Abstract
Background
Lymph node metastasis is a key driver of poor outcomes in cervical cancer. However, the molecular mechanisms of circular RNAs (circRNAs) driving cervical cancer lymph node metastasis remain unclear.
Methods
We identified circZFR, fatty acid synthase (FASN) and YTH N6-methyladenosine RNA binding protein F3 (YTHDF3) protein expression in the cervical cancer patients with long and short disease-free survival (DFS). Functional experiments were performed to investigate the function of circZFR, FASN and YTHDF3 on cell migration and invasion. MeRIP-qPCR, RNA pulldown, RNA Immunoprecipitation (RIP), and Co-Immunoprecipitation (Co-IP) assays were executed to investigate the mechanism of circZFR regulating FASN protein expression.
Results
Our study reveals that elevated FASN protein is closely linked to metastasis and reduced survival, and identified a regulatory mechanism involving circular RNAs. We identified circZFR as a crucial regulator, significantly enhancing FASN protein expression. CircZFR overexpression was significantly correlated with accelerated lymph node metastasis and shortened DFS. Mechanistically, circZFR binds to the m6A reader protein YTHDF3, facilitating m6A recognition on FASN mRNA and recruiting the translation initiator eIF4A3, thereby boosting FASN translation.
Conclusions
These findings establish circZFR as a pivotal driver of cervical cancer progression and highlight its inhibition as a promising therapeutic strategy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12943-025-02424-5.
Keywords: Prognostic markers, Lymph node metastasis, YTH N6-methyladenosine RNA binding protein F3 (YTHDF3), Fatty acid synthase (FASN)
Background
Cervical cancer remains a major global health concern and led to 348,189 cancer-related deaths by world area among women in 2022, with lymph node metastasis serving as a critical determinant of poor prognosis [1, 2]. According to the updated 2018 FIGO staging system, patients with lymph node metastasis are classified as stage IIIC, reflecting their high risk of relapse and poor survival outcomes [3]. Despite advancements in radical surgery, radiotherapy, and chemotherapy, therapeutic options for recurrent or metastatic cervical cancer remain limited.
Aberrant lipid metabolism, including excessive fatty acid (FA) synthesis, is a hallmark of cancer progression. Fatty acid synthase (FASN), a key enzyme in FA synthesis, has been implicated in promoting tumor progression, chemoresistance, and metastasis across various cancers, including breast, colorectal, and prostate cancers [4–11]. In cervical cancer, elevated FASN expression has been associated with poor survival and lymph node metastasis [12]. However, the molecular mechanisms driving FASN upregulation in cervical cancer remain largely unexplored.
N6-Methyladenosine (m6A) RNA modification of messenger RNA is prevalent in higher eukaryotes [13, 14]. Recent research highlights the role of m6A RNA modifications in regulating mRNA translation, stability, and degradation [14, 15]. The m6A “reader” protein YTH N6-methyladenosine RNA binding proteins 3 (YTHDF3) has been shown to facilitate the translation of m6A-modified transcripts, contributing to metastasis in multiple cancers [16–21]. Notably, YTHDF3 has been linked to lymph node metastasis and poor survival in cervical cancer, though its mechanistic role in metastatic progression remains unclear [22]. Circular RNAs (circRNAs) have special structures and affect various pathogenesis [23, 24]. CircZFR, as one of circRNAs, has function in promoting cervical cancer cells proliferation [25]. Emerging evidence also suggests that circRNAs can interact with m6A readers to regulate translation, yet the functional implications of circRNAs in FASN regulation and cervical cancer metastasis are not well defined [26].
Here, we hypothesize that circZFR, through a potential interaction with YTHDF3, drives FASN upregulation and facilitates lymph node metastasis in cervical cancer. This study investigates the circZFR-YTHDF3-FASN axis, aiming to unravel its molecular mechanisms and evaluate its potential as a therapeutic target for metastatic cervical cancer.
Methods
Bioinformatic analysis
We downloaded a total of 491 genes associated with poor survival in cervical cancer patients from the Human Protein Atlas (https://www.proteinatlas.org/). From this list, 223 genes were identified as unfavorable prognostic markers based on both RNA and protein expression data. To identify m⁶A-modified, protein-coding transcripts in HeLa cells, we cross-referenced these genes with the RMVar database (http://rmvar.renlab.cn). Differential expression and survival analyses for FASN were conducted using GEPIA2 (http://gepia.cancer-pku.cn), based on TCGA and GTEx data. Default cutoffs in GEPIA2 were used for log₂ fold-change (|log₂FC| >1) and adjusted P-value (< 0.01). Kaplan-Meier survival plots for overall survival (OS) and disease-free survival (DFS) were generated from TCGA datasets, stratified by median FASN expression. Analyses were performed using GEPIA2 (v2.0) and R (v4.3.1) with the ‘survival’ and ‘ggplot2’ packages.
We conducted molecular docking analysis to predict the potential binding sites of circZFR on the YTHDF3 protein. Firstly, the circZFR 3D structure was predicted using 3dRNA v2.0 (http://biophy.hust.edu.cn/3dRNA) [27]. Secondly, the YTHDF3 protein 3D structure was predicted using AlphaFold (https://alphafold.ebi.ac.uk) [28]. Moreover, the RNA binding motifs of YTHDF3 were searched by CLIPdb (http://clipdb.ncrnalab.org) [29]. Finally, the best docking model between circZFR and YTHDF3 protein was predicted using HDOCK web server (http://hdock.phys.hust.edu.cn/) [30]. The cut-off docking score was set as −200. PyMOL (Schrödinger, New York, NY, USA) was used to generate molecular docking model.
Human tissue specimen
Human cervical cancer tissue samples were obtained from the Liaoning Cancer Hospital & Institute between January 10, 2018, and March 13, 2024. Patient follow-up was completed by December 1, 2024. Inclusion criteria for the study were as follows: all cases were histologically confirmed as cervical cancer via preoperative biopsy; staging was performed by at least two experienced gynecologic oncologists in accordance with the 2018 FIGO clinical staging system (stages 1B1, 1B2, 1B3, 2A1, or 2A2); all tissue samples were obtained from patients who underwent surgical resection without any prior chemotherapy, radiotherapy, or immunotherapy. Cases confirmed as FIGO stage 3C1p or 3C2p based on postoperative pathological findings of lymph node metastasis were included. However, patients staged as 3C1r or 3C2r based solely on preoperative imaging were excluded. Survival data were obtained through the institutional follow-up registry.
