Abstract
The Mitf-Tfe family of basic helix–loop–helix-leucine zipper (bHLH-Zip) transcription factors encodes four family members: Mitf, Tfe3, Tfeb, and Tfec. In vitro, each protein in the family can bind DNA as a homo- or heterodimer with other family members. Mutational studies in mice have shown that Mitf is essential for melanocyte and eye development, whereas Tfeb is required for placental vascularization. Here, we uncover a role for Tfe3 in osteoclast development, a role that is functionally redundant with Mitf. Although osteoclasts seem normal in Mitf or Tfe3 null mice, the combined loss of the two genes results in severe osteopetrosis. We also show that Tfec mutant mice are phenotypically normal, and that the Tfec mutation does not alter the phenotype of Mitf, Tfeb, or Tfe3 mutant mice. Surprisingly, our studies failed to identify any phenotypic overlap between the different Mitf–Tfe mutations. These results suggest that heterodimeric interactions are not essential for Mitf-Tfe function in contrast to other bHLH-Zip families like Myc/Max/Mad, where heterodimeric interactions seem to be essential.
Basic helix–loop–helix-leucine zipper (bHLH-Zip) proteins regulate gene expression by binding to the E-box (CANNTG) as hetero- or homodimers; some dimers activate gene expression whereas others repress it. The prototypic bHLH-Zip family is Myc/Max/Mad. Work from many laboratories suggests that these proteins function through a complex network of interacting proteins (1). The ubiquitous Max protein is at the heart of this network. When Myc concentration is high, it complexes with Max, resulting in increased gene expression with concomitant effects on the cell. In contrast, when the concentration of a Mad family member is high, Max complexes with Mad, resulting in reduced gene expression. Mad-mediated repression results from the interaction of Mad proteins with mSin3 proteins, which in turn interact with the histone deacetylases to moderate gene expression (2). The emerging complexity of this network suggests that the specificity of this protein family is because of protein–protein interactions as well as different target gene specificity (3). It has been difficult, however, to confirm these results in vivo, as a result of the complexity of this family and that many of the mutations are embryonic-lethal.
Another well studied bHLH-Zip protein family is Mitf-Tfe. Four Mitf-Tfe family members have been identified: microphthalmia (Mitf), Tfe3, Tfeb, and Tfec. Mutations in Mitf were recognized as early as 1942 and since then over 20 spontaneous or induced mutations have been identified at the locus (4). Interestingly, about half of the mutations are semidominantly inherited (i.e., they show a partial phenotype in the heterozygous condition) and about half are recessively inherited. All Mitf mutations result in defects in neural crest-derived melanocytes, manifested by reduction or lack of pigment in the coat and inner ear. Many of the mutations also affect differentiation of the neuroepithelial-derived retinal pigment epithelium of the eye, ultimately resulting in microphthalmia, and some Mitf mutations result in a reduction in mast cell numbers. Thus, the Mitf mutations are pleiotropic, affect many different cell types, and can be arranged in an allelic series (4).
A mutation has been made in the Tfeb gene (TfebFcr) and results in embryonic lethality because of defects in placental vascularization; the labyrinthine cells fail to express vascular endothelial growth factor, the embryonic vasculature is unable to invade the placenta, and the embryos degenerate as a result of hypoxia (5). Unfortunately, the embryos die before melanocytes or mast cells are formed thus it is unclear whether Tfeb is important for the development of these cell types as well.
Molecular analysis of the semidominant Mitf mutations shows that they affect the basic or transcriptional activation domains of the protein (6–8). These mutant proteins cannot bind DNA; however, they can still dimerize with proteins such as Tfe3 and thereby interfere with DNA binding of the wild-type partner (9). The dominant negative behavior of these mutant proteins likely accounts for the phenotype seen in heterozygous mice. Consistent with this hypothesis, the recessive Mitf mutations affect the dimerization domain of the protein or lack Mift expression (6, 7, 10).
Interestingly, a few of the strong semidominant Mitf mutations also produce osteopetrosis because of osteoclast defects, which is not seen in Mitf mutant mice carrying loss of function mutations at the locus. This phenotype is limited to homozygous animals indicating that the mutations are recessive with respect to the osteoclasts. Bone marrow transplantation experiments performed on the original Mitfmicrophthalmia (Mitfmi) mutation (one of the semidominant Mitf mutations that produces osteopetrosis) showed that osteopetrosis can be rescued by transplanting cell suspensions from wild-type spleen and bone marrow to the mutant animals (11). Similarly, transplantation of bone marrow from Mitfmi mice to lethally irradiated wild-type mice induces osteopetrosis (12). Ultrastructural analysis shows that osteoclasts from Mitfmi mice are smaller than normal, lack ruffled borders (13), and exhibit fusion disability (14). These strong dominant negative Mitf alleles therefore seem to induce osteopetrosis by affecting osteoclast differentiation; the defect is cell-autonomous with respect to osteoclasts.
