Abstract
We have focused on stability of the peptide-MHC complex as a determining factor of ligand potency for thymocytes and peripheral CD4+ T cell responses. MHC variant peptides that have low affinities and fast dissociation rates are different in that they stimulate proliferation and cytolysis of mature T cells (classifying the variant peptides as weak agonists) but do not induce thymocyte negative selection. The MHC variant weak agonists require significant receptor reserve, because decreasing the level of T cell receptor on mature T cells blocks the proliferative response. These results demonstrate that peripheral T cells are more sensitive to MHC variant ligands by virtue of increased T cell receptor expression; in addition, the data support a T cell model of the spare receptor theory.
The pivotal interaction between T cell receptor (TCR)–peptide–MHC ternary complexes mediates the development and activation of T cells. Mature T cells respond to antigenic peptides that can induce variable responses. Altered peptide ligands (APLs) are analog peptides with substitutions at TCR contact residues that have no change in affinity for the MHC molecule, and can be classified according to their ability to activate T cells, from agonist to partial agonist to antagonist to null (1–3). Investigation of the interactions of APLs with TCR has correlated dissociation, a kinetic parameter of peptide–MHC interaction, with the resulting T cell response (4–8). TCRs that dissociate very quickly from peptide-MHC ligands may result in no response, whereas ligands that bind for an extended duration lead to activation of the T cell. These data support the kinetic discrimination or kinetic proofreading models that suggest that the half-life of the interaction between peptide–MHC and TCR may determine the agonist or antagonist properties of a ligand (9, 10). A recent article expanded this model to state that any TCR half-life above or below an optimal dwell time, or length of TCR–peptide–MHC interaction, results in poor activation of the T cell (11). Because of the focus on classic APL with amino acid changes at TCR contacts, none of these models specifically address the effect of variation in the kinetics of peptide–MHC interactions on the potency of ligand and the resulting T cell response.
Thymocytes, like peripheral T cells, are exquisitely sensitive to subtle changes in the peptide sequence. Early experiments with fetal thymic organ culture as a model for selection indicated that antagonists, weak agonists, and partial agonists induce positive selection of CD8 thymocytes (12–14), whereas CD4 T cell antagonists induce negative selection (15, 16). Together, these data imply that thymocytes are more sensitive in that they respond by positive/negative selection to APLs that fail to activate peripheral T cells. This increased sensitivity is consistent with the requirement of thymocytes to interact with self-peptide MHC complexes during selection to generate a T cell repertoire that responds to foreign antigen yet is tolerant to self (17–19). The quantitative, or avidity, model of selection suggests that low avidity interactions between the TCR and peptide-MHC complexes lead to positive selection while high avidity ligands lead to negative selection, or deletion of thymocytes (20).
Although multiple models of T cell activation and selection have been proposed, ligand–receptor models are well described in pharmacology and may be equally applicable to TCR–peptide–MHC interactions. The relationship between the concentration of an agonist and response was initially determined to be directly proportional to the extent of receptor occupancy (21). In 1956, R. P. Stephenson modified the receptor theory to account for his observations of a receptor reserve (22). A key concept of his spare receptor model is that a maximal response can be achieved by an agonist occupying only a small proportion of the receptors. However, weak/partial agonists must bind to more receptors to produce a maximum response. A receptor reserve allows for responses that are extremely rapid in onset and termination, yet at the same time are sensitive to low, transient ligand concentrations and low-affinity interactions. Several properties of T cells are compatible with this pharmacological theory, including a vast excess of TCRs, variable affinities for ligand, and sensitivity to low concentrations of agonists (6, 23, 24).
Although the classical definition of APL has typically excluded analog peptides possessing major differences in peptide affinity for MHC, we sought to determine the effect of the altered stability of a peptide for the MHC class II molecule on CD4+ peripheral T cells. MHC variant peptides that form unstable complexes are weak agonists for peripheral T cell responses, yet these weak agonists fail to induce negative selection of double-positive thymocytes. This observation diverges from the prevailing theory that thymocytes are more sensitive and indicates that peripheral T cells respond better than thymocytes to weak agonists of poor stability. When TCR levels are masked on peripheral T cells, to equivalent levels as on thymocytes, the mature T cells no longer proliferate to the MHC variant peptides, demonstrating that the peripheral response to the weak agonists was dependent on amount of expressed TCR. Thus, receptor reserve dissociates peripheral T cell response and thymocyte negative selection to MHC variants supporting a spare receptor model for TCR interaction with ligand.
Materials and Methods
Mice.
AND TCR transgenic mice [TgN(TcrAND)53Hed] (H-2b) and B10.A/Cr (H-2a) mice were purchased from The Jackson Laboratory and the National Cancer Institute (Frederick, MD), respectively. All mice were housed and maintained at the Emory University Department of Animal Resources facility.