At the end of follow-up, 40 patients had experienced relapse but were still alive. These cases were classified as Cohort 1, and RNA was extracted from their FFPE tissue samples for circZFR expression analysis. The median follow-up duration for Cohort 1 was 62.00 months, with a median disease-free survival (DFS) of 29.77 months.
Since the establishment of the institutional biobank in August 2021, fresh frozen cervical cancer tissue samples from 104 patients were collected and classified as Cohort 2. These specimens were snap-frozen in liquid nitrogen and stored at − 80 °C immediately after surgical resection. The median follow-up for Cohort 2 was 29.33 months, and all patients remained recurrence-free at the end of the follow-up period. Clinicopathological characteristics of all enrolled patients are detailed in Supplementary Table S1.
Tissue microarrays (TMAs) and immunohistochemistry (IHC)
TMAs were constructed using primary tumor samples from 19 cervical cancer patients with DFS < 2 years and 21 patients with DFS > 2 years. Tissue sections were deparaffinized, rehydrated through an alcohol gradient, and subjected to antigen retrieval in Tris/EDTA buffer (pH 9.0) for 3 min. Blocking was performed followed by incubation with primary antibodies for 90 min at room temperature [25]. Details of the primary antibodies are provided in Supplementary Table S2.
Staining intensity and the percentage of positive tumor cells were evaluated independently by two investigators blinded to clinical data. Intensity was scored from 0 to 3: 0 for no staining, 1 for barely detectable staining, 2 for moderate staining, and 3 for strong staining. The percentage of positive cells was scored on a scale from 1 to 4: 1 for ≤ 25%, 2 for ≤ 50%, 3 for ≤ 75%, and 4 for > 75%. The intensity and percentage were then multiplied (Intensity x Percentage) to derive the final staining score. Then the score 8 was chosen as cut off value, which samples with a final staining score ≥ 8 were determined as high expression and the samples with a final staining score < 8 were determined as low expression. And the agreement of FASN and YTHDF3 protein expression assessments between two different pathologists were evaluated with Cohen’s kappa coefficient.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was extracted from fresh frozen tissues using TRIzol Reagent (Invitrogen) and from FFPE tissues using the RNeasy FFPE Kit (QIAGEN). Complementary DNA (cDNA) synthesis was performed with 1 µg of RNA using the PrimeScript RT reagent kit (Takara). qRT-PCR was carried out using the SYBR Premix Ex Taq II kit (Takara), with relative gene expression normalized to GAPDH and calculated using the 2-∆∆CT method [25]. Primer sequences are provided in Supplementary Table S3.
Pull-down assay with in vitro circZFR
The biotin-labeled probes were synthesized by GenePharma (China). The sequence of circZFR probes is listed in Supplementary Table S4. Equal amounts of sense and antisense circRNAs were denatured and renatured. The pull-down assay was performed using Pierce Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher Scientific). The bound proteins were subjected to immunoblot analysis.
Cell culture, stable transfectants, and transfection
Cervical cancer cell lines (HeLa, C33A, and Caski) were obtained from ATCC and maintained in DMEM (Gibco) supplemented with 10% FBS (Biological Industries) at 37 °C in a 5% CO2 atmosphere. Stable cell lines overexpressing circZFR or an empty vector were established as described previously [25]. The site-specific mutants of circZFR were constructed according to molecular docking analysis between circZFR and YTHDF3 protein. The sequences of plasmids are provided in Supplementary Table S5.
The full-length YTHDF3 gene (NM_152758) was cloned into a GV657 vector (Shanghai Genechem Co., Ltd.) to generate wild-type YTHDF3 plasmids. Site-specific mutants were constructed with amino acid substitutions (W438A, W492A, or both) [20]. The sequences of plasmids are provided in Supplementary Table S6.
For transient transfections, siRNAs were introduced using Lipofectamine RNAiMAX (Invitrogen) following the manufacturer’s protocol. Cells were analyzed 48 to 72 h post-transfection. Details of siRNAs are listed in Supplementary Table S7.
Protein extraction and western blotting
Proteins were extracted, concentrated, separated by SDS-PAGE, transferred to PVDF membranes, blocked, and incubated with primary antibodies according to the previous protocol [25]. After the incubation with secondary antibody, blots were visualized with a ChemiDoc imaging system (Bio-Rad) and quantified using Image Lab software. Antibodies used are listed in Supplementary Table S2.
Cell proliferation assays
For CCK-8 assays, HeLa and C33A cells were seeded into 96-well plates at 2000 cells per well and cultured overnight. CCK-8 reagent (10 µL) was added, and absorbance at 450 nm was measured at specific time points [25]. For EdU assays, HeLa, C33A, and Caski cells were seeded at 3000 cells per well in 96-well plates. EdU labeling was conducted following the manufacturer’s protocol (Elabscience). Each group was set up in triplicate wells, and each experiment was independently repeated three times.
Transwell migration and invasion assays
Cell migration and invasion were performed according to the previous protocol [25]. Each condition was tested in triplicate wells, and the experiment was repeated three times.
Gene-specific meRIP-qPCR
M6A modifications were assessed using Magna MeRIP Kits (Millipore). Fragmented RNA was incubated with anti-m6A or control IgG antibodies conjugated to magnetic beads. Bound RNA was eluted with free N6-methyladenosine and analyzed by qRT-PCR. Primers are listed in Supplementary Table S8.
RNA immunoprecipitation (RIP) assay
RIP was performed using the Magna RIP Kit (Millipore). Cell lysates were incubated with beads conjugated to YTHDF3 or eIF4A3 antibodies (Proteintech). RNA was purified from input and immunoprecipitated fractions and analyzed by qRT-PCR.
RNA stability assay
Cells were treated with actinomycin D (5 µg/mL) for various time points (0 h, 1 h, 3 h, 4 h, and 5 h), and RNA stability was analyzed using qRT-PCR.
Protein stability assay
Cells were treated with cycloheximide (100 µg/mL) for the indicated durations (0 h, 4 h, 8 h, and 12 h), and protein levels were evaluated by western blotting.
Proteasome assay
Cells were treated with MG132 (20 µM) for 4 h, and protein levels were evaluated by western blotting.
