Abstract
Recently, insulin-like growth factor binding proteins (IGFBPs) have been found to be primary mediators of the anti-proliferative actions of the nuclear hormone 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], but dependent on cellular context IGFBPs can also have a mitogenic effect. In this study, we performed expression profiling of all six human IGFBP genes in prostate and bone cancer cells and demonstrated that IGFBP1, 3 and 5 are primary 1α,25(OH)2D3 target genes. In silico screening of the 174 kb of genomic sequence surrounding all six IGFBP genes identified 15 candidate vitamin D response elements (VDREs) close to or in IGFBP1, 2, 3 and 5 but not in the IGFBP4 and 6 genes. The putative VDREs were evaluated in vitro by gelshift assays and in living cells by reporter gene and chromatin immuno-precipitation (ChIP) assays. Of these 10 VDREs appear to be functional. ChIP assays demonstrated for each of these an individual, stimulation time-dependent association profile not only with the vitamin D receptor, but also with first heterodimeric partner the retinoid X receptor, other regulatory complex components and phosphorylated RNA polymerase II. Some of the VDREs are located distantly from the transcription start sites of IGFBP1, 3 and 5, but all 10 VDREs seem to contribute to the regulation of the genes by 1α,25(OH)2D3. In conclusion, IGFBP1, 3 and 5 are primary 1α,25(OH)2D3 target genes that in intact cells are each under the control of multiple VDREs.
INTRODUCTION
The biologically most active vitamin D metabolite, 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], is essential for mineral homeostasis and skeletal integrity (1). However, it also has important roles in the control of the growth and differentiation in normal tissues and malignant cells derived from prostate, breast and bone (2). 1α,25(OH)2D3 mainly acts as a nuclear hormone and mediates its genomic effects via the nuclear receptor vitamin D receptor (VDR) (3). The anti-proliferative effects of 1α,25(OH)2D3 include induction of a G1/G0 cell cycle arrest and stimulation of apoptosis, which are mediated by the up-regulation of tumor suppressors, such as the cyclin-dependent kinase inhibitory proteins p21 and p27 (4), and the down-regulation of oncogene products including Bcl-2 (5) and Myc (6). Mitogens, such as the insulin-like growth factors (IGFs), have also been reported to be down-regulated by 1α,25(OH)2D3 (7). In addition, also the up-regulation of factors which control the actions of mitogens, such as IGF binding proteins (IGFBPs), have important anti-cancer effects (8). Unfortunately, many of the genes involved in these processes, such as the p27 and bcl-2, are secondary 1α,25(OH)2D3 targets (9). However, the IGFBP3 gene was shown to be a primary VDR target (10) and is therefore of special interest for understanding the mechanisms of the cell-regulatory actions of 1α,25(OH)2D3. IGFBP3 belongs to the six-member IGFBP family that bind IGFs with high affinity and specificity (11). IGFBPs also mediate IGF-independent actions, including inhibition of cell growth and induction of apoptosis (12). Since gene families often encode products, which have overlapping functions, we wondered if this was also true at the level of responsiveness to a particular ligand. Therefore, we focused in this study on the regulation of the IGFBP gene family by 1α,25(OH)2D3.
An essential prerequisite for the direct modulation of transcription by 1α,25(OH)2D3 is the location of at least one activated VDR protein close to the transcription start site (TSS) of the respective primary 1α,25(OH)2D3 target gene. This is achieved through the specific binding of VDR to a vitamin D response element (VDRE) (13). In detail, the DNA-binding domain of the VDR contacts the major groove of a double-stranded hexameric DNA sequence with the optimal RGKTCA (R = A or G, K = G or T) core binding sequence. VDR binds as a dimer to DNA and in most cases the nuclear receptor retinoid X receptor (RXR) is its heterodimeric partner (13). Simple VDREs are therefore formed by two hexameric core binding motifs in a direct repeat (DR) or everted repeat (ER) orientation. The optimal spacing for DR-type VDREs was found to be 3 and 4 nt (DR3 and DR4) (14,15) and that of ER-type VDREs 7 to 9 nt (ER7, ER8 and ER9), respectively (16,17). Although individual VDREs have been shown to be able to induce transactivation on their own, the presence of multiple VDREs in any given regulatory region will allow a more flexible and complex regulation of the respective gene. Recently, we have shown that the primary 1α,25(OH)2D3 target genes CYP24 (18) and cyclin C (19) each contain four functional VDREs within the first 10 kb of their promoter region. In the promoter of the human IGFBP3 gene only one DR3-type VDRE so far has been reported, which is located ∼3.2 kb upstream of the TSS (10). However, this does not exclude the presence of additional VDREs.
Binding of VDR to a VDRE is a central step in 1α,25(OH)2D3 signaling. In the absence of ligand, corepressor proteins, such as SMRT, NCoR and Alien (20), link DNA-bound VDR to enzymes with histone deacetylase activity that cause chromatin condensation (21). This provides VDR with intrinsic repressive properties comparable with both retinoic acid and thyroid hormone receptors (20). Ligand binding to the VDR causes a conformational change within its ligand-binding domain that results in the replacement of corepressors by coactivator proteins of the p160-family, such as SRC-1, TIF2 and RAC3 (22). These coactivator proteins link ligand-activated VDR to enzymes displaying histone acetyltransferase activity, that cause chromatin relaxation and thereby reversing the action of unliganded VDR (23). In a subsequent step, ligand-activated VDR changes rapidly from interacting with p160-coactivators to those of the mediator complex, such as TRAP220/DRIP205 (24). The mediator complex acts as a bridge from activated VDR to the basal transcriptional machinery (25). In this way ligand-activated VDR executes two tasks, the modification of chromatin and the regulation of transcription.
In this study, we performed expression profiling of all six human IGFBP gene family members in prostate and bone cancer cells and demonstrated that the genes IGFBP1, 3 and 5 are primary 1α,25(OH)2D3 targets. In silico screening of all IGFBP genes identified 15 candidate VDREs close to IGFBP1, 2, 3 and 5, but none within IGFBP4 and 6. Of these 10 VDREs seem to be functional and chromatin immuno-precipitation (ChIP) assays demonstrated that for each of them an individual, ligand-stimulation time-dependent association profile with VDR, RXR, SMRT, SRC-1, TRAP220 and phosphorylated RNA polymerase II (P-Pol II). Some of the VDREs are located rather distantly from the TSS of the IGFBP1, 3 and 5, but all 10 functional VDREs appear to contribute to their regulation by 1α,25(OH)2D3.
MATERIALS AND METHODS
Cell culture
The human prostate cancer cell line PC-3 and the human osteosarcoma cell line SaOS-2 were cultured in DMEM containing 10% fetal bovine serum (FBS), while for the human breast cancer cell line MCF-7, α-MEM supplemented with 5% FBS was used. Both media also contained 2 mM l-glutamine, 0.1 mg/ml streptomycin and 100 U/ml penicillin and the cells were kept in a humidified 95% air/5% CO2 incubator. FBS was stripped of lipophilic compounds, such as endogenous 1α,25(OH)2D3, by stirring it with 5% activated charcoal (Sigma–Aldrich, St Louis, MO) for 3 h at room temperature. Charcoal was then removed by centrifugation and sterile filtration. Prior to mRNA or chromatin extraction, cells were grown overnight in phenol red-free DMEM supplemented with 5% charcoal-stripped FBS to reach a density of 50–60% confluency. Cells were treated then with solvent (ethanol, 0.1% final concentration) or 10 nM 1α,25(OH)2D3 (kindly provided by Dr Lise Binderup, LEO Pharma, Ballerup, Denmark) for up to 24 h for RNA extractions and for up to 4 h for chromatin preparations.
