Abstract
Calcineurin is a calcium–calmodulin-regulated, serine–threonine phosphatase that functions as a key inducer of stress responsive gene expression in multiple cell types through a direct activation of nuclear factor of activated T cells and myocyte enhancer factor 2 transcription factors. In cardiomyocytes, calcineurin signaling has been implicated in the regulation of the hypertrophic response caused by pressure overload or neuroendocrine stimulation. Three separate genes encode the catalytic subunit of calcineurin in mammalian cells, CnAα, CnAβ, and CnAγ. To evaluate the necessary function of calcineurin as a hypertrophic regulatory factor, the CnAβ gene was disrupted in the mouse. CnAβ-deficient mice were viable, fertile, and overtly normal well into adulthood, but displayed a 80% decrease in calcineurin enzymatic activity in the heart that was associated with a 12% reduction in basal heart size. CnAβ-deficient mice were dramatically impaired in their ability to mount a productive hypertrophic response induced by pressure overload, angiotensin II infusion, or isoproterenol infusion. Analysis of marker genes associated with the hypertrophic response revealed a partial defect in the molecular program of hypertrophy. Collectively, these data solidify the hypothesis that calcineurin functions as a central regulator of the cardiac hypertrophic growth response in vivo.
Calcineurin is a serine–threonine protein phosphatase that is activated in response to sustained elevations in intracellular calcium through direct binding of calmodulin (reviewed in refs. 1 and 2). Once activated, calcineurin directly dephosphorylates members of the nuclear factor of activated T cells (NFAT) family, resulting in their translocation from the cytosol to the nucleus, where they participate in regulating inducible gene expression in multiple cell types (1). Activated calcineurin is a multicomponent complex consisting of a catalytic A subunit (59–62 kDa), a regulatory B subunit (19 kDa), and calmodulin. Three separate catalytic subunit genes have been identified in vertebrate species, referred to as CnAα, CnAβ, and CnAγ. CnAα and CnAβ are ubiquitously expressed and comprise nearly all of the enzymatic activity in most tissues, whereas CnAγ is restricted to the testis and a limited region of the brain (1, 3). In the heart, mRNA and protein levels of CnAβ, but not CnAα, were significantly increased by stress or agonist stimulation, suggesting a role for this specific isoform in regulating the hypertrophic growth response (4, 5).
Calcineurin-mediated translocation of NFAT transcription factors is an important signaling event involved in T lymphocyte activation in response to antigen stimulation. Indeed, the widely used immunosuppressive agents cyclosporine A and FK506 largely function by inhibiting calcineurin, thereby preventing NFAT nuclear translocation and the subsequent induction of cytokine genes (1–3). More recently, a conserved role for calcineurin–NFAT was identified in the heart (6). Specifically, transgenic mice overexpressing activated calcineurin or a nuclear localized NFATc4 mutant protein in the heart each demonstrated a dramatic hypertrophy response (6). A number of different inhibitory strategies have also suggested a necessary role for calcineurin signaling in the regulation of cardiomyocyte hypertrophy. Specifically, cyclosporine A and FK506 treatment reduced cardiac hypertrophy in many, but not all rodent models (reviewed in ref. 7). More recently, overexpression of multiple calcineurin inhibitory protein domains or a dominant-negative calcineurin protein in the hearts of transgenic mice attenuated agonist- or pressure-overload-induced cardiac hypertrophy (8–10).
Although a large number of studies have proposed a central regulatory role for calcineurin as a mediator of cardiac hypertrophy, other studies have disputed such a role (7). To further evaluate the importance of calcineurin as a necessary hypertrophic regulatory factor in the heart, the calcineurin Aβ (CnAβ) gene was inactivated by gene targeting. CnAβ-null mice were viable well into adulthood, but were significantly compromised in their ability to mount a hypertrophic response after aortic banding, angiotensin II (AngII) infusion, or isoproterenol (Iso) infusion. These results definitively establish the hypothesis that calcineurin is a necessary regulator of cardiomyocyte hypertrophy in vivo.
