Abstract
Bone remodeling, comprising resorption of existing bone and de novo bone formation, is required for the maintenance of a constant bone mass. Prostaglandin (PG)E2 promotes both bone resorption and bone formation. By infusing PGE2 to mice lacking each of four PGE receptor (EP) subtypes, we have identified EP4 as the receptor that mediates bone formation in response to this agent. Consistently, bone formation was induced in wild-type mice by infusion of an EP4-selective agonist and not agonists specific for other EP subtypes. In culture of bone marrow cells from wild-type mice, PGE2 induced expression of core-binding factor α1 (Runx2/Cbfa1) and enhanced formation of mineralized nodules, both of which were absent in the culture of cells from EP4-deficient mice. Furthermore, administration of the EP4 agonist restored bone mass and strength normally lost in rats subjected to ovariectomy or immobilization. Histomorphometric analysis revealed that the EP4 agonist induced significant increases in the volume of cancellous bone, osteoid formation, and the number of osteoblasts in the affected bone of immobilized rats, indicating that activation of EP4 induces de novo bone formation. In addition, osteoclasts were found on the increased bone surface at a density comparable to that found in the bone of control animals. These results suggest that activation of EP4 induces bone remodeling in vivo and that EP4-selective drugs may be beneficial in humans with osteoporosis.
Bones undergo continuous remodeling through repeated cycles of destruction and rebuilding (1). This remodeling is mediated by the well balanced actions of osteoclasts, which resorb old bones, and osteoblasts, which form new bones. However, in the elderly, especially in postmenopausal women, the extent of bone resorption far exceeds that of bone rebuilding, resulting in osteoporosis and the associated increases in bone fragility and susceptibility to fractures (2). About 100 million people are estimated to suffer from this debilitating disease worldwide. Several drugs have been developed to treat osteoporosis, with most inhibiting bone resorption and only a few promoting bone formation (3). Such modulation of only one of the two processes in bone remodeling renders these drugs of limited efficacy in restoring the normal balance and bone mass.
Recently, significant advances have been made in our understanding of molecular mechanisms of osteoclast and osteoblast differentiation (4), but such knowledge has not been exploited fully to develop a drug that corrects the imbalance and restores normal bone remodeling. Prostaglandins (PGs) are a group of lipid mediators that are produced from arachidonic acid in a variety of tissues under various physiological and pathophysiological conditions and serve to maintain local homeostasis (5). Among them, PGs of the E type work bimodally in bone metabolism (6). PGE2 potently induces bone resorption in bone organ cultures, whereas repeated injection of this compound in vivo induces bone formation in a variety of animals including humans. However, the use of PGE2 as a therapeutic agent in the treatment of bone loss has been hindered by unwanted actions that systemically applied PGE2 exerts in the body. It is not understood, either, the mechanism by which it induces bone formation or how this in vivo effect is related to its bone resorbing activity in vitro.
PGE2 exerts its effects through interaction with specific cell surface receptors (5). Four subtypes of PGE receptors—EP1, EP2, EP3, and EP4—have been identified. These receptors are encoded by distinct genes and are expressed differentially in the body. With the use of homologous recombination, we have generated mice that lack each of the four EP subtypes individually (7–9). We also have screened compounds on a panel of the cloned receptors and developed drugs that act specifically at each EP subtype (10). With these tools, we now have investigated which EP subtype mediates the bone-forming activity of PGE2 and how activation of this receptor induces bone formation. We have also used ovariectomized or immobilized rats as models of diseases with bone loss and examined the efficacy of EP-subtype-specific drugs in the treatment of this condition.
Materials and Methods
Mice.
Mice deficient in each EP subtype (7–9) were backcrossed for more than five generations into C57BL/6CrSlc (Japan SLC, Hamamatsu, Japan). Males of the F2 progenies of N10 EP1−/− mice, N5 EP2−/− mice, and N5 EP3−/− mice were used. Because EP4−/− mice do not survive in the C57BL/6 background because of patent ductus arteriosus (8), survivors of F2 progenies in the mixed genetic background of 129/Ola × C57BL/6 were intercrossed and the resulting male survivors were used. Mice were treated according to the guidelines for the protection of experimental animals of Kyoto University and Ono Pharmaceutical.
Chemicals.
DI-004, AE1–259, AE-248, and AE1–329, agonists for EP1, EP2, EP3, and EP4, respectively, were described (10). A new EP4 agonist, ONO-4819, methyl 7-[(1R, 2R, 3R)-3-hydroxy-2-[(E)-(3S)-3-hydroxy-4-(m-methoxymethylphenyl)-1-butenyl]-5-oxocyclopentyl]-5-thiaheptanoate (Patent Cooperation Treaty publish no. WO 00/03980), shows inhibition constant values of 0.7, 56, and 620 nM for radioligand binding to EP4, EP3, and EP2, respectively, and values of more than 10 μM for EP1 and receptors for PGD2, PGF2α, PGI2, or thromboxane A2. This compound was administered as an inclusion complex with α-cyclodextrin.
