Abstract
Progesterone-induced blocking factor 1 (PIBF1) is linked to pregnancy-induced immunity and tumor evasion of maternal immunity. PIBF1 is overexpressed in several cancers, including breast, cervical, and lymphoma. However, limited research is available on the role of PIBF1 in breast cancer and its clinical outcomes. Therefore, we investigated the relationship between PIBF1 expression, prognosis, and its impact on chemotherapy response. Samples from 231 patients with high-risk triple-negative breast cancer (TNBC) who underwent surgery between 2008 and 2013 with lymph node metastasis and underwent taxane-based adjuvant chemotherapy were collected. Additionally, 238 non-TNBC patients matched to TNBC patients were selected. Immunohistochemical detection of the PIBF1 protein in tissues was conducted using a cut-off value of 3 (intensity plus proportion). Kaplan–Meier survival analysis assessed the probability of overall survival (OS). Using the clonogenic unit assay and knockdown methodologies in breast cancer cell lines, we examined the correlation between PIF1 expression and chemosensitivity. In a study of 469 patients with breast cancer, non-TNBC (n = 238) and TNBC (n = 231), those with PIBF1 expression manifested a lower histologic grade (p < 0.001), reduced p53 (p < 0.001) and decreased Ki-67 (p < 0.001) compared with their non-expressing counterparts. A significant difference in OS for patients with PIBF1 was observed, with non-TNBC patients showing superior outcomes. PIBF1 expression showed a relation with a better prognosis, and the statistical significance was borderline (hazard ratio = 0.44, 95% confidence interval = 0.18–1.11, p = 0.082). A correlation between PIBF1 expression in breast cancer cell lines (BT549, HCC70, BT20, and HS578T) and their sensitivity to paclitaxel was shown in vitro, with certain cell lines showing significant viability reductions and also resisting the treatment after PIBF1 knockdown. We observed a correlation between PIBF1 expression and improved prognosis in breast cancer patients with nodal metastasis undergo taxane-based chemotherapy, particularly in the non-TNBC cohort. We discerned a relationship between PIBF1 and chemosensitivity in our in vitro studies. These findings suggest the potential usefulness of PIBF1 as a predictive marker for guiding therapeutic approaches.
Keywords: Breast cancer, Progesterone-induced blocking factor, Chemotherapy, Response, In vitro
Subject terms: Biochemistry, Cancer, Biomarkers, Oncology
Introduction
Breast cancer remains a global health concern, impacting many individuals each year. Its complex and diverse pathology means that patients present with a broad range of clinical manifestations and prognoses, and it is a clear challenge to stratify patients into the most suitable treatment option, with each therapeutic approach bearing its own unique set of toxicities1. This emphasizes the necessity for developing and validating new tumor markers, which could provide insights into refining treatment strategies and prognosis determination1–3.
Initially identified as a secretory product of lymphocytes during human pregnancy, progesterone-induced blocking factor 1 (PIBF1) has been characterized as an immunoregulatory molecule crucial for maintaining pregnancy. Current research has revealed an elevated expression of PIBF1 in tumor cells compared with their normal counterparts across diverse malignancies. Despite its potential significance, comprehensive studies about the molecular mechanisms of PIBF1 remain sparse. Hypothesized mechanisms through which PIBF1 might influence tumorigenesis include modulating anti-neoplastic immune responses, instigating apoptosis via p53 upregulation, and orchestrating cell cycle dynamics4. The 35-kDa secretory variant of PIBF1 is predominant during pregnancy. In contrast, the 90-kDa full-length version, which is associated with centrosomes, is prevalent in cancerous states. This observation becomes significant considering the association of various tumorigenic proteins with centrosomes, leading to compromised centrosome functionality and resulting chromosomal missegregation5. Also, previous cell-line studies have indicated that the PIBF1 protein plays an oncogenic role by influencing tumor cell proliferation and migration through the ATR/CHK1 signaling pathway. Moreover, the genomic mapping of the PIBF1 gene on chromosome 13 corresponds to a region implicated in breast cancer susceptibility. Breast carcinoma cells exhibit autonomous PIBF1 production, independent of progesterone, manifesting a pronounced expression compared with unaltered breast tissues3,5,6.
PIBF1, primarily recognized for its immune modulation activities, appears to influence cancer cell growth. Studies on its exact mechanism revealed its involvement in regulating cytokine production, which may affect cellular processes such as proliferation and apoptosis2,3. Its involvement in several other malignancies, such as cervical, lymphoma, and leukemia, has been the subject of ongoing investigations, with preliminary findings underscoring its potential as a valuable tumor marker7–9. Particularly in cervical cancer, the study of Li et al. demonstrated that PIBF1 acts as a regulatory protein through HPV integration, contributing to carcinogenesis10. These findings unveiled the oncogenic role of PIBF1, providing fresh insights into its functional characteristics and associated molecular mechanisms in breast cancer.
With the advancement of chemotherapeutic agents and their efficacy, the role of chemotherapy is becoming increasingly significant in breast cancer management. The number of patients undergoing chemotherapy is on the rise, especially among those with axillary metastasis. Even in cases without metastasis, genetic testing enabled some patients to receive chemotherapy as adjuvant therapy, which was beneficial for preventing recurrence. Consequently, investigating factors associated with chemotherapy, such as chemosensitivity or predictive response markers, is crucial for tailoring appropriate therapeutic strategies.
This study aimed to explore the clinicopathological attributes of PIBF1 in breast cancer and to assess its therapeutic implications by examining the association between PIBF1 and chemosensitivity through a comprehensive approach, employing immunohistochemistry (IHC) and cell line experiments. We aimed to unearth the utility of PIBF1 as a prognostic tool within the breast cancer spectrum, potentially aiding in anticipating the treatment response and consequently optimizing patients’ management5,6.
Methods
Patients
In this retrospective study, tissue specimens were procured from a cohort comprising 469 high-risk patients. These patients underwent surgical interventions at the Asan Medical Center, Seoul, Korea, from January 2008 to December 2013. All included patients exhibited pathologically positive lymph nodes and subsequently received taxane-based adjuvant systemic chemotherapy. For analytical purposes, 231 patients with TNBC were selected, and 238 non-TNBC patients were matched using propensity score matching. Concerning the systemic chemotherapy administered, a taxane-based regimen was universally adopted for all patients. Standard therapeutic interventions were adhered to: individuals underwent either breast-conserving surgery or mastectomy coupled with axillary procedures. Patients diagnosed with hormone receptor-positive breast cancer received treatments with tamoxifen or aromatase inhibitors, with optional ovarian function suppression. Additionally, patients identified with human epidermal growth factor receptor 2 (HER2) + neoplasms received adjuvant targeted therapies, while those opting for breast-conserving surgeries underwent subsequent radiotherapy. Due to the retrospective nature of this study, the patient’s information remains anonymous. Therefore, need for informed consent was waived by"the Institutional Review Board of the Asan Medical Center (Approval No. 2021–0004)”. Given the retrospective nature of the data underpinning this study, the need for informed consent was dispensed.