Protein co-immunoprecipitation (Co-IP)
Co-IP was performed using the Pierce Crosslink IP Kit (Thermo). Cell lysates were incubated with the antibodies crosslinked to protein A/G agarose beads. Immunoprecipitated proteins were eluted and analyzed by western blotting.
Animal experiments
Female BALB/c nude mice (4–6 weeks old) were purchased from Beijing HFK Bioscience Co. Forty mice were randomly assigned to four experimental groups (n = 10 per group). Each mouse was subcutaneously injected at the footpad with 1 × 10⁶ HeLa cells stably expressing circZFR, empty vector, sh-circZFR, or negative control, respectively. After a 3-week observation period, the popliteal lymph nodes were excised and photographed. Lymph node volume was calculated using the formula: volume = (length × width²)/2. Randomization was performed using a computer-generated sequence. The investigators assessing lymph node size and imaging were blinded to the group allocation to reduce bias.
Statistical analysis
All statistical analyses were performed using SPSS v25.0 (RRID: SCR_002865) and GraphPad Prism v8 (RRID: SCR_002798). Fisher’s exact test was used to analyze the correlation between circZFR and clinical parameters. Kaplan-Meier survival analysis with log-rank tests was used to evaluate DFS. The median expression of circZFR was used to classify patients into low- and high-expression groups. Comparison analysis were performed by two tailed paired Student’s t-test or one-way ANOVA test (NS, P > 0.05, *P < 0.05, **P < 0.01, and ***P < 0.001), as indicated in figures and legends.
Results
CircZFR overexpression promotes lymph node metastasis and shortens DFS in cervical cancer
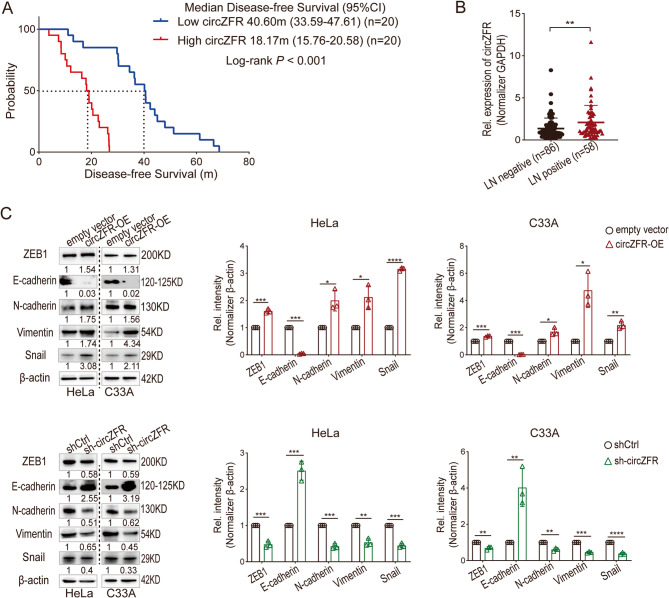
In our previous study, circZFR was identified as upregulated in cervical cancer using circRNA array data and GEO database analysis [25]. To investigate its clinical significance, we analyzed the survival data of 40 cervical cancer patients with median follow-up of 62 months and found that high circZFR expression shortened 22.43 months of DFS of cervical cancer patients (Median DFS: high circZFR group 18.17 months [95%CI, 15.76 to 20.58] vs. low circZFR group 40.60 months [95%CI, 33.59 to 47.61], P < 0.001) (Fig. 1A). We further analyzed the survival data of 144 cases with median follow-up of 33.30 months and validated that overexpression of circZFR increased the relapse risk for cervical cancer patients (Hazard ratio for relapse 1.54, 95%CI 1.30–1.82, P < 0.001) (Fig. S1A and B). Lymph node metastasis, a critical factor in cervical cancer relapse, was examined in relation to circZFR expression. Among 86 lymph node-negative and 58 lymph node-positive patients, circZFR levels were markedly higher in the lymph node-positive group (P = 0.0084) (Fig. 1B). Mechanistically, circZFR was found to promote epithelial-mesenchymal transition (EMT) by upregulating ZEB1, N-cadherin, Vimentin, and Snail while downregulating E-cadherin at the protein level (Fig. 1C). Thus, elevated circZFR contributes to lymph node metastasis and EMT activation, leading to shorter DFS in cervical cancer patients.
Fig. 1.
CircZFR Overexpression Drives Lymph Node Metastasis and Shortens DFS in Cervical Cancer Patients. A Kaplan-Meier analysis for disease-free survival of 40 cervical cancer patients with primary tumors expressing high or low levels of circZFR, median circZFR value was set as cut-off. P < 0.001 by log rank test. B Expression of circZFR in primary tumors of lymph nodes positive (n = 58) and negative (n = 86) cervical cancer patients. LN, lymph node. Lines show mean value and SD, student t test, **P < 0.01. C Western blot analysis of protein levels of the epithelial-mesenchymal transition (EMT) marker genes in HeLa and C33A empty vector, circZFR overexpression, negative control, and knockdown cells. circZFR-OE, circZFR-overexpression; shCtrl, sh-Control. Data in C is mean value ± standard error of the mean (SEM) of three biological replicates, student t test, *P < 0.05, **P < 0.01, ***P < 0.001
CircZFR binds to YTHDF3 and enhances FASN expression in cervical cancer cells
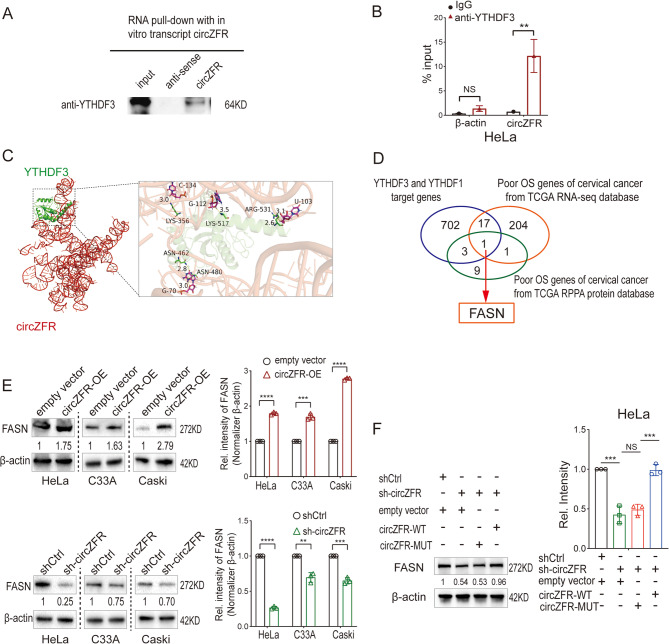
To explore how circZFR promotes cervical cancer metastasis, we utilized circRNA pulldown assays followed by mass spectrometry to identify its binding proteins (Fig. S1C). Among RNA-binding proteins implicated in m6A modifications, YTHDF3 was confirmed as a direct interactor of circZFR, validated by both in vitro pulldown and RIP assays (Fig. 2A and B; Fig. S1D). YTHDF3, an m6A reader, is known to recognize m6A-modified mRNAs and regulate their translation. Binding regions on circZFR by YTHDF3 protein were predicted (Fig. 2C).