Local Infusion of PGE2 and EP Agonists.
Eight-week-old mice with body weights of 22–25 g were anesthetized. Their right femora were exposed, and the periosteum was removed around their shaft 8 mm in length. A polyvinyl catheter was fixed distally to the exposed bone surface and was connected proximally to an Alzet 1002 miniosmotic pump (Alza) implanted s.c. in the back and containing PGE2 or EP agonists in ethanol/propylene glycol (40:60, vol/vol). The infusion was performed at a rate of 0.25 μl/hr for 6 weeks, with reservoir replacement every other week. The mice then were killed. Soft x-rays of the femur were taken as described (11). The anteroposterior and lateral diameters of the femur were measured in a callus-containing region of the treated bone and in the corresponding region of the contralateral femur. Each bone volume was calculated assuming the horizontal section of the femur to be elliptical, and the volume of the callus was obtained by subtraction. Histology of the femur was analyzed as described (12).
Bone Marrow Cell Culture.
Bone marrow cells were obtained from femora of 8-week-old mice and cultured in the medium containing PGE2 or vehicle as described (13). The medium was replaced every 3 days. After 21 days, the cells were rinsed with PBS, fixed in a 1:1:1.5 solution of 10% formalin/methanol/water for 2 h, and stained with the von Kossa method for mineralization. Nuclei were counterstained with neutral red. Photographs were taken with transmitted light, and the black-stained area of the mineralized nodules was measured with nih image.
Immunoblot was performed as described with 100 μg protein of cell lysates and antibodies to core-binding factor α1 (Cbfa1) (14). Bound antibodies were detected with ECL Plus reagents (Amersham Pharmacia). Northern blot analysis was performed with 0.6 μg of poly(A) RNA. Hybridization was performed with random-primed 32P-labeled probes prepared, with the 0.8-kb EcoRI-HindIII fragment of mouse Cbfa1 cDNA as a template (15).
Administration of ONO-4819 to Ovariectomized Rats.
Fifteen-week-old female Crj:CD(SD)IGS (IGS) rats were anesthetized, and both ovaries were removed. ONO-4819 was dissolved in saline and administered either by i.v. infusion through a catheter into the right jugular vein or by s.c. injection in the back. Infusion was performed at a rate of 4 ml/kg per hr for 2 h twice per day. Seventy days after surgery, rats were killed and both femora and the fourth lumbar body were isolated. The right femur was fixed in 10% formalin and subjected to analysis with Micro Focus X-ray-Computed Tomography (MCT-CB100MF; Hitachi, Tokyo). The density of cancellous bone was measured in the left femur in a 0.77-mm-thick slice at a distance of 3 mm from the epiphysial growth plate by peripheral quantitative-computed tomography (Stratec Medizintechnik, Pforzeim, Germany) with a voxel size of 0.12 mm at a tube voltage of 50 kV. The lumbar body was used for compression testing, which was performed with a bone compression machine (MZ-500D; Maruto, Tokyo).
Immobilized Rat Model and Histomorphometric Analysis of Bone Tissues.
Five- to six-week-old male IGS rats were anesthetized, and the left sciatic and femoral nerves each were resected at a length of 10 mm. Sham-operated rats received a similar operation without nerve resection. The rats then were systematically infused i.v. either with vehicle or ONO-4819 for 2 h twice a day. For bone density measurement, the rats were killed after 14 days, and the density of trabeculae of the left tibia was examined 4 mm from the proximal end as described above. For histomorphometric analysis, the infusion of ONO-4819 at 100 ng/kg per min or vehicle for 2 h twice a day continued for 28 days. On days 24 and 27, the rats received injection with tetracycline and calcein, respectively. After sacrifice on day 29, the left tibia was isolated. The sagittal block of the metaphysis was obtained, incubated with the Villanueva bone stain for 7 days, dehydrated, and embedded in methylmethacrylate. Sections of 4-μm thickness were prepared and subjected to the analysis by using a microscope coupled to the computerized bone morphometry analysis system (Luzex F Bone system, NIRECO, Tokyo). The primary parameters were measured as recommended (16) in an area between 1 and 1.2 mm from the growth plate and 200 μm apart from the cortical bone and were used to calculate the secondary parameters shown in Table 1.
Table 1.