Several observations suggest that Mitf-Tfe proteins mediate their effects as heterodimers, like Myc/Max/Mad. For example, the Mitf-Tfe family members are coexpressed in several cell types, including cells affected by Mitf mutations such as osteoclasts (15) and melanocytes (16). Furthermore, all possible combinations of Mitf-Tfe homo- and heterodimers can form in vitro (9), and Mitf-Tfe heterodimers have been shown to exist in cell cultures (15). Taken together, these data suggest that the absence of any one protein would produce a phenotype that is at least partially overlapping with one or more of the other family members. Here, we produce germ-line null mutations in Tfe3 and Tfec and show that the osteopetrosis associated with the dominant negative Mitf alleles is most likely a result of dominant negative interference with Tfe3. Surprisingly, none of the Mitf-Tfe phenotypes studied overlap, suggesting that the homodimer, not the heterodimer, is the essential functional unit of the Mitf-Tfe family.
Materials and Methods
Targeted Disruption of Tfe3 and Tfec.
Tfe3 and Tfec genomic clones were isolated from a 129/Sv mouse library (Stratagene) by using human Tfe3 and rat Tfec cDNA probes, respectively (17, 18). The X-chromosome origin (19) of the most strongly hybridizing Tfe3 clone (clone Tfe3-1) was confirmed by interspecific backcross mapping. Similarly, the chromosomal origin of the most strongly hybridizing Tfec clone (clone Tfec4-1) was confirmed by mapping. To generate the Tfe3 targeting vector, two NotI fragments from clone Tfe3-1 were subcloned into pBluescript. A 5.5-kb ClaI-NotI fragment, containing sequences located upstream of the known Tfe3 coding region, was excised and subcloned. Next, a 1.8-kb fragment from the end of the gene was generated by PCR, using primers P1 (5′-gcaacgctccaaagacctggggatccggcagcgg) and P2 (5′-ccagtgccaggactagttggactggcaattccc) and fused with the 5.5-kb clone in the correct orientation. A PgkNeo cassette (20) was inserted between these fragments in the opposite transcriptional orientation, and a tk gene was placed at the 5′ end of the targeting construct. To generate the Tfec targeting construct, a 1.4-kb EcoRI-EcoRV fragment from clone Tfec4-1 was cloned into pBluescript. A 6.0-kb EcoRV-SacI fragment containing the 3′ end of the gene was ligated onto the 1.4-kb clone in the correct orientation, and a PgkNeo cassette was inserted between these fragments in the same transcriptional orientation. Finally, the tk gene was placed at the 5′ end of the construct. The mutant constructs were linearized with NotI and electroporated into CJ7 embryonic stem (ES) cells as described (21). DNAs derived from G418/FIAU-resistant ES clones were identified by hybridization. For Tfe3, recombinant clones containing the predicted rearranged bands were obtained at a frequency of 2/179 or 1.1%. For Tfec, a single positive clone was obtained from 300 clones analyzed and its structure confirmed by using an internal probe. The Tfe3 and Tfec mutant clones were injected into C57BL/6J blastocysts, and the resulting chimeras were identified by agouti coat color. Chimeras that transmitted the Tfe3 and Tfec mutations through the germ line were mated to both 129/Sv and C57BL/6J females to establish lines. For genotyping purposes, DNA was isolated from ES cells and from mouse tails as described (22, 23) and processed for Southern analysis (24). The genotypes of MitfMi-wh and Mitfmi-ew animals were ascertained by PCR analysis.
Mice.
The mutant strains C57BL/6J- Tfe3Fcr, C57BL/6J- TfecFcr, NAW-Mitfmi-ew/Mitfmi-ew, C57BL/6J-MitfMi-wh/MitfMi-wh, and C57BL/6J-Mitfmi/Mitfmi were maintained and propagated in the Mammalian Genetics Laboratory (Advanced BioScience Laboratories–Basic Research Program, Frederick, MD), and the Mitfmi-vga9/Mitfmi-vga9 mice were maintained and propagated at the National Institutes of Health.
Results
Tfe3 and Tfec Mutations.
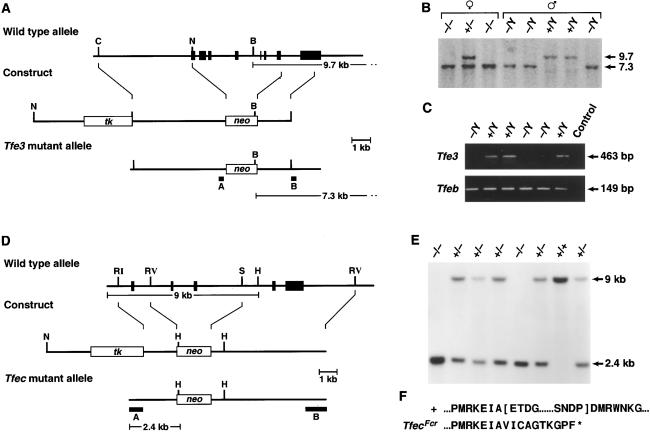
To begin to analyze the functions of Tfe3 and Tfec in vivo and determine whether any of these functions overlap with Mitf and Tfeb, we made germ-line null mutations in both genes by means of gene targeting in mouse embryonic stem cells. Part, or all, of the bHLH-Zip domain of each protein was replaced with pGKNeo (Fig. 1 A and D), and the mutations, designated Tfe3Fcr and TfecFcr, respectively, were introduced into the mouse germ line by standard techniques (Fig. 1 B and E). As expected, the Tfec message expressed in homozygous TfecFcr mice is missing 132 bp of the Tfec bHLH-Zip domain and contains a frameshift mutation, which deletes the remainder of the protein (Fig. 1F). In contrast, the Tfe3Fcr mutation destabilizes the Tfe3Fcr message, and no stable mRNA is made in homozygous Tfe3Fcr mice (Fig. 1C). Surprisingly, homozygous Tfe3Fcr and TfecFcr mice are indistinguishable from their wild-type littermates; they are viable and fertile, normally pigmented, have normal eyes and mast cells, and show no osteopetrosis (Fig. 2F, Table 1, and data not shown). Furthermore, detailed analysis of homozygous Tfe3Fcr mice (C. Tunyaplin and K. Calame, unpublished observations) shows that B-cell development is normal in contrast to what was observed in Tfe3:Rag2 chimeric mice (25).