Peptides.
Peptides were synthesized with use of standard 9-fluorenylmethyloxycarbonyl chemistry on a Symphony/Multiplex Peptide Synthesizer and analyzed by HPLC (Rainin Instruments) and mass spectrometry at the Emory University Department of Chemistry Core Facility. The sequences of the peptides used were as follows: MCC 88–103 (ANERADLIAYLKQATK), GGQM (ANERADLGAYGKQATM), GEEK (ANERADLGAYEKEATK), 98E (ANERADLIAYEKQATK), and 99G APL (ANERADLIAYLGQATK).
Cells and Reagents.
Transgenic T cell lines were obtained by stimulating AND splenocytes with 1 μM MCC 88–103. T cell lines were restimulated every 7 days in a 24-well plate with MCC and 5 × 106 gamma-irradiated spleen cells (2,000 rads) from a B10.A mouse. Cell culture media consisted of RPMI medium 1640 supplemented with 10% FBS (Atlanta Biologicals, Norcross, GA), 2 mM L-glutamine, 0.01 M Hepes buffer, 100 μg/ml Gentamycin (Mediatech, Herndon, VA), and 2 × 10−5 M 2-ME (Sigma). The following antibodies were used: CD8, CD4 (Caltag, Burlingame, CA), CD69, and Vβ3 (KJ25, PharMingen). Quantum R-PE microbeads 500 to 50,000 MESF (Bangs Laboratories, Fishers, IN) carrying known numbers of phycoerythrin molecules were used as quantitative fluorescent standards to directly quantitate the absolute numbers of TCR on AND T cells and thymocytes. The data were analyzed by using the calibration platform from the FLOWJO software (Tree Star, San Carlos, CA).
Competition ELISA.
Purified IEk class II molecules (0.5 μM), high-affinity biotinylated reference peptide (MCC 88–103), and various concentrations of unlabeled competitor peptide were incubated at 37°C in 0.1 M citrate phosphate buffer (pH 5.0) with 0.2% Nonidet P-40 containing protease inhibitors. After 48 h the peptide–MHC complexes were captured on microtiter plates coated with the IEk capture antibody, 14-4-4S, which was purified from culture supernatants of cells obtained from the American Type Culture Collection (Rockville, MD). The biotinylated peptide–MHC complexes were detected by an alkaline-phosphatase-dependent color change. The data are expressed as relative IC50 or the amount of peptide needed to inhibit the binding of a high-affinity biotinylated reference peptide by 50%, normalized to a value of one for the immunogenic peptide.
High-Performance Size Exclusion Chromatography (HPSEC).
Soluble IEk from CH27 cells was purified on an affinity column coupled to anti-IEk. The protein was eluted from the antibody column with a solution of 100 mM glycine/0.15 M NaCl/0.5% n-octyl-β-D-thioglucopyranoside, pH 11.5; and protein concentrations were determined by using a bicinchoninic acid assay. HPSEC was performed as described (25). Soluble purified IEk (1 μM) was incubated at 37°C with fluorescein-labeled peptide for 48–72 h. Free, unbound peptide was removed on a Sephadex G-50 column, and 100-fold excess of unlabeled peptide was added. At various times, samples were removed and IEk-FITC-peptide complexes were quantified with a Tosohaas TSK G3000SW high-performance size exclusion column (30 cm × 7.5 mm) (Toso Biosep LLC, Montgomeryville, PA) with a guard column (7.5 cm × 7.5 mm), dissociation was monitored by a Shimadzu RF-10A fluorometer. The peak height of the bound peptide–MHC complexes was measured.
Proliferation Assay.
AND×B10.A splenocytes (3 × 105 per well) were incubated with the indicated peptide in a 96-well plate. After 72 h in culture, cells were labeled with 0.4 μCi/well of [3H]thymidine, and after another 18 h, the plates were harvested and analyzed on a Matrix 96 Direct Beta Counter (Packard). In some assays, various concentrations of KJ-25 (Vβ3) Fab fragments were added to mask TCR.
Cytolytic Assay.
AND T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes). Labeled T cells (1 × 105), CH27 cells (5 × 104), and various concentrations of peptide were cultured for 24 h in a 96-well flat-bottomed plate. Cells were stained with 7-amino actinomycin D (Sigma), and the percentage of dead CH27 cells was determined by flow cytometry on a Becton Dickinson FACSCalibur after gating out the CFSE+ T cells.
CD69 Up-Regulation.
