Abstract
Adult naive T cells, which are at rest in normal conditions, proliferate strongly when transferred to lymphopenic hosts. In neonates, the first mature thymocytes to migrate to the periphery reach a compartment devoid of preexisting T cells. We have extensively analyzed the proliferation rate and phenotype of peripheral T cells from normal C57BL/6 and T cell antigen receptor transgenic mice as a function of age. We show that, like adult naive T cells transferred to lymphopenic mice, neonatal naive T cells proliferate strongly. By using bone-marrow transfer and thymic-graft models, we demonstrate that the proliferation of the first thymic emigrants reaching the periphery requires T cell antigen receptor-self-peptide/self-MHC interactions and is regulated by the size of the peripheral T cell pool.
Peripheral T cells expand strongly after transfer to lymphopenic hosts (1, 2). This proliferation has been considered as a homeostatic mechanism that fills the peripheral T cell pool. It was initially suggested that homeostatic T cell proliferation was restricted to activated/memory T cells. Indeed, most authors inferred that naive and memory T cell pools would be regulated independently. The memory T cell pool would be renewed and filled by proliferation of preexisting activated/memory cells, whereas only continuous output of naive T cells by the thymus could generate a full compartment of truly naive T cells (3). This theory is supported by the observation that a significant proportion of memory T cells proliferate in normal mice, whereas naive T lymphocytes do not cycle in normal conditions (4–8).
Nevertheless, using monoclonal naive T cells from T cell antigen receptor (TCR) transgenic mouse strains and purified polyclonal naive T cells from normal mice, it has recently been shown that naive T cells proliferate when transferred to lymphopenic hosts (8–21). Such peripheral T cell expansion would be depend on self-peptide-specific, low-affinity interactions similar to those required for positive selection in the thymus (10–12), although other reports do not support this view (13). By following the long-term fate of naive T cells transferred to lymphopenic hosts (rag-deficient, CD3ɛ-deficient, and irradiated normal mice), we and others have observed that the transferred cells fail to fill the peripheral naive T cell pool. Indeed, absolute numbers of recovered T cells are far below those found in the full peripheral naive T cell pool of a normal mouse. Moreover, injected naive T cells acquire a memory-like phenotype that remains stable with time, despite the absence of foreign antigenic stimulation, and their functional capacities are modified, enhanced, or abolished (18–21). Recent studies have demonstrated a role of IL-7, IL-12, and p56lck in the proliferation of naive T cells in lymphopenic mice (22–25), but the mechanisms underlying this process are not fully understood.
Furthermore, it is crucial to show the physiological relevance of this mechanism. Indeed, injection of purified naive T cells to irradiated or genetically modified adult mice could reveal particular T cell reactivities that would never occur in unmanipulated healthy animals. In neonates, the first thymic emigrants reach a peripheral compartment devoid of preexisting T cells. Therefore, the proliferation/activation of naive T cells transferred to lymphopenic hosts might also be expected to apply to the first thymic emigrants in neonates.
In this study, we analyzed the absolute number, proliferation rate, and phenotype of peripheral T cells from normal C57BL/6 and TCR transgenic mice as a function of age. We show that peripheral naive T cells proliferate strongly in neonates. By using bone-marrow transfer and thymic-graft models, we demonstrate that the proliferation of peripheral naive T cells in neonates requires TCR-self-peptide/self-MHC interactions and is regulated by the size of the peripheral T cell pool.
Materials and Methods
Mice.
H-2b and H-2k AND TCR transgenic rag-20/0 mice (21, 26), 5CC7 TCR transgenic rag-20/0 mice (27, 28), normal C57BL/6 mice, and C57BL/6 Ba mice (Thy 1.1) were maintained in our animal facilities. C57BL/6 CD3ɛ-deficient mice (29) and C57BL/6 nude mice were obtained from the Centre de Développement des Techniques Avancées pour l'Experimentation Animale (Orléans, France). C57BL/6 CD3ɛ-deficient mice were crossed with C57BL/6 Aβ-deficient mice (30) to generate CD3ɛ/Aβ double-deficient mice (CD3ɛKO IIKO) (KO, knockout; ref. 21). The resulting mice were crossed with C57BL/6 Aβ/β2m double-deficient mice (31, 32) to obtain CD3ɛ/β2m/Aβ triple-deficient mice (CD3ɛKO IKO IIKO). P14 TCR transgenic rag-20/0 mice (33) and HY TCR transgenic rag-20/0 mice (34, 35) were generously provided by A. Freitas (Unité de Biologie des Populations Lymphocytaires) and B. Rocha (Institut National de la Santé et de Recherche Médicale, U345), respectively.
Thymus Grafts.
Thymuses from 2- to 3-day-old normal C57BL/6 mice or from 2- to 3-day-old H-2b AND mice were grafted under the kidney capsule of C57BL/6 Ba mice, CD3ɛ−/− mice with normal MHC expression, and CD3ɛ−/− mice deficient for MHC class II or MHC classes I and II molecule expression. Spleens of these mice were recovered 1 week after grafting.
Adoptive Transfer of Bone Marrow (BM) Cells.
Six- to eight-week-old CD3ɛ-deficient mice were irradiated (1,000 rad) and injected i.v. with 5 × 106 CD4- and CD8-depleted BM cells from H-2b AND TCR transgenic rag-20/0 mice (Thy 1.2). In some experiments, 20 × 106 lymph node T cells from C57BL/6 Ba mice (Thy 1.1) were coinjected. Spleens of these mice were recovered 14–28 days after BM transfer.