RNA extraction and real-time quantitative PCR
Total RNA was extracted using the Mini RNA Isolation II kit (Zymo Research, HiSS Diagnostics, Freiburg, Germany) and cDNA synthesis was performed for 1 h at 37°C using 1 µg of total RNA as a template, 100 pmol oligodT18 primer and 40 U RT (Fermentas, Vilnius, Lithuania). Real-time quantitative PCR was performed in an IQ-cycler (BioRad, Hercules, CA) using the dye SybrGreen I (Molecular Probes, Leiden, The Netherlands). For each reaction, 1 U Hot Start Taq polymerase (Fermentas) and 3 mM MgCl2 were used and the PCR cycling conditions were: 45 cycles of 30 s at 95°C, 30 s at 62°C and 40 s at 72°C. The gene-specific primer pairs (and product sizes) were as follows: IGFBP1 gene, forward 5′-CACAGGAGACATCAGGAGAAG-3′ and reverse 5′-GAGCTTTGGAAGAGCAGAAATG-3′ (507 bp); IGFBP2 gene, forward 5′-GATGACCACTCAGAAGGAG-3′ and reverse 5′-CTGCTGCTCATTGTAGAAGAG-3′ (257 bp); IGFBP3 gene, forward 5′-AAGTTGACTACGAGTCTCAG-3′ and reverse 5′-AATCAGTTCACCACAAACAGA-3′ (439 bp); IGFBP4 gene, forward 5′-CACGAGGACCTCTACATCATC-3′ and reverse 5′-CAGGACTCAGACTCAGACTC-3′ (297 bp); IGFBP5 gene, forward 5′-GTCAAGATCGAGAGAGACTC-3′ and reverse 5′-GAAGGTTTGCACTGCTTTCTC-3′ (370 bp); IGFBP6 gene, forward 5′-GTGTCCAAGACACTGAGATG-3′ and reverse 5′-CAACACCAACACTCTTTCCAAC-3′ (404 bp); p27 gene, forward 5′-GAGAAGCACTGCAGAGACAT-3′ and reverse 5′-AAGAATCGTCGGTTGCAGGT-3′ (242 bp) and acidic riboprotein P0 (ARP0, also known as 36B4) control gene, forward 5′-AGATGCAGCAGATCCGCAT-3′ and reverse 5′-GTGGTGATACCTAAAGCCTG-3′ (318 bp). The control gene ARP0 was checked against a second control gene RPL13A and neither gene was found to be affected by any of the treatments at the time points used (data not shown). PCR product quality was monitored using post-PCR melt curve analysis. Fold inductions were calculated using the formula 2−(ΔΔCt), where ΔΔCt is the ΔCt(1α,25(OH)2D3) − ΔCt(Ethanol), ΔCt is Ct(IGFBPn or p27) − Ct(ARP0) and Ct is the cycle at which the threshold is crossed.
DNA constructs
Full-length cDNAs for human VDR (15) and human RXRα (26) were subcloned into the T7/SV40 promoter-driven pSG5 expression vector (Stratagene, LaJolla, CA). The same constructs were used for both T7 RNA polymerase-driven in vitro transcription/translation of the respective cDNAs and for viral promoter-driven overexpression in mammalian cells. Each two copies of the VDREs derived from the rat atrial natriuretic factor (ANF) (27) and the 15 candidate response elements (REs) of the IGFBP genes (for sequence see Table 1) were fused with the thymidine kinase promoter driving the firefly luciferase reporter gene. All constructs were verified by sequencing.
Table 1.
Sequence and position of putative REs within the human IGFBP1, 2, 3 and 5 genes
| RE | Type | Gene | Position | Strand | Gene area | Sequence |
|---|---|---|---|---|---|---|
| rANF | DR3 | rat ANF | −907 | + | promoter | agAGGTCAtgaAGGACA |
| RE1 | DR3 | IGFBP1 | −4533 | − | promoter | tcGGTTCTggaAGATCA |
| RE2 | ER7 | IGFBP1 | −3111 | + | promoter | TGACCCctgccagGGGGCA |
| RE3 | DR3 | IGFBP1/3 | +5327 | + | intergenic | ctGGGGCAggcGGGGCA |
| RE4 | ER7 | IGFBP1/3 | +15359 | + | intergenic | TGAACTtggagatAGGTCA |
| RE5 | ER7 | IGFBP1/3 | +18886 | + | intergenic | TGAACTccacagaAGTTCA |
| RE6 | DR3 | IGFBP3 | −387 | − | promoter | cgGGGTCAaggAGATCG |
| RE7 | DR3 | IGFBP3 | −396 | − | promoter | taAGGGCGgcgGGGTCA |
| RE8 | DR3 | IGFBP3 | −3347 | + | promoter | gaGGTTCAccgGGTGCA |
| RE9 | DR3 | IGFBP2 | +17676 | + | 1st intron | aaAGGTCAgagGGGGCA |
| RE10 | DR4 | IGFBP2 | +24655 | − | 1st intron | agGGTTCAttgaGGGGCA |
| RE11 | DR3 | IGFBP2/5 | +35665 | − | intergenic | ctGGTTCAttcAGATCA |
| RE12 | DR3 | IGFBP2/5 | +37171 | + | intergenic | gtGGGTTAtatGGGTCG |
| RE13 | ER9 | IGFBP5 | +7775 | + | 1st intron | TGACCCatcacagccAGTTCA |
| RE13+ | ER11 | IGFBP5 | +7775 | + | 1st intron | TGACCCacatcacagccAGTTCA |
| RE14 | DR4 | IGFBP5 | −4679 | + | promoter | atAGGGCAgagcAGGGCA |
| RE15 | ER8 | IGFBP5 | −7384 | + | promoter | TGAACCgaaagctgGGTTCA |
The known DR3-type VDRE of the rat ANF gene (28) serves as a comparison. Capital letters indicate hexameric core binding sites. The position of the RE is given relative to the TSS of the indicated gene.
Transfection and luciferase reporter gene assays
MCF-7, PC-3 and SaOS-2 cells were seeded into 6-well plates (105 cells/ml) and grown overnight in phenol red-free DMEM supplemented with 5% charcoal-stripped FBS. Plasmid DNA containing liposomes were formed by incubating a reporter plasmid and the expression vector for human VDR (each 1 µg) with 10 µg N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (DOTAP, Roth, Karlsruhe, Germany) for 15 min at room temperature in a total volume of 100 µl. After dilution with 900 µl phenol red-free DMEM, the liposomes were added to the cells. Phenol red-free DMEM supplemented with 500 µl 15% charcoal-stripped FBS was added 4 h after transfection. At this time, 100 nM 1α,25(OH)2D3 or solvent were also added. The cells were lysed 16 h after onset of stimulation using reporter gene lysis buffer (Roche Diagnostics, Mannheim, Germany). The constant light signal luciferase reporter gene assay was performed as recommended by the supplier (Canberra-Packard, Groningen, The Netherlands). Luciferase activities were normalized with respect to protein concentration and induction factors were calculated as the ratio of luciferase activity of ligand-stimulated cells to that of solvent controls.
