, 1b, 2 and 3 transcripts were the least abundant in all tissues analyzed, perhaps with the exception of AtORC3 in cauline leaves (Figure 2A). AtORC4 and 5 transcripts were abundant, relative to the amount of AtORC1a, in the aerial part and the root system of seedlings as well as in young leaves, that contains a significant amount of proliferating cells (Figure 2A). These observations complement previous expression studies (9) and reveal that flowers at different stages of development show the highest levels of AtORC genes.
Figure 2.
Organ- and cell cycle-dependent expression of AtORC genes. (A) Expression pattern of AtORC genes in different organs. Measurements were normalized to the amount of UBQ10 or ACT2 and, then all the AtORC values made relative to the amount of AtORC1a present in the sample of aerial part of these seedlings (the lowest of all). Samples were prepared from aerial parts and root system of 12 day-old seedlings, young and mature rosette leaves, cauline leaves, stems, flowers at different stages or growth. (B) A.thaliana MM2d suspension cultured cells were sucrose-starved for 24 h and the amount of different AtORC mRNAs was determined by real-time RT–PCR, using the normalization procedure described for panel A. (C–D) A.thaliana MM2d suspension cultured cells, sucrose-starved for 24 h, were stimulated to re-enter the cell cycle, as described (19). The amount of mRNA of several cell cycle marker genes (31) was determined at the indicated times after sucrose addition by real-time RT–PCR, as described in panel A. CYCD3;1 and CYCD2;1 were used as G1 markers, CYCA3;1 and histone H4, as S-phase markers and CYCB1;1, as a G2/M marker (panel C). The mRNA levels of each AtORC gene (panel D) were determined at the indicated times after sucrose addition by real-time RT–PCR, as described in panel A. Numbers on top of the bars in panels C and D indicate the fold increase at the maximum level of expression relative to the value, in each case, obtained at time zero (arrested cells). In all cases, the RT–PCR measurements were repeated, at least, 2–3 times but error bars have been omitted for simplicity.
To study in detail whether AtORC expression depends on different cell cycle stages we determined the mRNA levels of each AtORC gene in Arabidopsis cell suspension cultures. Sucrose starvation is known to arrest cell proliferation which is resumed synchronously upon sucrose addition (19). RT–PCR determination of AtORC mRNA levels in arrested cells indicated that the amount of AtORC1a transcripts was the lowest (Figure 2B). To establish whether AtORC gene expression was subjected to cell cycle regulation we determined mRNA levels at 2 h intervals after releasing from a sucrose-deprivation cell cycle block. Cell cycle progression during the recovery period was assessed by following the expression pattern of well-known cell cycle genes that were used as markers of the different cell cycle phases (30). This analysis confirmed a proper cell cycle progression as indicated by the expression of CYCD3;1 and CYCD2;1 (30), G1 markers that peak at 7–9 h after sucrose addition, CYCA3;1 (30) and histone H4 (31), S-phase markers with a maximum of ∼9 h after sucrose addition and CYCB1;1 (32), a G2/M marker with a maximum of 13 h after sucrose addition (Figure 2C). Transcription of all six AtORC genes, except for AtORC5, was rapidly stimulated after cell cycle reactivation by sucrose addition, and the mRNA levels reached a maximum of ∼7–9 h after (Figure 2D), coinciding with the time of expression of the G1 marker genes and clearly before that of S-phase genes. AtORC5 reached a maximum accumulation at 13 h after sucrose addition (Figure 2D). The largest increase in mRNA levels corresponded to AtORC1a, AtORC1b and AtORC6 (42-, 24- and 29-fold, respectively), although the rest also showed a significant up-regulation during re-entry into the cell cycle AtORC2, AtORC3, AtORC4 and AtORC5 (13-, 8-, 5- and 6-fold, respectively). This indicates that transcriptional regulation of AtORC genes seems to be temporally coordinated during cell cycle progression, but not at the same stage in different AtORC genes. A comparable situation occurs in animal cells in culture where ORC transcripts are not abundant in serum-starved, quiescent cells (13,33).
E2F/DP binding sites in AtORC gene promoters
The identification of the bona fide translation start sites for each AtORC gene allowed us a direct sequence analysis of the individual upstream regions which likely cover the putative promoters. In silico studies revealed the presence of the minimal consensus binding sites for the E2F/DP transcription factors (TTTSSCGS, S being C or G). We also included in the search degenerated residues in the three T, since they have also been shown to mediate E2F/DP binding both in animals and plants (24,34,35). All AtORC genes, except AtORC5, contains at least one consensus E2F binding site relatively close to the ATG, the most common being TTTCCCGC (Figure 3A). The location of these putative E2F binding sites has been considered a strong indication that members of the E2F family may actually regulate AtORC gene expression (24), as suggested by their cell cycle expression pattern. Also consistent with this is the lack of an E2F binding site in the AtORC5 promoter.
Figure 3.