Histomorphometric parameters in the tibia of sham-operated rats and immobilized rats with or without ONO-4819 treatment
| Parameter* | Sham-operated rats (n = 4) | Control immobilized rats (n = 6) | ONO-4819-treated immobilized rats (n = 5) |
|---|---|---|---|
| Bone volume (BV/TV), % | 7.82 ± 0.50 | 1.04 ± 0.38 | 13.4 ± 2.51† |
| Bone formation rate (BFR/TV), %/year | 120.02 ± 7.77 | 17.01 ± 7.78 | 187.21 ± 42.97† |
| Bone surface (BS/TV), μm/μm2 × 103 | 3.49 ± 0.26 | 0.60 ± 0.19 | 6.37 ± 0.81† |
| Osteoid volume (OV/TV), % | 0.39 ± 0.08 | 0.05 ± 0.02 | 1.17 ± 0.30† |
| Osteoid surface (OS/BS), % | 30.39 ± 5.44 | 22.45 ± 5.18 | 44.51 ± 5.22† |
| Mineralizing surface (MS/OS), % | 138.86 ± 19.68 | 78.41 ± 21.52 | 64.03 ± 11.01 |
| Osteoblast surface (Ob.S/BS), % | 11.90 ± 3.28 | 9.29 ± 3.01 | 22.38 ± 4.43† |
| Osteoclast surface (Oc.S/BS), % | 13.81 ± 0.94 | 19.87 ± 4.40 | 15.09 ± 2.93 |
| Osteoclast number (N.Oc/BS), no./mm | 2.35 ± 0.33 | 3.22 ± 0.86 | 2.11 ± 0.48 |
| Mineral apposition rate (MAR), μm/day | 2.46 ± 0.11 | 2.20 ± 0.75 | 2.86 ± 0.12 |
| Mineralization lag time (Mlt), day | 1.14 ± 0.18 | 0.71 ± 0.23 | 2.48 ± 0.50† |
Statistical Analysis.
Data are presented as mean ± SE and were analyzed by using either Student's t test or, after variation analysis, Welch's t test or the Dunnett multiple comparison test. An associated probability (P value) of <0.05 was considered significant.
Results
Lack of PGE2-Induced Bone Formation in EP4−/− Mice.
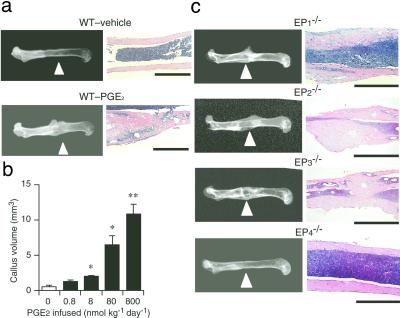
By using a miniosmotic pump, we continuously infused PGE2 into the periosteal region of the femur of wild-type C57BL/6 mice or mice deficient in each EP subtype. After 6 weeks, the femur was isolated and bone formation was examined by both radiographic and histological analyses. Radiography revealed that PGE2 induced extensive callus formation on the femur at the site of infusion in wild-type mice (Fig. 1a). The extent of callus formation depended on the dose of PGE2 between 0.8 and 800 nmol/kg per day (Fig. 1b). Histological analysis showed marked thickening of the femoral cortex, with a large amount of woven bone as well as substantial accumulation of cells and bone matrix (Fig. 1a). Little bone formation was evident in the wild-type mice infused with vehicle alone. When PGE2 was infused to mice deficient in each EP receptor subtype, the callus formation of as much extent as that seen in wild-type mice was observed in the cortex of the femora of EP1−/−, EP2−/−, and EP3−/− mice (Fig. 1c). This effect again depended on the doses of PGE2 (data not shown). In contrast, no callus formation was detected in EP4−/− mice. On histology, massive formation of woven bone, similar to that apparent in wild-type animals infused with PGE2, was seen in PGE2-treated EP1−/−, EP2−/−, or EP3−/− mice but not in EP4−/− mice.
Figure 1.
Absence of PGE2-induced bone formation in EP4−/− mice. (a) PGE2-induced bone formation in wild-type mice. Typical radiographs (Left; arrowheads indicate the site of infusion) and histological preparations (Right; bars = 1 mm) of eight mice injected with vehicle or with PGE2 at a dose of 800 nmol/kg per day are shown. (b) The dose dependence of the effect of PGE2 on callus formation in wild-type mice. Data are values from six mice per dose. *, P < 0.01; **, P < 0.001 vs. vehicle-treated control mice. (c) The effect of PGE2 on bone formation in EP-deficient mice. Typical radiographs (Left) and hematoxylin/eosin staining (Right) of the treated femur from each mouse strain infused with PGE2 at a dose of 800 nmol/kg per day for 6 weeks are shown. Six mice per each strain were used in the analysis with reproducible results. (Bars = 1 mm.)
PGE2 Induces Osteoblast Differentiation in Vitro Through EP4 Receptor Activation.