Fig. 2.
CircZFR Binds to YTHDF3 and Enhances FASN Expression in Cervical Cancer Cells. A Western blot analysis of in vitro circZFR pulldown assay indicated that circZFR could bind with YTHDF3 protein. B RIP-qPCR showing the association of YTHDF3 protein with circZFR in HeLa cells. C Left: Simulated model of the ternary complex formation between circZFR and YTHDF3 protein. Right: Schematic diagram highlighting the predicted binding regions between YTHDF3 and circZFR. D Schematic workflow of circZFR-YTHDF3 downstream target analysis. E. Western blot analysis of FASN protein levels in HeLa, C33A, and Caski cells with circZFR overexpression and knockdown. F. Western blot analysis of FASN protein levels in HeLa cells transfected with either wild-type or mutant circZFR plasmids after circZFR knockdown. Data in E and F are mean value ± standard error of the mean (SEM) of three biological replicates, student t test, *P < 0.05, **P < 0.01, ***P < 0.001
Using publicly available data, we first retrieved 491 genes associated with poor survival from the Human Protein Atlas and filtered this list to 223 protein-coding genes supported by both RNA and protein evidence. To identify m6A-modified genes potentially regulated by YTHDF3, we cross-referenced these 223 genes with the RMVar database, which catalogs RNA modifications. Additionally, we intersected this filtered gene set with previously published YTHDF1/3 target datasets, including PAR-CLIP and RIP-seq data [17]. FASN emerged as a high-confidence candidate, as it met all criteria: (1) unfavorable prognosis in cervical cancer; (2) presence of validated m6A modification sites; and (3) binding evidence by YTHDF3 (Fig. 2D; Tables S9-11). FASN expression was subsequently validated by Western blotting in circZFR overexpression and knockdown models (Fig. 2E). Importantly, circZFR mutants lacking YTHDF3 binding failed to restore FASN protein levels, reinforcing the mechanistic link between circZFR, YTHDF3, and FASN translation (Fig. 2F).
FASN expression correlates with lymph node metastasis and poor DFS
To investigate the clinical relevance of FASN in cervical cancer, we first analyzed TCGA data using GEPIA2. High FASN mRNA expression was significantly associated with advanced tumor stage and poor overall survival (OS) and disease-free survival (DFS) (Fig. 3A and B). These in silico results support our experimental findings that FASN promotes disease progression and lymph node metastasis. Additionally, by intersecting the unfavorable prognostic genes from the Human Protein Atlas with m⁶A-modified transcripts in the RMVar database, we identified FASN as a strong candidate downstream of m⁶A regulation via YTHDF3. Our cohort 1 data analysis confirmed that high FASN protein expression associates with a shorter DFS of 22.03 months in cervical cancer patients (Fig. 3C and D), and high YTHDF3 protein expression associates with a shorter DFS of 18.67 months in cervical cancer patients (Fig. 3E-F; Fig. S2-S3). Inter-observer agreement for IHC scoring was strong for both proteins (FASN: Kappa = 0.90, P < 0.001; YTHDF3: Kappa = 0.85, P < 0.001).
Fig. 3.
Elevated FASN Levels Correlate with Lymph Node Metastasis and Poor Survival Outcomes in Cervical Cancer. A Kaplan-Meier survival analysis of TCGA CESC data showed that the cervical cancer patients with higher FASN mRNA expression (n = 146) had shorter survival time than the cases with lower FASN mRNA expression (n = 146). Median value of FASN mRNA expression was set as cut-off. Left: survival curve of DFS (HR = 2, Log-rank P = 0.021); Right: survival curve of overall survival (HR = 2, Log-rank P = 0.0033). B Analysis of TCGA CESC data showed that the advanced stage of cervical cancer had higher FASN mRNA expression (n = 292, P = 0.0289). C and E. Immunohistochemistry of FASN and YTHDF3 protein levels in the primary tumors of cervical patients with long or short DFS. The cut-off value is 2 years. Left: representative immunohistochemistry (three samples in each group) of FASN and YTHDF3 protein levels. Right: histograms of FASN and YTHDF3 protein levels in the patients with DFS < 2 years (n = 19) and DFS > 2 years (n = 21). Lines show mean value and SD, student t test, ***P < 0.001. D and F. Kaplan-Meier survival analysis showed significant differences in DFS between the patients with high FASN protein expression (IHC score ≥ 8, n = 23) and low FASN protein expression (IHC score < 8, n = 17). And the patients with high YTHDF3 protein expression (IHC score ≥ 8, n = 22) had shorter DFS than low YTHDF3 protein expression (IHC score < 8, n = 18). G. The level of FASN and YTHDF3 protein in lymph nodes positive patients was higher than lymph nodes negative cases. Left: representative western blot of FASN and YTHDF3 protein levels in the primary tumors of the patients with (n = 6) or without (n = 6) lymph nodes metastasis. Right: histograms of FASN and YTHDF3 protein relative intensity in the patients with (n = 39) or without (n = 65) lymph nodes metastasis. Lines show mean value and SD, student t test, ***P < 0.001. H. Positive correlation existed between FASN and YTHDF3 proteins. N = 104, Pearson r = 0.664, P < 0.001
Moreover, FASN and YTHDF3 protein levels were significantly higher in lymph node-positive patients than in those without metastasis (Fig. 3G; Fig. S4). A positive correlation was observed between FASN and YTHDF3 protein levels (Pearson r = 0.664, P < 0.001) (Fig. 3H). Collectively, these data suggest that elevated FASN expression, driven by YTHDF3, is closely linked to lymph node metastasis and poor prognosis in cervical cancer.