E2F binding to the AtORC gene promoters. (A) Summary of the location of consensus E2F DNA-binding sites in the AtORC promoters, relative to the ATG. Note that in the case of AtORC2, the transcription initiation start site (12) is indicated (arrow). E2F binding sites (oligonucleotides used in panel B are in parenthesis) are: TTTCCCGC (1a, 1b, 2.2, 3 and 4), TTTCCCGG (2.1), TTTGGCGG (6.1) and ATTCGCGG (6.2). (B) EMSA with purified AtE2Fc/AtDPb using the oligonucleotide indicated at the top. C, control using an oligonuclotide known to interact with E2F/DP (8). M, assay using the same probe but containing two point mutations that abolish E2F/DP binding (24). Arrow points to the DNA–protein complexes and the asterisk to the free DNA probe. The lanes lacking AtE2Fc/AtDPb proteins for each probe have been omitted.
To determine whether these E2F sites can mediate E2F binding we used EMSA using oligonucleotide probes containing the actual genomic sequences around the E2F binding sequences present in the AtORC promoters. Figure 3B shows that recombinant Arabidopsis E2Fc/DPb complexes bound specifically to oligonucleotide probes containing different E2F binding sites. These data indicate that AtORC1a, 1b, 2, 3, 4 and 6 promoter sequences can direct binding of E2F/DP. This together with their cell cycle expression pattern strongly suggests that they may be E2F target genes.
E2F/DP-regulated expression of AtORC genes in planta
To determine whether expression of AtORC genes is regulated by E2F in planta, we used first transgenic Arabidopsis plants expressing a truncated version DP [DPΔBD;(24)], the heterodimerization partner of E2Fa, b and c (36,37). This truncated DP, which lacks part of the DNA-binding domain, has been shown to bind efficiently to E2F but to prevent binding of the E2F/DP heterodimer to DNA (24), thus behaving as a dominant negative of the DP-dependent E2F activity. We determined by real-time RT–PCR the mRNA levels of each AtORC gene in control and transgenic plants. The results, summarized in Figure 4A, indicate that the amount of mRNA of most AtORC genes diminished in plants expressing the truncated DP isoform. The differences were statistically significant except for the AtORC1a, AtORC2 and AtORC5 genes. As an internal control, we also determined the levels of AtCDC6a, a known E2F target gene (3,8), that also showed differences between control and DPΔBD-expressing plants that were statistically significant.
Figure 4.
E2F-mediated regulation of AtORC gene expression in planta. (A) Levels of mRNA for each AtORC gene and for AtCDC6 were determined by real-time RT–PCR in extracts of 10–12 day-old seedlings of plants expressing a dominant negative version of DP (24) and in control plants transformed with an empty vector. Measurements were carried out as described in Materials and Methods and, then the AtORC values made relative to that of AtORC1a in control plants. Asterisks indicate that the differences between the mean relative values of control and DPΔBD-expressing plants were statistically significant (P ≤ 0.025). (B) Levels of mRNA for each AtORC gene and for AtCDC6 were determined by real-time RT–PCR in extracts of 10 day-old seedlings of plants expressing the wild-type geminivirus RepA protein (RepAwt) or the same protein bearing the E198K point mutation (RepAE198K), and in control plants transformed with an empty vector. Measurements were carried out 7 h after induction of RepA protein by treatment with 1 µM dexamethasone and in each case the values were made relative to those obtained in the control plants. Asterisks indicate that the differences between the mean relative values in plants expressing RepAwt and RepAE198K were statistically significant.
To complement the analysis we determined AtORC mRNA levels in plants where E2F activity was increased. This was achieved by targeted inactivation of the RBR protein after expressing the geminivirus RepA protein under the control of a dexamethasone-responsive promoter (B. Desvoyes et al., manuscript submitted). The RepA interacts efficiently with RBR through a L×C×E amino acid motif (25,38,39). The interaction of virus RepA with RBR bypasses the normal activity of CDK/cyclin complexes that phosphorylate RBR and release E2F activity (40). We have shown that, after RepA induction, the endogenous set of AtE2Fa/b/c, normally bound by RBR, is released (B. Desvoyes, E. Ramirez-Parra, Q. Xie, N.-H. Chua and C. Gutierrez, manuscript submitted). Cell extracts of plants induced to express RepAwt contain increased levels of all AtORC mRNAs, except for AtORC5 (Figure 4B). This indicated that these AtORC genes respond positively to the increased E2F activity achieved by RBR inactivation. Furthermore, this effect was specific for the release of RBR-bound E2F as revealed by the lack of a significant change in AtORC mRNA levels after inducing a RepA protein that contains the E198K point mutation that almost completely abolish the interaction with RBR (25). It should be kept in mind that AtORC messages, except for AtORC5, increase before S-phase upon cell cycle reactivation, and that AtORC genes, except AtORC5, contain E2F binding sites in their promoters. Therefore, all these data together led us to conclude that expression of all AtORC genes, except AtORC5, is regulated by the RBR/E2F pathway. This situation is different from that found in animal cells where only the ORC1 gene has been demonstrated to respond to E2F (13,14).