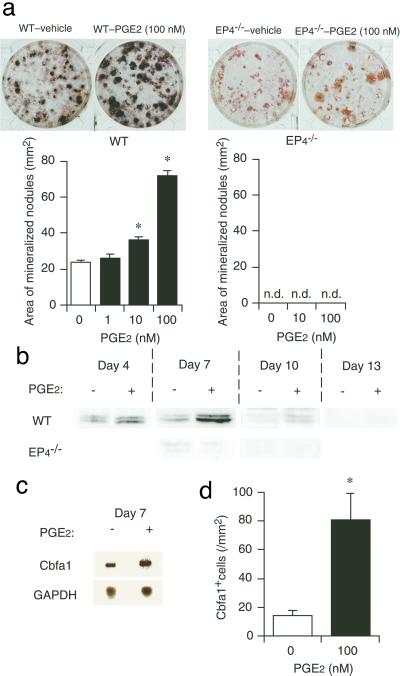
The bone-forming activity of PGE2 can be evaluated in vitro in a primary culture of rat bone marrow cells by measuring the formation of mineralized nodules (13). By application of this system to mice, we examined the mechanism of EP4-induced bone formation. Bone marrow cells were harvested from either wild-type C57BL/6 mice or EP4−/− mice and were cultured with PGE2 for 3 weeks. Mineralized nodules were stained black by the von Kossa method, and their areas were summed. Culture of wild-type cells with vehicle alone resulted in the formation of mineralized nodules, and this was enhanced by the addition of PGE2 in a concentration-dependent manner (Fig. 2a). In contrast, neither the basal level of mineralization nor its enhancement by PGE2 was detected in cell culture derived from EP4−/− mice. These results suggested that PGE2 might promote osteoblast differentiation from precursor bone marrow cells. To test this possibility, we examined the effect of PGE2 on the expression of Cbfa1 in these cells. Cbfa1 is an osteoblast-specific transcription factor and an important determinant of osteoblast differentiation (4, 17). Immunoblot analysis with mouse mAb αA8G5 to Cbfa1 (14) detected two bands of ≈65 kDa in lysates prepared from vehicle-treated, wild-type cells on day 4 or 7 (Fig. 2b). The amounts of these proteins were markedly increased, and the duration of their expression was prolonged in cells cultured with 100 nM PGE2. In contrast, only a very little amount of Cbfa1 was detected in vehicle-treated EP4−/− cells, and the abundance was not enhanced by the addition of PGE2. These results suggest that PGE2 increases the amount of Cbfa1 via EP4. This effect of PGE2 appears to be exerted at the mRNA level, given that Northern blot analysis revealed that culture of wild-type cells with PGE2 increased the level of Cbfa1 mRNA (Fig. 2c). Immunostaining of wild-type cultures with anti-Cbfa1 antibody revealed that incubation with PGE2 induced a significant increase in the number of cells containing Cbfa1 immunoreactivity (Fig. 2d), indicating that PGE2 increased the number of Cbfa1-expressing cells and not simply increased the amount of Cbfa1 in a fixed number of cells. These results taken together identify EP4 as the EP receptor subtype that mediates the bone-forming activity of PGE2, and suggest that EP4 achieves this effect through induction of osteoblast differentiation.
Figure 2.
EP4 mediates mineralized nodule formation and Cbfa1 expression in cultured bone marrow cells. (a) EP4-mediated enhancement of mineralized nodule formation by PGE2. (Upper) von Kossa staining of mineralized nodules in bone marrow cell culture from wild-type C57BL/6 (WT) and EP4−/− mice in the presence of either vehicle or 100 nM PGE2. Nuclei were counterstained with neutral red in the EP4−/− cell cultures. Typical results of six independent cultures are shown. (Lower) The concentration dependence of the effect of PGE2 on mineralized nodule formation. Data are values from six experiments. *, P < 0.001 vs. vehicle-treated control. n.d., not detected. (b) Immunoblot analysis for Cbfa1 expression in bone marrow cells. Results of wild-type (WT) and EP4−/− cells cultured for indicated days in the absence or presence of 100 nM PGE2 are shown. (c) Northern blot analysis for Cbfa1 mRNA abundance. Wild-type bone marrow cells cultured in the absence or presence of 100 nM PGE2 for 7 days were subjected to Northern blot with probes specific to Cbfa1 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNAs. (d) Effect of PGE2 on the number of Cbfa1-positive cells. Bone marrow cells from wild-type mice cultured for 4 days in the absence or presence of 100 nM PGE2 were stained with anti-mouse Cbfa1 antibody and visualized by the ABC method. The number of positive cells per culture was determined (n = 10). *, P < 0.001 vs. vehicle-treated culture.
An EP4-Selective Agonist Potently Stimulates Bone Formation in Vivo.