Gelshift assay
In vitro translated VDR and RXR proteins were generated by coupled in vitro transcription/translation using their respective pSG5-based full-length cDNA expression constructs and rabbit reticulocyte lysate as recommended by the supplier (Promega, Madison, WI). Protein batches were quantified by test translation in the presence of [35S]methionine. The specific concentration of the receptor proteins was adjusted to ∼4 ng/µl (10 ng corresponds to ∼0.2 pmol) after taking the individual number of methionine residues per protein into account. Gelshift assays were performed with 10 ng of the appropriate in vitro translated proteins. The proteins were incubated for 15 min in a total volume of 20 µl binding buffer (150 mM KCl, 1 mM DTT, 0.2 µg/µl poly(dI-C), 5% glycerol and 10 mM HEPES, pH 7.9). Constant amounts (1 ng) of [32P]-labeled double-stranded oligonucleotides (50 000 c.p.m.) containing one copy of the respective REs (Table 1) were then added and incubation was continued for 20 min at room temperature. Protein–DNA complexes were resolved by electrophoresis through 8% non-denaturing polyacrylamide gels (mono- to bisacrylamide ratio 19:1) in 0.5x TBE (45 mM Tris–HCl, 45 mM boric acid and 1 mM EDTA, pH 8.3) for 105 min at 200 V and quantified on a FLA-3000 reader (Fuji, Tokyo, Japan) using ScienceLab99 software (Fuji).
ChIP assays
Nuclear proteins were crosslinked to genomic DNA by adding formaldehyde for 15 min directly to the medium to a final concentration of 1%. Crosslinking was stopped by adding glycine to a final concentration of 0.125 M and incubating for 5 min at room temperature on a rocking platform. The medium was removed and the cells were washed twice with ice-cold phosphate-buffered saline (PBS) (140 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4 and 8.1 mM Na2HPO4•2H2O). The cells were collected by scraping in ice-cold PBS supplemented with a protease inhibitor cocktail (Roche Diagnostics). After centrifugation the cell pellets were resuspended in lysis buffer (1% SDS, 10 mM EDTA, protease inhibitors and 50 mM Tris–HCl, pH 8.1) and the lysates were sonicated to result in DNA fragments of 300–1000 bp in length. Cellular debris was removed by centrifugation and the lysates were diluted 1:10 in ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM NaCl, protease inhibitors and 16.7 mM Tris–HCl, pH 8.1). Non-specific background was removed by incubating the chromatin resuspension with a salmon sperm DNA/protein A agarose slurry (Upstate Biotechnology, Lake Placid, NY) for 30 min at 4°C with agitation. The samples were centrifuged and the recovered chromatin solutions were incubated with 5 µl of indicated antibodies overnight at 4°C with rotation. The antibodies against VDR (sc-1008), RXRα (sc-553), SMRT (sc-1610), SRC-1 (sc-7216), TRAP220 (sc-5334), P-Pol II (sc-13583), IgG (sc-2027) and p53 (sc-6243) were obtained from Santa Cruz Biotechnologies (Heidelberg, Germany). The immuno-complexes were collected with 60 µl of protein A agarose slurry (Upstate Biotechnology) for 2 h at 4°C with rotation. The beads were pelleted by centrifugation for 1 min at 4°C at 100× g and washed sequentially for 5 min by rotation with 1 ml of the following buffers: low salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 150 mM NaCl and 20 mM Tris–HCl, pH 8.1), high salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 500 mM NaCl and 20 mM Tris–HCl, pH 8.1) and LiCl wash buffer (0.25 mM LiCl, 1% Nonidet P-40, 1% sodium deoxycholate, 1 mM EDTA and 10 mM Tris–HCl, pH 8.1). Finally, the beads were washed twice with 1 ml TE buffer (1 mM EDTA and 10 mM Tris–HCl, pH 8.0). The immuno-complexes were then eluted by adding 250 µl elution buffer (1% SDS and 100 mM NaHCO3) and incubation for 15 min at room temperature with rotation. After centrifugation, the supernatant was collected and the elution was repeated. The supernatants were combined and the cross-linking was reversed by adding NaCl to final concentration of 200 mM and incubating overnight at 65°C. The remaining proteins were digested by adding proteinase K (final concentration 40 µg/ml) and incubation for 1 h at 45°C. Genomic DNA fragments were recovered using the Qiaex II Gel Extraction Kit (Qiagen, Hilden, Germany).
PCR of chromatin templates
For each of the 14 candidate RE-containing genomic regions and the TSS of the genes IGFBP1, 2, 3 and 5 primer pairs were designed (Table 2), optimized and controlled by running PCRs with 25 ng genomic DNA (input) as a template. When running immuno-precipitated DNA (output) as a template, following PCR profile was used: preincubation for 5 min at 94°C, 40 cycles of 30 s at 95°C, 30 s at 60°C and 30 s at 72°C and one final incubation for 10 min at 72°C. The PCR products were separated by electrophoresis through 2.0% agarose. Dye SybrGreen (1 µl) (1:2500 dilution) was added to each sample before loading. Gel images were scanned on a Fuji FLA3000 reader using ScienceLab99 software.
Table 2.
Genomic PCR primers
| Region (gene) | Location | Primer sequences (5′–3′) |
|---|---|---|
| 1 (IGFBP1) | −4371 to −4156 | CTCTACATCTTTGAGGGTGGG |
| GACGTGGGAGAATCGTTTGAG | ||
| 2 (IGFBP1) | −3246 to −3062 | CTGCACTTCCCAGAGCTGAG |
| GTTACAGCCGATGATGTGAC | ||
| 3 (IGFBP1/3) | +5077 to +5415 | CTGTGGTGATGTTGCCACCTG |
| GAGGATGGGGACTAATGGAGAG | ||
| 4 (IGFBP1/3) | +15255 to +15576 | GAGAACTAAATGGAAATGCTGGAG |
| CTGGAATAAATCCCACTGATCATG | ||
| 5 (IGFBP1/3) | +18796 to +19008 | CTTACTAGGCAGCGTTCTCATG |
| CTGTCTACCAGCGACAACCAAC | ||
| 6/7 (IGFBP3) | −280 to −483 | GTCACCTTGTCGTCTACAAG |
| CACGAGGTACACACGAATGC | ||
| 8 (IGFBP3) | −3286 to −3465 | CTGGAGTGACTCACCAGAGTC |
| CTTGCCTGCCTCTCTCAGCTG | ||
| 9 (IGFBP2) | +17599 to +17734 | GAATGTGTCCTGAGTGCCAG |
| GTCCTGAGACTTTGTGGGTG | ||
| 10 (IGFBP2) | +18965 to +19136 | GTTACTCCATGTTGGTCAGACTG |
| GAGATTCACTCACGAGTCAGAAC | ||
| 11 (IGFBP2/5) | +35559 to +35740 | CATGAACTGCAACTGTGATGTC |
| CAATAAGCCACCAGGTGCTAG | ||
| 12 (IGFBP2/5) | +37095 to +37295 | GAGACAGGAGTGGAGTAGTC |
| CACACTGCCTGGAGCAGGTG | ||
| 13 (IGFBP5) | +7887 to +7635 | CTGTCTGTTCCTCATACATTC |
| CTCCCTTGTTTGGTCCCTAG | ||
| 14 (IGFBP5) | −4572 to −4740 | GAGCCAGCTGTGACGCAGAG |
| GAACATGCCAGATCCCATGATC | ||
| 15 (IGFBP5) | −7275 to −7535 | GATGGCTCTGTACTCAAGGAG |
| CTCAGAGGATAAGTCCTGAGTC | ||
| IGFBP1 TSS | −100 to +153 | GAACACTCAGCTCCTAGCGTG |
| CTGACATCTCCAGGCGCGAG | ||
| IGFBP3 TSS | −129 to +92 | GAGCAGCACCAGCAGAGTC |
| CAGGGATGGGGCGACAGTAC | ||
| IGFBP2 TSS | −62 to +117 | GAAGGGAGTGGTCTCCAAAAG |
| CACTCTCGGCAGCATGCTG | ||
| IGFBP5 TSS | −32 to +92 | GTTCTACGCGAAGTCCGGAG |
| CTCCTTGGCATCCTTGCCTG |
Sequence and location of the primer pairs used to detect genomic regions containing putative VDREs or TSS within the IGFBP1 and 3 and IGFBP2 and 5 gene pairs. The location is indicated in relation to the closest TSS.