The two AtORC1 genes are differentially expressed in proliferating and endoreplicating cells
One the most striking features of the AtORC gene set is the presence of two genes encoding ORC1 homologues (AtORC1a, At4g14700; AtORC1b, At4g12620), which are >80% similar in amino acid sequence. The high amino acid identity strongly suggests that they likely have a very similar role in ORC activity. However, while the coding sequences have not diverged too much, the two promoter sequences do not show a significant similarity. An attractive possibility is that this may confer differences in the expression pattern of the two genes.
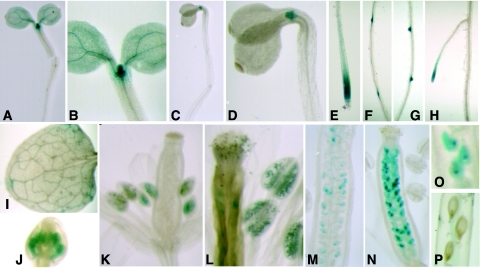
To determine the functional relevance of the two AtORC1 genes we first analyzed the individual expression pattern of individual AtORC1 genes. We set up to study the spatial activity of each promoter in transgenic plants expressing the GUS reporter gene under the control of each of the AtORC1 promoters. We used genomic regions upstream the predicted ATG fused in frame to the GUS gene. Histochemical analysis of the pORC1b:GUS transgenic seedlings (3–4 day-old) showed a strong GUS activity in highly proliferating cells located in the shoot (Figure 5A and B) and root apical meristems (Figure 5E and H). AtORC1b promoter activity in dark-grown seedlings was restricted to shoot (Figure 5C and D) and root (data not shown) apical meristems while the cotyledons and the hypocotyl appeared negative. In older seedlings, GUS activity was largely restricted to the lateral root primordia (Figure 5F and G) and meristems (Figure 5H). In two week-old rosette leaves, GUS staining was negative, indicating that the promoter was no longer active after cell proliferation ceased (Figure 5I). We also found AtORC1b promoter activity in young flower buds (Figure 5J), developing anthers (Figure 5K) and mature pollen (Figure 5L). Interestingly, this expression pattern in the developing flowers is similar to that of the Arabidopsis AtE2Fc gene (22) and AtE2Ff (20), reinforcing the idea that AtORC1b is an E2F target gene. Later in development, the AtORC1b promoter is active again during embryogenesis (Figure 5M–O) and inactivated in mature embryos (Figure 5P). Altogether these data demonstrate that AtORC1b promoter activity correlates strongly with the presence of highly proliferating cells.
Figure 5.
Expression pattern of AtORC1b. The activity of the AtORC1b promoter was monitored by histochemical detection of the marker GUS gene in different organs during development. (A and B) Four day-old seedlings grown in the light or (C and D) in the dark. B and D are details of the shoot apical region in each case. (E) Primary root or (F–H) lateral roots at different stages of growth in 10 day-old seedlings. (I) Mature leaf. (J and K) Flowers at two stages of development. (L) Detail showing the anthers and pollen grains. (M–O) Pistils with embryos at different stages of development and (P) seeds in a mature silique.
Unfortunately, and for unknown reasons, we have been to date unsuccessful in generating transgenic plants expressing the GUS reporter gene under the control of the AtORC1a promoter. This problem was not solved even after several independent transformation trials, including a variety of constructs containing different promoter and coding sequence regions fused to GUS. To overcome this problem, we used in situ hybridization with a probe that detects both AtORC1a and AtORC1b messages. AtORC1 mRNAs were abundant in the SAM as well as in cells of the apical hook of dark-grown seedlings (Figure 6A and B), known to develop at least one extra endoreplication cycle (41). This pattern indicated that the AtORC1 genes were expressed in locations where proliferating and endoreplicating cells occur, very similar to what was observed for AtCDC6a gene (8). To corroborate this, we used the Arabidopsis ctr1 mutant. Hypocotyl cells of seedlings carrying a mutation in the CTR1 (constitutive triple response 1) gene, involved in ethylene signaling (42), undergo one extra endoreplication round in a large proportion of cells (41). AtORC1 gene expression was much higher in the apical hook of dark-grown ctr1 mutant seedlings (Figure 6C and D). In the absence of direct data from AtORC1a:GUS tansgenic plants it is difficult to draw a clear cut conclusion. However, our data are suggestive that the occurrence of extra endocycles during hypocotyl growth in the dark could be associated with increased expression of AtORC1 genes, as it was shown to be the case with AtCDC6a (8). Finally, we complemented this analysis by determining the levels of AtORC1a and AtORC1b mRNAs by RT–PCR in the aerial part of young seedlings under light of dark conditions. Results are summarized in Figure 6E where it is clearly shown that whereas the level of AtORC1b messages were independent on the light regime, AtORC1a expression was up regulated in the aerial parts of dark-grown seedlings.
Figure 6.