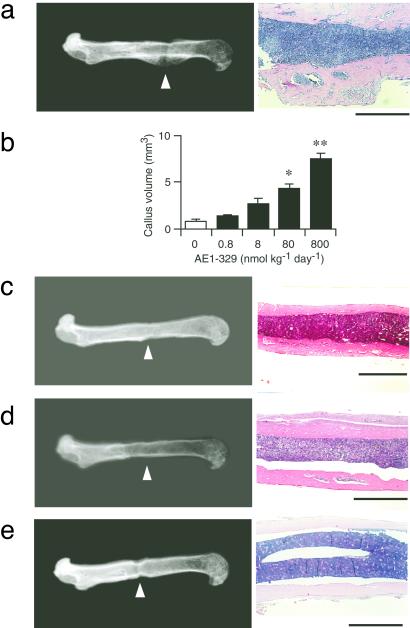
To corroborate these observations pharmacologically, we next applied agonists DI-004, AE1–259, AE-248, and AE1–329, which specifically target EP1, EP2, EP3, and EP4, respectively (10). Wild-type mice were infused continuously with one of these compounds for 6 weeks, and their in vivo bone-forming activities were assessed. Infusion of the selective EP4 agonist, AE1–329, markedly increased bone formation, as detected both radiographically and histologically, in a dose-dependent manner (Fig. 3 a and b). In contrast, the femora of mice infused with each of the agonists specific for the other three EP subtypes did not appear to differ from those of animals infused with vehicle (Fig. 3 c–e). We also tested the in vitro activities of these agonists on the formation of mineralized nodules in primary cultures of bone marrow cells. AE1–329 again was the only one of four compounds capable of inducing mineralized nodule formation (data not shown).
Figure 3.
Selective induction of bone formation by an EP4 agonist. Radiograph and histology of the femora treated with either the EP4 agonist AE1–329 (a), EP1 agonist DI-004 (c), EP2 agonist AE1–259 (d), or EP3 agonist AE-248 (e) are shown. Typical findings with a dose of 800 nmol/kg per day are shown. (Bars = 1 mm.) In b, the dose dependence of the effects of AE1–329 is shown. Data are from four animals per each dose. *, P < 0.01; **, P < 0.001 vs. vehicle-treated control.
Administration of an EP4 Agonist Prevents Bone Loss and Restores Bone Mass and Strength in Rats Subjected to Ovariectomy and Immobilization.
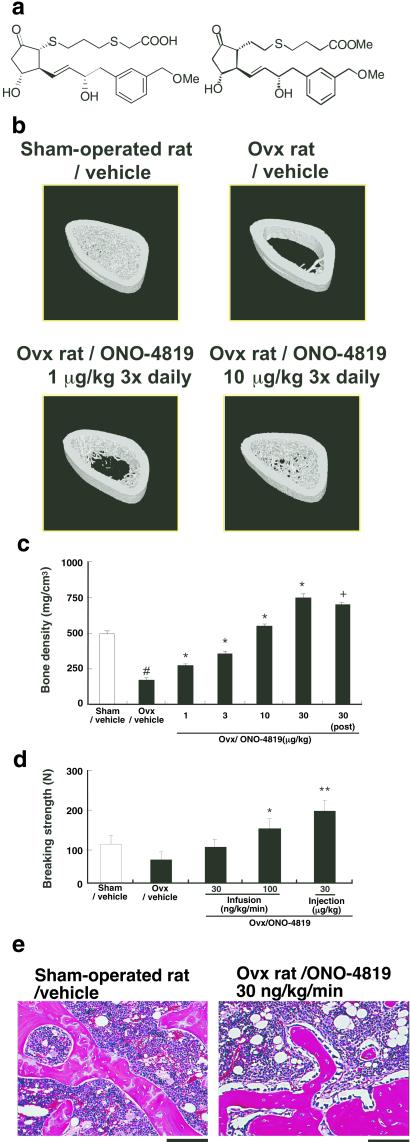
Given that the EP4 agonist is a potent inducer of bone formation in healthy animals, we next investigated whether this type of drugs might exert a similar action in animals with bone loss and, thereby, restore bone mass and strength. To this end, we modified AE1–329 to increase its chemical stability (Fig. 4a). The resulting compound, ONO-4819, was administered to ovariectomized (OVX) rats, a model for human postmenopausal osteoporosis. Both ovaries were removed from 15-week-old female rats, and the effect of daily administration of ONO-4819 was examined. Seventy days after ovariectomy, vehicle-treated control OVX rats exhibited marked osteoporosis, as was apparent from the almost complete loss of bone trabeculae (Fig. 4b). s.c. injection of ONO-4819 three times per day beginning on the day of ovariectomy inhibited bone loss in a dose-dependent manner (Fig. 4 b and c); at a dose of 10 μg/kg, the drug completely prevented the bone loss. Importantly, the full restoration of bone density also was obtained when the injection was initiated 20 days after ovariectomy. We next evaluated the effect of the EP4 agonist on bone strength by subjecting the fourth lumbar body from these animals to the compression test. Ovariectomy resulted in a marked reduction in bone strength. This fragility was prevented by treatment of animals with ONO-4819 (Fig. 4d). In animals injected with ONO-4819 at 30 μg/kg three times per day or infused at 100 ng/kg per min for 2 h twice per day, bone strength was significantly greater than that in vehicle-treated OVX rats. Histology of bones revealed that trabeculae similar in architecture and appearance to those found in the sham-operated rats and lined with osteoblasts were formed in the OVX rats treated with ONO-4819 (Fig. 4e).