Assay performed as described by Davey et al. (26). Thymocytes (5 × 105) or purified CD4+ splenocytes from AND mice, 105 CH27 cells, and various concentrations of peptide were incubated for 3 h at 37°C in a round-bottom 96-well plate. After incubation, the cells were stained for CD4, CD8, Vβ3, and CD69 expression. The CD69 levels at 100 μM MCC were set at 100% and the data were normalized by dividing the mean fluorescence intensity (MFI) at that peptide dilution by the at 100 μM MCC and multiplying by 100.
Fetal Thymic Organ Culture.
The matings between AND male mice and B10.A female mice were timed, and the thymic lobes were removed from a day 16 embryonic fetus. The lobes were placed on a 0.45 μM filter (Millipore) that was resting on a gelfoam sponge (Amersham Pharmacia and Upjohn) in a well of a six-well culture dish containing the indicated peptide and 2 ml of cell culture media. Media and peptide were exchanged daily, and the lobes were harvested after 7 days in culture. Single-cell suspensions were prepared and stained for analysis by flow cytometry.
Results
Peptide–MHC Affinity.
To characterize the effects of altering the stability of peptide–MHC interactions on T cell responses, we altered the MHC anchor residues of an antigenic peptide, moth cytochrome C 88–103 (MCC) restricted by the MHC class II molecule IEk. Crystal structure and functional data have delineated the MHC anchor residues of the IEk peptide-binding motif to be located at positions 1, 4, 6, and 9 of the peptide, which correspond to amino acids isoleucine (95), leucine (98), glutamine (100), and lysine (103) for MCC (88–103) (27, 28). Unfavorable amino acid substitutions were deliberately chosen at the MHC anchor positions to destabilize the peptide–MHC interaction. For example, a nonpolar amino acid, leucine, was exchanged for a negatively charged residue, glutamic acid, at the hydrophobic pocket 4 to generate the 98E variant peptide. The GGQM peptide has three MHC anchor changes at P1 (I→G), P4 (L→G), and P9 (K→M) and the GEEK peptide has altered residues at three MHC anchors, P1 (I→G), P4 (L→E), and P6 (Q→E). We have also synthesized a classical APL, 99G APL, which substitutes a glycine for a lysine at the P5 primary TCR contact residue. Murine class II associated invariant chain peptide was included as a negative control because of its demonstrated low affinity and fast dissociation rate for IEk (29, 30).
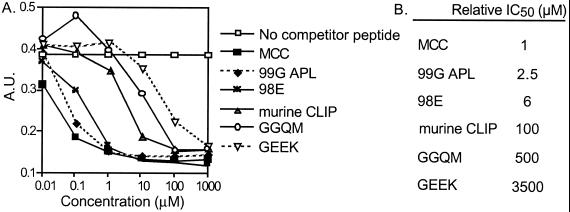
After synthesis of the MHC variant peptides, the affinity of each peptide for the MHC complex, IEk, was determined by using a competition ELISA. MCC and 99G APL can compete efficiently with the biotinylated indicator peptide for binding to IEk indicating that they have a similar affinity (Fig. 1A). The amino acid side chain of the primary TCR contact residue does not interact with the MHC molecule and has no effect on the binding of the peptide to the MHC class II molecule. As is often true for single amino acid changes at MHC anchor residues (28, 31), 98E had an affinity similar to MCC. The two MHC variant peptides, GGQM and GEEK containing multiple changes in anchor residues, bind weakly to IEk, resulting in very high relative IC50 values of 500 and 3,500, respectively (Fig. 1B). The multiple MHC anchor substituted peptides have a poor affinity for IEk, whereas MCC, 99G APL, and 98E have equivalent and higher affinities.
Figure 1.
Peptide–MHC affinity of the MHC variant ligands. (A) Purified IEk and biotinylated reference peptide, MCC 88–103, were incubated at 37°C for 48 h. This reaction mixture and the indicated concentrations of competitor peptides were added to a 96-well plate, coated overnight with an IEk capture antibody. (B) Relative IC50 is the amount of peptide needed to inhibit the binding of a high affinity biotinylated reference peptide by 50%, normalized to a value of one for MCC.
Peptide–MHC Dissociation.