Clinicopathological assessments
Clinicopathological data encompassing factors such as age, surgical approach to the breast and axilla, and tumor, node, and metastasis staging were meticulously collated. Histopathological parameters, including histologic grading, lympho-vascular invasion status, breast cancer subtyping, and Ki-67, were obtained. Additionally, the use of radiotherapy as an adjuvant therapeutic modality was documented.
Tissue specimens were fixed using 10% buffered formalin (Sigma-Aldrich, St. Louis, Missouri) and subsequently embedded in paraffin (Sigma-Aldrich). In each case, a singular tissue block embedded in paraffin was procured. These blocks were then sectioned into 4-µm thick slices. Following paraffin removal, these sections underwent rehydration and were subsequently treated with a target retrieval solution. Treatment with 3% H2O2 (Sigma-Aldrich) was employed to quench endogenous peroxidase activity, followed by blocking of nonspecific immunoglobulin binding using 10% goat serum (Sigma-Aldrich). Section incubation utilized primary rabbit polyclonal anti-PIBF antibody (Sigma-Aldrich, AE030801) was utilized for section incubation at a dilution of 1:300. Post-incubation, sections were washed using phosphate-buffered saline (Sigma-Aldrich), followed by incubation with secondary antibodies (Sigma-Aldrich) and 3,3’-diaminobenzidine (Sigma-Aldrich). Counterstaining was accomplished by employing hematoxylin and eosin (Sigma-Aldrich). Expert pathologists meticulously evaluated each section using polarized light microscopy (Nikon Eclipse Ni-E; Nikon, Japan). For analytical considerations, sections displaying the zenith of tumor cell staining were chosen. Expression levels of PIBF were quantified using the quick score, which integrates both general staining intensities (0: negative; 1 + : mild intensity; 2 + : moderate intensity; 3 + : intense staining) and percentages of positive tumor cell staining (1 + : 1%–20%; 2 + : 21%–50%; 3 + : > 50%). The preparations were digitally documented using a camera (Nikon DS-Fi2; Nikon).
Cell lines with stable overexpression
Protein extracts were prepared using human breast cancer cell lines, including BT549, MM231, HCC70, MCF7, BT20, ZR-75–1, SK-BR-3, HCC1395, and HS578T cells, obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum (Gibco® Life Technologies, USA) and 1% penicillin/streptomycin solution (Gibco® Life Technologies, USA) in a humidified incubator at 37 °C and 5% CO2.
The human PIBF1 cDNA (GeneBank accession No. NM_001349655) was PCR amplified, and the entire nucleotide sequences were cloned into the pCMV delta R8.2. The vector was transformed in primary embryonic kidney cells (293FT; Invitrogen) and used for packaging lentiviruses (cotransfection of pRSV-Rev, pMDLg/pRRE, and pMD2.G; 3rd generation transfer plasmids, Addgene) for 36 h. Viral particles were then concentrated from 293FT host cells using a Lenti-X™ concentrator (Clontech). HS578T cells were infected with the particles to establish cell lines with stable overexpression of PIBF1.
Assays
The immunoblotting was performed using antibodies against PIBF1 (1:1000, Cat ab72118, Abcam, Cambridge, UK) and β-actin (1:5000, Santa Cruz, California, USA). Protein expression was visualized using an enhanced chemiluminescence system (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK). Moreover, a 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyl-tetrazolium bromide assay (TACS MTT kit, #4890–25-02, TREBIGEN® Instruction) was used to assess cell viability according to the manufacturer’s instructions. For colony formation assay, untreated control and 2 nM paclitaxel-treated cells were plated at a low density (200–600 cells/well) in RPMI1640 medium. Fresh medium with 2 nM paclitaxel was added once every three days. After two weeks of culture, cells were fixed with 4% formaldehyde for 10 min and stained with 0.05% crystal violet for 2 h.
Statistical analysis
We established an IHC cut-off score of 3 (comprising intensity and proportion), which was delineated as the median value for categorization. Scores at or above this threshold were classified as high-PIBF1 expression, while scores below this were considered representative of low-PIBF1 expression.
To understand the implications of PIBF1 in breast cancer, we examined two critical outcomes: overall survival (OS) and disease-free survival (DFS) among TNBC and non-TNBC patient group, categorized according to PIBF1 expression. Although PIBF1 originates from the progesterone gene, it is expressed and functions independently of the progesterone protein. However, since the exact mechanism has not yet been fully elucidated, we conducted analysis across all breast cancer subtypes. DFS was delineated as the interval from the surgery date to the earliest manifestation of local or regional recurrence, distant metastasis, or any mortality. Conversely, OS signifies the duration from the breast cancer diagnosis date leading up to any mortality, irrespective of its association with breast cancer.
Baseline variables, segregated by the presence or absence of PIBF1, underwent rigorous statistical analyses. We employed the chi-squared, Fisher’s exact, and Mann–Whitney U tests to ascertain the significance of our findings. Survival outcomes, namely OS and DFS, were graphically represented through the Kaplan–Meier product-limit method, accompanied by the computation of the log-rank p-value. To assess the prognostic implications of clinicopathological factors, hazard ratios, 95% confidence intervals, and p-values were obtained using the Cox proportional hazards model. All statistical evaluations were two-tailed, and an alpha level below 0.05 was designated as the threshold for statistical significance. All computational analyses were orchestrated using the Statistical Package for the Social Sciences (ver. 20, Armonk, NY, USA).
Clonogenic unit assay and knockdown of breast cancer cell lines
Cell lines were cultivated in 24-well plates supplemented with complete RPMI-1640 medium (10% fetal bovine serum and 1% antibiotic). After 24-h incubation, cells were subjected to 2 or 5 nM paclitaxel and a combination of both for five days at 37 °C, 5% CO2 in a water-jacketed incubator. After air-drying the cells for 60 min at room temperature, they were stained with crystal violet solution for 5 min. Excess dye was gently rinsed with water, and the plates were air-dried inverted. Imaging was performed, and colonies with a diameter exceeding 0.5 mm were enumerated. The clonogenic assay was replicated thrice across all groups.