Figure 1.
Generation of Tfe3Fcr and TfecFcr mutant mice. (A) The Tfe3 knockout. The exon-intron organization of Tfe3 is shown at the top with exons indicated as black boxes. A 5.5-kb genomic fragment containing seven Tfe3 exons, the 5′ end of Tfe3, and the bHLH-Zip domains was replaced with PgkNeo. The first exon corresponds to nucleotides 14–153 of mouse Tfe3 (19). (B) Identification of Tfe3Fcr mutant animals. Tail DNA from the progeny of a heterozygous Tfe3Fcr intercross was digested with BamHI and analyzed by Southern analysis with probe B, which detects 9.7- (wild-type) and 7.3-kb (mutant) alleles. Tfe3Fcr males only transmit the mutant allele to their daughters, confirming the X chromosome linkage of Tfe3 (19). (C) Tfe3 expression in mutant animals. Kidney RNA from Tfe3Fcr and wild-type males was reversed-transcribed and PCR-amplified by using Tfe3 primers 5′-ccaagctggcttcccaggctctcac and 5′-gttaatgttgaatcgcctgcgtcg. The wild-type 463-bp fragment corresponds to nucleotides 245–708 of Tfe3 (19). The same samples were also amplified with Tfeb primers 5′-ccctgtctagcagccacctgaacg and 5′-gccgctccttggccagggctctgctc as a control. Primer pairs spanned exon-intron boundaries to eliminate false positives. (D) The Tfec knockout. A 5-kb fragment containing two Tfec bHLH exons was replaced with PgkNeo. Deleted exons correspond to nucleotides 507–639 of rat Tfec (18). (E) Identification of TfecFcr mutant animals. Tail DNA from the progeny of a heterozygous mutant intercross was digested with HindIII and analyzed by Southern analysis with probe A, which detects 9.0- (wild-type) and 2.4-kb (mutant) alleles. (F) Sequence of the TfecFcr mutant transcript. Kidney RNA from wild-type and mutant animals was reverse-transcribed and PCR-amplified with Tfec primers 5′-cagtgatgctggctgtgc and 5′-gtagccacttgatgtagtcc. Sequencing of the mutant transcript showed that it lacked two conserved Tfec functional domains (indicated by brackets in the wild-type sequence) and is out of frame for the rest of the coding region. B, BamHI; C, ClaI; N, H, HindIII; RI, EcoRI; N, NotI; RV, EcoRV; S, SacI.
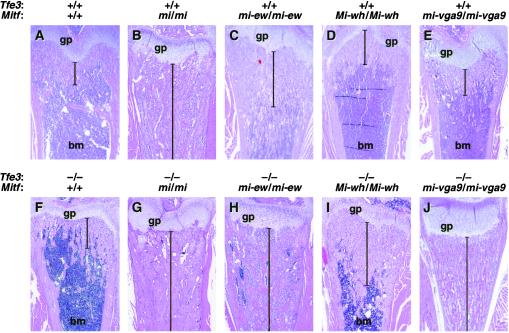
Figure 2.
Osteopetrosis in Mitf and Tfe3Fcr mutant animals. (A–J) Sections through femurs of wild-type and mutant mice showing different levels of osteopetrosis. The genotypes of the different animals are shown at the top of each image. Brackets indicate the extent of osteopetrosis. gp, growth plate; bm, bone marrow.
Table 1.
A summary of phenotypes of Mitf and Tfe mutant mice
| Genotype | Phenotype
|
||||
|---|---|---|---|---|---|
| Eyes | Pigment | Mast cells | Osteoclasts | Placenta | |
| Single mutants | |||||
| Tfe3Fcr/Tfe3Fcr | — | — | — | — | — |
| TfecFcr/TfecFcr | — | — | — | — | — |
| TfebFcr/TfebFcr | ND | ND | ND | ND | ++++ |
| Mitfmi-vga9/Mitfmi-vga9 | ++++ | ++++ | + | — | — |
| Mitfmi/Mitfmi | ++++ | ++++ | + | ++++ | — |
| MitfMi-wh/MitfMi-wh | ++ | ++++ | + | — | — |
| Mitfmi-ew/Mitfmi-ew | ++++ | ++++ | + | + | — |
| Double and triple mutants | |||||
| MitfMi-wh/MitfMi-wh; TfecFcr/TfecFcr | ++ | ++++ | + | — | — |
| MitfMi-wh/MitfMi-wh; Tfe3Fcr/Tfe3Fcr | ++ | ++++ | + | +++ | — |
| Mitfmi/Mitfmi; TfecFcr/TfecFcr | ++++ | ++++ | + | ++++ | — |
| Mitfmi/Mitfmi; Tfe3Fcr/Tfe3Fcr | ++++ | ++++ | + | ++++ | — |
| Tfe3Fcr/Tfe3Fcr; TfecFcr/TfecFcr | — | — | — | — | — |
| Mitfmi-ew/Mitfmi-ew; Tfe3Fcr/+ | ++++ | ++++ | + | +++ | — |
| Mitfmi-ew/Mitfmi-ew; Tfe3Fcr/Tfe3Fcr | ++++ | ++++ | + | ++++ | — |
| Mitfmi-vga9/Mitfmi-vga9; Tfe3Fcr/Tfe3Fcr | ++++ | ++++ | + | ++++ | — |
| MitfMi-wh/MitfMi-wh; TfecFcr/TfecFcr, Tfe3Fcr/Tfe3Fcr | ++ | ++++ | + | +++ | — |
| Tfeb double mutants | |||||
| Tfe3Fcr/Tfe3Fcr; TfebFcr/+ | — | — | — | — | — |
| TfecFcr/TfecFcr; TfebFcr/+ | — | — | — | — | — |
| MitfMi-wh/MitfMi-wh; TfebFcr/+ | ++ | ++++ | + | — | — |
—, no change; +, affected; ++++, severely affected. ND, not determined because of embryonic lethality.