Cell Surface Staining and Flow Cytometry.
Surface staining.
Thymuses, lymph nodes, and spleens were homogenized with a nylon cell strainer (Falcon) in PBS/5% FCS/0.2% NaN3 and then distributed in 96-well U-bottom microplates (4 × 106 cells per well). Staining was performed on ice for 30 min per step.
Abs were purchased from PharMingen unless otherwise indicated. The following Ab combinations were used: for four-color analysis, phycoerythrin (PE) anti-CD44 (1M781), FITC anti-CD8 (53-6-7), PercP anti-CD4 (RM4–5), and biotinylated anti-TCRβ (H57–597) or anti-Vβ3 (KJ25), with allophycocyanin-streptavidin revelation (PharMingen); for detection and characterization of DNA-synthesizing cells, PE anti-CD8 (53-6-7), PercP anti-CD4 (RM4-5), and biotinylated anti-TCRβ (H57-7597) or anti-Vβ3 (KJ25), with allophycocyanin-streptavidin revelation (PharMingen). To analyze chimeras, cell suspensions were stained for Thy 1.2 (30-H12), CD4 or CD8, TCRβ or Vβ3, and CD44 expression or BrdUrd incorporation.
BrdUrd labeling.
One milligram of BrdUrd (Sigma) was injected i.p. twice, at a 30-min interval. To detect and characterize DNA-synthesizing cells, spleens were taken 30 min after the second injection. Surface-stained cells were fixed and permeabilized in PBS containing 1% paraformaldehyde plus 0.01% Tween 20 for 48 h at 4°C and then submitted to the BrdUrd DNase detection technique as described (36, 37), using FITC-conjugated anti-BrdUrd Ab (Becton Dickinson).
Flow cytometry.
Four-color immunofluorescence was analyzed with a FACScalibur cytometer (Becton Dickinson). List-mode data files were analyzed with cell quest software (Becton Dickinson).
Results
Peripheral T Cells Proliferate Strongly in Neonates.
The absolute number, proliferation rate, and phenotype of peripheral T cells from normal C57BL/6 mice were studied 2–10 days after birth and compared with the results obtained in adult mice (6–8 weeks of age). To detect and characterize DNA-synthesizing cells, BrdUrd was injected i.p. twice, at a 30-min interval; spleens were taken 30 min after the second injection, cell-counted, and stained for CD4, CD8, TCR, and CD44 expression or BrdUrd incorporation.
CD4+ and CD8+ T cells were detectable in the spleen 2 days after birth. Such cells were absent from nude mice of the same age, and were therefore recent thymic emigrants (Fig. 1a). Some CD4+ cells are reported not to express TCR in the periphery of newborn mice (38). To exclude these cells, the analysis was restricted to CD4+ TCRhi and CD8+ TCRhi cells (Fig. 1 b and c).
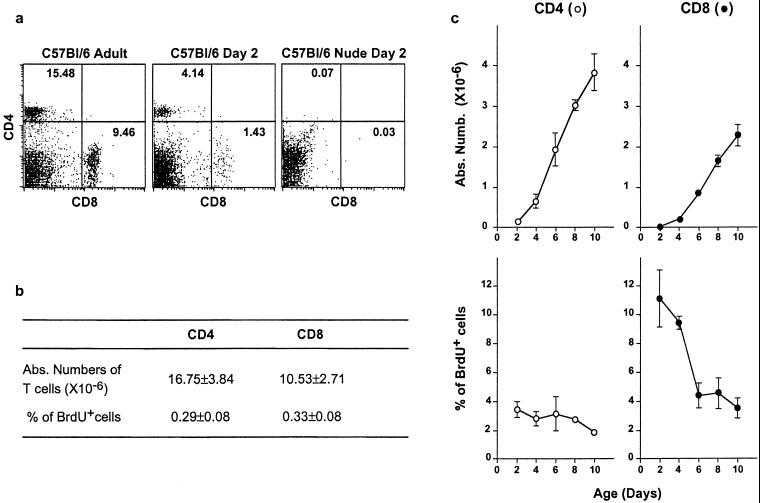
Figure 1.
Peripheral T cells proliferate strongly in neonates. The absolute number and proliferation rate of peripheral T cells from normal C57BL/6 mice were studied from 2 to 10 days after birth and in adult control mice. To detect and characterize DNA-synthesizing cells, BrdUrd was injected i.p. twice, at a 30-min interval. Spleens were taken 30 min after the second injection, cell-counted, and stained for CD4, CD8, TCR surface expression, and BrdUrd incorporation. (a) CD4/CD8 fluorescence dot-plots of adult and day-2 normal and nude C57BL/6 mice. (b) Absolute numbers of splenic CD4+ and CD8+ TCRhi cells and percentages of BrdUrd+ cells among these subsets in adult mice. (c) Absolute numbers of splenic CD4+ and CD8+ TCRhi cells and percentages of BrdUrd+ cells among these subsets as a function of age.
Splenic CD4+ and CD8+ T cell compartments grew strongly during the first days after birth but still represented only one-fourth of the corresponding adult compartments 10 days after birth (Fig. 1 b and c). By contrast to adult T cells, T cells from neonates proliferated strongly. Indeed, more than 3% of splenic CD4+ T cells and 10% of splenic CD8+ T cells from 2-day-old C57BL/6 mice incorporated BrdUrd (Fig. 1c), whereas only 0.3% of splenic CD4+ and CD8+ T cells proliferated in adult mice (Fig. 1b). CD4+ and CD8+ T cell proliferation rates fell rapidly with age but were still higher than in adult mice 10 days after birth.