To further elucidate this association, PIBF1 knockdown was achieved using 200 pmol pooling served-three siRNAs of PIBF1 (NM_001349655.1: cat no. 10464–1, 10,464–2, and 10,464–3; Bioneer, Korea). H578T cells were transfected using the Lipofectamine® RNAiMAX (Invitrogen, Carlsbad, CA, USA) reagent according to the manufacturer’s instructions.
Cell viability was ascertained using the EZ-Cytox Cell Viability Assay Kit, measured at 450 nm. The implications of PIBF1 manipulation were assessed by comparing the relative cell viability of the knockdown cohort to its control counterpart.
Results
Patients’ characteristics
Table 1 provides a comparison of clinical and histopathological parameters in a cohort of 469 patients with breast cancer, categorized into non-TNBC (n = 238) and TNBC (n = 231). The age distribution was remarkably consistent across both groups, with a mean age of 47.4 ± 9.1 years. In terms of surgical interventions, breast-conserving surgery was opted for by 55.0% of the non-TNBC population, in contrast to a larger 70.0% in the TNBC category. In contrast, 45.0% of non-TNBC patients elected for a total mastectomy, compared with a lesser 30.0% in the TNBC. This divergence in surgical choices was statistically significant (p = 0.001). Significant discrepancies (p = 0.015) were observed in tumor stages. T2 tumors were predominantly diagnosed in the TNBC subset (60.2%) compared with 48.3% in their non-TNBC counterparts. In the non-TNBC category exclusively, receptor statuses showed that 77.3% were estrogen receptor (ER)-positive, 60.9% were progesterone receptor (PGR)-positive, and a significant 66.7% were HER2-negative. A rigorous analysis of tumor grades unveiled that patients with TNBC predominantly had G3 histologic (82.0%) and nuclear (82.5%) grades, in stark contrast to the 35.3% and 36.6%, respectively, in the non-TNBC category. These grading variations bore statistical significance (p < 0.001). Expression analyses showed a marked upregulation of p53 in TNBC, with a pronouncedly strong expression in 43.0% of cases, dwarfing the 14.9% observed in non-TNBC. These expression differentials were statistically significant (p < 0.001). Furthermore, elevated Ki-67 expression (> 20%) was predominantly observed in the TNBC group at 83.3%, overshadowing the 43.2% in the non-TNBC group (p < 0.001). In therapeutic interventions, radiotherapy was administered to 84.0% of patients with TNBC, which was significantly higher than the 74.4% in the non-TNBC group (p = 0.010).
Table 1.
Comparison of patient characteristics between the non-triple-negative breast cancer (TNBC) and TNBC groups.
| Characteristics | Total (n = 469) | non-TNBC (n = 238) | TNBC (n = 231) | p-value |
|---|---|---|---|---|
| Age (yr) (Mean ± SD) | 47.35 ± 9.12 | 47.24 ± 8.98 | 47.46 ± 9.28 | 0.999 |
| Breast operation | 0.001 | |||
| Breast-conserving surgery | 292(62.4) | 131(55.0) | 161(70.0) | |
| Total mastectomy | 176(37.6) | 107(45.0) | 69(30.0) | |
| Unknown | 1 | 0 | 1 | |
| Axillary operation | 0.322 | |||
| No | 2(0.4) | 2(0.8) | 0(0.0) | |
| Sentinel node biopsy | 67(14.3) | 36(15.1) | 31(13.4) | |
| Axillary lymph node dissection | 400(85.3) | 200(84.0) | 200(86.6) | |
| pT stage | 0.015 | |||
| T0/is | 4(0.9) | 0(0.0) | 4(1.7) | |
| T1 | 178(38.0) | 105(44.1) | 73(31.6) | |
| T2 | 254(54.2) | 115(48.3) | 139(60.2) | |
| T3 | 30(6.4) | 16(6.7) | 14(6.1) | |
| T4 | 3(0.6) | 2(0.8) | 1(0.4) | |
| pN stage | 0.412 | |||
| N0 | 7(1.5) | 4(1.7) | 3(1.3) | |
| N1 | 326(69.5) | 167(70.2) | 159(68.8) | |
| N2 | 388(17.5) | 45(18.9) | 37(16.0) | |
| N3 | 360(11.5) | 22(9.2) | 32(13.9) | |
| Pathologic stage | 0.992 | |||
| I | 17(3.6) | 8(3.4) | 9(3.9) | |
| II | 298(63.5) | 152(63.9) | 146(63.2) | |
| III | 152(32.4) | 77(32.4) | 75(32.5) | |
| IV | 2(0.4) | 1(0.4) | 1(0.4) | |
| ER | - | - | ||
| Negative | 54(22.7) | 54(22.7) | - | - |
| Positive | 184(77.3) | 184(77.3) | - | - |
| PGR | - | - | ||
| Negative | 93(39.1) | 93(39.1) | - | - |
| Positive | 145(60.9) | 145(60.9) | - | - |
| HER2 status | - | - | ||
| Negative | 148(66.7) | 148(66.7) | - | - |
| Positive | 74(33.3) | 74(33.3) | - | - |
| Unknown | 16 | 16 | - | - |
| Histologic grade | < 0.001 | |||
| G1/2 | 195(41.8) | 154(64.7) | 41(18.0) | |
| G3 | 271(58.2) | 84(35.3) | 187(82.0) | |
| Unknown | 3 | 0 | 3 | |
| Nuclear grade | < 0.001 | |||
| G1/2 | 191(41.0) | 151(63.4) | 40(17.5) | |
| G3 | 275(59.0) | 87(36.6) | 188(82.5) | |
| Unknown | 3 | 0 | 3 | |
| Lymphovascular invasion | 0.539 | |||
| Absent | 276(60.3) | 139(58.9) | 137(61.7) | |
| Present | 182(39.7) | 97(41.1) | 85(38.3) | |
| Unknown | 11 | 2 | 9 | |
| P53 | < 0.001 | |||
| Negative | 275(59.1) | 168(71.5) | 107(46.5) | |
| Weak | 31(6.7) | 21(8.9) | 10(4.3) | |
| Intermediate | 25(5.4) | 11(4.7) | 14(6.1) | |
| Strong | 134(28.8) | 35(14.9) | 99(43.0) | |
| Unknown | 4 | 3 | 1 | |
| Ki-67 | < 0.001 | |||
| Low (≤ 20%) | 72(35.5) | 54(56.8) | 18(16.7) | |
| High(> 20%) | 131(64.5) | 41(43.2) | 90(83.3) | |
| Unknown | 266 | 143 | 123 | |
| Radiotherapy | 0.010 | |||
| No | 98(20.9) | 61(25.6) | 37(16.0) | |
| Yes | 371(79.1) | 177(74.4) | 194(84.0) |
Data shown are number (%), when not otherwise specified. SD, standard deviation; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PGR, progesterone receptor; G, grade.