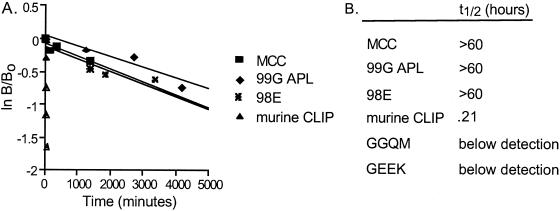
The dissociation of the TCR from its ligand and the stability of the ligand itself should also affect the duration of TCR–peptide–MHC ternary interaction; therefore, we analyzed the dissociation, or half-life, of our peptides from IEk. Soluble IEk molecules preloaded with fluorescein-labeled peptides were incubated for various times with excess unlabeled peptide then analyzed by HPSEC to determine the quantity of bound fluorescein peptide (25). In accordance with the affinity values, the immunogenic peptide, MCC, had a very long half-life of greater than 60 h on IEk (Fig. 2B). 99G APL and 98E, which had similar affinities to the wild-type peptide (Fig. 1B), also created stable peptide–MHC complexes with half-lives of greater than 60 h. Murine class II associated invariant chain peptide, which had a similar affinity for IEk as GGQM, had a very short half-life of only 13 min. GGQM and GEEK, the MHC variant peptides with substitutions at three MHC anchors, form transient complexes with half-life values below our detection levels. These data demonstrate that the MHC variant peptides have a very fast dissociation from IEk as compared with the antigenic peptide, MCC. Thus, the substitution of MHC anchor residues resulted in very unstable peptide-MHC complexes as determined by both affinity and half-life measurements.
Figure 2.
Half-life of MCC variant peptides from soluble IEk. (A) Fluorescein-labeled peptides and soluble IEk complexes were incubated for various times at 37°C with excess unlabeled peptide then analyzed by HPSEC. The peak height of the bound fluorescein peptide-MHC complexes was determined. The data are graphed as (natural log of Bound at time x/Bound at time 0) versus time. (B) Half-life values for each peptide as derived from the slope of the graph, t1/2 = natural log 2/slope of curve.
Peripheral CD4 T Cell Response to Unstable Peptide–MHC Complexes.
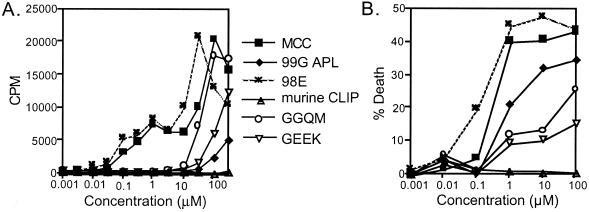
We examined the ability of the MHC variant peptides to induce effector functions of T cells to determine the ligand classification. The wild-type and 98E peptides induced comparable levels of proliferation in a manner consistent with their similar affinities for MHC (Fig. 3A). The 99G APL, which contains a glycine for lysine amino acid change at the primary TCR contact, stimulated minimal proliferation of AND T cells although it too has a similar affinity for MHC. GGQM and GEEK, the fast half-life peptides, stimulated significant proliferation, although they were weaker in potency, as compared with MCC. The cytolytic response of CD4+ T cells to the variant ligands was also determined. MCC and 99G APL induce death, or cytolysis, of CH27 cells (Fig. 3B). Because 99G APL can stimulate cytolysis and minimal proliferation, it behaves as a partial agonist. GGQM and GEEK are classified as weak agonists, because they stimulate proliferative and cytolytic responses. In summary, for peripheral T cells, MCC and 98E are full agonists, GGQM and GEEK are weak agonists, and 99G APL is a partial agonist.
Figure 3.
AND T cells proliferate and induce cytolysis to MHC variant ligands, but not APLs. (A) AND×B10.A transgenic splenocytes (3 × 105) were incubated with the indicated doses of peptides for 72 h and proliferation was measured by [3H]thymidine incorporation. (B) CFSE-labeled AND T cells (105) and CH27 APCs (5 × 104), an effector to target ratio of 2:1, were incubated with the indicated concentrations of peptides for 24 h, then the cells were stained with 7-amino actinomycin D (7-AAD). The percentage of dead CH27 cells (7-AAD+) were determined by flow cytometry after gating out CFSE+ T cells.
Negative Selection of AND Thymocytes.
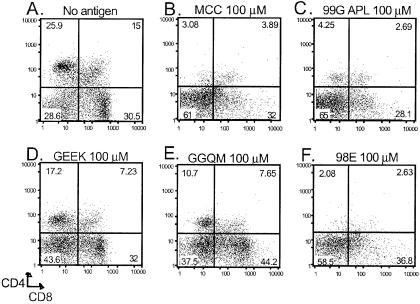
Negative selection of thymocytes was examined by fetal thymic organ culture to determine whether thymocytes have an increased sensitivity to unstable weak agonist peptide–MHC complexes as compared with mature T cells. Day 16 AND embryonic thymic lobes were cultured with the various peptides for 7 days and then assessed by flow cytometry for CD4 and CD8 surface expression (Fig. 4). MCC stimulated thymocytes were nearly all double-negative (CD4−CD8−) with very few double-positive or single-positive cells remaining (Fig. 4B). 98E and 99G APL were identical to MCC, with complete negative selection of AND thymocytes (Fig. 4 C and F). The poor binding variant peptides, GGQM and GEEK (Fig. 4 D and E), revealed a distribution of cells with minimal negative selection, much like those in the no-antigen control (Fig. 4A). The effects of the MHC anchor-substituted peptides were also confirmed by using an in vivo model of negative selection (32) and the in vitro dulling assay (33) (data not shown). In summary for thymocytes, the less stable GGQM and GEEK variants induced little to no negative selection, whereas 98E, 99G APL, and MCC are very potent stimulators of negative selection.