RESULTS
Basal expression of IGFBP gene family members in prostate and bone cancer
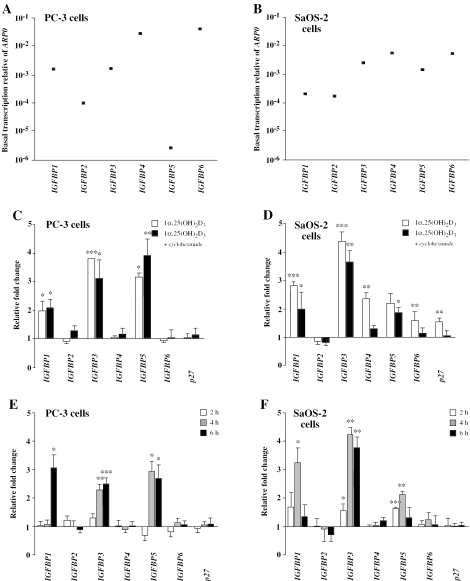
The basal mRNA expression levels of all six members of the IGFBP gene family were monitored by real-time quantitative PCR in relation to the control gene ARP0 in PC-3 human prostate cancer (Figure 1A) and SaOS-2 human osteosarcoma cells (Figure 1B). In both cell lines the genes IGFBP4 and 6 showed the highest expression, but in SaOS-2 cells these maximal levels were ∼10-fold lower than in PC-3 cells. In PC-3 cells the mRNA amount of IGFBP1 and 3 was ∼15-fold lower, that of IGFBP-2 >300-fold lower and that of IGFBP5 even 12 000-fold lower than that of IGFBP4 and 6 (Figure 1A). In contrast, in SaOS-2 cells the differences in the expression of the IGFBP gene family members showed less pronouced differences, since the mRNA levels of IGFBP3 and 5 were only ∼3-fold lower and that of IGFBP1 and 2 only 30-fold lower than that of IGFBP4 and 6 (Figure 1B).
Figure 1.
Expression profiling of the human IGFBP gene family in prostate and bone cancer cells. Real-time quantitative PCR was used to determine the ratio of the basal mRNA expression of the six IGFBP genes relative to the control gene ARP0 in PC-3 (A) and SaOS-2 cells (B). A logarithmic scale is employed on the Y-axis to better present the data. In the same cell lines, the induction of mRNA levels of the six IGFBP genes and of the known secondary 1α,25(OH)2D3 target gene p27 after a 24 h treatment with 10 nM 1α,25(OH)2D3 was determined in the absence and presense of 10 µM cycloheximde (C and D). The early time course (2 to 6 h) of the mRNA expression of all seven genes in response to 10 nM 1α,25(OH)2D3 was also measured in PC-3 (E) and SaOS-2 (F) cells. Data points (A and B) and columns (C–F) indicate the means of at least three independent cell treatments and the bars represent standard deviations. The standard deviations in (A and B) are too small to be visible in relation to the data points. Two-tailed Student's t-tests were performed to determine the significance of the mRNA induction by 1α,25(OH)2D3 in reference to solvent controls (*P < 0.05, **P < 0.01, ***P < 0.001).
IGFBP1, 3 and 5 are primary 1α,25(OH)2D3 target genes
PC-3 and SaOS-2 cells were treated for 24 h with 10 nM 1α,25(OH)2D3 in the absence or presence of the protein translation inhibitor cycloheximide in order to differentiate between primary and secondary effects of the VDR ligand. The relative fold induction of the mRNA amounts of the six IGFBP genes and of the known secondary 1α,25(OH)2D3 target gene p27 (9) were determinded by real-time quantitative PCR (Figure 1C and D). In PC-3 cells the genes IGFBP1, 3 and 5 were found to be 2- to 4-fold induced by 1α,25(OH)2D3 and this induction was not significantly modulated by the presence of cycloheximide (Figure 1C). The three other IGFBP gene family members and the p27 gene showed in this cell line no response to the natural VDR ligand. In SaOS-2 cells the genes IGFBP1, 3 and 5 were also 2- to 4-fold inducible by 1α,25(OH)2D3 without being significantly affected by cycloheximide (Figure 1D). Interestingly, in this cell line even the genes IGFBP4, IGFBP6 and p27 responded 1.5- to 2.2-fold to 1α,25(OH)2D3 treatment, but this induction disappeared in the presence of cycloheximide. This observation indicates that the IGFBP4 and 6 gene are only secondary 1α,25(OH)2D3 targets as is known for the p27 gene. The IGFBP2 gene did not respond at all to ligand treatment.
Next, we performed in both cell lines a time course analysis of the early mRNA expression of all six IGFBP genes and the p27 gene in response to 1α,25(OH)2D3 over 2, 4 and 6 h (Figure 1E and F). In confirmation of the results obtained in Figure 1C and D, only the IGFBP1, 3 and 5 gene, but neither the genes IGFBP2, 4 and 6 nor the p27 gene responded within this time frame to the natural VDR ligand. After 4 and 6 h of ligand treatment the response of the IGFBP3 gene showed a stable 2.5- and 4-fold induction in PC-3 and SaOS-2 cells, respectively. In contrast, the expression of the genes IGFBP1 and 5 demonstrated a more cell-specific profile (compare Figure 1E and F). In PC-3 cells IGFBP1 mRNA was induced 3-fold after 6 h of stimulation with 1α,25(OH)2D3, while in SaOS-2 cells it showed a comparable peak already after 4 h. Moreover, in PC-3 cells IGFBP5 mRNA expression was after 4 and 6 h constantly 3-fold increased, while in SaOS-2 cells it displayed a smaller peak of only 2-fold induction after 4 h stimulation with ligand. Taken together, in both cell lines the genes IGFBP1, 3 and 5 but not IGFBP2, 4 and 6 are primary 1α,25(OH)2D3 target genes.
In silico screening for putative VDREs
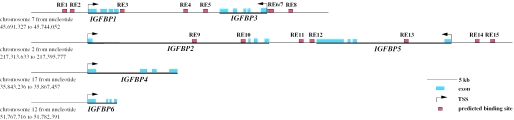
Based on a list of >15 known natural VDREs (28) we obtained the consensus hexameric core sequence RGDKYR (R = G or A, D = A,G or T, K = G or T, Y = C or T) and used it to screen in silico the gene area (defined as 10 kb upstream of the TSS until the end of the last exon) of each of the six human IGFBP genes for putative VDREs. Since the genes IGFBP1 and 3 as well as IGFBP2 and 5 are found adjacent to each other in the genome (Figure 2), we also screened the intergenic area between these two gene pairs. For the specific search for VDREs we considered only hexameric core sequence pairs in DR3, DR4, ER6, ER7, ER8 or ER9 orientation. Moreover, we kept only RE candidates formed of hexamers with maximal two deviations from the optimal RGKTCA core binding sequence in our list and excluded putative VDREs being located in Alu repetitive sequence (representing ∼12% of the 174 kb genomic sequence screened in total). These restrictions finally resulted in 15 putative VDREs, of which two (REs 1 and 2) were located in the area of the IGFBP1 gene, three (REs 3, 4 and 5) between the genes IGFBP1 and 3, three (REs 6, 7 and 8) in the IGFBP3 gene, two (REs 9 and 10) in the IGFBP2 gene, two (REs 11 and 12) between the genes IGFBP2 and 5 and three (REs 13, 14 and 15) in the IGFBP5 gene (Figure 2). In contrast, no REs were found in the genes IGFBP4 and 6.