Upon categorizing patients based on PIBF1 status (Table 2), no statistically significant variations were observed in the tumor, node, and metastasis stages. However, notable differences in receptor status, tumor grade, and molecular markers, particularly p53 and Ki-67, were evident concerning PIBF1 status. The ER and PGR were less prevalent in low-PIBF1 patients, documented at 10.3% and 6.9%, respectively. This contrasted with high-PIBF1 patients with substantially higher figures: 61.3% for ER and 49.2% for PGR. Both distinctions were statistically significant (p < 0.001). Furthermore, the HER2-negative phenotype was more commonly exhibited among low-PIBF1 patients, at 45.2% compared with 71.7% in the high-PIBF1 cohort. This discrepancy was statistically significant (p = 0.001). Regarding histologic gradation, low-PIBF1 patients showed a notable inclination towards G3 histologic (78.5%) and nuclear (79.0%) grades, significantly higher than those in high-PIBF1 patients: 42.9% and 44.0% (p < 0.001). Moreover, molecular marker assessment revealed heightened strong p53 expression in low-PIBF1 patients (39.3%) compared with their high-PIBF1 counterparts (20.8%), with a significant difference indicted by a p-value < 0.001. Additionally, high Ki-67 expression (> 20%) was predominantly found in low-PIBF1 patients (82.4%) compared to high-PIBF1 patients (46.5%), a statistically significant distinction (p < 0.001).
Table 2.
Patients characteristics categorized by progesterone-induced blocking factor 1 (PIBF1) status.
| Characteristics | Total (n = 469) | PIBF1 | p-value | |
|---|---|---|---|---|
| Low (n = 203) | High (n = 266) | |||
| Age (yr) (Mean ± SD) | 47.35 ± 9.12 | 47.76 ± 8.87 | 47.03 ± 9.32 | 0.953 |
| Breast operation | 0.705 | |||
| Breast-conserving surgery | 292(62.4) | 128(63.4) | 164(61.7) | |
| Total mastectomy | 176(37.6) | 74(36.6) | 102(38.3) | |
| Unknown | 1 | 1 | ||
| Axillary operation | 0.849 | |||
| No | 2(0.4) | 1(0.5) | 1(0.4) | |
| Sentinel node biopsy | 67(14.3) | 31(15.3) | 36(13.5) | |
| Axillary lymph node dissection | 400(85.3) | 171(84.2) | 229(86.1) | |
| pT stage | 0.387 | |||
| T0/is | 4(0.9) | 3(1.5) | 1(0.4) | |
| T1 | 178(38.0) | 70(34.5) | 108(40.6) | |
| T2 | 254(54.2) | 113(55.7) | 141(53.0) | |
| T3 | 30(6.4) | 15(7.4) | 15(5.6) | |
| T4 | 3(0.6) | 2(1.0) | 1(0.4) | |
| pN stage | 0.725 | |||
| N0 | 7(1.5) | 3(1.5) | 4(1.5) | |
| N1 | 326(69.5) | 140(69.0) | 186(69.9) | |
| N2 | 388(17.5) | 33(16.3) | 49(18.4) | |
| N3 | 360(11.5) | 27(13.3) | 27(10.2) | |
| Pathologic stage | 0.428 | |||
| I | 17(3.6) | 8(3.9) | 9(3.4) | |
| II | 298(63.5) | 127(62.6) | 171(64.3) | |
| III | 152(32.4) | 66(32.5) | 86(32.3) | |
| IV | 2(0.4) | 2(1.0) | 0(0.0) | |
| ER | < 0.001 | |||
| Negative | 285(60.8) | 182(89.7) | 103(38.7) | |
| Positive | 184(39.2) | 21(10.3) | 163(61.3) | |
| PGR | < 0.001 | |||
| Negative | 324(69.2) | 189(93.1) | 135(50.8) | |
| Positive | 144(30.8) | 14(6.9) | 131(49.2) | |
| HER2 status | 0.001 | |||
| Negative | 148(66.7) | 19(45.2) | 129(71.7) | |
| Positive | 74(33.3) | 23(54.8) | 51(28.3) | |
| Unknown | 16 | 1 | 15 | |
| Histologic grade | < 0.001 | |||
| G1/2 | 195(41.8) | 43(21.5) | 152(57.1) | |
| G3 | 271(58.2) | 157(78.5) | 114(42.9) | |
| Unknown | 3 | 3 | 0 | |
| Nuclear grade | < 0.001 | |||
| G1/2 | 191(41.0) | 42(21.0) | 149(56.0) | |
| G3 | 275(59.0) | 158(79.0) | 117(44.0) | |
| Unknown | 3 | 3 | 0 | |
| Lymphovascular invasion | 0.128 | |||
| Absent | 276(60.3) | 126(64.6) | 150(57.3) | |
| Present | 182(39.7) | 70(35.7) | 112(42.7) | |
| Unknown | 11 | 7 | 4 | |
| P53 | < 0.001 | |||
| Negative | 275(59.1) | 97(48.3) | 178(67.4) | |
| Weak | 31(6.7) | 13(6.5) | 18(6.8) | |
| Intermediate | 25(5.4) | 12(6.0) | 13(4.9) | |
| Strong | 134(28.8) | 79(39.3) | 55(20.8) | |
| Unknown | 4 | 2 | 2 | |
| Ki-67 | < 0.001 | |||
| Low (≤ 20%) | 72(35.5) | 18(8.9) | 54(53.5) | |
| High(> 20%) | 131(64.5) | 84(82.4) | 47(46.5) | |
| Unknown | 266 | 101 | 165 | |
| Radiotherapy | 0.214 | |||
| No | 98(20.9) | 37(18.2) | 61(22.9) | |
| Yes | 371(79.1) | 166(81.8) | 205(77.1) | |
Data shown are number (%), when not otherwise specified. SD, standard deviation; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PGR, progesterone receptor; G, grade.
In stratifying patients into either TNBC or PIBF expression, some characteristics showed even clearer statistical significance in the non-TNBC subgroup (Table 3). In the non-TNBC cohort, PIBF1 expression was related to a lower nodal stage (p = 0.025), lower pathologic stage (p = 0.024), and a lower histologic grade (p = 0.006). However, these differences were not distinct in the TNBC subgroup.
Table 3.