Figure 4.
Fetal thymic organ culture of AND thymocytes. Thymic lobes from a day 16 AND×B10.A fetus are removed and cultured on gelfoam sponges with media and the indicated concentrations of peptide. After 7 days of culture, the lobes are disrupted, stained for CD4 and CD8, and analyzed by flow cytometry. A no antigen (A) dot plot is shown as a negative control.
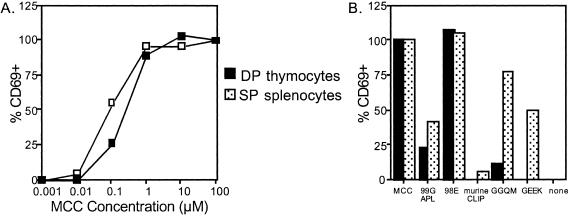
CD69 up-regulation was measured to compare directly the peripheral T cell and thymocyte response to ligand. Double-positive thymocytes and CD4+ splenocytes demonstrate equal expression of CD69 and similar antigen sensitivity to MCC (Fig. 5A). 98E induced maximal up-regulation of CD69 for both populations, whereas 99G APL stimulated minimal levels of CD69 on peripheral T cells but considerable CD69 expression on thymocytes (Fig. 5B). GGQM and GEEK induced CD69 up-regulation for the splenocytes but not the double-positive thymocytes. Thus, CD69 expression confirmed the differential sensitivity between peripheral T cells and thymocytes for the MHC variant peptides.
Figure 5.
CD69 up-regulation on AND DP thymocytes and CD4+ splenocytes. Thymocytes and splenocytes were incubated with (A) MCC or (B) 100 μM of the indicated peptides for three hours, then stained for CD69 expression. The data have been normalized such that CD69 up-regulation was set at 100% for 100 μM MCC for each cell population.
Decreased TCR Expression Ablates Proliferation.
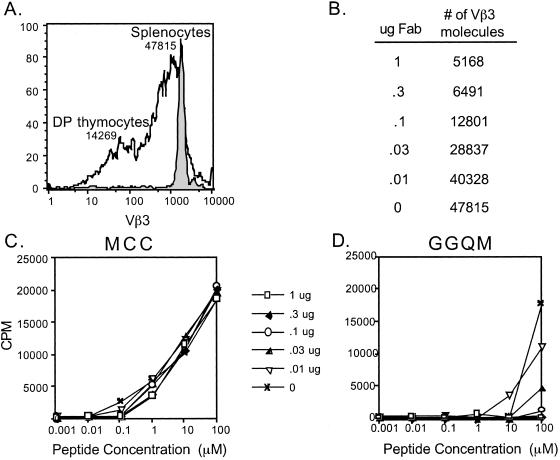
Normal, nontransgenic mice express lower levels of TCR on double-positive thymocytes than peripheral T cells (23). As compared with AND splenocytes, AND double-positive thymocytes have a more heterogeneous surface expression of Vβ3, expressing on average 70% fewer, 14,269 vs. 47,615 (Fig. 6A). If the excess TCRs, or receptor reserve, are critical for responses to weak ligands than the response to the weak agonist MHC variant peptides may be more sensitive to TCR levels as compared with the strong agonists. To address whether T cells fit into the spare receptor model, TCR level was decreased on peripheral T cells with Fab fragments specific for Vβ3.
Figure 6.
Blocking the TCR on AND splenocytes ablates the T cell response to the MHC variant peptide, GGQM. (A) Splenocytes and thymocytes from AND×B10.A mice were stained with Vβ3. The histogram shown is gated on double-positive (CD4+CD8+) thymocytes (black line) and AND CD4+ splenocytes (shaded). The number above each histogram corresponds to the molecules of Vβ3 TCR expressed on each cell population as quantitated by microbeads. (B) The number of Vβ3 molecules remaining on AND CD4+ splenocytes after incubation with the indicated amount of KJ-25 (Vβ3) Fab fragments as determined by quantitation with microbeads. (C, D) AND×B10.A transgenic splenocytes (3 × 105) were incubated with various concentrations of MCC (C) or GGQM (D) and the indicated concentrations of Fab fragments to block TCR for 72 h. Proliferation was determined by [3H]thymidine incorporation.