CircZFR-YTHDF3 axis promotes proliferation, migration, and invasion of cervical cancer cells
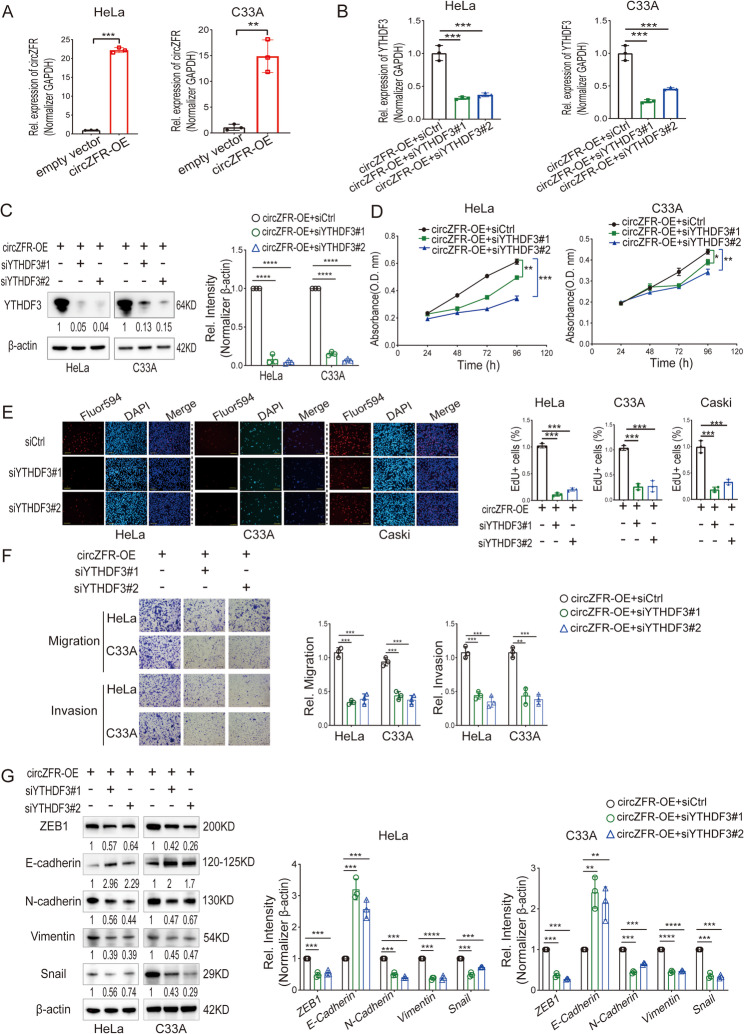
To study functional effects, circZFR was stably overexpressed in HeLa and C33A cells (Fig. 4A). Knocking down YTHDF3 in circZFR-overexpressing cells using siRNAs effectively reduced its expression (Fig. 4B and C). Cell proliferation was significantly inhibited upon YTHDF3 knockdown, as shown by CCK8 and EdU assays (Fig. 4D and E). Similarly, YTHDF3 knockdown reduced the migration and invasion capacities of cervical cancer cells (Fig. 4F). EMT marker analysis revealed that YTHDF3 knockdown restored E-cadherin levels and decreased ZEB1, N-cadherin, Vimentin, and Snail protein expression (Fig. 4G). These data confirmed that circZFR promotes cervical cancer cell proliferation, migration, and invasion through YTHDF3-mediated regulation of EMT.
Fig. 4.
CircZFR-YTHDF3 Axis Promotes Proliferation, Migration, and Invasion in Cervical Cancer Cells. A RT-qPCR analysis of circZFR expression in HeLa and C33A cells with circZFR stable overexpression. circZFR-OE, circZFR-overexpression. B RT-qPCR analysis of YTHDF3 mRNA expression in HeLa and C33A cells treated with two independent siRNAs against YTHDF3. siCtrl, Si-Control. C Western blot analysis of YTHDF3 protein expression in HeLa and C33A cells treated with two independent siRNAs against YTHDF3. D Cell counting kit-8 (CCK-8) assay of HeLa and C33A cells treated with siRNAs against YTHDF3. E EdU assay of HeLa, C33A, and Caski cells treated with siRNAs against YTHDF3. Left: representative immunofluorescence of Fluro594 (red), DAPI (blue), and merge. Scale bars: 50 μm. Right: percentages of EdU-incorporated Hela, C33A, and Caski cells. F Migratory and invasive ability assessed by Transwell assay of HeLa and C33A cells treated with siRNAs against YTHDF3. Scale bars: 50 μm. G Representative western blot of EMT markers protein levels in HeLa and C33A cells treated with siRNAs against YTHDF3. Data in A, B, C, D, E, F, and G are mean value ± SEM of three biological replicates, student t test, *P < 0.05, **P < 0.01, ***P < 0.001
CircZFR facilitates FASN translation via YTHDF3
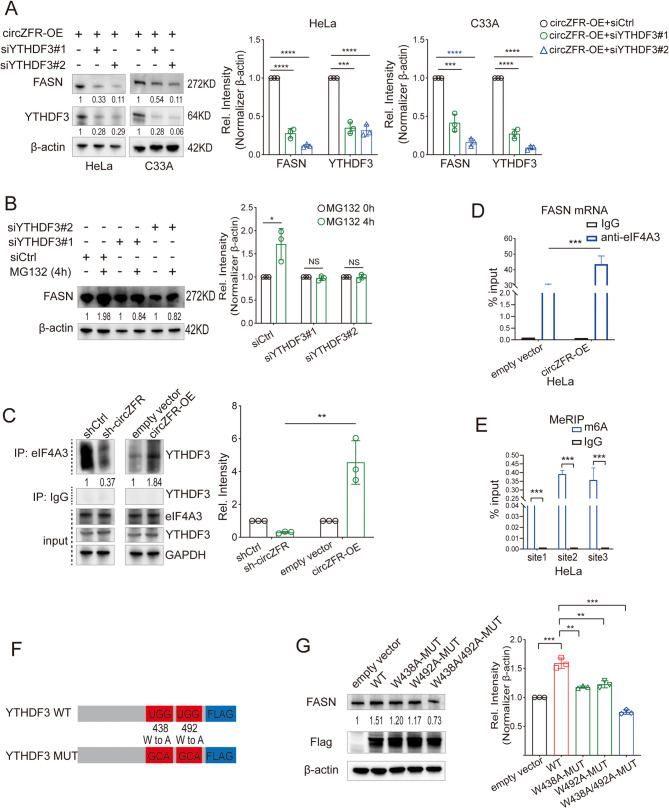
Further investigation into the regulation of FASN by circZFR-YTHDF3 showed that YTHDF3 knockdown reduced FASN protein expression without altering FASN mRNA levels or stability, or protein stability (Fig. 5A-B; Fig. S5A-C). This suggested that YTHDF3 influences FASN translation. Additional experiments confirmed that YTHDF3 promotes FASN mRNA translation by recognizing m6A-modified sites and interacting with translation initiation factor eIF4A3 (Fig. 5C-E; Fig. S5D-G). Mutating residues W438 and W492, critical for m6A binding in YTHDF3, impaired FASN protein synthesis and cell metastatic capacity (Fig. 5F and G; Fig. S5H and I). CircZFR enhances FASN protein translation by leveraging YTHDF3’s m6A recognition capability, contributing to cancer cell aggressiveness.