Figure 2.
Overview on the genomic organization and location of the six human IGFBP family members. Human genomic DNA (174 kb) (thin black line) from four different chromosomes comprising all six human IGFBP genes were screened in silico for putative VDREs. Fifteen candidate REs (red boxes) were identified in the genes IGFBP1, 2, 3 and 5 but none in IGFBP4 and 6. The sequences of the REs are indicated in Table 1.
The majority (eight) of the REs are of DR3-type. There were also two DR4-type, three ER7-type, one ER8-type and one ER9-type REs (Table 1). The DR3-type RE8 in the promoter of the IGFBP3 gene has already been described as a functional VDRE (10), whereas the 14 remaining putative VDREs are novel. Interestingly, REs 6 and 7 form a composite RE of three hexameric binding motifs. Moreover, all REs were screened in the database dbSNP (www.ncbi.nlm.nih.gov/SNP) for known small nucleotide polymorphisms (SNPs) and for RE13 a 2 nt insertion variation was found (RE13+ in Table 1), which was included in the following RE evaluation. In summary, in silico screening resulted in 15 putative VDREs in the genes IGFBP1, 2, 3 and 5, but none in the genes IGFBP4 and 6. This result is mostly in agreement with the observation that the genes IGFBP1, 3 and 5 but not IGFBP2, 4 and 6 are primary 1α,25(OH)2D3 targets.
Functionality of putative VDREs within the IGFBP genes
The functionality of the 15 putative VDREs identified in silico, the SNP variation of RE13 (RE13+) and the reference DR3-type VDRE of the rat ANF gene (27) was assessed by reporter gene assays in transiently transfected MCF-7 breast cancer cells (Figure 3A), PC-3 cells (Figure 3B) and SaOS-2 cells (Figure 3C). MCF-7 cells represent an established 1α,25(OH)2D3 responding cell line (19) and were chosen as reference to other studies. Two copies of each of the 15 putative VDREs, RE13+ and the rat ANF VDRE were fused with the thymidine kinase promoter driving the firefly luciferase reporter gene. The response to 1α,25(OH)2D3 of the three different cell lines transfected with these constructs was monitored after 16 h. As expected, MCF-7 cells showed the highest inducibilty by 1α,25(OH)2D3: compared with the 16.9-fold inducibility of the reference VDRE, REs 8, 2 and 13 were even more potent with a 51.4-, 28.5- and 22.4-fold induction of reporter gene activity (Figure 3A). The 13.2-, 9.9-, 9.5-, 6.9-, 5.0- and 4.9-fold induction mediated by two copies of the REs 5, 9, 4, 3, 10 and 14, respectively, indicate that they are able to function as VDREs. In contrast, the SNP variation RE13+ lost significantly its 1α,25(OH)2D3 responsiveness and therefore represents a less efficient VDRE. Moreover, neither the absolute reporter gene activity nor the inducibility of REs 1, 6, 7, 11, 12 and 15 was sufficient for a functional VDRE.
Figure 3.
Functionality of putative VDREs derived from human IGFBP genes. Reporter gene assays were performed with extracts from MCF-7 cells (A), PC-3 cells (B) and SaOS-2 cells (C) that were transiently transfected with luciferase reporter constructs each containing two copies of one of the 15 candidate VDREs and the SNP variation of RE13 (RE13+) or of the rat ANF DR3-type VDRE and an expression vector for human VDR. Cells were treated for 16 h with either solvent or 100 nM 1α,25(OH)2D3. Relative luciferase activity is shown and fold inductions are indicated above the columns. Columns represent means of at least three experiments and bars indicate SDs. Two-tailed Student's t-tests were performed to determine the significance of reporter gene induction by 1α,25(OH)2D3 in reference to solvent controls (*P < 0.05, **P < 0.01, ***P < 0.001).
The inducibility of PC-3 (Figure 3B) and SaOS-2 cells (Figure 3C) were in average 5- and 10-fold lower than that of MCF-7 cells, but concerning relative inducibility all three cell lines provided similar results. RE8 was in all cells more inducible than the reference VDRE, RE13 showed a comparable inducibility than the reference VDRE and also REs 2, 4, 5, 9 and 14 demonstrated a statistically significant inducibility by 1α,25(OH)2D3 in all tested cell lines. However, owing to cell-specific variations in the basal activity of the reporter gene constructs carrying the different REs, the absolute activities of the REs do not match perfectly between the three different cell lines.
In vitro characterization of putative VDREs within the IGFBP genes
To assess the relative VDR–RXR heterodimer binding, gelshift assays were performed using in vitro translated VDR and RXR proteins either alone or in combination and the 15 putative VDREs of the IGFBP genes (Figure 4). The conditions were identical to our earlier DR3-type VDRE comparative study (28) and the DR3-type VDRE of the rat ANF gene was chosen again as reference. The ER7-type REs 2 and 5 bound 3.5-fold more effectively VDR–RXR heterodimers than the reference DR3-type VDRE and also the ER9-type RE13 and the ER7-type RE4 were 1.5- and 1.2-fold stronger, respectively. Moreover, the DR3-type REs 9, 8 and 11 showed with 132, 110 and 75% of the binding of the reference RE good affinity for VDR–RXR heterodimers. Finally, the ER8-type RE15 (67% heterodimer binding capacity) and the DR4-type RE10 (44%) can be considered as VDREs as well. Interestingly, the ER11-type SNP variation of RE13 (RE13+) still showed 67% binding capacity of the reference VDRE (which equals 44% of RE13). In contrast, the binding of VDR–RXR heterodimers to the REs 1, 3, 6, 7, 12 and 14 was too low in our stringent assay conditions to consider them as efficient VDREs. Taken together, according to standardized in vitro criteria developed in our previous studies (28), 7 (REs 4, 8, 9, 10, 11, 13 and 15) of the 15 candidate REs show 0.5- to 1.5-fold of DNA-binding capacity of the reference VDRE and can be considered as good VDREs. In addition, two REs (REs 2 and 5) even demonstrated a 3.5-fold higher DNA-binding affinity than the reference VDRE and should be considered as high affinity VDREs.
Figure 4.
In vitro analysis of the putative VDREs derived from the human IGFBP gene areas. Gelshift experiments were performed with in vitro translated human VDR and human RXR alone or in combination and in the presence of different [32P]-labeled REs representing the 15 candidate VDREs and the SNP variation of RE13 (RE13+) of the human IGFBP genes and the rat ANF DR3-type reference VDRE. Protein–DNA complexes were resolved from free probe through non-denaturing 8% polyacrylamide gels. Representative gels are shown. The relative amount of VDR–RXR heterodimer complex formation was quantified on a FLA-3000 reader in relation to the reference VDRE. Numbers below the gels indicate the means of at least three independent gel shift experiments and their SDs are given in brackets. NS indicates non-specific complexes.