Comparison of progesterone-induced blocking factor 1 (PIBF1) expression characteristics across triple-negative breast cancer (TNBC) subgroups.
| Characteristics | Non-TNBC | TNBC | ||||
|---|---|---|---|---|---|---|
| No PIBF1 | PIBF1 | p-value | No PIBF1 | PIBF1 | p-value | |
| Age (yr) (Mean ± SD) | 49.91 ± 7.95 | 46.65 ± 9.11 | 0.370 | 47.19 ± 9.03 | 48.07 ± 9.86 | 0.967 |
| Breast operation | 0.009 | 0.827 | ||||
| Breast-conserving surgery | 16(37.2) | 115(59.0) | 112(70.4) | 49(69.0) | ||
| Total mastectomy | 27(62.8) | 80(41.0) | 47(29.6) | 22(31.0) | ||
| Unknown | 0 | 0 | 1 | 0 | ||
| Axillary operation | 0.480 | 0.290 | ||||
| No | 1(2.3) | 1(0.5) | 0(0.0) | 0(0.0) | ||
| Sentinel node biopsy | 7(16.3) | 29(14.9) | 24(15.0) | 7(9.9) | ||
| Axillary lymph node dissection | 35(81.4) | 165(84.6) | 136(85.0) | 64(90.1) | ||
| pT stage | 0.315 | 0.865 | ||||
| T0/is | 0(0.0) | 0(0.0) | 3(1.9) | 1(1.4) | ||
| T1 | 17(39.5) | 88(45.1) | 53(33.1) | 20(28.2) | ||
| T2 | 20(46.5) | 95(48.7) | 93(58.1) | 46(64.8) | ||
| T3 | 5(11.6) | 11(5.6) | 10(6.3) | 4(5.6) | ||
| T4 | 1(2.3) | 1(0.5) | 1(0.6) | 0(0.0) | ||
| pN stage | 0.025 | 0.224 | ||||
| N0 | 0(0.0) | 4(2.1) | 3(1.9) | 0(0.0) | ||
| N1 | 26(60.5) | 141(72.3) | 114(71.3) | 45(63.4) | ||
| N2 | 8(18.6) | 37(19.0) | 25(15.6) | 12(16.9) | ||
| N3 | 9(20.9) | 13(6.7) | 18(11.3) | 14(19.7) | ||
| Pathologic stage | 0.024 | 0.276 | ||||
| I | 0(0.0) | 8(4.1) | 8(5.0) | 1(1.4) | ||
| II | 23(53.5) | 129(66.2) | 104(65.0) | 42(59.2) | ||
| III | 19(44.2) | 58(29.7) | 47(29.4) | 28(39.4) | ||
| IV | 1(2.3) | 0(0.0) | 1(0.6) | 0(0.0) | ||
| ER | < 0.001 | |||||
| Negative | 22(51.2) | 32(16.4) | - | - | ||
| Positive | 21(48.8) | 163(83.6) | - | - | ||
| PGR | < 0.001 | |||||
| Negative | 29(67.4) | 64(32.8) | - | - | ||
| Positive | 14(32.6) | 131(67.2) | - | - | ||
| HER2 status | 0.001 | |||||
| Negative | 19(45.2) | 129(71.7) | - | - | ||
| Positive | 23(54.8) | 51(28.3) | - | - | ||
| Unknown | 1 | 15 | - | - | ||
| Histologic grade | 0.006 | 0.051 | ||||
| G1/2 | 20(46.5) | 134(68.7) | 23(14.6) | 18(25.4) | ||
| G3 | 23(53.5) | 61(31.3) | 134(85.4) | 53(74.6) | ||
| Unknown | 0 | 0 | 3 | 0 | ||
| Nuclear grade | 0.004 | 0.088 | ||||
| G1/2 | 19(44.2) | 132(67.7) | 23(14.6) | 17(23.9) | ||
| G3 | 24(55.8) | 63(32.3) | 134(85.4) | 54(76.1) | ||
| Unknown | 0 | 0 | 3 | 0 | ||
| Lymphovascular invasion | 0.359 | 0.285 | ||||
| Absent | 28(65.1) | 111(57.5) | 98(64.1) | 39(56.5) | ||
| Present | 15(34.9) | 82(42.5) | 55(35.9) | 30(43.5) | ||
| Unknown | 0 | 2 | 7 | 2 | ||
| P53 | 0.048 | 0.529 | ||||
| Negative | 24(57.1) | 144(74.6) | 73(45.9) | 34(47.9) | ||
| Weak | 4(9.5) | 17(8.8) | 9(5.7) | 1(1.4) | ||
| Intermediate | 2(4.8) | 9(4.7) | 10(6.3) | 4(5.6) | ||
| Strong | 12(28.6) | 23(11.9) | 67(42.1) | 32(45.1) | ||
| Unknown | 1 | 2 | 1 | 0 | ||
| Ki-67 | 0.049 | 0.015 | ||||
| Low (≤ 20%) | 9(39.1) | 45(62.5) | 9(11.4) | 9(31.0) | ||
| High (> 20%) | 14(60.9) | 27(37.5) | 70(88.6) | 20(69.0) | ||
| Unknown | 20 | 123 | 81 | 42 | ||
| Radiotherapy | 0.706 | 0.807 | ||||
| No | 12(27.9) | 49(25.1) | 25(15.6) | 12(16.9) | ||
| Yes | 31(72.1) | 146(74.9) | 135(84.4) | 59(83.1) | ||
Data shown are number (%), when not otherwise specified. SD, standard deviation; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PGR, progesterone receptor; G, grade.
Survival outcomes according to PIBF
Figure 1 shows the results of PIBF1 IHC in various tumor tissues. Figure 1a and 1b display low expression in non-TNBC and TNBC, respectively, while 1c and 1 d show high expression. Figure 2 displays the Kaplan–Meier curves for OS and DFS, stratified by the high or low PIBF1 expression within the total study population. Significant differences in OS between the two groups were observed (p = 0.010). Additionally, while there was a result of better DFS in the high-PIBF1 group, its statistical significance approached the threshold of validation (p = 0.055). Among the 105 patients who exhibited recurrence, 15 events of local recurrence, 11 events of regional recurrence, and 79 events of distant metastasis were reported within a median follow-up period of 98.2 months (5.5–140.7 months). Moreover, 76 patients died during this period. We also found that patients with PIBF1 had a five-year OS rate of 92.5% compared with those without PIBF1, who had an OS rate of 84.7% (p = 0.010) (Fig. 2a). The five-year DFS rate was 92.7% for patients with PIBF1 and 86.7% for patients without PIBF1 (p = 0.055). (Fig. 2b).
Fig. 1.