The CD4+ T cell proliferation to MCC is not affected by any concentration of Vβ3 Fab tested, even when TCR was reduced by 90% (Fig. 6C). In contrast, the response stimulated by the MHC variant, GGQM, was blocked when TCR levels were reduced by 40% (Fig. 6 B and D). The AND T cells do not proliferate to GGQM even at 0.1 μg concentration of Vβ3 Fab, where the TCR level is equivalent to that seen in double-positive thymocytes. Fab fragments were similarly potent when used with the weaker agonist, GEEK (data not shown). For both MHC variant weak agonists, the peripheral T cell response required expression of more TCR than is found in the thymus (28,000 vs. 14,000). Thus, the MHC variant weak agonists require many more TCRs than full agonists, a receptor reserve, to induce proliferation of mature T cells.
Discussion
Previous studies have demonstrated that thymocytes are more sensitive to ligand than peripheral T cells, ensuring that mature T cells will not be able to respond by proliferation and effector functions to self-ligands (26, 34, 35). However, all of these studies examined the effect of peptides with alterations at TCR contact residues (APLs) that possessed a high affinity for the MHC molecule. In confirmation of the previous reports, we found that 99G APL is a partial agonist that is much weaker than the MHC variants in stimulating mature T cells, yet it can induce negative selection of thymocytes. 99G APL has been characterized as a partial agonist based on its ability to make T cells competent for activation induced cell death (36). Our finding is an extension of this analysis to include the MHC variant peptides, GGQM and GEEK, that have a low affinity for MHC molecules and form unstable complexes with rapid dissociation rates. For mature T cells, GGQM and GEEK are weak agonists as they can induce proliferation, mediate cytolysis, and induce CD69 up-regulation. However, these MHC variant peptides are unable to induce negative selection of thymocytes. Thus, we have identified that the destabilization of peptide–MHC interactions dissociates the peripheral T cell response from thymocyte negative selection.
Peripheral T cells and thymocytes share many features in their response to ligand. Maximal response (proliferation or negative selection, respectively) requires costimulation and similar levels of peptide–MHC ligand to achieve sufficient threshold levels of activation (24, 37–41). One critical difference between mature CD4+ T cells and thymocytes is the amount of TCR on the cell surface. Double-positive thymocytes, which are undergoing positive and negative selection, have a lower overall level of TCR. This low level of TCR could prevent the thymocyte from productively interacting with the weak agonist peptide–MHC complexes. To decrease the level of TCR on our peripheral CD4+ T cells to an equivalent level as on thymocytes, we masked the TCR with Fab fragments (Fig. 6). MCC, a full agonist, requires very few TCRs (<4,000) to achieve maximal proliferation. Unlike the response to MCC, a 40% reduction in TCR level blocked the response to GGQM and GEEK. Thus, the T cell response to the MHC variant weak agonists was sensitive to the decreased level of TCR expression, and may suggest a possible mechanism for low thymocyte sensitivity to MHC variants. We observed a diminution of Vβ3hi DP thymocytes during fetal thymic organ culture with GGQM and GEEK (data not shown), although these peptides allow maturation of T cells as evidenced by the CD4 SP thymocyte population.
Mature T cells commonly express 30,000–100,000 TCRs, yet very few TCRs are actually required for activation and induction of effector functions by strong agonists (42, 43). Because of this large receptor reserve, T cells seem to be a functional model of the spare receptor theory. In this model, a full agonist does not need to occupy all receptors to achieve a maximal response, whereas partial agonists/weak agonists require more receptors to respond (22, 44). Our data are a demonstration of a spare receptor system, where the full agonist peptide, MCC requires significantly less TCR as compared with the GGQM and GEEK MHC variants, which can no longer stimulate proliferation when even 24,000 TCR remain (Fig. 6). Double-positive thymocytes express a mean of 15,000 TCRs on their surface suggesting that the number of TCRs is insufficient for adequate response to weaker ligands such as GGQM and GEEK. The receptor reserve on peripheral T cells provides a mechanism for effector responses to agonists with fast dissociation rates and peptides with low affinities for MHC. Thus, excess TCR on peripheral T cells increases the optimal sensitivity to antigen to include less stable ligands.