Detection of AtORC1 mRNA in hypocotyl cells. (A–D) AtORC1 messages were revealed by whole-mount in situ hybridization in wild-type (A and B) and ctr1 mutant (C and D) 4 day-old seedlings grown in the dark. A and C correspond to the signal obtained with antisense (as) probe and B and D with the sense (s) probe. (E) Measurement of the mRNA levels of each AtORC1a and AtORC1b by real-time RT–PCR in extracts of hypocotyl cells of 4 day-old seedlings grown under light or dark conditions. Note than AtORC1a, but not AtORC1b, mRNAs increases in the dark, coinciding with occurrence of extra endoreplication cycles.
DISCUSSION
In this work we have isolated cDNAs encoding all six subunits of the Arabidopsis ORC. We have also analyzed their expression pattern. We have found that AtORC genes are preferentially, although not exclusively, expressed in proliferating tissues, where they behave as E2F/DP targets. We have also focused on understanding the significance of the presence of two AtORC1 genes. Based on their expression pattern we propose that they respond differently to signals present in either proliferating or endoreplicating cells.
The AtORC cDNA sequences isolated in our study generally matched those appeared during the course of this work (6,9). However, in some cases the differences are striking. Our AtORC4 sequence likely coincides with the cDNA isolated by Masuda et al. (9) but not with that reported by Collinge et al. (6) which contains in the C-terminus one extra nucleotide residue that leads to a different and longer C-terminal amino acid sequence. These differences may reflect either errors in cDNA isolation or a complex expression pattern (9). It should be kept in mind that alternative splicing variants have also been reported for human ORC5 (43). Significant differences were also observed in the identification of residues potentially involved in regulating the degradation of AtORC polypeptides. Overall, a much higher similarity was detected with animal than with yeast ORC homologues, a situation that also extends to CDC6 (8) and CDT1 (3).
We have detected strong interactions among the AtORC2-3-4-5 subunits. Although they should be confirmed in vivo in the future, they seem to be largely similar to what has been proposed for the architecture of human (44–46), mouse (47) and maize (5) ORC, suggesting that the basic interactions have been conserved through evolution. The interaction between ORC1 and 6 seems to be labile in both the human and the Arabidopsis complex. However, differences seem to have evolved even between maize and Arabidopsis regarding ORC1 interactions with core ORC2-5 components since they seem to be mediated by ORC4 in Arabidopsis and by ORC5 in maize (5). We can speculate that AtORC3, which is larger than AtORC2, may have replaced the role of ORC2 in other systems. In this regard, and based on the available information on potential CDK phosphorylation sites in the ORC subunits of Arabidopsis and other model systems (2), it is tempting to speculate that CDK phosphorylation may be important for the regulation of AtORC1 and AtORC3 (instead of ORC2) function.
Arabidopsis ORC genes are preferentially expressed in proliferating tissues. This is comparable to the situation in cultured animal cells where ORC transcripts are not abundant in serum-starved, quiescent cells (13,33). Furthermore, it is likely that they are also required during gametogenesis and early embryogenesis, which has been clearly shown by AtORC2 in situ hybridization (6). However, AtORC gene expression is not restricted to these tissues since, at least some of them are expressed at relatively high levels in organs largely composed of post-mitotic cells. In this regard, the high abundance of AtORC5 and 6 in cauline and mature rosette leaves (Figure 2A) is a striking example [see also ref. (6)]. These observations strongly suggest that these AtORC subunits may play important roles also in differentiated organs, most likely not directly related to DNA replication events. Similar conclusions have been derived for several ORC subunits in other organisms. Thus, in yeast, ORC plays a crucial role in heterochromatin silencing at the HM loci through its interaction with Sir1, a component of the silencing machinery (48–53). Likewise, ORC also plays a role in heterochromatin silencing in Drosophila (54,55). In addition, Drosophila ORC3 (56,57) also seem to play some role in neuronal development and behavior. Human ORC4 and ORC5 are expressed in differentiated tissues (43) such as spleen and ovaries. A role for ORC6 in cytokinesis has also been reported (58,59). Finally, the observation that different ORC subunits may interact with other cellular proteins (44,60) further support the idea of additional functions of ORC. The availability of all Arabidopsis ORC genes and tools should facilitate in the future the identification of possible non-DNA replication-related roles of Arabidopsis ORC subunits.