Overall there is good correlation between the reporter gene assay results (Figure 3) and the in vitro analysis (Figure 4). REs 2 and 13 are confirmed as very good VDREs and REs 1, 6, 7 and 12 showed no response in either of the two assays. The response of RE8 in the functional assay was higher and that of RE5 lower than expected, but both REs appeared to be very effective VDREs. Finally, REs 4 and 9 were also confirmed as VDREs. In summary, a number of VDREs (REs 2, 4, 5, 8, 9 and 13) were shown to respond effectively to 1α,25(OH)2D3 and its receptor in both evaluation series.
Functionality of putative VDREs in chromatin context of living cells
We next examined whether VDR was located to the genomic regions containing these REs in living cells. Chromatin was extracted from PC-3 cells which had been grown overnight in the presence of 5% charcoal-treated FBS, stimulated for 0, 0.5, 1, 2, 3 and 4 h with 10 nM 1α,25(OH)2D3 and then crosslinked for 15 min in the presence of formaldehyde. ChIP assays were performed using an antibody against VDR. The genomic DNA fragments that were recovered from reverse-crosslinked chromatin served as templates for PCRs with primers specific for the regions containing the 15 putative VDREs (Table 2). Since REs 6 and 7 are in the same region, they cannot be detected separately and therefore represented together. Representative agarose gels of the PCR products from all treatment times are shown (Figure 5A). The input lane serves as a reference for comparable detection sensitivity for the 14 genomic regions within the IGFBP1 and 3 and IGFBP2 and 5 gene tandems (Figure 2) and ChIP assays using IgG or anti-p53 antibody served as controls. Interestingly, with the exception of regions 11, 12 and 15, on the remaining 11 genomic regions VDR binding could be detected (Figure 5A). On some of the latter regions, such as regions 2, 3, 6/7, 8, 9, 10, 13 and 14, VDR binding was found in the absence of ligand. Moreover, 1α,25(OH)2D3 treatment did not significantly affect the binding of its receptor to these genomic regions, but on some regions (2, 8, 9 and 10) the tendency of a maximal binding after 2 h could be observed. Only on regions 1, 4 and 5 a significant 1α,25(OH)2D3-dependent modulation of the VDR association could be detected.
Figure 5.
Association of RE-containing genomic regions and proximal promoters of IFGBP genes with VDR and other nuclear proteins. Chromatin was extracted from PC-3 cells that had been treated for indicated time periods with 10 nM 1α,25(OH)2D3. ChIP experiments were performed with anti-VDR, anti-RXR, anti-SMRT, anti-SRC-1, anti-TRAP220 or anti-P-Pol II antibodies. Anti-IgG and anti-p53 antibodies were used as non-specific controls. These display some background signal for the regions 9, 10 and 14. The association of VDR and its partner proteins was monitored on the 14 RE-containing genomic regions (A and B) of the genes IGFBP1, 2, 3 and 5 and their proximal promoters (C). Representative agarose gels are shown.
For a deeper analysis of the 11 VDR-associated regions of the IGFBP1/3 and 2/5 gene cluster, further ChIP assays were performed with antibodies against RXR, the corepressor SMRT, the coactivator SRC-1, the mediator protein TRAP220 and P-Pol II (Figure 5B). With the exception of region 1, on the remaining 10 genomic regions RXR association was found. This RXR binding was not significantly modulated by 1α,25(OH)2D3 treatment in most cases. In the latter aspect, the binding of SMRT to the 10 VDR- and RXR-positive genomic regions provided a clearer picture. In the absence of ligand SMRT binding was detected on all 10 regions containing a putative VDRE with the exception of regions 9 and 10. This SMRT binding was diminished after 30–120 min of 1α,25(OH)2D3 treatment. Interestingly, on some of these eight regions (regions 4, 5, 13 and 14) SMRT binding was reconstituted after 1 to 3 h of ligand application, while for regions 2, 3, 6/7 and 8 the measurement window of 4 h was apparently too short to observe the return of the corepressor. This indicates that for each region an idiosyncratic corepressor association profile exists. A similar individual profile is observed concerning the binding of SRC-1 to the 10 genomic regions. On none of the regions SRC-1 was observed to bind in the absence of ligand and it took 30–240 min of 1α,25(OH)2D3 treatment to observe coactivator association. Moreover, the duration of the SRC-1 association varied between ∼1 h for the genomic regions 2, 4, 13 and 14 to >3 h for regions 9 and 10. The association of the mediator TRAP220 and P-Pol II with the 10 genomic regions appeared to be timely coordinated and showed for most regions a peak of ∼1 h duration after 1–2 h of ligand treatment. Taken together, the IGFBP1/3 and 2/5 gene cluster contain 10 VDR- and RXR-associated regions (2, 3, 4, 5, 6/7, 8, 9, 10, 13 and 14), which each shows an individual, ligand-dependent profile of SMRT, SRC-1, TRAP220 and P-Pol II binding.
The IGFBP2 gene is not transcriptionally regulated by 1α,25(OH)2D3
The REs 9 and 10 are located within the large first intron of the IGFBP2 gene but are already >17 and 24 kb downstream of the TSS (Figure 2). This raised the question, whether they are involved in the regulation of the IGFBP2 gene or whether they may regulate the IGFBP5 gene, although the TSS of the latter gene is even more distant. We tried to answer this question indirectly by assessing via ChIP assays a 1α,25(OH)2D3-dependent association of VDR with the TSS of the genes IGFBP1, 2, 3 and 5 (Figure 5C). From this it was found that VDR interacts with the proximal promoter of the genes IGFBP1, 3 and 5 but not to that of IGFBP2. The VDR interaction with the TSS of the IGFBP1 gene was clearly ligand-dependent with a peak after 1 h stimulation, whereas that on the IGFBP3 and 5 gene TSS was only weakly modulated by 1α,25(OH)2D3. These findings confirm the real-time quantitative PCR results (Figure 1) that the genes IGFBP1, 3 and 5 but not the IGFBP2 gene are regulated by VDR and its ligand. This observation also suggests that the association of VDR to REs 9 and 10 is not related to a regulation of the gene, IGFBP2, in which they are located.
DISCUSSION
This study describes the regulation of multiple IFGBP gene family members by the hormone 1α,25(OH)2D3. We confirmed a previous study (10) that IGFBP3 is a primary VDR target gene and found in addition that its direct genome neighbor, the IGFBP1 gene, is also a primary target of 1α,25(OH)2D3. Moreover, we even could identify a third family member, the IGFBP5 gene, as a primary VDR target, whereas the mRNA expression of its tandem partner, the IGFBP2 gene, was not regulated by the nuclear receptor and its ligand. We observed these primary ligand responses in prostate and bone cancer cells, but it can be assumed that these three genes may also be VDR targets in other 1α,25(OH)2D3 responsive tissues. The 2- to 4-fold induction of IGFBP1, 3 and 5 is not a very strong up-regulation, but it is in the order of what was observed with most other 1α,25(OH)2D3 target genes (29,30). The genes IGFBP4 and 6 showed in both tested cell lines the highest basal expression of the six IGFBP genes, so that a further up-regulation of their expression through 1α,25(OH)2D3 may not be necessary. However, in SaOS-2 cells, which show a significantly lower IGFBP4 and 6 mRNA expression than PC-3 cells, both genes were shown to be secondary 1α,25(OH)2D3 targets.