What are the consequences of the apparent excess of TCRs on T cell immunity? Several articles have reported the identification of low-affinity self-peptides for MHC Class II in autoimmunity or cancer models (45, 46). In the MBP Ac 1–9 model of EAE, this peptide has an extremely low affinity and undetectable half-life for the IAu restriction element yet activates peripheral T cells. Further analysis of this system by Wraith and colleagues demonstrates a lack of thymocyte-negative selection that could be compensated by changing an MHC anchor residue to create a superagonist (47). Less stable peptide–MHC complexes could also occur as cell types differ in their ability to process and present antigen. H-2O+ cells could allow for a broader range of peptides, including low-affinity peptides, to be presented by MHC Class II molecules through inhibition of the peptide-editing function of H-2M (48, 49). Although the potential for escape of autoimmune T cells from selection exists, by virtue of their increased expression of TCRs, peripheral T cells are more responsive to less stable peptide–MHC complexes and some level of autoimmunity may be an acceptable consequence to achieve maximal sensitivity to ligand.
In conclusion, our findings support a T cell model of the spare receptor theory where the excess level of TCRs on peripheral T cells are required for responses to peptides with altered kinetics for the MHC molecule. Although it is possible that our unstable peptide-MHC ligands represent a hole in thymocyte selection that results in autoimmunity in the periphery, the MHC variant peptides represent an intrinsic feature of a spare receptor system allowing for increased sensitivity of peripheral T cells to a broader range of ligands.
Acknowledgments
We thank Dr. Gilbert J. Kersh, Dr. Peter E. Jensen, and members of the Evavold laboratory for critical reading of the manuscript. This work was supported by American Cancer Society Grant RPG-00-314.
Abbreviations
- APL
altered peptide ligand
- TCR
T cell receptor
- MCC
moth cytochrome C
- HPSEC
high-performance size exclusion chromatography
- CFSE
carboxyfluorescein diacetate succinimidyl ester
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Evavold B D, Sloan-Lancaster J, Allen P M. Immunol Today. 1993;14:602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- 2.Sloan-Lancaster J, Allen P M. Annu Rev Immunol. 1996;14:1–28. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Jameson S C, Bevan M J. Immunity. 1995;2:1–11. doi: 10.1016/1074-7613(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 4.Kersh G J, Kersh E N, Fremont D H, Allen P M. Immunity. 1998;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 5.Alam S M, Travers P J, Wung J L, Nasholds W, Redpath S, Jameson S C, Gascoigne N R J. Nature (London) 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 6.Lyons D S, Lieberman S A, Hampl J, Boniface J J, Chien Y, Berg L J, Davis M M. Immunity. 1996;5:53–62. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- 7.Matsui K, Boniface J J, Steffner P, Reay P A, Davis M M. Proc Natl Acad Sci USA. 1994;91:12862–12866. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sykulev Y, Brunmark A, Jackson M, Cohen R J, Peterson P A, Eisen H N. Immunity. 1994;1:15–22. doi: 10.1016/1074-7613(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 9.Rabinowitz J D, Beeson C, Lyons D S, Davis M M, McConnell H M. Proc Natl Acad Sci USA. 1996;93:1401–1405. doi: 10.1073/pnas.93.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeithan T W. Proc Natl Acad Sci USA. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalergis A M, Boucheron N, Doucey M A, Palmieri E, Goyarts E C, Vegh Z, Luescher I F, Nathenson S G. Nat Immunol. 2001;2:229–234. doi: 10.1038/85286. [DOI] [PubMed] [Google Scholar]
- 12.Ashton-Rickardt P G, Bandeira A, Delaney J R, Van Kaer L, Pircher H P, Tonegawa S. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 13.Hogquist K A, Jameson S C, Heath W R, Howard J L, Bevan M J, Carbone F R. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 14.Sebzda E, Wallace V A, Mayer J, Yeung R S M, Mak T W, Ohashi P S. Science. 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 15.Spain L M, Jorgensen J L, Davis M M, Berg L J. J Immunol. 1994;152:1709–1717. [PubMed] [Google Scholar]
- 16.Page D M, Alexander J, Snoke K, Appella E, Sette A, Hedrick S M, Grey H M. Proc Natl Acad Sci USA. 1994;91:4057–4061. doi: 10.1073/pnas.91.9.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen P M. Cell. 1994;76:593–596. doi: 10.1016/0092-8674(94)90497-9. [DOI] [PubMed] [Google Scholar]