Double-Mutant Combinations.
To examine functional redundancy among the Mitf-Tfe family members, we generated mice that were double homozygous for all possible combinations of Mitf (three alleles, described in Table 2), TfecFcr, and Tfe3Fcr, as well as mice that were triple homozygous for MitfMi-white (MitfMi-wh), TfecFcr, and Tfe3Fcr. In addition, we generated mice that were simultaneously heterozygous for TfebFcr and homozygous for MitfMi-wh, TfecFcr, or Tfe3Fcr (the TfebFcr mutation was kept heterozygous because the TfebFcr mutation is lethal when homozygous). The results are summarized in Table 1. Except for the double- and triple-mutant combinations involving Mitf and Tfe3, no functional redundancy was observed. For example, double homozygous Tfe3Fcr;TfecFcr mice are phenotypically normal, double homozygous MitfMi-wh;TfecFcr mice are identical to homozygous MitfMi-wh mice, homozygous Mitfmi;TfecFcr or Mitfmi;Tfe3Fcr mice are identical to Mitfmi mice, and triple homozygous MitfMi-wh;TfecFcr;Tfe3Fc mice are identical to Mitfmi mice (Table 1). Similarly, no genetic interactions were observed between TfebFcr in the heterozygous condition and Tfe3Fcr, TfecFcr, and MitfMi-wh in the homozygous condition (Table 1). In contrast, mice homozygous for Tfe3Fcr and Mitfmi-vga9 (an Mitf null allele) developed severe osteopetrosis, a phenotype not produced by loss of function mutations in either gene alone (compare Fig. 2 J to E and F; Table 1). In the double mutants, the bone marrow cavity is completely filled with bony material (Fig. 2J), tooth eruption is delayed, and the animals die within 3 weeks of birth.
Table 2.
Mitf alleles used in this study
| Allele symbol | Phenotype
|
Molecular defect | |
|---|---|---|---|
| Heterozygote | Homozygote | ||
| Mitfmi | Iris pigment less than in wild type; occasional spots on belly, head, or tail | White coat; microphthalmia; incisors fail to erupt; osteopetrosis | Deletion of R216; basic domain |
| MitfMi-or | Slight dilution of coat color; pale ears and tail; belly streak or head spot | White coat; microphthalmia; incisors fail to erupt; osteopetrosis | R216K; basic domain |
| MitfMi-wh | Dilute coat color; eyes dark ruby; white spots on feet, tail, and belly | White coat; eyes small and slightly pigmented | I212N; basic domain |
| Mitfmi-ew | Normal | White coat; microphthalmia; hyperosteosis | 25 amino acid deletion; basic domain |
| Mitfmi-vga9 | Normal | White coat; microphthalmia | Insertion and deletion; regulatory |
Mitf-Associated Osteopetrosis.
A number of the semidominant Mitf mutations, including Mitfmi, Mitfmi-eyeless white (Mitfmi-ew), and MitfMi-oak ridge (MitfMi-or), induce osteopetrosis in the homozygous condition (Table 1). In each case, these mutations produce dominant negative proteins, which can interfere with the function of other Mitf-Tfe proteins (9). Thus, it has been proposed that the osteopetrosis observed is because of dominant negative interference with one or more of the Mitf-Tfe proteins in osteoclasts. The most likely target for down-regulation is Tfe3, because Tfe3 and Mitf are both expressed in osteoclasts where they are known to form stable heterodimers (15). Our results confirm this speculation and support the hypothesis that osteopetrosis results from mutations in Mitf and the associated down-regulation of Tfe3 activity.