Materials and Methods
CnAβ Gene Targeting.
To construct the CnAβ targeting vector, a genomic clone was isolated from a Sv129 λ genomic library and the intron–exon boundaries were defined by restriction enzyme site mapping and nucleotide sequence analysis. The targeting vector consisted of a 2.5-kb genomic fragment 5′ to exon 2 (short arm), the neomycin-resistance gene (neo), and a 4.0-kb genomic fragment 3′ to exon 2 (long arm). The long and short arms were cloned into the ClaI and XhoI sites of the vector pTK-NEO.
The linearized targeting vector was introduced into KG1 embryonic stem (ES) cells (Sv129) by electroporation and cells were grown on STO feeder fibroblasts with media containing G418 and 1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-5-iodouracil (FIAU). Drug-resistant clones were picked 10 days after selection, and targeted clones were identified by Southern blot analysis of genomic DNA. Two targeted clones were obtained from a total of 400, and both were injected into the blastocysts of C57BL/6 mice to generate chimeric mice. Chimeric offspring were bred with C57BL/6 mice to generate heterozygous targeted mice.
Western Immunoblot Analysis.
Protein samples were separated by SDS/PAGE according to the method described by Laemmli (11). Protein extracts were prepared from cardiac tissue, loaded onto 10% polyacrylamide gels, electrophoresed in the presence of 25 mM Tris, pH 8.3/2 mM glycine/0.1% SDS at room temperature, and transferred electrophoretically onto Hybond-P membranes (Amersham Pharmacia). Antibodies used were CnAα, CnAβ (Santa Cruz Biotechnology), and Pan CnA (Chemicon). Immunoreactivity was detected using the enhanced chemifluorescence system (Amersham Pharmacia) according to kit instructions. Immunoreactive bands were digitized and quantified for fluorescence with a Storm 860 PhosphoImager (Molecular Dynamics).
Calcineurin Activity Assay.
Phosphatase activity was measured using the calcineurin assay kit (Biomol, Plymouth Meeting, PA) according to manufacturer's instructions. Cardiac extracts from either wild-type or null mice were prepared, and calcineurin activity was measured as the dephosphorylation rate of a synthetic phosphopeptide substrate (RII peptide). The amount of PO4 released was determined colorimetricaly with the BIOMOL GREEN reagent.
Histological Analysis.
Hearts were collected at the indicated times, fixed in 10% formalin containing PBS, and embedded in paraffin. Serial 10-μm heart sections from each group were analyzed. Samples were stained with hematoxylin/eosin and Masson's trichrome on longitudinal histological sections to evaluate gross morphology, fiber integrity, and fibrosis.
RNA Quantitation.
Cardiac gene expression of hypertrophic markers was assessed by RNA dot blot analysis as described (12). In brief, RNA was extracted from murine ventricular tissue using Trizol reagent (Gibco/BRL), and 2 μg was blotted to nitrocellulose filters using a dot-blot filtration manifold (BioRad) to generate blots for subsequent hybridization and mRNA quantification. Cardiac gene expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels. The sequence of the oligonucleotide probes used has been described elsewhere (12, 13).
Murine Abdominal Aorta Constriction and Agonist Infusion.
To induce cardiac pressure overload in mice, the abdominal aorta was constricted as described previously (9). To evaluate cardiac hypertrophy induced by cardiac acting agonists, Alzet osmotic minipumps (model 2002; Alza) that contained either Iso (60 mg/kg/day), AngII (432 μg/kg/day), or PBS were implanted dorsally and s.c. under isoflurane anesthesia. All mice (CnAβ−/− and CnAβ+/+ strain- and age-matched) were evaluated at 8–10 weeks of age for a total of 14 days of stimulation.
Echocardiography and Measurement of Blood Pressure.