The expression of AtORC genes, except AtORC5, seems to be dependent on E2F regulation. This conclusion is based on our results derived from three complementary lines of evidence. First, AtORC expression peaks at the G1/S transition as deduced from analysis in synchronized cells. These data suggested that AtORC gene expression may coincide with the RBR inactivation, and concomitant release of E2F activity, that takes place before the G1/S transition. Second, the presence of E2F binding sites in the promoter region of all AtORC genes, except AtORC5 which does not show a peak of expression at G1/S. This observation was reinforced by the ability of purified E2F/DP heterodimers to form specific complexes in vitro with DNA probes containing the sequences present in the AtORC promoter regions. Third, the level of AtORC mRNAs is very sensitive to changes in the level of E2F activity in planta. In the case of plants expressing a dominant negative version of DP, most AtORC transcripts were reduced while in some cases reduction was not statistically significant. In spite of the presence of E2F binding sites in AtORC1a and AtORC2, the lack of significant reduction in their mRNA levels may be due to the action of other E2F known to act independently of DP (20,37). Although results using a dominant negative approach should always be taken with caution, they are consistent with an E2F-dependent effect on AtORC gene expression. The analysis of AtORC gene expression in plants with increased E2F activity obtained by targeted inactivation of RBR nicely complemented the study. Inactivation of RBR that leads to the release of endogenous RBR-bound E2F activity (B. Desvoyes, E. Ramirez-Parra, Q. Xie, N.-H. Chua and C. Gutierrez, manuscript submitted) produced a significant increase of all AtORC transcripts, except in the case of AtORC5, an effect that was not observed in plants expressing a RepA protein containing a point mutation that abolish almost completely binding to RBR. Therefore, all our data together strongly support the conclusion that E2F/DP complexes may participate in regulating the expression of AtORC genes both in cultured cells and in planta. It must be emphasized that this situation is different from that found in animal cells where only the ORC1 gene has been demonstrated to respond to E2F (13,14).
Finally, a sophisticated regulatory mechanism seem to have evolved in Arabidopsis regarding the expression of the two AtORC1 genes. Based on the in situ localization data shown above, AtORC1 transcripts are present in both proliferating and endoreplicating cells. However, AtORC1b promoter activity was detected in actively proliferating cells but not in locations containing endoreplicating cells, e.g. dark-grown hypocotyl cells or trichomes. Therefore, we can reasonably conclude that the AtORC1b promoter is active exclusively in proliferating cells while that of AtORC1a could be preferentially, perhaps specifically, active in endoreplicating cells. In the absence of direct data derived from AtORC1a:GUS transgenic plants we cannot conclude whether AtORC1a expression is specific for endoreplicating cells. These finding represent a first example of duplication of an ORC gene in which the coding sequence is maintained virtually unchanged while the two promoters may have diverged significantly to activate gene expression in a tissue- and developmental-stage-specific manner. Interestingly, other pre-RC genes such as AtCDC6 (8) and AtCDT1 (3) are also duplicated in the Arabidopsis genome, although in these cases the occurrence of a cell type-specific regulation of the expression of each member remains to be studied.
Acknowledgments
Authors are indebted to J.A.H. Murray and Bayer Cropscience for the A.thaliana MM2d cell suspension culture, to R. Solano for the ctr1 seeds, and to Sergio Llorens and Ana I. Díaz for technical support. This work has been partially supported by grants BMC2003-2131 (MCyT) and 07B/053/2002 (CAM), and by an institutional grant from Fundación Ramón Areces. Funding to pay the Open Access publication charges for this article was provided by grant BMC2003-2131 (MycT).
Conflict of interest statement. None declared.
REFERENCES
- 1.Kelly T.J., Brown G.W. Regulation of chromosome replication. Annu. Rev. Biochem. 2000;69:829–880. doi: 10.1146/annurev.biochem.69.1.829. [DOI] [PubMed] [Google Scholar]
- 2.Bell S.P., Dutta A. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 3.Castellano M.M., Boniotti M.B., Caro E., Schnittger A., Gutierrez C. DNA replication licensing affects cell proliferation or endoreplication in a cell type-specific manner. Plant Cell. 2004;16:2380–2393. doi: 10.1105/tpc.104.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura S., Ishibashi T., Hatanaka M., Sakakibara Y., Hashimoto J., Sakaguchi K. Molecular cloning and characterization of a plant homologue of the origin recognition complex 1 (ORC1) Plant Scig. 2000;158:33–39. doi: 10.1016/s0168-9452(00)00297-1. [DOI] [PubMed] [Google Scholar]