Among the three semidominant mutations, Mitfmi has the most severe osteopetrosis, with extensive accumulation of unresorbed endochondral bone and no bone marrow cavity, as seen in sections through femur (Fig. 2B), tibia, ribs, and head bone such as maxillae of homozygous 21-day-old mice (not shown). Homozygotes are half-normal size and do not live past 3 weeks of age. Animals carrying the Mitfmi-ew mutation show the mildest bone phenotype. Homozygotes are white and severely microphthalmic yet show no signs of growth retardation at 3 weeks of age. However, detailed histopathological analysis of bones from 21-day-old Mitfmi-ew/Mitfmi-ew mice shows clear signs of hyperosteosis, with extensions of bony trabeculae that reach further into the bone marrow cavity than in wild type (Fig. 2C). This phenotype resembles the hyperosteosis associated with Camurati–Engelmann disease (26). Although the Mitfmi-ew protein acts in a strong dominant negative fashion in vitro (9), only a small fraction of the mutant protein translocates to the nucleus (27). Thus, the mild phenotype is consistent with dominant negative action of the few nuclear Mitfmi-ew proteins. The osteopetrosis observed in the MitfMi-or mutation is more severe than that observed in Mitfmi-ew homozygotes but less severe than in Mitfmi animals (28). Thus, the three Mitf alleles form an allelic series with respect to osteopetrosis.
Osteopetrosis is not observed in all dominant negative Mitf mutations. For example, the MitfMi-wh mutation has normal bone development in the homozygous condition (Fig. 2D). Similarly, the dominant negative MitfMi-brownish (MitfMi-b) mutation is normal with respect to bone development (8). Although both of these mutations behave in a dominant negative fashion in vitro, they still retain DNA-binding activity under certain conditions (9). This behavior is in sharp contrast to the Mitfmi, Mitfmi-ew, and MitfMi-or proteins, which cannot bind DNA under any conditions.
Mitf and Tfe3 Interaction Is Allele-Specific.
To determine whether the Mitf–Tfe3 interaction is allele-specific, we crossed Tfe3Fcr mice to Mitfmi-ew and MitfMi-wh mice. In contrast to the single homozygotes, Mitfmi-ew;Tfe3Fcr double-mutant mice show severe osteopetrosis (Fig. 2H, Table 1). The double-mutant animals are smaller than their single-mutant littermates [Mitfmi-ew;Tfe3Fcr 6.1 ± 0.8g (n = 6); Mitfmi-ew 10.4 ± 1.3g (n = 9); Tfe3Fcr 11.3 ± 1.3g (n = 8)], tooth eruption is delayed, and the animals die by 3 weeks of age (data not shown). This effect varies with respect to the Tfe3 concentration as the osteopetrosis in Mitfmi-ew/Mitfmi-ew,Tfe3Fcr/+ animals is more severe than in Mitfmi-ew homozygotes but less severe than in the double homozygotes (Table 1). No such concentration-dependence is observed in Mitfmi-vga9/Mitfmi-vga9,Tfe3Fcr/+ animals, suggesting that this effect is caused by dominant negative action of the Mitfmi-ew protein against Tfe3.
Although the coat and eye phenotype associated with the MitfMi-wh mutation is unchanged in MitfMi-wh,Tfe3Fcr double mutants, the mice are smaller and more runted than their single-mutant littermates [5.6 ± 1.3g (n = 17) vs. 10.0 ± 1.2g (n = 8)]. Although most of these mice die at 3–4 weeks of age, some survive to adulthood. In all cases, bones show clear signs of osteopetrosis with intermediate extensions of bony trabeculae (Fig. 2I), similar to that seen in MitfMi-or mice (28). The residual activity of the MitfMi-wh protein is probably responsible for the fact that this osteopetrosis is less severe than that seen in the other double mutants analyzed. In all cases, the effects of the Mitf;Tfe3 mutant combinations are specific to osteoclasts, because in no case do they alter the mast cell, eye, or pigmentation defects of Mitf mice (Table 1).
Unlike Tfe3Fcr, TfecFcr does not induce osteopetrosis when combined with MitfMi-wh or with any of the other mutations tested (Table 1), nor does it exacerbate the osteopetrosis observed in MitfMi-wh;Tfe3Fcr;TfecFcr triple-mutant mice (Table 1). Although the embryonic lethality of TfebFcr precludes analysis of osteoclasts in homozygotes, MitfMi-wh/MitfMi-wh,TfebFcr/+ animals do not develop osteopetrosis, and reducing the Tfeb gene dosage does not change the appearance of these mice in any other way (Table 1). Furthermore, explant culture and chimera analysis strongly indicate that Tfeb is not necessary for melanocyte development (data not shown).
Effects on Osteoclasts.
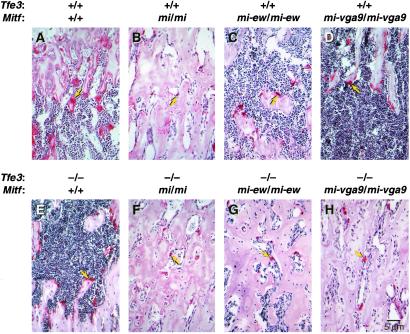
Staining for osteoclast activity with tartrate-resistant alkaline phosphatase (TRAP) shows that Mitfmi homozygotes (Fig. 3B) have smaller osteoclasts than either wild-type (Fig. 3A) (13), Tfe3Fcr (Fig. 3E), Mitfmi-ew (Fig. 3C), or Mitfmi-vga9 (Fig. 3D) mice. Consistent with their bone morphology, animals double homozygous for Tfe3Fcr and Mitfmi (Fig. 3F) or Mitfmi-ew (Fig. 3G) or Mitfmi-vga9 (Fig. 3H) have smaller osteoclasts resembling those of Mitfmi (Fig. 3B) homozygotes. The different effects of the various Mitf alleles on osteoclast function are likely the results of different effects on target gene expression. Although osteoclasts are smaller in the mutants, TRAP activity can still be detected (Fig. 3) indicating that, although the Mitf and Tfe3 proteins may regulate the expression of the Trap gene in vitro (29), their role can be substituted for by other factors in vivo.