Our finding that three members of the IFGBP gene family respond to 1α,25(OH)2D3 increases the impact of IGF-1 and the regulation of its circulating amounts by IGFBPs in models of the anti-proliferative action of 1α,25(OH)2D3 and its synthetic analogs (31). In addition, IGFBPs mediate IGF-independent actions, including the activation of the p21 gene, causing cell cycle arrest or cell death through induction of apoptosis (32). However bound to cellular membranes, IGFBPs can have mitogenic, IGF-dependent effects on cellular growth (33,34). For example, the effect of IGFBP5 induction has been described mitogenic, when induced by androgens in prostate cells (35). This complicates the interpretation of the up-regulation of the IGFBP family members by 1α,25(OH)2D3. The differential regulation of several other primary and secondary 1α,25(OH)2D3 target genes, such as the down-regulation of IGF-1 and the up-regulation of the p21 gene, has to be considered in context of the response of IFGBPs. Since the mitogenic effect of IGFBPs is IGF-dependent, 1α,25(OH)2D3 seems to promote the IGF-independent pathway of IGFBPs.
The in silico screening performed in this study involved a defined six-member gene family and 174 kb genomic sequence. After a number of carefully selected restrictions the screen resulted in 15 candidate VDREs. ChIP assays indicated that 10 of these 15 REs are bound by VDR in intact PC-3 cells. This represents a 67% success rate of the in silico prediction of VDR binding sites, which is much higher than in comparable screenings (36). Moreover, non-ligand responsive genes, such as IGFBP4 and 6, did not contain any VDRE candidates, although 24 and 15 kb, respectively, of their genomic sequence were screened. The other non-ligand responsive gene, IGFBP2, contains two VDREs (REs 9 and 10) within its first intron. However, ChIP analysis of the proximal promoter region of the IGFBP2 gene indicated that these VDREs cannot be in contact with the TSS of this gene. It is therefore more probable that they are involved in the regulation of the neighboring IGFBP5 gene. The high success rate of our combined in silico screen/ChIP analysis method suggests that its application on even larger screenings involving megabases of genomic sequence is possible.
It is important to note that our in silico screening was not restricted to regulatory regions that comprise only maximal 2 kb of sequence upstream and downstream of the TSS, as in a recent whole genome screen for regulatory elements (37), but involved up to 10 kb upstream sequence, intronic and even intergenic sequences. Therefore, our method reveals candidate REs that are located at >30 kb distannce from the TSS that is regulated by this VDRE. Based on the present understanding of enhancers, DNA looping and chromatin units being flanked by insulators or matrix attachment sites (38) these distances are no limitations. However, such large distances between RE and TSS cannot be assessed by older experimental approaches, such as transiently transfected promoter reporter gene fusion constructs and the validation of critical promoter regions via truncations or point mutations. In contrast, ChIP assays omit the need of transient transfections and allow the analysis of every region in a genome in vivo or in situ.
The observation that the IGFBP1/3 gene cluster contains six functional VDR associated regions (2, 3, 4, 5, 6/7 and 8) and the IGFBP5 gene area four such regions (9, 10, 13 and 14) supports the model of multiple VDREs per primary 1α,25(OH)2D3 target gene, which we developed during our analysis of the CYP24 and cyclin C gene (18,19). Interestingly, the VDR-associated genomic regions contain three ER7-type (REs 2, 4 and 5) and one ER9-type (RE 13) VDREs. All four REs showed to bind VDR–RXR heterodimers strongly in vitro and are potent VDREs in reporter gene assays. Although synthetic ER7-type VDREs have been known since more than a decade (16), this study demonstrates for the first time the existence of functional ER7-type VDREs in vivo. Similarly, the ER9-type RE13 is one of the first natural VDREs of this type. Interestingly, the SNP variation that increases the spacing of this VDRE by 2 nt, forming the ER11-type RE13+, significantly decreases its ability to function as a VDRE in vitro and in reporter gene assays. Unfortunately, the SNP variation occurs only with a frequency of 3% in the population, so that we presently have no cellular system testing its effect in ChIP assays.
In conclusion, our study has provided insight into the regulation of the IGFBP gene family by 1α,25(OH)2D3. We demonstrated that IGFBP1, 3 and 5 are primary 1α,25(OH)2D3 target genes. Each of these three genes contains multiple functional VDR associated regions in their genomic area suggesting a complex regulation by 1α,25(OH)2D3.
Acknowledgments
We would like to thank Dr Lise Binderup for 1α,25(OH)2D3 and Dr Thomas W. Dunlop for critical reading of the manuscript. Grants from the Academy of Finland, the Finnish Cancer Organisation and the Finnish Technology Agency TEKES supported this research. Funding to pay the Open Access publication charges for this article was provided by Academy of Finland.
Conflict of interest statement. None declared.
REFERENCES
- 1.Sutton A.L., MacDonald P.N. Vitamin D: more than a ‘bone-a-fide’ hormone. Mol. Endocrinol. 2003;17:777–791. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 2.Mørk Hansen C., Binderup L., Hamberg K.J., Carlberg C. Vitamin D and cancer: effects of 1,25(OH)2D3 and its analogs on growth control and tumorigenesis. Front. Biosci. 2001;6:D820–D848. doi: 10.2741/hansen. [DOI] [PubMed] [Google Scholar]
- 3.Jones G., Strugnell S.A., DeLuca H.F. Current understanding of the molecular actions of vitamin D. Physiol. Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 4.Liu M., Lee M.-H., Cohen M., Bommakanti M., Freedman L.P. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 5.Xu H.M., Tepper C.G., Jones J.B., Fernandez C.E., Studzinski G.P. 1,25-Dihydroxyvitamin D3 protects HL60 cells against apoptosis but down-regulates the expression of the bcl-2 gene. Exp. Cell Res. 1993;209:367–374. doi: 10.1006/excr.1993.1322. [DOI] [PubMed] [Google Scholar]
- 6.Pan Q., Simpson R.U. c-myc intron element-binding proteins are required for 1,25-dihydroxyvitamin D3 regulation of c-myc during HL-60 cell differentiation and the involvement of HOXB4. J. Biol. Chem. 1999;274:8437–8444. doi: 10.1074/jbc.274.13.8437. [DOI] [PubMed] [Google Scholar]
- 7.Xie S.P., Pirianov G., Colston K.W. Vitamin D analogues suppress IGF-I signalling and promote apoptosis in breast cancer cells. Eur. J. Cancer. 1999;35:1717–1723. doi: 10.1016/s0959-8049(99)00200-2. [DOI] [PubMed] [Google Scholar]
- 8.Colston K.W., Perks C.M., Xie S.P., Holly J.M. Growth inhibition of both MCF-7 and Hs578T human breast cancer cell lines by vitamin D analogues is associated with increased expression of insulin-like growth factor binding protein-3. J. Mol. Endocrinol. 1998;20:157–162. doi: 10.1677/jme.0.0200157. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y.C., Chen J.Y., Hung W.C. Vitamin D3 receptor/Sp1 complex is required for the induction of p27Kip1 expression by vitamin D3. Oncogene. 2004;23:4856–4861. doi: 10.1038/sj.onc.1207621. [DOI] [PubMed] [Google Scholar]