- 5.Witmer X., Alvarez-Venegas R., San-Miguel P., Danilevskaya O., Avramova Z. Putative subunits of the maize origin of replication recognition complex ZmORC1-ZmORC5. Nucleic Acids Res. 2003;31:619–628. doi: 10.1093/nar/gkg138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collinge M.A., Spillane C., Kohler C., Gheyselinck J., Grossniklaus U. Genetic interaction of an origin recognition complex subunit and the Polycomb group gene MEDEA during seed development. Plant Cell. 2004;16:1035–1046. doi: 10.1105/tpc.019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens R., Grelon M., Vezon D., Oh J., Meyer P., Perennes C., Domenichini S., Bergounioux C. A CDC45 homolog in Arabidopsis is essential for meiosis, as shown by RNA interference-induced gene silencing. Plant Cell. 2004;16:99–113. doi: 10.1105/tpc.016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellano M.M., del Pozo J.C., Ramirez-Parra E., Brown S., Gutierrez C. Expression and stability of Arabidopsis CDC6 are associated with endoreplication. Plant Cell. 2001;13:2671–2686. doi: 10.1105/tpc.010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda H.P., Ramos G.B., de Almeida-Engler J., Cabral L.M., Coqueiro V.M., Macrini C.M., Ferreira P.C., Hemerly A.S. Genome based identification and analysis of the pre-replicative complex of Arabidopsis thaliana. FEBS Lett. 2004;574:192–202. doi: 10.1016/j.febslet.2004.07.088. [DOI] [PubMed] [Google Scholar]
- 10.Blow J.J., Hodgson B. Replication licensing—defining the proliferative state? Trends Cell Biol. 2002;12:72–78. doi: 10.1016/s0962-8924(01)02203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DePamphilis M.L. The ‘ORC cycle’: a novel pathway for regulating eukaryotic DNA replication. Gene. 2003;310:1–15. doi: 10.1016/s0378-1119(03)00546-8. [DOI] [PubMed] [Google Scholar]
- 12.Gavin K.A., Hidaka M., Stillman B. Conserved initiator proteins in eukaryotes. Science. 1995;270:1667–1671. doi: 10.1126/science.270.5242.1667. [DOI] [PubMed] [Google Scholar]
- 13.Ohtani K., DeGregori J., Leone G., Herendeen D.R., Kelly T.J., Nevins J.R. Expression of the HsOrc1 gene, a human ORC1 homolog, is regulated by cell proliferation via the E2F transcription factor. Mol. Cell Biol. 1996;16:6977–6984. doi: 10.1128/mcb.16.12.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asano M., Wharton R.P. E2F mediates developmental and cell cycle regulation of ORC1 in Drosophila. EMBO J. 1999;18:2435–2448. doi: 10.1093/emboj/18.9.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez C. Coupling cell proliferation and development in plants. Nature Cell Biol. 2005;7:535–541. doi: 10.1038/ncb0605-535. [DOI] [PubMed] [Google Scholar]
- 16.Hulskamp M., Schnittger A., Folkers U. Pattern formation and cell differentiation: trichomes in Arabidopsis as a genetic model system. Int. Rev. Cytol. 1999;186:147–178. doi: 10.1016/s0074-7696(08)61053-0. [DOI] [PubMed] [Google Scholar]
- 17.Kondorosi E., Roudier F., Gendreau E. Plant cell-size control: growing by ploidy? Curr. Opin. Plant Biol. 2000;3:488–492. doi: 10.1016/s1369-5266(00)00118-7. [DOI] [PubMed] [Google Scholar]
- 18.Larkins B.A., Dilkes B.P., Dante R.A., Coelho C.M., Woo Y.M., Liu Y. Investigating the hows and whys of DNA endoreduplication. J. Exp. Bot. 2001;52:183–192. [PubMed] [Google Scholar]
- 19.Menges M., Murray J.A. Synchronous Arabidopsis suspension cultures for analysis of cell-cycle gene activity. Plant J. 2002;30:203–212. doi: 10.1046/j.1365-313x.2002.01274.x. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez-Parra E., Lopez-Matas M.A., Frundt C., Gutierrez C. Role of an atypical E2F transcription factor in the control of Arabidopsis cell growth and differentiation. Plant Cell. 2004;16:2350–2363. doi: 10.1105/tpc.104.023978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez-Parra E., Gutierrez C. Characterization of wheat DP, a heterodimerization partner of the plant E2F transcription factor which stimulates E2F-DNA binding. FEBS Lett. 2000;486:73–78. doi: 10.1016/s0014-5793(00)02239-0. [DOI] [PubMed] [Google Scholar]
- 22.del Pozo J.C., Boniotti M.B., Gutierrez C. Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCF(AtSKP2) pathway in response to light. Plant Cell. 2002;14:3057–3071. doi: 10.1105/tpc.006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clough S.J., Bent A.F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez-Parra E., Frundt C., Gutierrez C. A genome-wide identification of E2F-regulated genes in Arabidopsis. Plant J. 2003;33:801–811. doi: 10.1046/j.1365-313x.2003.01662.x. [DOI] [PubMed] [Google Scholar]