Figure 3.
Osteoclast defects in Mitf and Tfe3Fcr mutant animals. (A–H) Sections through femurs of wild-type and mutant mice showing different levels of osteoclast activity as determined by tartrate-resistant alkaline phosphatase staining. Arrows point to osteoclasts.
Discussion
Here we describe the generation of knockout mutations in Tfe3 and Tfec, two members of the Mitf-Tfe family of bHLH-Zip transcription factors. We report the phenotype of these mice in the heterozygous and homozygous condition and when combined with each other and with mutations in Mitf and Tfeb, two other family members. Our studies show that Tfec is not essential for mammalian development; TfecFcr mutant mice are phenotypically normal and the TfecFcr mutation does not exacerbate the phenotype of mutations in genes of the other family members. In contrast, we uncover an important but functionally redundant role for Tfe3 and Mitf in osteoclasts, the cells required for bone remodeling. Although osteoclasts appear normal in Mitf and Tfe3Fcr null mice, their combined loss results in severe osteopetrosis. These results explain previous genetic data showing that some strong dominant negative alleles of Mitf induce osteopetrosis on their own. Our results indicate that dominant negative mutations of Mitf down-regulate Tfe3 activity and that the combined loss of Mitf and Tfe3 results in osteopetrosis.
Two human deafness and pigmentation disorders, Waardenburg Syndrome type 2A (WS2A) (30) and Tietz Syndrome (31), are caused by mutations in MITF. Individuals with Tietz syndrome have profound congenital deafness and generalized hypopigmentation, whereas individuals with WS2A have milder manifestations of deafness and pigmentation disturbances. Both diseases are dominant. To date, osteopetrosis has not been observed in any individuals with WS2A or Tietz. However, osteopetrosis is seen only in a few mouse Mitf mutations that encode strong dominant negative proteins and only when they are homozygous. It thus remains possible that individuals with WS2A or Tietz will be identified who suffer from osteopetrosis.
Mitf mutations have also been observed in rats (15, 32), hamsters (33), quail (34), and zebrafish (35). Molecular analyses of these mutations show that they are loss of function mutations rather than dominant negative mutations. Despite this, osteopetrosis has been reported as a phenotype associated with the quail silver mutation and the rat Mitfmib mutation (36, 37). In both cases the osteopetrosis is mild. For example, newborn rats show sclerosis that is reduced significantly during the first months, with bone marrow cavities shaping to near-wild-type levels. The Mitfmib mutation is caused by a deletion, but the full extent of this deletion is unknown. It is possible that this mild osteopetrosis is caused by the removal of an as yet unidentified flanking gene that is important for osteoclast function. It is also possible this phenotype reflects species differences in Mitf function in rats or genetic background. In this light, it will be of interest to investigate Tfe3 expression, function, and sequence in normal and Mitfmib rats. The quail mutation is unusual because it carries two alterations in the gene, a histidine to arginine change in the basic domain, and a stop codon shortly after the zipper domain. Although the transactivation potential of this mutation is somewhat reduced as compared with wild type (34), its dominant negative activity has not yet been determined.
Mutations in cathepsin K (Ctsk) also produce an osteopetrotic phenotype. Ctsk-deficient mice survive and are fertile but display excessive trabeculation of the bone marrow space (38). Ctsk-deficient osteoclasts manifest a modified ultrastructural appearance: their resorptive surface is poorly defined with a broad demineralized matrix fringe containing undigested fine collage fibrils, their ruffled borders lack crystal-like inclusions, and they are devoid of collagen-fibril-containing cytoplasmic vacuoles. In humans, CTSK deficiency induces pycnodysostosis (39), a rare inherited osteochondrodysplasia that is characterized by osteosclerosis, short stature, and acroosteolysis of the distal phalanges. Interestingly, in vitro expression studies show that Ctsk expression can be up-regulated by Mitf and Tfe3 (40). Mitf and Tfe3 may therefore be important activators of Ctsk expression in vivo, and loss of Ctsk expression in Mitf;Tfe3Fcr double-mutant mice could, in part, explain the osteopetrosis we observe in these animals.
Immunohistochemical studies show that carbonic anhydrase II, another potential target of Mitf, is expressed in osteoclasts of Mitfmi homozygous mice (41). Electron microscopic studies of osteoclasts from these mice show close contact with bone and some ability to resorb calcified matrix (14). Furthermore, the cells have the capacity to adhere to and spread from surfaces and form lamellipodia (42). These observations suggest that the Mitf and Tfe3 mutations do not eliminate osteoclast function altogether. Perhaps Mitf and Tfe3 are required for the expression of genes that enhance the bone resorption ability of osteoclasts. Alternatively, an as yet unidentified protein(s) in osteoclasts, a cell type that seems to have developed genetic redundancy as an important evolutionary theme, could substitute for their function.