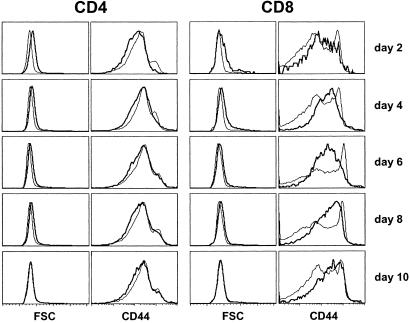
Most splenic CD4+ and CD8+ T cells were blasts on day 2 after birth; cell size decreased with time, becoming similar to that of adult T cells 10 days after birth (Fig. 2). We then investigated whether the observed proliferation was associated with a parallel increase in CD44 expression. CD44 expression by CD4+ T cells from neonates was not increased relative to that of adult CD4+ T cells. Only weak CD44 up-regulation was found on CD8+ T cells 4–10 days after birth (Fig. 2). Most CD8+ T cells from adult mice express little or no CD44, whereas some express high levels of this activation marker; these latter cells are considered as activated/memory CD8+ T cells. Four to eight days after birth, all CD8+ T cells expressed intermediate densities of CD44, and this expression increased with time, reaching on day 10 the level observed on adult activated/memory CD8+ T cells. In addition, although a large proportion of CD4+ and CD8+ T cells proliferated during the neonatal period, they did not express CD69 (data not shown).
Figure 2.
CD44 expression and size of neonatal spleen CD4+and CD8+ T cells. From 2 to 10 days after birth, spleens from normal C57BL/6 mice were taken and stained for CD4, CD8, TCR, and CD44 surface expression. Forward scatter (FSC) and CD44 fluorescence histograms of CD4+ and CD8+ TCRhi cells from 2- to 10-day-old mice (thick lines) in comparison with FSC and CD44 fluorescence histograms of adult mouse CD4+ and CD8+ T cells (thin lines).
Peripheral T cells proliferated strongly in neonates, but this proliferation was not matched closely by an increase in activation marker expression. It was thus important to determine the mechanism underlying this proliferation, particularly its dependence on TCR/MHC interactions.
Peripheral T Cells Proliferate in Neonates in Response to TCR/MHC Interactions.
T cells are not produced in MHC-deficient mice (30–32). Therefore, to study the proliferation rate of neonatal thymic emigrants in an MHC-deficient environment, we used the following experimental system. Thymuses from 2- to 3-day-old C57BL/6 mice were engrafted under the kidney capsule of CD3ɛ−/− mice with normal MHC expression and similar mice lacking MHC class II or MHC classes I and II molecule expression. One week after grafting, the spleen and thymus were removed, cell-counted, and stained for CD4, CD8, TCR, and CD44 expression or BrdUrd incorporation. This experimental system allowed us to determine whether the proliferation rate of thymic emigrants from neonates depended on the MHC expression in the periphery.
As shown in Fig. 1b, very few peripheral CD4+ and CD8+ T cells proliferated in adult mice (C57BL/6 adult, Fig. 3a). By contrast, after thymus grafting into recipients expressing MHC classes I and II molecules but lacking endogenous T cell production (CD3ɛ-deficient mice), a large proportion of thymus graft-derived CD4+ T cells and CD8+ T cells proliferated in the spleen (Fig. 3a). CD44 expression by these cells was strongly up-regulated (Fig. 3b). This up-regulation of CD44 expression was restricted to peripheral T cells, as no difference in the surface expression of this marker was observed between engrafted and normal adult mature thymocytes (Fig. 3c).
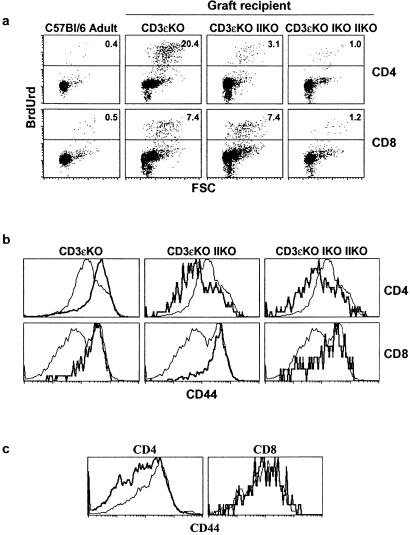
Figure 3.
Peripheral T cells proliferate in neonates in response to TCR/MHC interactions. Thymuses from 2- to 3-day-old C57BL/6 mice were engrafted under the kidney capsule of normal CD3ɛ−/− mice and CD3ɛ−/− mice lacking MHC class II molecules or MHC classes I and II molecules. One week after grafting, spleens and thymuses were removed, cell-counted, and stained for CD4, CD8, TCR, and CD44 expression or BrdUrd incorporation. (a) FSC/BrdUrd fluorescence dot-plots of both spleen CD4+ and CD8+ TCRhi cells. (b) CD44 fluorescence histograms of graft-derived CD4+ and CD8+ TCRhi spleen cells (thick lines) in comparison with CD44 expression on adult CD4+ and CD8+ spleen T cells (thin lines). (c) CD44 fluorescence histograms of graft-derived CD4+ and CD8+ TCRhi thymocytes (thick lines) in comparison with CD44 expression on adult CD4+ and CD8+ TCRhi thymocytes (thin lines).