- 25.Xie Q., Suarez-Lopez P., Gutierrez C. Identification and analysis of a retinoblastoma binding motif in the replication protein of a plant DNA virus: requirement for efficient viral DNA replication. EMBO J. 1995;14:4073–4082. doi: 10.1002/j.1460-2075.1995.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwart M., Hirner B., Hummel S., Frommer W.B. Differential expression of two related amino acid transporters with differing substrate specificity in Arabidopsis thaliana. Plant J. 1993;4:993–1002. doi: 10.1046/j.1365-313x.1993.04060993.x. [DOI] [PubMed] [Google Scholar]
- 27.Ludevid M.D., Freire M.A., Gomez J., Burd C.G., Albericio F., Giralt E., Dreyfuss G., Pages M. RNA binding characteristics of a 16 kDa glycine-rich protein from maize. Plant J. 1992;2:999–1003. [PubMed] [Google Scholar]
- 28.Jefferson R.A., Bevan M., Kavanagh T. The use of the Escherichia coli beta-glucuronidase as a gene fusion marker for studies of gene expression in higher plants. Biochem. Soc. Trans. 1987;15:17–18. doi: 10.1042/bst0150017. [DOI] [PubMed] [Google Scholar]
- 29.Perkins G., Diffley J.F. Nucleotide-dependent prereplicative complex assembly by Cdc6p, a homolog of eukaryotic and prokaryotic clamp-loaders. Mol. Cell. 1998;2:23–32. doi: 10.1016/s1097-2765(00)80110-0. [DOI] [PubMed] [Google Scholar]
- 30.Menges M., de Jager S.M., Gruissem W., Murray J.A. Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. Plant J. 2005;41:546–566. doi: 10.1111/j.1365-313X.2004.02319.x. [DOI] [PubMed] [Google Scholar]
- 31.Reichheld J.P., Chaubet N., Shen W.H., Renaudin J.P., Gigot C. Multiple A-type cyclins express sequentially during the cell cycle in Nicotiana tabacum BY2 cells. Proc. Natl Acad. Sci. USA. 1996;93:13819–13824. doi: 10.1073/pnas.93.24.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doerner P., Jorgensen J.E., You R., Steppuhn J., Lamb C. Control of root growth and development by cyclin expression. Nature. 1996;380:520–523. doi: 10.1038/380520a0. [DOI] [PubMed] [Google Scholar]
- 33.Springer J., Kneissl M., Putter V., Grummt F. Identification and characterization of MmORC4 and MmORC5, two subunits of the mouse origin of replication recognition complex. Chromosoma. 1999;108:243–249. doi: 10.1007/s004120050374. [DOI] [PubMed] [Google Scholar]
- 34.Tao Y., Kassatly R.F., Cress W.D., Horowitz J.M. Subunit composition determines E2F DNA-binding site specificity. Mol. Cell Biol. 1997;17:6994–7007. doi: 10.1128/mcb.17.12.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouellette M.M., Chen J., Wright W.E., Shay J.W. Complexes containing the retinoblastoma gene product recognize different DNA motifs related to the E2F binding site. Oncogene. 1992;7:1075–1081. [PubMed] [Google Scholar]
- 36.Kosugi S., Ohashi Y. Interaction of the Arabidopsis E2F and DP proteins confers their concomitant nuclear translocation and transactivation. Plant Physiol. 2002;128:833–843. doi: 10.1104/pp.010642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mariconti L., Pellegrini B., Cantoni R., Stevens R., Bergounioux C., Cella R., Albani D. The E2F family of transcription factors from Arabidopsis thaliana. Novel and conserved components of the retinoblastoma/E2F pathway in plants. J. Biol. Chem. 2002;277:9911–9919. doi: 10.1074/jbc.M110616200. [DOI] [PubMed] [Google Scholar]
- 38.Grafi G., Burnett R.J., Helentjaris T., Larkins B.A., DeCaprio J.A., Sellers W.R., Kaelin W.G., Jr A maize cDNA encoding a member of the retinoblastoma protein family: involvement in endoreduplication. Proc. Natl Acad. Sci. USA. 1996;93:8962–8967. doi: 10.1073/pnas.93.17.8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Q., Sanz-Burgos A.P., Hannon G.J., Gutierrez C. Plant cells contain a novel member of the retinoblastoma family of growth regulatory proteins. EMBO J. 1996;15:4900–4908. [PMC free article] [PubMed] [Google Scholar]
- 40.Gutierrez C., Ramirez-Parra E., Castellano M.M., del Pozo J.C. G(1) to S transition: more than a cell cycle engine switch. Curr. Opin. Plant Biol. 2002;5:480–486. doi: 10.1016/s1369-5266(02)00301-1. [DOI] [PubMed] [Google Scholar]
- 41.Gendreau E., Orbovic V., Hofte H., Traas J. Gibberellin and ethylene control endoreduplication levels in the Arabidopsis thaliana hypocotyls. Planta. 1999;209:513–516. doi: 10.1007/PL00008123. [DOI] [PubMed] [Google Scholar]
- 42.Chang C., Shockey J.A. The ethylene-response pathway: signal perception to gene regulation. Curr. Opin. Plant Biol. 1999;2:352–358. doi: 10.1016/s1369-5266(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 43.Quintana D.G., Thome K.C., Hou Z.H., Ligon A.H., Morton C.C., Dutta A. ORC5L, a new member of the human origin recognition complex, is deleted in uterine leiomyomas and malignant myeloid diseases. J. Biol. Chem. 1998;273:27137–27145. doi: 10.1074/jbc.273.42.27137. [DOI] [PubMed] [Google Scholar]