A possible explanation for our results lies in the expression patterns of the Mitf-Tfe genes combined with tissue-specific threshold requirements. In melanocytes and labyrinthine cells of the placenta, which express high levels of Mitf and Tfeb, respectively, only the highly expressed family member may be able to meet the threshold requirement. In osteoclasts, Tfe3 and Mitf are expressed at similar levels (15), the threshold requirement could be lower, and either protein may be able to replace the other. The sequences of the Mitf-Tfe genes differ outside of their basic and HLHZip domains, however, and these nonconserved sequences may be responsible for the different phenotypes of mutations in Tfe3 and Mitf. Consistent with this explanation, the Mitf targets Tyrp1 and Tyr can both be stimulated by overexpression of Tfe3 in vitro, whereas Tfeb can stimulate only expression of Tyrp1 (16).
In summary, our results indicate that the functional importance of heterodimeric interactions varies dramatically among bHLH-Zip families. Although a number of the Myc/Max/Mad heterodimers are thought to provide critical and essential functions to this transcription factor network, this does not seem to be true for the Mitf-Tfe family. At least three Mitf-Tfe family members (Mitf, Tfe3, and Tfec) do not serve as essential heterodimerization partners, although Mitf-Tfe3 dimers clearly form in osteoclasts (15). It remains possible, however, that Tfeb is an essential dimerization partner for Mitf in mast cells or for Mitf or Tfe3 in osteoclasts, as the effects of the TfebFcr mutation have been fully explored only in melanocytes. In short, our results indicate that the outcome of heterodimerization differs dramatically among different bHLH-Zip families.
Acknowledgments
We thank Susan Reid, Jan Flynn, Debby Swing, Bryn Eagleson, Lisa Secrest, Joanne Dietz, Fran Dorsey, and Linda Cleveland for expert technical assistance; Miriam Anver, Becky Oden, and the staff of the histopathology laboratory (Science Applications International Corporation, Frederick, MD) for help with histology. We also thank Francesca Pignoni, Nick Short, Pamela Schwartzberg, and Lynn Hudson for comments on the manuscript; and Richard Frederickson for illustrations. This work was supported by the National Cancer Institute, the Department of Health and Human Services (N.G.C. and N.A.J.), by a North Atlantic Treaty Organization science fellowship (E.S.), and by the Icelandic Research Council (E.S.).
Abbreviation
- bHLH
basic helix–loop–helix
References
- 1.Schreiber-Agus N, DePinho R A. BioEssays. 1998;20:808–818. doi: 10.1002/(SICI)1521-1878(199810)20:10<808::AID-BIES6>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 2.Knoepfler P S, Eisenman R N. Cell. 1999;99:447–450. doi: 10.1016/s0092-8674(00)81531-7. [DOI] [PubMed] [Google Scholar]
- 3.O'Hagan R C, Schreiber-Agus N, Chen K, David G, Engelman J A, Schwab R, Alland L, Thomson C, Ronning D R, Sacchettini J C, et al. Nat Genet. 2000;24:113–119. doi: 10.1038/72761. [DOI] [PubMed] [Google Scholar]
- 4.Moore K J. Trends Genet. 1995;11:442–448. doi: 10.1016/s0168-9525(00)89143-x. [DOI] [PubMed] [Google Scholar]
- 5.Steingrímsson E, Tessarollo L, Reid S W, Jenkins N A, Copeland N G. Development (Cambridge, UK) 1998;125:4607–4616. doi: 10.1242/dev.125.23.4607. [DOI] [PubMed] [Google Scholar]
- 6.Hodgkinson C A, Moore K J, Nakayama A, Steingrímsson E, Copeland N G, Jenkins N A, Arnheiter H. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- 7.Steingrímsson E, Moore K J, Lamoreux M L, Ferré-D'Amaré A R, Burley S K, Zimring D C, Skow L C, Hodgkinson C A, Arnheiter H, Copeland N G, Jenkins N A. Nat Genet. 1994;8:256–263. doi: 10.1038/ng1194-256. [DOI] [PubMed] [Google Scholar]
- 8.Steingrímsson E, Nii A, Fisher D E, Ferré-D'Amaré A R, McCormick R J, Russell L B, Burley S K, Ward J M, Jenkins N A, Copeland N G. EMBO J. 1996;15:6280–6289. [PMC free article] [PubMed] [Google Scholar]
- 9.Hemesath T J, Steingrímsson E, McGill G, Hansen M J, Vaught J, Hodgkinson C A, Arnheiter H, Copeland N G, Jenkins N A, Fisher D E. Genes Dev. 1994;8:2770–2780. doi: 10.1101/gad.8.22.2770. [DOI] [PubMed] [Google Scholar]
- 10.Hughes M J, Lingrel J B, Krakowsky J M, Anderson K P. J Biol Chem. 1993;268:20687–20690. [PubMed] [Google Scholar]
- 11.Walker D G. Science. 1975;190:784–785. doi: 10.1126/science.1105786. [DOI] [PubMed] [Google Scholar]
- 12.Walker D G. Science. 1975;190:785–786. doi: 10.1126/science.1198094. [DOI] [PubMed] [Google Scholar]
- 13.Holtrop M E, Cox K A, Eilon G, Simmons H A, Raisz L G. Metab Bone Dis Rel Res. 1981;3:123–129. doi: 10.1016/0221-8747(81)90030-8. [DOI] [PubMed] [Google Scholar]