Mice from either genotype were anesthetized with isoflurane, and echocardiography was performed with a Hewlett Packard 5500 instrument with a 15-MHz microprobe. Ventricular measurements were taken on M mode as reported previously (9, 13). The quantitative measurements represent the average of 5 mice in each cohort (3 readings per mouse).
Statistical Analysis.
Statistical analyses between the experimental groups were performed using a Student's t test or one-way ANOVA when comparing multiple groups. Data are reported as mean ± SEM. P values ≤ 0.05 were considered significant.
Results
Generation of CnAβ-Deficient Mice.
Analysis of calcineurin A isoform expression in agonist-stimulated cardiomyocytes demonstrated a significant increase in CnAβ mRNA and protein, whereas CnAα expression was unaffected and CnAγ was not detected (4). These data suggested an important role for the CnAβ gene isoform in regulating the hypertrophic growth of cardiomyocytes. To evaluate the function of CnAβ as a necessary regulator of cardiac hypertrophy in vivo, gene targeting was used in ES cells to permit generation of nullizygous mice. The CnAβ gene was inactivated by homologous recombination with a targeting vector designed to delete the exon encoding the majority of the catalytic domain of calcineurin (Fig. 1A). Southern blot analysis identified two independent ES clones that had undergone proper targeting, which were injected into C57BL/6 blastocysts to generate chimeric mice. Germline-containing progeny from chimeric mice were interbred to generate CnAβ homozygous null mice, as well as strain-matched wild-type mice for comparison (Fig. 1B). The functional disruption of the CnAβ gene was confirmed by Western blot analysis from brain tissue using a CnAβ-specific antibody (Fig. 1C). CnAβ homozygous null mice were fertile and largely indistinguishable from wild-type littermate mice with no overt signs of pathology. In addition, assessment of cardiovascular function by echocardiography or invasive catheterization revealed no change in fractional shortening, heart rate, mean arterial blood pressure, or an AngII-induced pressor response in vivo (Table 1). However, male CnAβ-null mice at 8 weeks of age demonstrated a ≈10% reduction in body weight and a further ≈12% reduction in heart weight normalized to body weight (Table 1). CnAβ-null mice also showed a trend toward smaller cardiac chamber dimensions, ventricular wall thickness, and septal wall thickness (Table 1). Lastly, analysis of other organs revealed a significant decrease in thymus weight (data not shown).
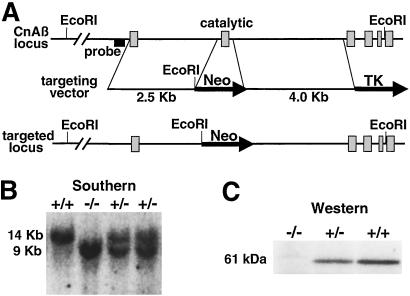
Figure 1.
Targeted disruption of the CnAβ gene. (A) Schematic representation of the CnAβ genomic locus, the targeting construct, and the result of homologous recombination that deletes the exon encoding the catalytic domain. (B) Southern blot analysis was performed using EcoRI-digested genomic DNA from four littermates obtained from CnAβ heterozygotes crosses, which show a wild-type (14 kb) and targeted fragment (9 Kb) of the predicted size. (C) Western blot analysis for CnAβ protein from brain extracts of wild-type, heterozygous, and null mice.
Table 1.