- 18.Jameson S C, Hogquist K A, Bevan M J. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 19.Page D M, Kane L P, Onami T M, Hedrick S M. Semin Immunol. 1996;8:69–82. doi: 10.1006/smim.1996.0010. [DOI] [PubMed] [Google Scholar]
- 20.Ashton-Rickardt P G, Tonegawa S. Immunol Today. 1994;15:362–366. doi: 10.1016/0167-5699(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 21.Furchgott R F. Annu Rev Pharmacol. 1964;4:21–50. [Google Scholar]
- 22.Stephenson R P. Br J Pharmacol. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roehm N, Herron L, Cambier J, DiGuisto D, Haskins K, Kappler J, Marrack P. Cell. 1984;38:577–584. doi: 10.1016/0092-8674(84)90512-9. [DOI] [PubMed] [Google Scholar]
- 24.Peterson D A, DiPaolo R J, Kanagawa O, Unanue E R. Immunity. 1999;11:453–462. doi: 10.1016/s1074-7613(00)80120-x. [DOI] [PubMed] [Google Scholar]
- 25.Kasson P M, Rabinowitz J D, Schmitt L, Davis M M, McConnell H M. Biochemistry. 2000;39:1048–1058. doi: 10.1021/bi9921337. [DOI] [PubMed] [Google Scholar]
- 26.Davey G M, Schober S L, Endrizzi B T, Dutcher A K, Jameson S C, Hogquist K A. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fremont D H, Hendrickson W A, Marrack P, Kappler J. Science. 1996;272:1001–1004. doi: 10.1126/science.272.5264.1001. [DOI] [PubMed] [Google Scholar]
- 28.Reay P A, Kantor R M, Davis M M. J Immunol. 1994;152:3946–3957. [PubMed] [Google Scholar]
- 29.Sette A, Southwood S, Miller J, Appella E. J Exp Med. 1995;181:677–683. doi: 10.1084/jem.181.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang M N, Beeson C, Mason K, McConnell H M. Int Immunol. 1995;7:1397–1404. doi: 10.1093/intimm/7.9.1397. [DOI] [PubMed] [Google Scholar]
- 31.Evavold B D, Williams S G, Hsu B L, Buus S, Allen P M. J Immunol. 1992;148:347–353. [PubMed] [Google Scholar]
- 32.Murphy K M, Heimberger A B, Loh D Y. Science. 1990;250:1720–1722. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 33.Swat W, Ignatowicz L, von Boehmer H, Kisielow P. Nature (London) 1991;351:150–153. doi: 10.1038/351150a0. [DOI] [PubMed] [Google Scholar]
- 34.Liu C P, Crawford F, Marrack P, Kappler J. Proc Natl Acad Sci USA. 1998;95:4522–4526. doi: 10.1073/pnas.95.8.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucas B, Stefanova I, Yasutomo K, Dautigny N, Germain R N. Immunity. 1999;10:367–376. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- 36.Combadiere B, Reis e Sousa C, Trageser C, Zheng L X, Kim C R, Lenardo M J. Immunity. 1998;9:305–313. doi: 10.1016/s1074-7613(00)80613-5. [DOI] [PubMed] [Google Scholar]
- 37.Harding C V, Unanue E R. Nature (London) 1990;346:574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- 38.Demotz S, Grey H M, Sette A. Science. 1990;249:1028–1030. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- 39.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Nature (London) 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 40.Amsen D, Revilla Calvo C, Osborne B A, Kruisbeek A M. Proc Natl Acad Sci USA. 1999;96:622–627. doi: 10.1073/pnas.96.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kishimoto H, Sprent J. J Exp Med. 1999;190:65–73. doi: 10.1084/jem.190.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schodin B A, Tsomides T J, Kranz D M. Immunity. 1996;5:137–146. doi: 10.1016/s1074-7613(00)80490-2. [DOI] [PubMed] [Google Scholar]
- 43.Labrecque N, Whitfield L S, Obst R, Waltzinger C, Benoist C, Mathis D. Immunity. 2001;15:71–82. doi: 10.1016/s1074-7613(01)00170-4. [DOI] [PubMed] [Google Scholar]
- 44.Furchgott R F, Bursztyn P. Ann NY Acad Sci. 1967;124:882–899. [Google Scholar]
- 45.Slansky J E, Rattis F M, Boyd L F, Fahmy T, Jaffee E M, Schneck J P, Margulies D H, Pardoll D M. Immunity. 2000;13:529–538. doi: 10.1016/s1074-7613(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 46.Fairchild P J, Wraith D C. Immunol Today. 1996;17:80–85. doi: 10.1016/0167-5699(96)80584-6. [DOI] [PubMed] [Google Scholar]
- 47.Liu G Y, Fairchild P J, Smith R M, Prowle J R, Kioussis D, Wraith D C. Immunity. 1995;3:407–415. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 48.van Ham M, van Lith M, Lillemeier B, Tjin E, Gruneberg U, Rahman D, Pastoors L, van Meijgaarden K, Roucard C, Trowsdale J, et al. J Exp Med. 2000;191:1127–1136. doi: 10.1084/jem.191.7.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen P E. Curr Biol. 1998;8:R128–R131. doi: 10.1016/s0960-9822(98)70988-1. [DOI] [PubMed] [Google Scholar]