Figure 4.
Prevention of bone loss and restoration of bone mass and strength by the EP4 agonist ONO-4819 in OVX rats. (a) Structure of AE1–329 (Left) and ONO-4819 (Right). (b) Bone architecture of OVX rats treated with ONO-4819. Seventy days after surgery, the right femur was isolated from a sham-operated rat as well as from OVX rats injected either with vehicle or two different doses of ONO-4819 and was subjected to analysis by x-ray-computed tomography. The three-dimensional structure was constructed by piling up images, and typical architecture of the metaphysis of each bone is presented. (c) Effects of ONO-4819 on bone density in OVX rats. OVX rats were injected s.c. with either vehicle or the indicated doses of ONO-4819 three times per day, either beginning on the day of surgery or day 20 after ovariectomy (post). Seventy days after the surgery, the left femur was isolated and subjected to analysis of bone density (n = 8 per group). #, P < 0.05 vs. the sham-operated group; *, P < 0.05 vs. control vehicle-treated OVX group (Dunnett test); +, P < 0.05 vs. control vehicle-treated OVX group (Student's t test). (d) Effects of ONO-4819 on bone strength in OVX rats. OVX rats were treated either by i.v. infusion of ONO-4819 at a rate of 30 or 100 ng/kg per min (≈15 and 50 nmol/kg per day) or by s.c. injection of the drug at a dose of 30 μg/kg three times per day (≈200 nmol/kg per day). Seventy days after surgery, rats were killed, and the fourth lumbar body was isolated and subjected to a compression test. No significant difference in the size of the bone was detected among the groups. Data are from eight rats per group. *, P < 0.05 vs. control vehicle-treated OVX group; *, P < 0.01 vs. control vehicle-treated OVX group. (e) Histology of the bone. Hematoxylin/eosin staining of the decalcified transverse sections of the epiphysis of the femur of sham-operated rats or OVX rats with ONO-4819 infusion is shown. (Bars = 100 μm.)
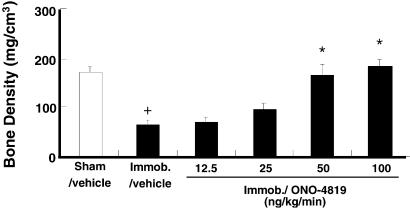
In healthy animals, bone remodeling is stimulated by the mechanical tension applied to each bone as a result of its daily use, and prolonged immobilization results in a reduction in bone mass and deterioration of bone architecture. We therefore examined the effect of ONO-4819 on bone loss of immobilized rats. Immobilization of a hind limb induced a significant reduction in bone density of the tibia of the affected leg in 2 weeks. The infusion of ONO-4819 inhibited this reduction in a dose-dependent manner, preventing it completely at higher doses tested (Fig. 5). We next used rats subjected to this model and performed histomorphometric analysis on the effects of the ONO-4819 treatment (Table 1). Because the immobilization in our model affected significantly the cancellous bone and not the cortical bone, we limited our description to the changes in the cancellous bone of the metaphysis. This analysis revealed significant reduction in the volume of cancellous bone in immobilized rats as determined by bone volume (BV/TV). The ONO-4819 infusion significantly increased the bone volume including the osteoid volume and completely restored it in the immobilized animals. This was reflected by the significant increase in bone formation rate in the ONO-4819-treated animals. The significant increases in bone surface (BS/TV) and in the bone surface lined with osteoblasts (Ob.S/BS) were also noted. These findings together with a similar density of osteoclasts on bone surface (N.Oc/BS) in ONO-4819-treated rats indicate that the total number of osteoclasts in the tissue markedly increased with the increase in the bone surface. On the other hand, the calcification rate as determined by double fluorescence labeling (mineral apposition rate) was not affected by this treatment.
Figure 5.