Fig. 5.
CircZFR Facilitates FASN Protein Translation via the YTHDF3-Dependent Mechanism. A Western blot analysis of FASN protein expression in HeLa and C33A cells treated with siYTHDF3#1 or siYTHDF3#2. B After treatment with MG132 for indicated times, protein levels of FASN were tested by Western blot analyses of HeLa cells treated with siCtrl, siYTHDF3#1, or siYTHDF3#2. C Coimmunoprecipitation by anti-eIF4A3 verified that the combination between YTHDF3 and eIF4A3 increased after overexpression circZFR and decreased after knocking down circZFR. D RIP assay using eIF4A3 antibody to test the combination between eIF4A3 protein and FASN mRNA in HeLa cells with or without circZFR overexpression. E MeRIP-qPCR assay validated the three m6A sites in FASN mRNA. F Schematic image of YTHDF3-WT plasmids, and YTHDF3-MUT plasmids containing m6A motif mutations in the CDS region. G Western blot assay tested the FASN protein expression in HeLa cells transfected with empty vector, YTHDF3-WT, or YTHDF3-MUT plasmids. WT, wild type; MUT, mutant. Data in A, B, C, D, E, and G are mean value ± SEM of three biological replicates, student t test, *P < 0.05, **P < 0.01, ***P < 0.001
FASN drives proliferation, migration, and invasion
Knocking down FASN in HeLa and C33A cells using siRNAs effectively silenced its expression (Fig. 6A and B). Functional assays demonstrated that FASN knockdown significantly inhibited cell proliferation, as shown by CCK8 and EdU assays (Fig. 6C and D). Additionally, FASN knockdown reduced cell migration and invasion capacities (Fig. 6E). EMT marker analysis revealed increased E-cadherin levels and reduced ZEB1, N-cadherin, Vimentin, and Snail expression following FASN knockdown (Fig. 6F). These data show that FASN is a key driver of proliferation, and metastatic behavior in cervical cancer cells.
Fig. 6.
FASN Enhances Proliferation, Migration, and Invasion in Cervical Cancer Cells. A RT-qPCR analysis of FASN mRNA expression in HeLa and C33A cells treated with two independent siRNAs against FASN. B Western blot analysis of FASN protein expression in HeLa and C33A cells treated with two independent siRNAs against FASN. C CCK-8 assay of HeLa and C33A cells treated with siRNAs against FASN. D EdU assay of HeLa and C33A cells treated with siRNAs against FASN. Up: representative immunofluorescence of Fluro594 (red), DAPI (blue), and merge. Scale bars: 50 μm. Down: percentages of EdU-incorporated Hela, C33A, and Caski cells. E Migratory and invasive ability assessed by Transwell assay of HeLa and C33A cells treated with siRNAs against FASN. Scale bars: 50 μm. F Representative western blot of EMT markers protein levels in HeLa and C33A cells treated with siRNAs against FASN. Data in A, B, C, D, E, and F are mean value ± SEM of three biological replicates, student t test, *P < 0.05, **P < 0.01, ***P < 0.001
CircZFR enhances lymph node metastasis in animal models
To confirm circZFR’s metastatic potential in vivo, HeLa cells with stable circZFR overexpression or empty vectors were injected into mice. Tumors in the circZFR-overexpression group metastasized more extensively to lymph nodes, growing faster and larger than those in the control group (P = 0.014) (Fig. 7A-C). Histological analysis further confirmed a higher density of tumor cells in the lymph nodes of the circZFR-overexpression group compared to controls (Fig. S6A). To further validate the role of circZFR, HeLa cells with stable sh-circZFR knockdown or negative control were injected in a similar manner. Lymph node metastasis was markedly suppressed in the sh-circZFR group (P < 0.0001) (Fig. 7A–C; Fig. S6A). FASN protein levels were also elevated in lymph nodes from the circZFR-overexpression group (empty vector vs. circZFR-OE, P < 0.0001; shCtrl vs. sh-circZFR, P < 0.0001) (Fig. 7D; Fig. S6B). These results demonstrate that circZFR promotes lymph node metastasis and tumor progression in vivo, likely by enhancing FASN protein translation through YTHDF3-mediated recognition of m6A-modified mRNA (Fig. 7E).
Fig. 7.