Representative pathologic images of PIBF1 expression in breast cancer IHC (Original magnification × 200) a. PIBF1 low expression in non-TNBC tumor b. PIBF1 low expression TNBC tumor c. PIBF1 high expression in non-TNBC tumor d. PIBF1 high expression in TNBC tumor.
Fig. 2.
Kaplan–Meier curves showing survival outcomes and disease-free survival according to PIBF1 expression in overall population. This demonstrates enhanced OS (a) and DFS (b) in the cohort with high PIBF1 expression but a statistically significant difference was only in OS (p = 0.010).
Based on the analysis segmented into TNBC and non-TNBC cohorts, the non-TNBC cohort mirrored the overall population outcomes. The group with PIBF1 exhibited superior outcomes in both OS and DFS, with statistically significant differences (p = 0.013 for OS and p = 0.025 for DFS), as illustrated in Figs. 3a and 3b. Conversely, in the TNBC cohort, no statistical significance was observed, as shown in Figs. 4a and 4b. And when analyzed according to Luminal A, B, HER2 subtypes, there were no significant differences in both OS and DFS (Supplement).
Fig. 3.
Kaplan–Meier curves showing survival outcomes and disease-free survival according to PIBF1 expression in non-TNBC cohort. This demonstrates enhanced OS (a) and DFS (b) in the cohort of non-TNBC patients. There were also improved OS and DFS with high PIBF1 expression and in this cohort both show statistically significant differences (OS: p = 0.013, DFS: p = 0.025).
Fig. 4.
Kaplan–Meier curves showing survival outcomes and disease-free survival according to PIBF1 expression in TNBC cohort. This demonstrates enhanced OS (a) and DFS (b) in the cohort of TNBC patients. There were no differences in both groups.
According to the univariate analysis of the non-TNBC cohort, pT, ER, HER2 status, and histologic grade were not statistically significant, but pN stage (hazard ratio = 5.70, 95% confidence interval = 2.44–13.33, p < 0.001), PGR (hazard ratio = 0.40, 95% confidence interval = 0.18–0.90, p = 0.026), lymphovascular invasion (hazard ratio = 4.68, 95% confidence interval = 1.86–11.79, p = 0.001), p53 (hazard ratio = 2.53, 95% confidence interval = 1.07–5.98, p = 0.035), and the presence of PIBF1 (hazard ratio = 0.35, 95% confidence interval = 0.15–0.83, p = 0.017) were statistically significant predictors of the OS (Table 4). In the multivariate analysis of the non-TNBC cohort, PIBF1 emerged as a favorable prognostic factor for OS, although this association did not reach statistical significance (hazard ratio = 0.44, 95% confidence interval = 0.18–1.11, p = 0.082).
Table 4.
Univariable and multivariable regression analysis of overall survival in the non-triple-negative breast cancer subset.
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | 1.011(0.966–1.059) | 0.630 | ||
| Breast operation | 0.359 | |||
| Breast-conserving surgery | 1(ref) | |||
| Total mastectomy | 1.457(0.652–3.253) | |||
| Axillary operation | 0.730 | |||
| No/SNB only | 1(ref) | |||
| Axillary lymph node dissection | 1.237(0.369–4.149) | |||
| pT stage | 0.283 | |||
| T0–1 | 1(ref) | |||
| T2–4 | 1.594(0.681–3.732) | |||
| pN stage | < 0.001 | 0.005 | ||
| N0–1 | 1(ref) | 1(ref) | ||
| N2–3 | 5.701(2.438–13.327) | 3.800(1.510–9.562) | ||
| ER | 0.375 | |||
| Negative | 1(ref) | |||
| Positive | 0.671(0.278–1.620) | |||
| PGR | 0.026 | 0.091 | ||
| Negative | 1(ref) | 1(ref) | ||
| Positive | 0.397(0.176–0.898) | 0.432(0.163–1.144) | ||
| HER2 status | 0.725 | |||
| Negative | 1(ref) | |||
| Positive | 1.167(0.494–2.760) | |||
| Histologic grade | 0.514 | |||
| G1/2 | 1(ref) | |||
| G3 | 1.311(0.582–2.952) | |||
| Nuclear grade | 0.598 | |||
| G1/2 | 1(ref) | |||
| G3 | 1.244(0.552–2.801) | |||
| Lymphovascular invasion | 0.001 | 0.001 | ||
| Absent | 1(ref) | 1(ref) | ||
| Present | 4.678(1.857–11.789) | 5.217(1.883–14.455) | ||
| P53 | 0.035 | 0.434 | ||
| Negative-Weak | 1(ref) | 1(ref) | ||
| Intermediate-Strong | 2.525(1.067–5.975) | 1.458(0.566–3.756) | ||
| Ki-67 | 0.896 | |||
| Low (≤ 20%) | 1(ref) | |||
| High (> 20%) | 1.092(0.293–4.069) | |||
| Radiotherapy | 0.058 | |||
| No | 1(ref) | |||
| Yes | 4.062(0.954–17.285) | |||
| PIBF1 expression | 0.017 | 0.082 | ||
| Low | 1(ref) | 1(ref) | ||
| High | 0.353(0.150–0.832) | 0.444(0.178–1.108) | ||
SNB, sentinal node biopsy; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PGR, progesterone receptor; G, grade; HR, hazard ratio; CI, confidence interval.
Association of PIBF1 with paclitaxel sensitivity: insights from breast cancer cell line experiments
All patients in the study underwent taxane-based chemotherapy, prompting our focus on the potential relationship between PIBF1 expression and chemotherapy sensitivity. To elucidate this, we conducted in vitro experiments using various breast cancer cell lines. Specifically, BT-549 and BT-20 (both from ATCC, Manassas, VA, USA) exhibited elevated PIBF1 expression, while HCC70 and HS578T (also from ATCC, Manassas, VA, USA) displayed reduced PIBF1 expression (Fig. 5).
Fig. 5.
PIBF1 expression in several cell lines In the cell line, BT 549 and BT20 showed elevated PIBF1 expression, where as HCC70 and HS578T showed reduced expression.
In the cell viability assays, upon treatment with 5 nM paclitaxel, BT549 and BT20 exhibited significant reductions in cell viability (BT549: 13.6%; BT20: 15.0%), whereas HCC70 and HS578T retained over 80% viability (Fig. 6).
Fig. 6.

Cell viability assays using paclitaxel BT549 and BT20 displayed reductions in cell viability, whereas HCC70 and HS578T retained viability.
In the clonogenic assays, the BT549 cell line displayed a 69.8% reduction in clonogenic count, while BT20 demonstrated complete colony eradication. Conversely, the HS578T cell line demonstrated a mere 26.7% decline, and the HCC70 cell line exhibited an increase in colony count (Fig. 7).