- 44.Dhar S.K., Dutta A. Identification and characterization of the human ORC6 homolog. J. Biol. Chem. 2000;275:34983–34988. doi: 10.1074/jbc.M006069200. [DOI] [PubMed] [Google Scholar]
- 45.Thome K.C., Dhar S.K., Quintana D.G., Delmolino L., Shahsafaei A., Dutta A. Subsets of human origin recognition complex (ORC) subunits are expressed in non-proliferating cells and associate with non-ORC proteins. J. Biol. Chem. 2000;275:35233–35241. doi: 10.1074/jbc.M005765200. [DOI] [PubMed] [Google Scholar]
- 46.Vashee S., Simancek P., Challberg M.D., Kelly T.J. Assembly of the human origin recognition complex. J. Biol. Chem. 2001;276:26666–26673. doi: 10.1074/jbc.M102493200. [DOI] [PubMed] [Google Scholar]
- 47.Kneissl M., Putter V., Szalay A.A., Grummt F. Interaction and assembly of murine pre-replicative complex proteins in yeast and mouse cells. J. Mol. Biol. 2003;327:111–128. doi: 10.1016/s0022-2836(03)00079-2. [DOI] [PubMed] [Google Scholar]
- 48.Dillin A., Rine J. Separable functions of ORC5 in replication initiation and silencing in Saccharomyces cerevisiae. Genetics. 1997;147:1053–1062. doi: 10.1093/genetics/147.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ehrenhofer-Murray A.E., Rivier D.H., Rine J. The role of Sas2, an acetyltransferase homologue of Saccharomyces cerevisiae, in silencing and ORC function. Genetics. 1997;145:923–934. doi: 10.1093/genetics/145.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iizuka M., Stillman B. Histone acetyltransferase HBO1 interacts with the ORC1 subunit of the human initiator protein. J. Biol. Chem. 1999;274:23027–23034. doi: 10.1074/jbc.274.33.23027. [DOI] [PubMed] [Google Scholar]
- 51.Sharp J.A., Krawitz D.C., Gardner K.A., Fox C.A., Kaufman P.D. The budding yeast silencing protein Sir1 is a functional component of centromeric chromatin. Genes Dev. 2003;17:2356–2361. doi: 10.1101/gad.1131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bose M.E., McConnell K.H., Gardner-Aukema K.A., Muller U., Weinreich M., Keck J.L., Fox C.A. The origin recognition complex and Sir4 protein recruit Sir1p to yeast silent chromatin through independent interactions requiring a common Sir1p domain. Mol. Cell Biol. 2004;24:774–786. doi: 10.1128/MCB.24.2.774-786.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox C.A., Ehrenhofer-Murray A.E., Loo S., Rine J. The origin recognition complex, SIR1, and the S phase requirement for silencing. Science. 1997;276:1547–1551. doi: 10.1126/science.276.5318.1547. [DOI] [PubMed] [Google Scholar]
- 54.Huang D.W., Fanti L., Pak D.T., Botchan M.R., Pimpinelli S., Kellum R. Distinct cytoplasmic and nuclear fractions of Drosophila heterochromatin protein 1: their phosphorylation levels and associations with origin recognition complex proteins. J. Cell Biol. 1998;142:307–318. doi: 10.1083/jcb.142.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shareef M.M., King C., Damaj M., Badagu R., Huang D.W., Kellum R. Drosophila heterochromatin protein 1 (HP1)/origin recognition complex (ORC) protein is associated with HP1 and ORC and functions in heterochromatin-induced silencing. Mol. Biol. Cell. 2001;12:1671–1685. doi: 10.1091/mbc.12.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rohrbough J., Pinto S., Mihalek R.M., Tully T., Broadie K. latheo, a Drosophila gene involved in learning, regulates functional synaptic plasticity. Neuron. 1999;23:55–70. doi: 10.1016/s0896-6273(00)80753-9. [DOI] [PubMed] [Google Scholar]
- 57.Pinto S., Quintana D.G., Smith P., Mihalek R.M., Hou Z.H., Boynton S., Jones C.J., Hendricks M., Velinzon K., Wohlschlegel J.A., et al. latheo encodes a subunit of the origin recognition complex and disrupts neuronal proliferation and adult olfactory memory when mutant. Neuron. 1999;23:45–54. doi: 10.1016/s0896-6273(00)80752-7. [DOI] [PubMed] [Google Scholar]
- 58.Prasanth S.G., Prasanth K.V., Stillman B. Orc6 involved in DNA replication, chromosome segregation, and cytokinesis. Science. 2002;297:1026–1031. doi: 10.1126/science.1072802. [DOI] [PubMed] [Google Scholar]
- 59.Chesnokov I.N., Chesnokova O.N., Botchan M. A cytokinetic function of Drosophila ORC6 protein resides in a domain distinct from its replication activity. Proc. Natl Acad. Sci. USA. 2003;100:9150–9155. doi: 10.1073/pnas.1633580100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du Y.C., Stillman B. Yph1p, an ORC-interacting protein: potential links between cell proliferation control, DNA replication, and ribosome biogenesis. Cell. 2002;109:835–848. doi: 10.1016/s0092-8674(02)00773-0. [DOI] [PubMed] [Google Scholar]