In this model, the proliferation of peripheral CD4+ T cells and concomitant CD44 up-regulation on these cells were abrogated in the absence of peripheral MHC class II molecule expression. Similar results were observed for peripheral CD8+ T cells when the hosts also lacked MHC class I expression (Fig. 3).
Thus, the high proliferation rates of CD4+ T cells and CD8+ T cells observed during the first days after birth depended on TCR interactions with self-MHC molecules, although they were not always matched by increased activation marker expression (Fig. 2). This proliferation could result from TCR interactions with environmental antigens, or from interactions with self-peptide/self-MHC complexes. To examine these possibilities, we investigated T cell proliferation rates in neonates of TCR transgenic mouse strains, in which encounters between T cells and their nominal antigens were highly unlikely.
Peripheral T Cells Proliferate in Neonates in Response to Self-Peptide/Self-MHC Complexes.
Monoclonal T cells from various TCR transgenic mouse strains were assessed for their capacity to proliferate in neonates (Tables 1 and 2). All of the studied TCR transgenic mouse strains were crossed with rag-2-deficient mice to avoid rearrangement of endogenous TCR genes resulting in dual TCR expression. The proliferation rate in day-2 splenic T cells from all of the tested TCR transgenic mouse strains was higher than in their adult counterparts. Furthermore, as in normal neonates, TCR transgenic CD8+ T cells exhibited higher proliferation rates than CD4+ T cells. With the exception of H-2b AND TCR transgenic T cells, the observed proliferation rates were not different between normal and TCR transgenic cells (Tables 1 and 2).
Table 1.
CD4 T cells
| TCR | Haplotype | Absolute no. of CD4 TCRhi cells, ×10−6
|
% of BrdUrd+ cells in CD4 TCRhi cells
|
||
|---|---|---|---|---|---|
| Day 2 | Adult | Day 2 | Adult | ||
| C57Bl/6 polyclonal | H-2b | 0.14 ± 0.08 | 16.75 ± 3.84 | 3.45 ± 0.54 | 0.30 ± 0.08 |
| AND | H-2b | 0.06 ± 0.02 | 9.30 ± 1.20 | 0.66 ± 0.32 | 0.24 ± 0.16 |
| AND | H-2k | 0.34 ± 0.17 | 13.40 ± 3.20 | 3.33 ± 1.32 | 0.12 ± 0.06 |
| 5CC7 | H-2k | 0.03 ± 0.01 | 5.01 ± 0.70 | 2.12 ± 1.11 | 0.10 ± 0.01 |
Table 2.
CD8 T cells
| TCR | Haplotype | Absolute no. of CD8 TCRhi cells, ×10−6
|
% of BrdUrd+ cells in CD8 TCRhi cells
|
||
|---|---|---|---|---|---|
| Day 2 | Adult | Day 2 | Adult | ||
| C57Bl/6 polyclonal | H-2b | 0.02 ± 0.02 | 10.53 ± 2.71 | 11.12 ± 1.97 | 0.33 ± 0.08 |
| H-Y | H-2b | 0.21 ± 0.11 | 1.28 | 9.31 ± 1.49 | 0.08 |
| P14 | H-2b | 0.03 ± 0.01 | 15.77 | 9.87 ± 2.26 | 0.20 |
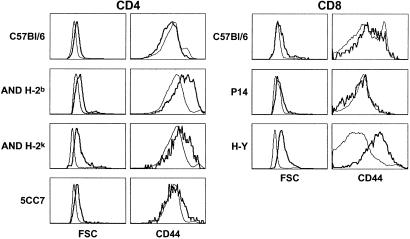
All tested day-2 TCR transgenic T cells contained a higher proportion of blasts than their adult counterparts (Fig. 4). Interestingly, CD44 expression was strongly up-regulated in day-2 AND CD4+ T cells (Fig. 4), as well as in day-2 HY CD8+ T cells (Fig. 4). By contrast, day-2 P14 CD8+ T cells did not express higher CD44 densities than their adult counterparts, and only a weak increase in CD44 expression was observed in day-2 5CC7 CD4+ T cells (Fig. 4). Moreover, as in normal neonates, CD69 expression was not significantly increased in neonatal peripheral T cells from TCR transgenic mice (data not shown). Thus, as in normal neonates, the observed proliferation of neonatal TCR transgenic T cells was not closely matched by an increase in activation marker expression.
Figure 4.
Peripheral naive T cells proliferate strongly in neonates. Two days after birth, spleens from different mouse strains were taken and stained for CD4, CD8, TCR, and CD44 surface expression. FSC and CD44 fluorescence histograms of CD4+ TCRhi spleen cells from 2-day-old mice of different strains (thick lines). FSC and CD44 fluorescence histograms of CD4+ TCRhi spleen cells from the corresponding adult mice are shown as controls (thin lines). FSC and CD44 fluorescence histograms of CD8+ TCRhi spleen cells from 2-day-old mice of different strains (thick lines). FSC and CD44 fluorescence histograms of CD8+ TCRhi spleen cells from the corresponding adult mice are shown as controls (thin lines).