Fig. 7.
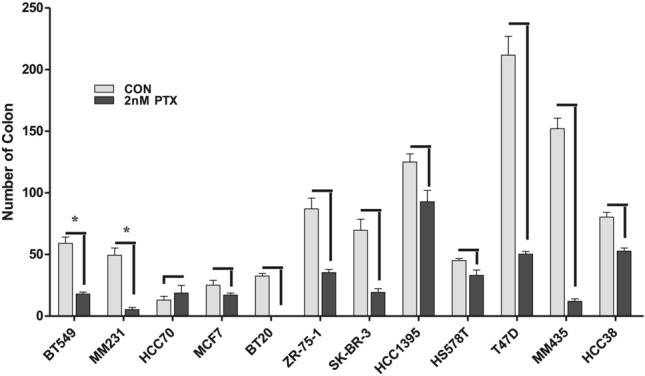
Colonogenic assays using paclitaxel. BT549 and BT20 displayed a substantial reduction in colonogenic count, but HS578T displayed a small decline, and in HCC70, there was even an increase in colony count.
To reinforce the association between PIBF1 expression and paclitaxel sensitivity, we performed a siRNA-mediated knockdown of PIBF1 in the BT20 cell line. Subsequent to this genetic intervention, the knockdown cell lines exhibited a reduced response to paclitaxel treatment compared with controls (control: 100 ± 51.5% decreasing to 32.43 ± 12.5%; knockdown: 99.9 ± 29.6% decreasing to 68.35 ± 18.7%) (Fig. 8).
Fig. 8.

PIBF1 knockdown and paclitaxel sensitivity. siRNA mediated knock-down of PIBF1 was conducted and the knock-down cell lines demonstrated a diminished response to paclitaxel.
Discussion
Breast cancer stands as a formidable adversary in the global fight against malignancies. The heterogeneity of its pathology presents both challenges and opportunities. Its diverse clinicopathological manifestations emphasize the paramount need for refining diagnostic and prognostic tools to aid clinicians in tailoring treatment modalities to individual patient profiles, thereby ensuring optimized therapeutic outcomes1. Our study concentrated on the potential of PIBF1 as such a marker.
Initially distinguished in pregnancy immunology, the PIBF1 transition to oncology research appeared surprising at first glance. However, its increased expression in various cancers compared with their normal tissue adds credence to its potential role in tumor progression and possibly in treatment modulation. The focal point of our study was to unravel the relevance of PIBF1 and its characteristics in the breast cancer panorama. Given the inherent chromosomal significance of its gene location and elevated expression in breast cancer cells, this protein has emerged as another probable keystone in breast cancer pathophysiology2,4,9.
The immune modulatory role of PIBF1, coupled with its interplay in cellular events such as proliferation and apoptosis, underpins its importance in tumorigenesis2,3,10. Although its exact mechanisms remain under exploration, preliminary findings from other malignancies set a promising stage for its implications in breast cancer7,8,11.
In our study, garnered from a sizable patient cohort, some insights were highlighted in PIBF1 and breast cancer. In the analysis of clinicopathological features, patients expressing PIBF1 exhibited an association with lower histologic and nuclear grade compared with those without PIBF1 expression. Additionally, PIBF1 expression was concurrently associated with a decrease in Ki-67 compared to the PIBF1-negative cohort. This pattern was accentuated within the non-TNBC cohort when categorizing patients into TNBC and non-TNBC subsets. Such findings underscore that PIBF1 expression potentially indicates more favorable clinicopathological attributes within breast cancer pathophysiology.
Additionally, PIBF1 expression was correlated with hormone receptor positivity, suggesting that its expression is not only associated with favorable prognostic indicators but may also be intrinsically linked to hormone-related characteristics of the cancer (Tables 2, 3). Within this study cohort, a quantitative evaluation was conducted on the relationship between the ER Allred score and PIBF1 expression, revealing a positive correlation. These findings potentially underscore the hormone-related nature of PIBF1, warranting more extensive research to fully elucidate this aspect.
In the survival analysis, the overall patient population showed enhanced OS with PIBF1 expression, reaching statistical significance (p = 0.010). Upon stratification into TNBC and non-TNBC subsets, the statistically significant improvement in survival was exclusively observed within the non-TNBC cohort (p = 0.013). Subsequent multivariable analysis concerning OS within the non-TNBC cohort indicated that PIBF1 expression might correlate with a reduced risk of adverse outcomes. While this association approached statistical significance, it did not conclusively attain it (hazard ratio = 0.44, 95% confidence interval = 0.18–1.11, p = 0.082). However, these associations may vary with the expansion of the study cohort to include a larger patient population. The survival analysis unveiled compelling distinctions predicated on PIBF1 expression, particularly prominent in the non-TNBC cohort. Collectively, our findings indicate that PIBF1 expression is favorably related to survival outcomes among patients with high-risk breast cancer who have been administered chemotherapy.
Perhaps one of the most enlightening aspects of this study was the exploration of PIBF in the context of taxane-based chemotherapy. With all patients in our cohort being subjected to this regimen, discerning a potential correlation with PIBF1 was paramount. Our in vitro assays, including viability and clonogenic assessments, revealed differential responses based on PIBF1 expression. Upon paclitaxel administration, the significant reductions in cell viability and colony formation in high-PIBF1-expressing cell lines, such as BT-549 and BT20, mirrored the inherent biology observed in the patient cohort. Moreover, our genetic knockdown experiments further reinforced the influence of PIBF1 on chemotherapy sensitivity.
Kim et al.12 identified that the larger isoform of PIBF1, primarily associated with the centrosome, functions as a pericentriolar satellite protein for the integrity of the mitotic spindle pole and have named this protein CEP90. Taxanes inhibit the dynamic behavior of microtubules, leading to the induction of multipolar mitotic spindles and the redistribution of the microtubule network from the centrosomes to the cell cortex13. Given the impact of PIBF1 on spindle pole conformation, it may exert a synergistic effect with taxane-based chemotherapy. This interaction could hypothetically contribute to the observed enhancement in the chemotherapy response and outcomes evidenced in the study.