CircZFR Accelerates Lymph Node Metastasis in Vivo in Preclinical Models. A HeLa cells stably expressing either shCtrl or sh-circZFR (1 × 10⁶ cells) were injected into the footpads of mice and harvested popliteal lymph nodes (upper panel). Moreover, HeLa cells stably expressing either empty control or circZFR-OE (1 × 10⁶ cells) were injected into footpads of mice and harvested popliteal lymph nodes (lower panel). B Tumor volume analysis: Left panel shows comparison between empty vector and circZFR-OE groups; right panel shows comparison between shCtrl and sh-circZFR groups. C Representative images of harvested popliteal lymph nodes from the shCtrl, sh-circZFR, empty vector, and circZFR-OE groups. D Western blot analysis of FASN protein levels in popliteal lymph nodes from all four groups. Left: Representative Western blot images. Right: Quantification of FASN protein expression. E Schematic illustration of circZFR molecular mechanism. Overexpression circZFR binds to the m6A reader protein YTHDF3, facilitating m6A recognition on FASN mRNA and recruiting the translation initiator eIF4A3, boosting FASN translation, thereby facilitating EMT of cervical cancer cells. Statistical analyses of data in B and D were conducted using Student’s t-test. Data are presented as mean ± SEM from independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
Cervical cancer patients with lymph node metastasis are categorized as having advanced-stage disease under updated clinical guidelines [3]. This classification stems from the significantly higher relapse risk these patients face after undergoing radical surgery, chemotherapy, or radiation therapy. Identifying the molecular mechanisms driving lymph node metastasis is critical for improving therapeutic outcomes. In this study, we found that FASN protein expression was markedly higher in patients with lymph node metastasis compared to those without, as evidenced by our cohort tissue samples. This observation aligns with findings from the TCGA database. Functional experiments further demonstrated that silencing FASN in circZFR-overexpressing cervical cancer cells significantly suppressed their proliferation, migration, and invasion. These results underscore the importance of unraveling the mechanisms underlying FASN upregulation in cervical cancer.
Circular RNA circZFR has emerged as a key player in cervical cancer progression, previously shown to promote migration and invasion. Through six years of follow-up, we established that circZFR is also associated with shorter DFS and lymph node metastasis. This prompted us to explore whether circZFR regulates FASN expression. CircRNA pulldown and mass spectrometry analyses revealed that circZFR binds to YTHDF3, an m6A reader protein. Prior studies have shown that FASN is a target of YTHDF3 and harbors m6A modifications on its mRNA. Evidence from PAR-CLIP, m6A-seq, and RIP-seq data further supports the role of YTHDF3 in facilitating the translation of target mRNAs [19]. Given that YTHDF3 collaborates with YTHDF1 to enhance translation by interacting with translation initiation factors, our findings suggest that circZFR may upregulate FASN expression by promoting its translation through the circZFR-YTHDF3 complex.
In this study, we confirmed that overexpressing circZFR increased FASN protein levels, whereas knocking down circZFR reduced them. Interestingly, YTHDF3 knockdown decreased FASN protein levels without affecting FASN mRNA expression or stability. RNA and protein half-life assays further validated that YTHDF3 does not influence FASN mRNA or protein stability. Instead, MeRIP-qPCR confirmed the presence of m6A modifications on FASN mRNA, and RIP assays validated the interaction between YTHDF3 and FASN mRNA. Notably, mutating the m6A recognition residues in YTHDF3 impaired FASN translation, further supporting the hypothesis that YTHDF3 drives FASN protein synthesis via m6A recognition.
To dissect this mechanism further, we investigated the interaction between FASN mRNA and translation initiation factors. Previous studies indicated that YTHDF3 and YTHDF1 enhance target translation by binding eIF4A3, a translation initiation factor, rather than eIF3A or eIF3B [14]. Consistent with this, our Co-IP assays demonstrated that circZFR promotes the interaction between eIF4A3 and FASN mRNA. Importantly, knocking down YTHDF3 in circZFR-overexpressing cells suppressed cell proliferation, migration, and invasion, emphasizing YTHDF3’s critical role in circZFR-driven cervical cancer metastasis.
Despite these compelling findings, our study has several limitations. Firstly, only patients with complete DFS information and available tissue samples were included to interpret the clinical significance of FASN and YTHDF3 protein expression, which may introduce selection bias. While we observed strong associations between protein expression levels and clinical outcomes, these correlations are inherently associative and do not establish causality. Confounding variables such as tumor stage, histological subtype, or treatment heterogeneity may influence both protein expression and patient outcomes. Although our cohorts were clinically well-annotated, future multivariate analyses in larger, prospectively collected cohorts are needed to validate these biomarkers and control for potential confounders. Secondly, while our findings establish the circZFR/YTHDF3/FASN axis as a critical pathway promoting lymph node metastasis in cervical cancer, we acknowledge that metastasis is a multifactorial process. Other signaling pathways—including those involving TGF-β, PI3K/AKT, Wnt/β-catenin, and immune modulation—may also contribute to metastatic dissemination. Our study focused on delineating one key axis and did not evaluate the interplay between circZFR and other pro-metastatic networks. Future studies incorporating transcriptomic or proteomic profiling of circZFR-modulated cells, or CRISPR-based combinatorial screens, will be necessary to comprehensively assess additional pathways that may synergize with or counteract the circZFR/YTHDF3/FASN signaling cascade.
Conclusions
In summary, our study identifies circZFR as a pivotal regulator of cervical cancer metastasis, significantly upregulating FASN protein expression. Mechanistically, circZFR binds to YTHDF3, facilitating its recognition of m6A-modified FASN mRNA and promoting translation through the recruitment of eIF4A3. This circZFR-YTHDF3-FASN axis accelerates lymph node metastasis and shortens DFS in cervical cancer patients, highlighting circZFR as a promising therapeutic target for halting disease progression.
Supplementary Information
Acknowledgements
Dr. Calin is the Felix L. Haas Endowed Professor in Basic Science.
Abbreviations
- Circular RNAs
circRNAs
- Disease-free survival
DFS
- Fatty acid synthase
FASN
- YTH N6-methyladenosine RNA binding protein F3
YTHDF3
- RNA Immunoprecipitation
: RIP
- Co-Immunoprecipitation
Co-IP
- Fatty acid
FA
- Tissue Microarrays
TMAs
- Immunohistochemistry
IHC
- Quantitative Reverse Transcription PCR
qRT-PCR
- RNA Immunoprecipitation
RIP
- Co-Immunoprecipitation
Co-IP
- Epithelial-mesenchymal transition
EMT
- Overall survival
OS
Authors’ contributions
MZ and DW designed this study. MZ, BG, LH, and YZ performed the experiments. MZ and YG performed the data analysis. BG, LH, and YZ reviewed the pathological data. MZ wrote the draft of the manuscript. FC, GC and DW reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
This project was supported by the grant from the National Natural Science Foundation of China (No.82103294) and Natural Science Foundation of Liaoning Province (No.2022-BS-064).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Ethics Committee of Liaoning Cancer Hospital & Institute (Approval No. 2021G0309). Animal experiments were approved by the Animal Ethical and Welfare Committee of China Medical University (Approval No. KT2021203). Informed consent was obtained from all participants, and the study adhered to the principles of the 1975 Declaration of Helsinki.