Proliferation of peripheral T cells in neonates was not restricted to polyclonal repertoires but was also seen, to a similar extent, in monoclonal T cells. Such proliferation did not result from interactions with environmental antigens, as tested TCR transgenic cells were unlikely to have encountered their known nominal antigens. Thus, proliferation of peripheral T cells in neonates did not depend on antigenic stimulation but rather involved TCR interactions with self-peptide/self-MHC complexes.
The Extent of Thymic Emigrant Proliferation Depends on the Size of the Peripheral T Cell Pool.
During the first days after birth, very few T cells can be recovered in the periphery. Therefore, in the neonatal period, most peripheral T cells are recent thymic emigrants. It was thus important to determine whether the proliferation of peripheral T cells in neonates depended on the “lymphopenic” state of the periphery in neonates or represented a property of recent thymic emigrants in general. Two experimental models were used to address this question.
First, thymuses from 2- to 3-day-old H-2b AND mice (Thy 1.2) were engrafted under the kidney capsule of C57BL/6 Ba mice (Thy 1.1), CD3ɛ−/− mice with normal MHC expression, and CD3ɛ−/− mice lacking MHC class II molecule expression. One week after grafting, splenocytes were harvested, counted, and stained for CD4, Thy 1.2, Vβ3, and CD44 expression or BrdUrd incorporation (Table 3).
Table 3.
The proliferation of peripheral T cells in neonates depends on the “lymphopenic” state of the periphery
| Studied parameter | AND H-2b/b Adult | Graft recipient
|
||
|---|---|---|---|---|
| CD3ɛKO | CD3ɛKO IIKO | C57Bl/6 Ba | ||
| Absolute no. of CD4+ T cells (×10−6) (Thy1.1 + Thy1.2) | 13.40 ± 3.20 | 0.12 ± 0.06 | 0.03 ± 0.01 | 10.41 ± 0.92 |
| % of BrdUrd+ cells in CD4+ Vβ3+ Thy1.2+ cells | 0.2 ± 0.1 | 8.0 ± 1.1 | 1.8 ± 0.5 | 1.5 ± 0.1 |
| % of CD44hi cells in CD4+ Vβ3+ Thy1.2+ cells | 2.5 ± 0.8 | 40.5 ± 9.7 | 9.7 ± 0.6 | 3.2 ± 3.0 |
KO, knockout.
After thymus grafting into recipients expressing MHC class II molecules but lacking endogenous T cell production (CD3ɛ-deficient mice), a large proportion of thymus graft-derived AND CD4+ T cells (8%) proliferated in the spleen. CD44 expression by these cells was strongly up-regulated. The proliferation of peripheral AND CD4+ T cells and concomitant CD44 up-regulation on these cells were not observed in the absence of peripheral MHC class II molecule expression (CD3ɛ-deficient IIKO mice) and when the host contained normal number of peripheral T cells (C57BL/6 Ba mice) (Table 3). Thus, both MHC class II molecule expression and a periphery devoid of endogenous preexisting T cells are required for the proliferation of neonatal AND CD4+ T cells.
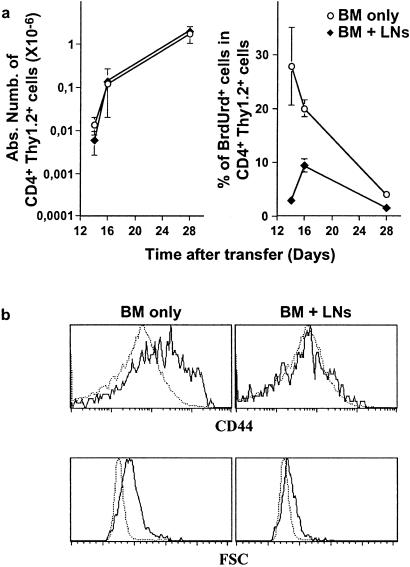
Finally, to confirm these results, H-2b CD3ɛ-deficient mice were irradiated and injected with CD4- and CD8-depleted BM cells from H-2b AND TCR transgenic mice. In this system, like in neonates, the first mature thymocytes to migrate from the thymus to the periphery reach a compartment devoid of preexisting T cells. Co-injection of lymph node T cells (Thy 1.1) at the same time as BM cells (Thy 1.2) allowed us to study the influence of the size of the peripheral T cell pool on the proliferation of thymic emigrants. The first CD4+ mature thymocytes and, therefore, the first thymic emigrants, are generated 14 to 16 days after transfer (37). Interestingly, 14 and 16 days after transfer, a significant percentage of splenic AND CD4+ T cells proliferated strongly (Fig. 5a). Moreover, they expressed high densities of CD44, and most of them were blast cells (Fig. 5b).
Figure 5.
The extent of thymic emigrant proliferation depends on the size of the peripheral T cell pool. H-2b CD3ɛ-deficient mice were irradiated and injected with CD4- and CD8-depleted BM cells from H-2b AND TCR transgenic mice (Thy 1.2). When indicated, 20 × 106 lymph node T cells from C57BL/6 Ba mice (Thy 1.1) were coinjected. Spleens of these mice were recovered 14–28 days after BM transfer. (a) Absolute numbers of splenic CD4+ Thy 1.2+ cells and percentages of BrdUrd+ cells among this subset as a function of time after transfer. (b) FSC and CD44 fluorescence histograms of day 16 after transfer of CD4+ Thy 1.2+ splenic cells (plain lines) in comparison with FSC and CD44 fluorescence histogram of CD4+ splenic cells from H-2b AND TCR transgenic adult mice (dotted line).