Effects of ONO-4819 on immobilization-induced bone loss. Rats immobilized in the left hind limb (Immob) were infused systematically for 2 h twice per day with vehicle or the indicated doses of ONO-4819. After 14 days, the left tibia was isolated and subjected to analysis of bone density (n = 9–14 per group). +, P < 0.01 vs. the sham-operated group; *, P < 0.01 vs. control vehicle-treated immobilized group.
In the above experiments, ONO-4819 administration caused no significant change in the serum concentrations of alkaline phosphatase, calcium, and inorganic phosphate in the animals (data not shown). Ectopic calcification in tissues such as cardiac valves or aorta was not found. No significant change in the blood pressure was detected in conscious rats by either infusion or injection of the drug at the doses described, although the infusion of the highest dose (100 ng/kg per min i.v.) induced less than 20% elevation of the heart rate.
Discussion
In this study, with both genetic and pharmacological approaches, we have unambiguously identified EP4 as the receptor that mediates the bone-forming activity of PGE2. The role of this receptor in bone formation was suggested previously only indirectly by the use of a limited repertory of EP-acting compounds (18). We have found that activation of EP4 induced callus formation on the femur of mice and restored the volume of cancellous bone in OVX or immobilized rats. The histomorphometric analysis in the latter model revealed increases in both the total volume of the bone and the volume of osteoid, suggesting that these effects of the EP4 activation are exerted by de novo bone formation. In vitro in the bone marrow cell culture, EP4 activation increased the number of Cbfa-1-positive cells, suggesting that EP4 exerts such an effect by inducing osteoblast differentiation. Consistently, the density of osteoblasts lining the bone surface (Ob.S/BS) increased with ONO-4819 treatment in the in vivo models. It is noteworthy that the callus induced by PGE2 in mice contained many fibrous tissues and that the bone of ONO-4819-treated rats showed a mineralizing surface (MS/OS) of about half of that seen in sham-operated animals. These results indicate that the osteoblasts induced by EP4 activation produce bone matrix at a rate exceeding that of calcification. This was reflected by the prolonged mineralization lag time (Mlt) with an unchanged mineral apposition rate. However, the trabeculae formed were well connected, and the majority was sufficiently calcified as shown in Fig. 4e and Table 1, yielding substantial strength to the bone of OVX animals (Fig. 4d).
Our current study thus suggests that EP4 activation induces osteoblasts and thereby stimulates de novo bone formation. Previously, we also noted in bone organ culture that EP4 in mature osteoblasts mediates PGE2-induced osteoclast differentiation (19, 20). We wondered how these two EP4 actions are coordinated in vivo in the bone of animals treated with the EP4 agonist. The bone morphometric analysis has shown that the EP4 agonist did not decrease the density of osteoclasts despite the increase in the bone surface, suggesting that it increased the number of osteoclasts in parallel with the de novo increase in bone. It is tempting to speculate that the PGE2-EP4 signaling first works in osteoblast precursors to induce osteoblasts for bone formation and then works in mature osteoblasts for induction of osteoclasts on newly formed bones. Both EP4 and EP2 respond to PGE2 and are coupled to activation of adenylate cyclase. Both also are implicated in PGE2-induced osteoclastogenesis (19–21). It is interesting in this respect that we have not observed any involvement of EP2 in PGE2-induced bone formation in mice (Figs. 1 and 3). We neither have observed any bone-forming effects of the EP2-selective agonist in OVX rats (data not shown). Although we cannot exclude a redundant role of EP2 in other species, these results indicate that EP4 is the only system mediating PGE2-induced bone formation at least in rodents. It is curious, therefore, that the skeleton of EP4−/− mice either alive to adulthood or dead in the neonatal period is apparently normal (19, 20), suggesting that some pathway(s) other than the PGE2 system works for physiological maintenance of bone. It remains to be clarified in what physiological or pathological context the PGE2-EP4 signaling is mobilized for bone formation.
Several types of drugs currently are used for the treatment of bone loss (3). These drugs either inhibit differentiation and functions of osteoclasts or activate osteoblasts. However, none of these drugs are able to restore the balance between bone formation by osteoblasts and bone resorption by osteoclasts. PGE2-EP4 appears to induce both osteoblastogenesis and osteoclastogenesis and to integrate the two actions temporarily and spatially in situ in bone remodeling. In this respect, the action of PGE2 may be similar to that of PTH, which also promotes both bone formation and resorption (1, 3, 22). Recently, clinical efficacy of PTH for postmenopausal osteoporosis has been reported (23). On the other hand, the numerous, unwanted effects of PGE2 injected systematically have precluded its use in therapeutics for bone loss. Because EP4 agonists are selective to only one subtype of EP receptors, they are expected to avoid several adverse actions caused by systemic administration of PGE2. Indeed, ONO-4819 lacks the uterine-contracting activity of PGE2. Furthermore, administration of ONO-4819 in rodents does induce diarrhea, hypotension, and thickening of intestinal epithelium but at higher doses than that required for bone formation. However, given various differences between the rodent models and human patients, it may be too early to conclude that EP4 agonists exert beneficial effects in humans. Their therapeutic potential will be tested rigorously in future studies.