Physical measurements in wild-type and CnAβ-null mice at 8 weeks of age
| Wild type | CnAβ−/− | P value | |
|---|---|---|---|
| Echocardiography (n =) | 5 | 5 | |
| Septum (mm) | 1.03 ± 0.05 | 0.92 ± 0.05 | NS |
| LV free wall (mm) | 1.00 ± 0.01 | 0.97 ± 0.02 | NS |
| LVEDD (mm) | 3.86 ± 0.22 | 3.38 ± 0.04 | NS |
| LVESD (mm) | 2.32 ± 0.19 | 1.94 ± 0.07 | NS |
| Fractional shortening (%) | 40.2 ± 2.00 | 42.6 ± 2.00 | NS |
| Gravimetry (n =) | 14 | 14 | |
| Body weight (g) | 29.7 ± 0.8 | 26.6 ± 0.7 | < 0.005 |
| Heart/body weight (mg/g) | 4.92 ± 0.1 | 4.35 ± 0.1 | < 0.001 |
| Hemodynamics (n =) | 4 | 4 | |
| MAP (mmHg) | 76 ± 8.9 | 87 ± 4.3 | NS |
| MAP + AngII (mmHg) | 124 ± 7.8 | 130 ± 4.0 | NS |
| Basal heart rate (bpm) | 414 ± 22 | 444 ± 24 | NS |
CnAβ-null mice were compared with age- and strain-matched control mice at 8 weeks of age by echocardiography, gravimetric analysis, and blood pressure analysis at baseline or after AngII infusion. For echocardiography, each mouse was measured 3 times (5 mice in each group). LV, left ventricle measured in systole; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; mm, millimeters. Fractional shortening was calculated as (LVEDD − LVESD)/LVEDD × 100. Mean arterial blood pressure (MAP) was measured through the femoral artery in anesthetized mice as previously described (31). Measurements were at baseline or after AngII infusion at 1 ng/g/min. NS, not significant; 1 mmHg = 133 Pa. All mean values are shown ± SEM.
Calcineurin Expression and Phosphatase Activity in CnAβ −/− Heart Tissue.
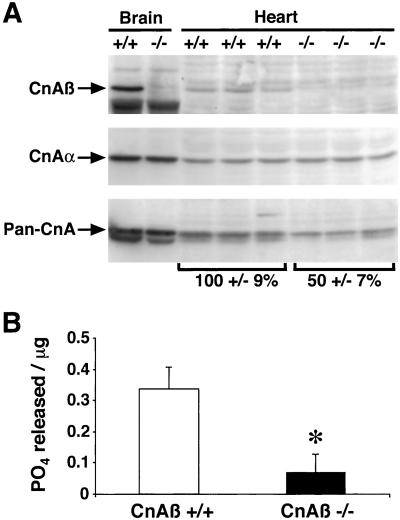
Calcineurin protein levels were measured by Western blot analysis of protein extracts from CnAβ−/− and CnAβ+/+ heart and brain tissues. CnAβ immunoreactivity was absent in CnAβ−/− heart and brain tissues, whereas CnAα protein levels were invariant, indicating no overt compensation in the absence of CnAβ (Fig. 2A). Analysis of CnAβ-null hearts also permitted a quantitative evaluation of relative isoform composition in the heart. When a pan CnA antibody was used, CnAβ was estimated to constitute approximately 50% of the total calcineurin catalytic protein content in the mouse heart (Fig. 2A). Interestingly, although protein levels were only reduced by half, examination of total calcineurin phosphatase activity from CnAβ−/− hearts demonstrated a 80% decrease from three independent experiments (P < 0.05) (Fig. 2B; see Discussion).
Figure 2.
Analysis of cardiac calcineurin protein/activity from wild-type and CnAβ−/− mice. (A) Protein extracts from 6-week-old mice of either genotype were prepared for Western blotting and analyzed using antibodies that recognize either all catalytic subunit gene isoforms (Pan-CnA), or specific isoforms (CnAβ or CnAα). (B) Cardiac extracts from wild-type and CnAβ−/− mice were assayed for calcineurin activity (okadaic acid-resistant and EGTA-sensitive phosphatase activity). ∗, P < 0.05.
CnAβ−/− Mice Are Defective in Agonist- and Load-Induced Cardiac Hypertrophy.