The proliferation of AND CD4+ T cells (Thy 1.2) was strongly diminished when polyclonal T cells (Thy 1.1) were injected at the same time as BM cells (Fig. 5a). In these conditions, fewer blasts and no CD44 up-regulation were observed in AND CD4+ T cells. These results suggest that an increase in the absolute size of the peripheral T cell pool abrogates the observed activation/proliferation of the first thymic emigrants to reach the periphery.
Discussion
Interactions with self-peptide/self-MHC complexes are required for the generation of naive T cells in the thymus and their subsequent maintenance in peripheral organs (35, 39–42). Such interactions have been shown to induce the proliferation of both memory and naive T cells after transfer to lymphopenic recipients (8–21). Indeed, although they do not cycle in normal conditions (unmanipulated healthy animals), naive T cells proliferate strongly when transferred to lymphopenic hosts (irradiated normal mice or rag- or CD3ɛ-deficient mice).
Here, we show that peripheral T cells proliferate strongly in neonates. Both CD4+ and CD8+ T cells from normal mice exhibited high proliferation rates in the first days after birth. In the neonatal period, most peripheral T cells are recent thymic emigrants, whereas the latter represent only a minor part of the peripheral T cell pool in adult mice. Nevertheless, peripheral T cell proliferation in neonates does not reflect a property of recent thymic emigrants in general. Indeed, several findings argue against this possibility. First, in adult mice, recent thymic emigrants do not contain a higher proportion of blast cells than the bulk of peripheral T cells (43, 44). Similarly, BrdUrd labeling of thymic emigrants detected by prior FITC intrathymic injection did not reveal higher proliferative activity in these cells than in total peripheral cells in adult mice (45). Finally, in the present study, the proliferation of the first T cells reaching the periphery after thymus graft and after BM transfer depended on the size of the peripheral T cell pool (Table 3 and Fig. 5).
By using a thymic-graft model, we found that the proliferation of peripheral T cells in neonates depended on TCR interactions with self-MHC molecules. Such proliferation could result from TCR interactions with environmental antigens, with stimulation of a minor proportion of thymic emigrants that strongly expands after antigenic stimulation. Alternatively, the observed proliferation might be because of interactions with self-peptide/self-MHC complexes and thus involve the vast majority of T cell clones migrating from the thymus to the periphery in neonates. Peripheral T cells from all tested TCR transgenic mouse strains strongly proliferated during the neonatal period, implying that reactivity against self-peptide/self-MHC complexes is certainly involved. Indeed, an encounter between TCR transgenic T cells and their nominal antigen is highly unlikely in this setting. As suggested by other groups regarding the observed proliferation of adult naive T cells transferred to lymphopenic hosts (10–12), the proliferation of peripheral T cells in neonates might depend on self-peptide-specific, low-affinity interactions similar to those required for positive selection in the thymus. We cannot exclude the possibility that, in normal mice, a proportion of peripheral T cells responds to exogenous antigens. Nevertheless, the fact that proliferation rates were not very different between normal and TCR transgenic cells suggests that, in normal mice, the bulk of proliferating T cells are responding to self-peptide/self-MHC complexes.
By contrast to adult CD4+ naive cells transferred to lymphopenic mice, cycling neonatal peripheral CD4+ T cells from normal mice did not convert to a memory-like phenotype. Only weak CD44 up-regulation was observed on CD8+ T cells on days 4–10 after birth. This difference between proliferating adult and neonatal naive T cells could result from the immaturity of the immune system in neonates. Indeed, although recent studies have shown that neonatal cells have the ability to mount adult-like T helper 1 and cytotoxic T lymphocyte responses (46–48), in standard conditions naive neonatal T cells are functionally deficient, both in vitro and in vivo (49, 50). More precisely, neonatal T cells respond poorly to antigens (49), and neonatal antigen-presenting cells (APCs) present antigens inefficiently (51–53). When day 2–3 neonatal thymuses were engrafted into adult lymphopenic mice, graft-derived T cells found in the periphery 1 week later proliferated strongly and showed marked CD44 up-regulation. In these conditions, newly produced T cells reached an adult peripheral environment. These data suggest that the absence of CD44 up-regulation in neonates is because of APC immaturity rather than T cell immaturity. Moreover, by contrast to polyclonal CD4+ T cells from normal mice, neonatal CD4+ T cells from TCR transgenic mouse strains showed CD44 up-regulation to the high levels characteristic of memory cells. This difference could reflect a higher affinity of TCR transgenic CD4+ T cells relative to the bulk of polyclonal CD4+ T cells, which might compensate for neonatal APC immaturity during encounters with self-peptide/self-MHC complexes. Only the results obtained with HY TCR transgenic CD8+ T cells are in conflict with such a theory. Indeed, neonatal HY TCR transgenic CD8+ T cells proliferated strongly (Table 2), whereas CD8+ T lymphocytes from adult HY TCR transgenic failed to proliferate after transfer to adult lymphopenic mice (4, 5, 8, 10, 35). Thus, the conditions required for neonatal naive T cell proliferation and for the proliferation of adult naive T cells transferred to lymphopenic mice might differ although they rely on a similar basis: they both depend on interactions with self-peptide/self-MHC complexes and on the “lymphopenic” state of the periphery.