- 10.Peng L., Malloy P.J., Feldman D. Identification of a functional vitamin D response element in the human insulin-like growth factor binding protein-3 promoter. Mol. Endocrinol. 2004;18:1109–1119. doi: 10.1210/me.2003-0344. [DOI] [PubMed] [Google Scholar]
- 11.Hwa V., Oh Y., Rosenfeld R.G. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr. Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 12.Lee K.W., Ma L., Yan X., Liu B., Zhang X.K., Cohen P. Rapid apoptosis induction by IGFBP-3 involves an insulin-like growth factor-independent nucleomitochondrial translocation of RXRalpha/Nur77. J. Biol. Chem. 2005;280:16942–16948. doi: 10.1074/jbc.M412757200. [DOI] [PubMed] [Google Scholar]
- 13.Carlberg C., Polly P. Gene regulation by vitamin D3. Crit. Rev. Eukaryot. Gene Expr. 1998;8:19–42. doi: 10.1615/critreveukargeneexpr.v8.i1.20. [DOI] [PubMed] [Google Scholar]
- 14.Quack M., Carlberg C. Ligand-triggered stabilization of vitamin D receptor/retinoid X receptor heterodimer conformations on DR4-type response elements. J. Mol. Biol. 2000;296:743–756. doi: 10.1006/jmbi.2000.3499. [DOI] [PubMed] [Google Scholar]
- 15.Carlberg C., Bendik I., Wyss A., Meier E., Sturzenbecker L.J., Grippo J.F., Hunziker W. Two nuclear signalling pathways for vitamin D. Nature. 1993;361:657–660. doi: 10.1038/361657a0. [DOI] [PubMed] [Google Scholar]
- 16.Schräder M., Müller K.M., Nayeri S., Kahlen J.P., Carlberg C. VDR-T3R receptor heterodimer polarity directs ligand sensitivity of transactivation. Nature. 1994;370:382–386. doi: 10.1038/370382a0. [DOI] [PubMed] [Google Scholar]
- 17.Schräder M., Nayeri S., Kahlen J.P., Müller K.M., Carlberg C. Natural vitamin D3 response elements formed by inverted palindromes: polarity-directed ligand sensitivity of vitamin D3 receptor–retinoid X receptor heterodimer-mediated transactivation. Mol. Cell Biol. 1995;15:1154–1161. doi: 10.1128/mcb.15.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Väisänen S., Dunlop T.W., Sinkkonen L., Frank C., Carlberg C. Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of 1alpha,25-dihydroxyvitamin D3. J. Mol. Biol. 2005;350:65–77. doi: 10.1016/j.jmb.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 19.Sinkkonen L., Malinen M., Saavalainen K., Väisänen S., Carlberg C. Regulation of the human cyclin C gene via multiple vitamin D3 -responsive regions in its promoter. Nucleic Acid Res. 2005;33:2440–2451. doi: 10.1093/nar/gki502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke L.J., Baniahmad A. Co-repressors 2000. FASEB J. 2000;14:1876–1888. doi: 10.1096/fj.99-0943rev. [DOI] [PubMed] [Google Scholar]
- 21.Polly P., Herdick M., Moehren U., Baniahmad A., Heinzel T., Carlberg C. VDR-Alien: a novel, DNA-selective vitamin D3 receptor–corepressor partnership. FASEB J. 2000;14:1455–1463. doi: 10.1096/fj.14.10.1455. [DOI] [PubMed] [Google Scholar]
- 22.Leo C., Chen J.D. The SRC family of nuclear receptor coactivators. Gene. 2000;245:1–11. doi: 10.1016/s0378-1119(00)00024-x. [DOI] [PubMed] [Google Scholar]
- 23.Castillo A.I., Jimenez-Lara A.M., Tolon R.M., Aranda A. Synergistic activation of the prolactin promoter by vitamin D receptor and GHF-1: role of coactivators, CREB-binding protein and steroid hormone receptor coactivator-1 (SRC-1) Mol. Endocrinol. 1999;13:1141–1154. doi: 10.1210/mend.13.7.0320. [DOI] [PubMed] [Google Scholar]
- 24.Fondell J.D., Guermah M., Malik S., Roeder R.G. Thyroid hormone receptor-associated proteins and general positive cofactors mediate thyroid hormone receptor function in the absence of the TATA box-binding protein-associated factors of TFIID. Proc. Natl Acad. Sci. USA. 1999;96:1959–1964. doi: 10.1073/pnas.96.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rachez C., Lemon B.D., Suldan Z., Bromleigh V., Gamble M., Näär A.M., Erdjument-Bromage H., Tempst P., Freedman L.P. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 26.Levin A.A., Sturzenbecker L.J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A., et al. 9-Cis retinoic acid stereoisomer binds and activates the nuclear receptor RXRα. Nature. 1992;355:359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- 27.Kahlen J.P., Carlberg C. Functional characterization of a 1,25-dihydroxyvitamin D3 receptor binding site found in the rat atrial natriuretic factor promoter. Biochem. Biophys. Res. Commun. 1996;218:882–886. doi: 10.1006/bbrc.1996.0157. [DOI] [PubMed] [Google Scholar]
- 28.Toell A., Polly P., Carlberg C. All natural DR3-type vitamin D response elements show a similar functionality in vitro. Biochem. J. 2000;352:301–309. [PMC free article] [PubMed] [Google Scholar]
- 29.Swami S., Raghavachari N., Muller U.R., Bao Y.P., Feldman D. Vitamin D growth inhibition of breast cancer cells: gene expression patterns assessed by cDNA microarray. Breast Cancer Res. Treat. 2003;80:49–62. doi: 10.1023/A:1024487118457. [DOI] [PubMed] [Google Scholar]
- 30.Palmer H.G., Sanchez-Carbayo M., Ordonez-Moran P., Larriba M.J., Cordon-Cardo C., Munoz A. Genetic signatures of differentiation induced by 1α,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003;63:7799–7806. [PubMed] [Google Scholar]
- 31.Rozen F., Pollak M. Inhibition of insulin-like growth factor I receptor signaling by the vitamin D analogue EB1089 in MCF-7 breast cancer cells: A role for insulin-like growth factor binding proteins. Int. J. Oncol. 1999;15:589–594. doi: 10.3892/ijo.15.3.589. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan A.V., Peehl D.M., Feldman D. The role of vitamin D in prostate cancer. Recent Results Cancer Res. 2003;164:205–221. doi: 10.1007/978-3-642-55580-0_15. [DOI] [PubMed] [Google Scholar]
- 33.Kelley K.M., Oh Y., Gargosky S.E., Gucev Z., Matsumoto T., Hwa V., Ng L., Simpson D.M., Rosenfeld R.G. Insulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamics. Int. J. Biochem. Cell Biol. 1996;28:619–637. doi: 10.1016/1357-2725(96)00005-2. [DOI] [PubMed] [Google Scholar]
- 34.Yin P., Xu Q., Duan C. Paradoxical actions of endogenous and exogenous insulin-like growth factor-binding protein-5 revealed by RNA interference analysis. J. Biol. Chem. 2004;279:32660–32666. doi: 10.1074/jbc.M401378200. [DOI] [PubMed] [Google Scholar]
- 35.Gregory C.W., Kim D., Ye P., D'Ercole A.J., Pretlow T.G., Mohler J.L., French F.S. Androgen receptor up-regulates insulin-like growth factor binding protein-5 (IGFBP-5) expression in a human prostate cancer xenograft. Endocrinology. 1999;140:2372–2381. doi: 10.1210/endo.140.5.6702. [DOI] [PubMed] [Google Scholar]
- 36.Wasserman W.W., Sandelin A. Applied bioinformatics for the identification of regulatory elements. Nature Rev. Genet. 2004;5:276–287. doi: 10.1038/nrg1315. [DOI] [PubMed] [Google Scholar]
- 37.Xie X., Lu J., Kulbokas E.J., Golub T.R., Mootha V., Lindblad-Toh K., Lander E.S., Kellis M. Systematic discovery of regulatory motifs in human promoters and 3′ UTRs by comparison of several mammals. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogata K., Sato K., Tahirov T.H. Eukaryotic transcriptional regulatory complexes: cooperativity from near and afar. Curr. Opin. Struct. Biol. 2003;13:40–48. doi: 10.1016/s0959-440x(03)00012-5. [DOI] [PubMed] [Google Scholar]