In an era of personalized medicine, with the diverse treatment approaches in breast cancer, the identification and validation of markers like PIBF1 could facilitate more tailored and individualized therapeutic strategies. This aligns with the overarching goal of optimizing patient outcomes in the complex landscape of breast cancer treatment. As a foundational study in this field, this research explores the relevance of the protein PIBF1 in breast cancer, which exhibits diverse molecular biology. In this study, we analyzed protein expression and conducted survival analysis in a specific cohort of patients who received taxane-based chemotherapy, dividing them into TNBC and non-TNBC groups. In future research, we plan to investigate the association of PIBF1 in patient groups who did not receive chemotherapy and examine its role in hormone-responsive breast cancer, which is another hypothesis under consideration. Should meaningful results emerge, we aim to apply these findings, along with those from this study, to real-world clinical applications. If there is one more limitation to mention, it would be, in the initial design of this cell-line study, we used breast cancer cell lines representing various subtypes, including HR + cell lines such as MCF7 and ZR-75–1, which exhibit high PIBF1 expression. Although ZR-75–1 showed significant results in the colony formation assay, these HR + cell lines generally had poor colony growth, making the experimental process challenging. This suggests the need for further discussion on methodological approaches for studying the impact of PIBF1 on breast cancer in HR + cell lines.
Our study establishes a preliminary but robust foundation for PIBF1’s significance in breast cancer prognosis and treatment strategies. However, this study had some limitations, primarily hinged on its retrospective nature and single-center design. Furthermore, given that the cohort predominantly consisted of high-risk patients who had received chemotherapy, there are inherent limitations in extrapolating the natural attributes of PIBF1 to the broader breast cancer population. Further investigation warranted to elucidate the oncogenic mechanisms of PIBF1 and its impact on patients, including those who have not received chemotherapy and those treated with other therapeutic modalities.
Conclusion
In conclusion, the identification and validation of biomarkers such as PIBF1 hold promise for advancing personalized medicine in breast cancer treatment. This offers a potential pathway to develop more nuanced and patient-specific therapeutic strategies. Our study adds to the body of evidence supporting the value of PIBF1 as a marker for breast cancer prognosis and prediction of chemotherapy response, paving the way for improved patient management in this complex disease landscape.
Supplementary Information
Acknowledgements
This work was supported by grants from the National Research Foundation of Korea (NRF) of the Republic of Korean government (MSIP: Ministry of Science and ICT) [grant number: NRF-2021R1A2C2008786] and Asan Medical Center (AMC) granted financial resource from Asan Institute for Life Science [grant number: 2021IL0026-1].
Abbreviations
- ATCC
American type culture collection
- CI
Confidence interval
- DFS
Diseade-free survival
- ER
Estrogen receptor
- G
Grade
- HER2
Human epidermal growth factor receptor 2
- HR
Hazard ratio
- OS
Overall survival
- PGR
Progesterone receptor
- PIBF1
Progesterone-induced blocking factor 1
- SD
Standard deviation
- SNB
Sentinal node biopsy
- TNBC
Triple-negative breast cancer
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Eunju Shin, Jewon Ryu, Tae-Kyung Yoo, Sae Byul Lee, Jisun Kim, Il Yong Chung, Beom Seok Ko, Hee Jeong Kim, Jong Won Lee, Jun Hyeong Lee, Kyunggon Kim, Sang-wook, Byung Ho Son. The first draft of the manuscript was written by Eunju Shin, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. We confirm that all authors listed in the manuscript have approved the order of authorship.
Funding
This work was supported by grants from the National Research Foundation of Korea (NRF) of the Republic of Korean government (MSIP: Ministry of Science and ICT) [grant number: NRF-2021R1A2C2008786] and Asan Medical Center (AMC) granted financial resource from Asan Institute for Life Science [grant number: 2021IL0026].
Data availability
The datasets analyzed during the current study are not publicly available, as the personal information of patients must be protected, but are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study received review and approval from the Institutional Review Board of the Asan Medical Center (2021–0004).
Consent to participate and publish
Due to the retrospective nature of this study, the patient’s information remains anonymous. Therefore, informed consent was not required.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sang-wook Lee, Email: lsw@amc.seoul.kr.
Byung Ho Son, Email: brdrson@korea.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-10546-5.
References
- 1.Walker, R. A. Immunohistochemical markers as predictive tools for breast cancer. J. Clin. Pathol.61(6), 689–696 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Check, J. H. & Check, D. Therapy aimed to suppress the production of the immunosuppressive protein progesterone induced blocking factor (PIBF) may provide palliation and/or increased longevity for patients with a variety of different advanced cancers – A review. Anticancer Res.39(7), 3365–3372 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Ro, E. J. et al. PIBF1 suppresses the ATR/CHK1 signaling pathway and promotes proliferation and motility of triple-negative breast cancer cells. Breast Cancer Res. Treat182(3), 591–600 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Balassa, T. et al. The effect of the Progesterone-Induced Blocking Factor (PIBF) on E-cadherin expression, cell motility and invasion of primary tumour cell lines. J. Reprod. Immunol.125, 8–15 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Szekeres-Bartho, J. & Polgar, B. PIBF the double edged sword. Pregnancy and tumor. Am. J. Reprod. Immunol.64(2), 77–86 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Lachmann, M. et al. PIBF (progesterone induced blocking factor) is overexpressed in highly proliferating cells and associated with the centrosome. Int. J. Cancer112(1), 51–60 (2004). [DOI] [PubMed] [Google Scholar]
- 7.González-Arenas, A. et al. Progesterone-induced blocking factor is hormonally regulated in human astrocytoma cells, and increases their growth through the IL-4R/JAK1/STAT6 pathway. J. Steroid. Biochem. Mol. Biol.144, 463–470 (2014). [DOI] [PubMed] [Google Scholar]
- 8.DiAntonio, G. et al. Serum levels of the immunomodulatory protein, the progesterone induced blocking factor (PIBF) which is found in high levels during pregnancy is not higher in women with progesterone (P) receptor (R) positive vs. negative breast cancer. Clin. Exp. Obstet. Gynecol.44(2), 187–189 (2017). [PubMed] [Google Scholar]
- 9.Kabel, A. M. Tumor markers of breast cancer: New prospectives. J. Oncol. Sci.3(1), 5–11 (2017). [Google Scholar]
- 10.Tang, H. et al. circKIF4A acts as a prognostic factor and mediator to regulate the progression of triple-negative breast cancer. Mol. Cancer18(1), 23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halasz, M. et al. Progesterone-induced blocking factor differentially regulates trophoblast and tumor invasion by altering matrix metalloproteinase activity. Cell Mol. Life Sci.70(23), 4617–4630 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, K. & Rhee, K. The pericentriolar satellite protein CEP90 is crucial for integrity of the mitotic spindle pole. J. Cell Sci.124(Pt 3), 338–347 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Hornick, J. E. et al. Live-cell analysis of mitotic spindle formation in taxol-treated cells. Cell Motil. Cytoskeleton65(8), 595–613 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are not publicly available, as the personal information of patients must be protected, but are available from the corresponding author upon reasonable request.