- 14.Thesingh C W, Scherft J P. Bone. 1985;6:43–52. doi: 10.1016/8756-3282(85)90406-5. [DOI] [PubMed] [Google Scholar]
- 15.Weilbaecher K N, Hershey C L, Takemoto C H, Horstmann M A, Hemesath T J, Tashjian A H, Fisher D E. J Exp Med. 1998;187:775–785. doi: 10.1084/jem.187.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verastegui C, Bertolotti C, Bille K, Abbe P, Ortonne J P, Ballotti R. Mol Endocrinol. 2000;14:449–456. doi: 10.1210/mend.14.3.0428. [DOI] [PubMed] [Google Scholar]
- 17.Beckmann H, Su L-K, Kadesch T. Genes Dev. 1990;4:167–179. doi: 10.1101/gad.4.2.167. [DOI] [PubMed] [Google Scholar]
- 18.Zhao G-Q, Zhao Q, Zhou X, Mattei M-G, DeCrombrugghe B. Mol Cell Biol. 1993;13:4505–4512. doi: 10.1128/mcb.13.8.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roman C, Matera A G, Cooper C, Artandi S, Blain S, Ward D C, Calame K. Mol Cell Biol. 1992;12:817–827. doi: 10.1128/mcb.12.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soriano P, Montgomery C, Geske R, Bradley A. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 21.Swiatek P J, Gridley T. Genes Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- 22.Laird P W, Zijderveld A, Linders K, Rudnicki M A, Jaenisch R, Berns A. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siracusa L D, Russell L B, Jenkins N A, Copeland N G. Genetics. 1987;117:85–92. doi: 10.1093/genetics/117.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins N A, Copeland N G, Taylor B A, Lee B K. J Virol. 1982;43:26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merrell K, Wells S, Henderson A, Gorman A, Alt F, Stall A, Calame K. Mol Cell Biol. 1997;17:3335–3344. doi: 10.1128/mcb.17.6.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makita Y, Nishimura G, Ikegawa S, Ishii T, Ito Y, Okuno A. Am J Med Genet. 2000;91:153–156. doi: 10.1002/(sici)1096-8628(20000313)91:2<153::aid-ajmg15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 27.Takebayashi K, Chida K, Tsukumoto I, Morii E, Munakata H, Arnheiter H, Kuroki T, Kitamura Y, Nomura S. Mol Cell Biol. 1996;16:1203–1211. doi: 10.1128/mcb.16.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nii A, Steingrímsson E, Copeland N G, Jenkins N A, Ward J M. Am J Pathol. 1995;147:1871–1882. [PMC free article] [PubMed] [Google Scholar]
- 29.Luchin A, Purdom G, Murphy K, Clark M-Y, Angel N, Cassady A I, Hume D A, Ostrowski M C. J Bone Miner Res. 2000;15:451–460. doi: 10.1359/jbmr.2000.15.3.451. [DOI] [PubMed] [Google Scholar]
- 30.Read A P, Newton V E. J Med Genet. 1997;34:656–665. doi: 10.1136/jmg.34.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amiel J, Watkin P M, Tassabehji M, Read A P, Winter R M. Clin Dysmorphol. 1998;7:17–20. [PubMed] [Google Scholar]
- 32.Opdecamp K, Vanvooren P, Riviere M, Arnheiter H, Motta R, Szpirer J, Szpirer C. Mamm Genome. 1998;9:617–621. doi: 10.1007/s003359900832. [DOI] [PubMed] [Google Scholar]
- 33.Hodgkinson C A, Nakayama A, Li H, Swenson L B, Opdecamp K, Asher J H, Jr, Arnheiter H, Glaser T. Hum Mol Genet. 1998;7:703–708. doi: 10.1093/hmg/7.4.703. [DOI] [PubMed] [Google Scholar]
- 34.Mochii M, Ono T, Matsubara Y, Eguchi G. Dev Biol. 1998;196:145–159. doi: 10.1006/dbio.1998.8864. [DOI] [PubMed] [Google Scholar]
- 35.Lister J A, Robertson C P, Lepage T, Johnson S L, Raible D W. Development (Cambridge, UK) 1999;126:3757–3767. doi: 10.1242/dev.126.17.3757. [DOI] [PubMed] [Google Scholar]
- 36.Cielinski M J, Marks S C. Bone. 1994;15:707–715. doi: 10.1016/8756-3282(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi N, Ono T, Mochii M, Noda M. Dev Dyn. 2001;220:133–140. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1095>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Saftig P, Hunziker E, Wehmyer O, Jones S, Boyde A, Rommerskirch W, Moritz J D, Schu P, von Figura K. Proc Natl Acad Sci USA. 1998;95:13453–13458. doi: 10.1073/pnas.95.23.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gelb B D, Shi G P, Chapman H A, Desnick R J. Science. 1996;273:1236–1238. doi: 10.1126/science.273.5279.1236. [DOI] [PubMed] [Google Scholar]
- 40.Motyckova G, Weilbaecher K N, Horstmann M, Rieman D J, Fisher D Z, Fisher D E. Proc Natl Acad Sci USA. 2001;98:5798–5803. doi: 10.1073/pnas.091479298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jilka R L, Rogers J I, Khalifah R G, Vaananen H K. Bone. 1985;6:445–449. doi: 10.1016/8756-3282(85)90222-4. [DOI] [PubMed] [Google Scholar]
- 42.Helfrich M H, Mieremet R H P. Bone. 1988;9:113–119. doi: 10.1016/8756-3282(88)90112-3. [DOI] [PubMed] [Google Scholar]