All of the neonatal TCR transgenic CD4+ T cells that we tested as well as neonatal HY TCR transgenic CD8+ T cells not only proliferated but also converted to a memory-like phenotype, whereas very few cells with such a phenotype could be detected in their adult counterparts. Such observations imply that neonatal cycling T cells either revert to a naive phenotype or are extremely diluted with time by newly migrating thymocytes and therefore represent a very limited proportion of the adult peripheral T cell pool that can barely be detected. The latter theory is supported by the results of Modigliani et al. (54) who have shown that the increase of the peripheral T cell pool during ontogeny essentially results from thymic output without peripheral expansion. Interestingly, in the present study, when lymph-node T cells were cotransferred with BM cells, proliferation of BM-derived thymic emigrants was profoundly impaired (Fig. 5), yet the increase in BM-derived peripheral T cells was not affected. Thus, the proliferation or not of recent thymic emigrants barely makes any difference in their accumulated numbers, suggesting that many of the proliferating peripheral T cells in neonates are destined to die. Altogether, these results suggest that “homeostatic” proliferation of recent thymic emigrants in neonates accounts only weakly for the filling of the peripheral T cell pool.
The mechanisms underlying the proliferation of naive T cells in neonates remain to be elucidated. In mice, regulatory T cells begin to exit the thymus only 3 days after birth (55, 56). Thus, during the first days after birth, the peripheral T cell pool would be devoid of regulatory T cells, and this could explain the ability of unregulated naive T cells to respond to self-peptide/self-MHC complexes. Mice thymectomized around day 3 of life develop autoimmune syndromes (57–61). Similarly, mice in which regulatory T cells are not maintained in the periphery [IL-2-deficient and IL-2-receptor-deficient mice (62–64)] develop inflammatory bowel disease and lymphadenopathy. Thus, regulatory T cells prevent the autoreactivity of the peripheral T cell pool. In neonates, the lack of such cells could explain the observed proliferation of naive T cells in response to self-peptide/self-MHC complexes.
Acknowledgments
We thank A. Banz and A. Sarukhan for illuminating discussions, and S. Ezine for surgical expertise and critical reading of the manuscript. This work was supported by the Agence Nationale de Recherches sur le SIDA. A.L.C. and F.L. were supported by Ph.D. fellowships from Ensemble Contre le SIDA.
Abbreviations
- FSC
forward scatter
- TCR
T cell antigen receptor
- BM
bone marrow
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tanchot C, Rosado M M, Agenes F, Freitas A A, Rocha B. Semin Immunol. 1997;9:331–337. doi: 10.1006/smim.1997.0090. [DOI] [PubMed] [Google Scholar]
- 2.Tanchot C, Rocha B. Immunol Today. 1998;19:575–580. doi: 10.1016/s0167-5699(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 3.Tanchot C, Rocha B. J Exp Med. 1997;186:1099–1106. doi: 10.1084/jem.186.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Boehmer H, Hafen K. J Exp Med. 1993;177:891–896. doi: 10.1084/jem.177.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tough D F, Sprent J. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sprent J, Tough D F. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 7.Tanchot C, Rocha B. Eur J Immunol. 1995;25:2127–2136. doi: 10.1002/eji.1830250802. [DOI] [PubMed] [Google Scholar]
- 8.Bruno L, von Boehmer H, Kirberg J. Eur J Immunol. 1996;26:3179–3185. doi: 10.1002/eji.1830261251. [DOI] [PubMed] [Google Scholar]
- 9.Oehen S, Brduscha-Riem K. Eur J Immunol. 1999;29:608–614. doi: 10.1002/(SICI)1521-4141(199902)29:02<608::AID-IMMU608>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 10.Ernst B, Lee D-S, Chang J M, Sprent J, Surh C D. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 11.Viret C, Wong F S, Janeway C A. Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 12.Goldrath A W, Bevan M J. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bender J, Mitchell T, Kappler J, Marrack P. J Exp Med. 1999;190:367–373. doi: 10.1084/jem.190.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kieper W C, Jameson S C. Proc Natl Acad Sci USA. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldrath A W, Bevan M J. Nature (London) 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira C, Barthlott T, Garcia S, Zamoyska R, Stockinger B. J Immunol. 2000;165:3689–3694. doi: 10.4049/jimmunol.165.7.3689. [DOI] [PubMed] [Google Scholar]
- 17.Goldrath A W, Bogatski L Y, Bevan M J. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murali-Krishna K, Ahmed R. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 19.Cho B K, Rao V P, Ge Q, Eisen H N, Chen J. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suhr C D, Sprent J. J Exp Med. 2000;192:F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanchot C, Le Campion A, Léaument S, Dautigny S, Dautigny N, Lucas B. Eur J Immunol. 2001;31:2256–2265. doi: 10.1002/1521-4141(200108)31:8<2256::aid-immu2256>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Schluns K S, Kieper W C, Jameson S C, Lefrançois L. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 23.Seddon B, Legname G, Tomlinson P, Zamoyska R. Science. 2000;290:127–131. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 24.Kieper W C, Prlic M, Schmidt C S, Mescher M F, Jameson S C. J Immunol. 2001;166:5515–5521. doi: 10.4049/jimmunol.166.9.5515. [DOI] [PubMed] [Google Scholar]