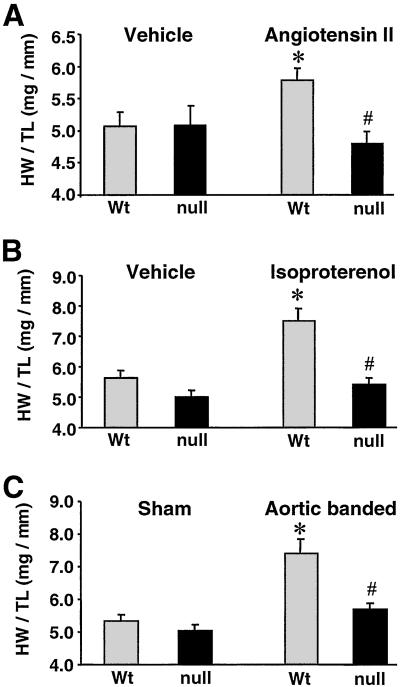
To investigate whether CnAβ−/− mice are capable of mounting a normal cardiac hypertrophic response, homozygous null and matched control mice were subjected to osmotic minipump infusion of AngII, Iso, or PBS vehicle for 14 days. Previous reports have shown that both AngII and Iso are potent stimulators of hypertrophy (14) and calcineurin activity in the heart (9). As shown in Fig. 3, analysis of heart weight/tibia length ratios demonstrated that both AngII and Iso induced a significant cardiac hypertrophic response in wild-type control mice compared with PBS infusion (14% and 33% increase, respectively) (Fig. 3 A and B). In contrast, CnAβ−/− mice did not exhibit a significant increase in heart weight/tibia length ratio in response to either AngII or Iso infusion (P < 0.05) (Fig. 3 A and B).
Figure 3.
Quantitation of the cardiac hypertrophic response in CnAβ−/− mice. (A) Heart weight/tibia length ratios after 14 days of AngII infusion (432 μg/kg/day) in 8-week-old CnAβ+/+ and CnAβ−/− mice (n = 6 mice in each cohort). (B) Heart weight/tibia length ratios after 14 days of Iso infusion (60 mg/kg/day) in 8-week-old CnAβ+/+ and CnAβ−/− mice (n = 6 mice in each cohort). (C) Heart weight/tibia length ratios after 14 days of pressure overload induced by stenosis of the abdominal aorta in 8-week-old CnAβ+/+ and CnAβ−/− mice (n = 5 banded mice and 6 sham mice for both wild-type and CnAβ−/−). ∗, P < 0.05 versus wild-type vehicle or sham group; #, P < 0.05 versus wild-type treated or banded mice; Wt, wild type.
To further investigate the role of calcineurin as a necessary regulator of cardiac hypertrophy, CnAβ+/+ and CnAβ−/− animals were subjected to a calibrated abdominal aortic constriction to induce pressure overload. Wild-type mice developed a 39% increase in heart weight/tibia length ratio compared with sham-operated wild-type mice (P < 0.05; Fig. 3C). In contrast, CnAβ−/− mice showed only a 13% increase in heart weight/tibia length ratio compared with sham-operated CnAβ−/− mice, suggesting a significant defect in load-induced hypertrophy in the absence of CnAβ (P < 0.05; Fig. 3C).