Consent for publication
Consent was obtained from each patient.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. [DOI] [PubMed] [Google Scholar]
- 2.Yun BS, Lee KB, Lee KH, Chang HK, Kim JY, Lim MC, Choi CH, Cho H, Kim DY, Kim YH, et al. Therapeutic effects of surgical debulking of metastatic lymph nodes in cervical cancer IIICR: a trial protocol for a phase III, multicenter, randomized controlled study (KGOG1047/DEBULK trial). J Gynecol Oncol. 2024;35(5):e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda M, Mabuchi S, Sakata M, Deguchi S, Kakubari R, Matsuzaki S, Hisa T, Kamiura S. Significance of tumor size and number of positive nodes in patients with FIGO 2018 stage IIIC1 cervical cancer. Jpn J Clin Oncol. 2024;54(2):146–52. [DOI] [PubMed] [Google Scholar]
- 4.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763–77. [DOI] [PubMed] [Google Scholar]
- 5.Xu L, Zhang Y, Lin Z, Deng X, Ren X, Huang M, Li S, Zhou Q, Fang F, Yang Q, et al. FASN-mediated fatty acid biosynthesis remodels immune environment in Clonorchis sinensis infection-related intrahepatic cholangiocarcinoma. J Hepatol. 2024;81(2):265–77. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Tsang WY, Fang XN, Zhang Y, Luo J, Gong LQ, Zhang BF, Wong CN, Li ZH, Liu BL, et al. FASN inhibition decreases MHC-I degradation and synergizes with PD-L1 checkpoint blockade in hepatocellular carcinoma. Cancer Res. 2024;84(6):855–71. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Hayashida T, Masugi Y, Oshikawa K, Hayakawa N, Itoh M, Nishime C, Suzuki M, Nagayama A, Kawai Y, et al. PRMT1 sustains de novo fatty acid synthesis by methylating PHGDH to drive chemoresistance in triple-negative breast cancer. Cancer Res. 2024;84(7):1065–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferraro GB, Ali A, Luengo A, Kodack DP, Deik A, Abbott KL, Bezwada D, Blanc L, Prideaux B, Jin X, et al. Fatty acid synthesis is required for breast cancer brain metastasis. Nat Cancer. 2021;2(4):414–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Piano M, Manuelli V, Zadra G, Otte J, Edqvist PD, Ponten F, Nowinski S, Niaouris A, Grigoriadis A, Loda M, et al. Lipogenic signalling modulates prostate cancer cell adhesion and migration via modification of Rho GTPases. Oncogene. 2020;39(18):3666–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang L, Xiao L, Sugiura H, Huang X, Ali A, Kuro-o M, Deberardinis RJ, Boothman DA. Metabolic reprogramming during TGFbeta1-induced epithelial-to-mesenchymal transition. Oncogene. 2015;34(30):3908–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaytseva YY, Rychahou PG, Gulhati P, Elliott VA, Mustain WC, O’Connor K, Morris AJ, Sunkara M, Weiss HL, Lee EY, et al. Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res. 2012;72(6):1504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du Q, Liu P, Zhang C, Liu T, Wang W, Shang C, Wu J, Liao Y, Chen Y, Huang J, et al. FASN promotes lymph node metastasis in cervical cancer via cholesterol reprogramming and lymphangiogenesis. Cell Death Dis. 2022;13(5):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–6. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo S, Tong L. Molecular basis for the recognition of methylated adenines in RNA by the eukaryotic YTH domain. Proc Natl Acad Sci U S A. 2014;111(38):13834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27(3):315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou Z, Sepich-Poore C, Zhou X, Wei J, He C. The mechanism underlying redundant functions of the YTHDF proteins. Genome Biol. 2023;24(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li A, Chen YS, Ping XL, Yang X, Xiao W, Yang Y, Sun HY, Zhu Q, Baidya P, Wang X, et al. Cytoplasmic m(6)a reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27(3):444–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang G, Shi L, Ye Y, Shi H, Zeng L, Tiwary S, Huse JT, Huo L, Ma L, Ma Y, et al. YTHDF3 induces the translation of m(6)A-Enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell. 2020;38(6):857–71. e857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y, He X, Wang S, Sun B, Jia R, Chai P, Li F, Yang Y, Ge S, Jia R, et al. The m(6)A reading protein YTHDF3 potentiates tumorigenicity of cancer stem-like cells in ocular melanoma through facilitating CTNNB1 translation. Oncogene. 2022;41(9):1281–97. [DOI] [PubMed] [Google Scholar]
- 22.Zhong S, Guo Q, Chen X, Luo X, Long Y, Chong T, Ye M, He H, Lu A, Ao K, et al. The inhibition of YTHDF3/m(6)A/LRP6 reprograms fatty acid metabolism and suppresses lymph node metastasis in cervical cancer. Int J Biol Sci. 2024;20(3):916–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pisignano G, Michael DC, Visal TH, Pirlog R, Ladomery M, Calin GA. Going circular: history, present, and future of circrnas in cancer. Oncogene. 2023;42(38):2783–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen B, Dragomir MP, Yang C, Li Q, Horst D, Calin GA. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct Target Ther. 2022;7(1): 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou M, Yang Z, Wang D, Chen P, Zhang Y. The circular RNA circZFR phosphorylates Rb promoting cervical cancer progression by regulating the SSBP1/CDK2/cyclin E1 complex. J Exp Clin Cancer Res. 2021;40(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen LL, Wang Y, et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27(5):626–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Wang J, Huang Y, Xiao Y. 3dRNA v2.0: an updated web server for RNA 3D structure prediction. Int J Mol Sci. 2019. 10.3390/ijms20174116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al. Highly accurate protein structure prediction with alphafold. Nature. 2021;596(7873):583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang YC, Di C, Hu B, Zhou M, Liu Y, Song N, Li Y, Umetsu J, Lu ZJ. CLIPdb: a CLIP-seq database for protein-RNA interactions. BMC Genomics. 2015;16(1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan Y, Zhang D, Zhou P, Li B, Huang SY. HDOCK: a web server for protein-protein and protein-DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res. 2017;45(W1):W365-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.