- 25.Tan J T, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg K I, Suhr C D. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaye J, Hsu M L, Sauron M E, Jameson J C, Gascoigne R J, Hedrick S M. Nature (London) 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 27.Miller C, Ragheb J A, Schwartz R H. J Exp Med. 1999;190:53–60. doi: 10.1084/jem.190.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanchot C, Barber D L, Chiodetti L, Schwartz R H. J Immunol. 2001;167:2030–2039. doi: 10.4049/jimmunol.167.4.2030. [DOI] [PubMed] [Google Scholar]
- 29.Malissen M, Gillet A, Ardouin L, Bouvier G, Trucy J, Ferrier P, Vivier E, Malissen B. EMBO J. 1995;14:4641–4650. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 31.Koller B H, Marrack P, Kappler J W, Smithies O. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 32.Chan S H, Cosgrove D, Waltzinger C, Benoist C, Mathis D. Cell. 1993;73:225–236. doi: 10.1016/0092-8674(93)90225-f. [DOI] [PubMed] [Google Scholar]
- 33.Pircher H, Bürki K, Lang R, Hengartner H, Zinkernagel R M. Nature (London) 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 34.Kisielow P, Blüthmann H, Staerz U W, Steimetz M, Von Boehmer H. Nature (London) 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 35.Tanchot C, Lemonnier F A, Pérarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 36.Vasseur F, Le Campion A, Pavlovitch J H, Pénit C. J Immunol. 1999;162:5164–5172. [PubMed] [Google Scholar]
- 37.Le Campion A, Vasseur F, Pénit C. Eur J Immunol. 2000;30:738–746. doi: 10.1002/1521-4141(200003)30:3<738::AID-IMMU738>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 38.Kelly K A, Scollay R. Eur J Immunol. 1992;22:329–334. doi: 10.1002/eji.1830220207. [DOI] [PubMed] [Google Scholar]
- 39.Takeda S, Rodewald H R, Arakawa H, Bluethmann H, Shimizu T. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 40.Kirberg J, Berns A, von Boehmer H. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rooke R, Waltzinger C, Benoist C, Mathis D. Immunity. 1997;7:123–134. doi: 10.1016/s1074-7613(00)80515-4. [DOI] [PubMed] [Google Scholar]
- 42.Brocker T. J Exp Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scollay R, Chen W-F, Shortman K. J Immunol. 1984;132:25–30. [PubMed] [Google Scholar]
- 44.Kelly K A, Scollay R. Int Immunol. 1990;2:419–425. doi: 10.1093/intimm/2.5.419. [DOI] [PubMed] [Google Scholar]
- 45.Pénit C, Vasseur F. J Immunol. 1997;159:4848–4856. [PubMed] [Google Scholar]
- 46.Ridge J P, Fuchs E J, Matzinger P. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 47.Sarzotti M, Robbins D S, Hoffman P M. Science. 1996;271:1726–1728. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- 48.Forsthuber T, Yip H C, Lehmann P V. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 49.Adkins B. Immunol Today. 1999;20:330–335. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 50.Garcia A M, Fadel S A, Cao S, Sarzotti M. Immunol Res. 2001;22:177–190. doi: 10.1385/IR:22:2-3:177. [DOI] [PubMed] [Google Scholar]
- 51.Le Bon A, Desaymard C, Papiernik M. Int Immunol. 1995;7:1897–1903. doi: 10.1093/intimm/7.12.1897. [DOI] [PubMed] [Google Scholar]
- 52.Trivedi H N, HayGlass K T, Gangur V, Allardice J G, Embree J E, Plummer F A. Hum Immunol. 1997;57:69–79. doi: 10.1016/s0198-8859(97)00202-4. [DOI] [PubMed] [Google Scholar]
- 53.Marshall-Clarke S, Reen D, Tasker L, Hassan J. Immunol Today. 2000;21:35–41. doi: 10.1016/s0167-5699(99)01548-0. [DOI] [PubMed] [Google Scholar]
- 54.Modigliani Y, Coutinho G, Burlen-Defranoux O, Coutinho A, Bandeira A. Eur J Immunol. 1994;24:1223–1227. doi: 10.1002/eji.1830240533. [DOI] [PubMed] [Google Scholar]
- 55.Sakaguchi S, Toda M, Asano M, Itoh M, Morse S S, Sakaguchi N. J Autoimmun. 1996;9:211–220. doi: 10.1006/jaut.1996.0026. [DOI] [PubMed] [Google Scholar]
- 56.Asano M, Toda M, Sakaguchi N, Sakaguchi S. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yunis E J, Hong R, Grewe M A, Martinez C, Cornelius E, Good R A. J Exp Med. 1967;125:947–966. doi: 10.1084/jem.125.5.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakaguchi S, Takahashi T, Nishizuka Y. J Exp Med. 1982;156:1565–1576. doi: 10.1084/jem.156.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakaguchi S, Takahashi T, Nishizuka Y. J Exp Med. 1982;156:1577–1586. doi: 10.1084/jem.156.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tung K S, Smith S, Matzner P, Kasai K, Oliver J, Feuchter F, Anderson R E. Am J Pathol. 1987;126:303–314. [PMC free article] [PubMed] [Google Scholar]
- 61.Tung K S, Smith S, Teuscher C, Cook C, Anderson R E. Am J Pathol. 1987;126:293–302. [PMC free article] [PubMed] [Google Scholar]
- 62.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Nature (London) 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 63.Willerford D M, Chen J, Ferry J A, Davidson L, Ma A, Alt F W. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 64.Papiernik M, de Moraes M L, Pontoux C, Vasseur F, Penit C. Int Immunol. 1998;10:371–378. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]