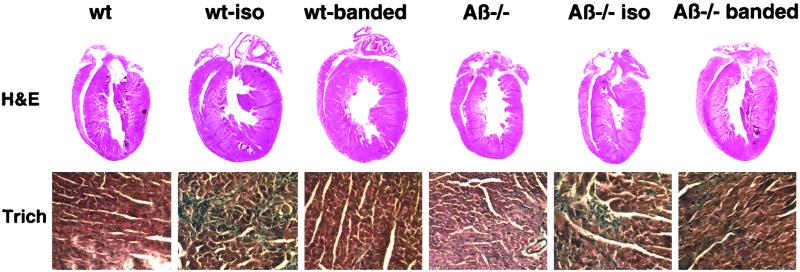
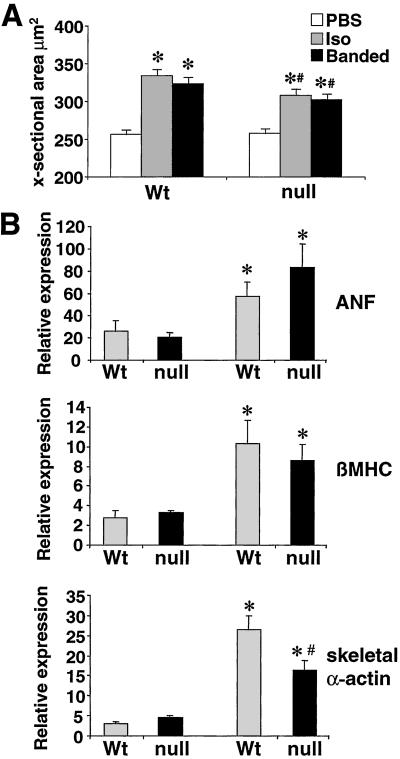
Histological analysis of Iso-infused and aortic-banded wild-type mice revealed a qualitative increase in heart size, which was attenuated in CnAβ−/− mice (Fig. 4). Microscopic analysis of hematoxylin/eosin- and trichrome-stained cardiac histological sections did not show significant signs of histopathology in any group after 14 days, though a mild increase in cardiac fibrosis was observed in all Iso-infused mice (Fig. 4). Myofibril cross-sectional areas were also quantified from histological sections stained with wheat germ agglutinin as previously described (15). Although both wild-type and CnAβ-null mice had increased myofibril cross-sectional areas in response to Iso-infusion or aortic banding, a significant attenuation was observed in the absence of CnAβ (Fig. 5A). These data indicate that the loss of CnAβ reduces stress-induced myofibrillar hypertrophy.
Figure 4.
Cardiac histological analysis of wild-type and CnAβ−/− mice subjected to 14 days of Iso infusion or pressure overload. (Upper) Macroscopic hematoxylin/eosin-stained histological section of hearts demonstrate a noticeable increase in heart size in wild-type mice, but not CnAβ−/− mice. (Lower) Microscopic analysis of trichrome-stained histological sections demonstrate comparable myofibrillar organization and interstitial fibrosis in wild-type (wt) and CnAβ−/− mice treated with Iso or by pressure overload.
Figure 5.
Myfibrillar cross-sectional areas and RNA dot blot analysis of hypertrophy associated genes from wild-type (Wt) and CnAβ−/− hearts. (A) Approximately 150 fibers were measured from each of the indicated groups to qunatify myofiber cross-sectional areas (∗, P < 0.05 versus unstimulated; #, P < 0.05 versus wild-type Iso-infused or banded). (B) Cardiac RNA levels quantified by dot blotting from mice subjected to 14 days of AngII infusion (432 μg/kg/day). RNA levels of atrial natriuretic factor (ANF), β-myosin heavy chain (βMHC), and skeletal α-actin, were analyzed (n = 4 each). Data are expressed as the percent of glyceraldehyde 3-phosphate dehydrogenase levels (∗, P < 0.05 versus vehicle-treated; #, P < 0.05 versus wild-type AngII-infused mice).
Hearts from AngII-infused wild-type and CnAβ−/− mice were also analyzed for hypertrophic marker gene expression by RNA dot blotting. Levels of atrial natriuretic factor (ANF), β-myosin heavy chain (βMHC), and skeletal α-actin mRNA were increased in both wild-type and CnAβ−/− mice after 14 days of AngII administration. A similar increase in ANF expression was observed in both groups after Iso-infusion or aortic banding (data not shown). However, the increase in skeletal α-actin mRNA levels was significantly reduced in CnAβ−/− mice compared with wild-type mice infused with AngII (Fig. 5B). This effect on skeletal α-actin mRNA induction was also observed in Cain transgenic mice, a previously described model with reduced cardiac calcineurin activity (9). Taken together, these results indicate that loss of CnAβ partially compromises the molecular program of hypertrophy in the heart.