Acknowledgments
We thank T. Komori for Cbfa1 cDNA, K. Deguchi for animal care and breeding, and T. Arai and H. Nose for secretarial assistance. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan and a grant from the Organization for Pharmaceutical Safety and Research.
Abbreviations
- PG
prostaglandin
- EP
PGE receptor
- Cbfa1
core-binding factor α1
- OVX
ovariectomized
References
- 1.Manolagas S C. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy. J Am Med Assoc. 2001;285:785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 3.Rodan G A, Martin T J. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 4.Karsenty G. Genes Dev. 1999;13:3037–3051. doi: 10.1101/gad.13.23.3037. [DOI] [PubMed] [Google Scholar]
- 5.Narumiya S, Sugimoto Y, Ushikubi F. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 6.Pilbeam C C, Harrison J R, Raisz L G. In: Principles of Bone Biology. Bilekizian J P, Raisz L G, Rodan G A, editors. San Diego: Academic; 1996. pp. 715–728. [Google Scholar]
- 7.Ushikubi F, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Segi E, Hizaki K, Ichikawa A, Tanaka T, Yoshida N, et al. Nature (London) 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 8.Segi E, Sugimoto Y, Yamasaki A, Aze Y, Oida H, Nishimura T, Murata T, Ushukubi F, Fukumoto M, Tanaka T, et al. Biochem Biophys Res Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 9.Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, Matsuoka T, Noda Y, Tanaka T, Yoshida N, et al. Proc Natl Acad Sci USA. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzawa T, Miyaura C, Inada M, Maruyama T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Suda T. Endocrinology. 2000;141:1554–1559. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita M, Tsuboyama T, Kasai R, Okumura H, Yamamuro T, Higuchi K, Higuchi K, Kohno A, Yonezu T, Utani A, et al. Am J Pathol. 1986;125:276–283. [PMC free article] [PubMed] [Google Scholar]
- 12.Okamoto Y, Takahashi K, Toriyama K, Takeda N, Kitagawa K, Hosokawa M, Takeda T. Anat Rec. 1995;242:21–28. doi: 10.1002/ar.1092420104. [DOI] [PubMed] [Google Scholar]
- 13.Weinreb M, Suponitzky I, Keila S. Bone. 1997;20:521–526. doi: 10.1016/s8756-3282(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y-W, Yasui N, Ito K, Huang G, Fujii M, Hanai J, Nogami H, Ochi T, Miyazono K, Ito Y. Proc Natl Acad Sci USA. 2000;97:10549–10554. doi: 10.1073/pnas.180309597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogawa E, Maruyama M, Kagoshima H, Inuzuka M, Lu J, Satake M, Shigesada K, Ito Y. Proc Natl Acad Sci USA. 1993;90:6859–6863. doi: 10.1073/pnas.90.14.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parfitt A M, Drezner M K, Glorieux F H, Kanis J A, Malluche H, Meunier P J, Ott S M, Recker R R. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 17.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson R T, Gao Y H, et al. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 18.Machwate M, Harada S, Leu C T, Seedor G, Labelle M, Gallant M, Hutchins S, Lachance N, Sawyer N, Slipetz D, et al. Mol Pharmacol. 2001;60:36–41. doi: 10.1124/mol.60.1.36. [DOI] [PubMed] [Google Scholar]
- 19.Sakuma Y, Tanaka K, Suda M, Yasoda A, Natsui K, Tanaka I, Ushikubi F, Narumiya S, Segi E, Sugimoto Y, et al. J Bone Miner Res. 2000;15:218–227. doi: 10.1359/jbmr.2000.15.2.218. [DOI] [PubMed] [Google Scholar]
- 20.Miyaura C, Inada M, Suzawa T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Suda T. J Biol Chem. 2000;275:19819–19823. doi: 10.1074/jbc.M002079200. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Okada Y, Pilbeam C C, Lorenzo J A, Kennedy C R, Breyer R M, Raisz L G. Endocrinology. 2000;141:2054–2061. doi: 10.1210/endo.141.6.7518. [DOI] [PubMed] [Google Scholar]
- 22.Dobnig H, Turner R T. Endocrinology. 1997;138:4607–4612. doi: 10.1210/endo.138.11.5505. [DOI] [PubMed] [Google Scholar]
- 23.Neer R M, Arnaud C D, Zanchetta J R, Prince R, Gaich G A, Reginster J-Y, Hodsman A B, Eriksen E F, Ish-Shalom S, Genant H K, et al. N Eng J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]