Discussion
The original description of calcineurin as a sufficient inducer of cardiac hypertrophy suggested a novel function for this calcium-sensitive signaling factor in the heart (6). This initial report was provocative, given the known importance of calcium as a fundamental regulatory molecule in the heart and the implications that available calcineurin inhibitory drugs might be used as a novel means of treating certain forms of heart disease. However, enthusiasm for the cardiac calcineurin hypothesis was dampened by a few reports that failed to observe a significant reduction in cardiac hypertrophy using the calcineurin inhibitory agents cyclosporine A and FK506 in rodent models of induced hypertrophy (reviewed in ref. 7). Here, analysis of CnAβ gene-targeted mice firmly establishes the importance of calcineurin as a regulator of the hypertrophic growth response in the adult heart.
Calcineurin Inhibitory Studies in the Heart.
The observation that CnAβ-null mice manifest reduced cardiac hypertrophy in response to agonist or pressure overload is consistent with approximately 15 reports in the literature that showed reduced cardiac hypertrophy or heart failure after cyclosporine and/or FK506 treatment (7, 10, 16–20). However, 5 equally credible reports have disputed the ability of cyclosporine or FK506 to significantly affect certain rodent models of induced cardiac hypertrophy, suggesting that calcineurin may not be a requisite mediator of this response (7, 21). Although drug studies can provide valuable insight into the biological functions of a given protein, a number of variables should be considered. With respect to the cardiac calcineurin hypothesis, negative results might have arisen because of ineffective inhibition of calcineurin with cyclosporine or FK506. Conversely, false positive results could have arisen because of nonspecific effects of cyclosporine or FK506 on other regulatory factors that also participate the hypertrophic response. In addition, almost 10-fold more cyclosporine is required to attenuate the cardiac hypertrophic response compared with immunosuppressive dosages (15), largely because cardiac myocytes have significantly more calcineurin than lymphocytes (1). Such higher dosages might further obscure the specificity of cyclosporine or enhance its toxicity on the kidney, which can produce secondary effects on the heart because of alterations in blood pressure or the release of endocrine factors (22).
More recently, three different groups reported four separate transgenic mouse models with inhibited cardiac calcineurin activity, each of which showed reduced cardiac hypertrophy in response to acute stimuli. In one study, transgenic mice were generated expressing the calcineurin inhibitory domains from Cain and AKAP79 in the heart (9). Both Cain and AKAP transgenic mice demonstrated a significant reduction in pressure overload (aortic banding) and agonist-induced (Iso infusion) cardiac hypertrophy (9). Calcineurin activity is also negatively regulated by the inhibitory proteins myocyte calcineurin inhibitory protein-1 and -2 (MCIP1 and -2, a.k.a. DSCR1 and ZAKI-4), which are each highly expressed in the heart and skeletal muscle (23, 24). Transgenic mice expressing the calcineurin inhibitory domain from MCIP1 also demonstrated reduced cardiac hypertrophy in response to stress stimulation (8). Lastly, cardiac-specific transgenic mice expressing a dominant negative mutant of calcineurin were also characterized by reduced stress-induced hypertrophic growth (10).
Although the transgenic studies discussed above strongly suggest the importance of calcineurin as a necessary regulator of adult-onset cardiac hypertrophy, each study is not without specific limitations. For example, the calcineurin inhibitory domain of Cain was also shown to directly inhibit the transcriptional activity of myocyte enhancer factor-2, suggesting an alternate mechanism whereby Cain might attenuate cardiac hypertrophy (25). In addition, although MCIP1 overexpression in the heart reduced cardiac hypertrophy (8), its mechanism of regulating the hypertrophic response is not understood. Specifically, endogenous MCIP1 expression is increased by hypertrophic stimulation in the heart, yet calcineurin activity is not down-regulated, but is instead augmented (5, 9, 15). Finally, overexpression of a dominant-negative calcineurin inhibitory protein in the heart raises a minor concern that other calcineurin-interacting proteins might be affected. Given these considerations discussed above, the description of reduced cardiac hypertrophy in CnAβ-null mice further establishes the importance of this signaling factor as a hypertrophic regulator in the heart and validates the specificity of previous transgenic inhibitory approaches as well as pharmacologic approaches.
Specific Role for CnAβ in the Heart.
Three separate calcineurin catalytic subunit genes have been identified in mammalian species, CnAα, CnAβ, and CnAγ. Here we demonstrated that CnAα and CnAβ proteins are expressed at equivalent levels in the heart, though disruption of CnAβ leads to an 80% decrease in total calcineurin activity. This subtle discrepancy between protein levels and activity suggests an unique regulatory role for the CnAβ isoform in the heart. Although CnAα and CnAβ are largely homologous to one another in primary amino acid sequence, approximately 20–30 aa of the N- and C-terminal sequences are divergent, which could account for differential interactions with substrates or regulatory proteins in the heart. In this regard, loss of CnAβ could alter the stoichiometry between endogenous calcineurin inhibitory factors such as MCIP1 and the remaining CnAα isoform, resulting in greater net inhibition. As a final and perhaps more important consideration, we have observed that hypertrophic agonist stimulation selectively up-regulates CnAβ protein content in cardiomyocytes, but not CnAα (4). This observation suggests a more pivotal role for CnAβ as a regulator of the hypertrophic response.
Interestingly, heart weight normalized to body weight was approximately 12% less in adult male CnAβ-null mice compared with matched control mice. This observation is consistent with the reported 5–10% reduction in normalized heart weight in calcineurin-inhibited MCIP1 transgenic mice (8) and in mice treated with cyclosporine (18). Collectively these observations suggest that calcineurin signaling either participates in regulating developmental hypertrophy of the heart or helps in regulating the steady-state mass of the myocardium in response to routine physiologic stimuli.
Multiple Necessary Signaling Pathways.
The observation that CnAβ-null mice fail to hypertrophy in response to acute stress stimuli indicates a central regulatory role for this calcium-dependent signaling pathway in the heart. Although calcineurin functions as a necessary regulator of the hypertrophic response, it does so in coordination with other intracellular signaling pathways. Calcineurin activation in cardiomyocytes has been associated with enhanced signaling through protein kinase Cα and θ, c-Jun N-terminal kinase, and extracellular-signal-regulated kinase (10, 26, 27), similar to cross-talking networks identified in T cells (28). Such observations underscore the complexity and interconnectivity that is inherent to intracellular signaling pathways in mammalian cells. Indeed, inhibition of multiple divergent classes of signaling factors in the adult heart by gene targeting, transgenesis, or adenoviral delivery of dominant negative factors has revealed important insights into the multifactorial nature of the hypertrophic response (7, 29). Although cooperation between divergent parallel pathways is required for productive hypertrophy, it is likely that certain signaling factors function as central regulators of the hypertrophic response. The manner in which calcineurin is regulated by sustained increases in intracellular calcium suggests that it might function as a central component in programming the cardiac hypertrophic response long-term (30).
Acknowledgments
This work was supported by National Institutes of Health grants and a Pew Scholar award (to J.D.M.). B.J.W. was supported by a M.D./Ph.D. Scholar Award from the University of Cincinnati Physician Scientist Training Program. O.F.B. was supported by National Institutes of Health Individual Research Service Award HL10336.
Abbreviations
- NFAT
nuclear factor of activated T cells
- MCIP1
myocyte calcineurin inhibitory protein 1
- AngII
angiotensin II
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Crabtree G R. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 2.Klee C B, Ren H, Wang X. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 3.Rusnak F, Mertz P. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 4.Taigen T, De Windt L J, Lim H W, Molkentin J D. Proc Natl Acad Sci USA. 2000;97:1196–1201. doi: 10.1073/pnas.97.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haq S, Choukroun G, Lim H, Tymitz K M, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin J D. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 6.Molkentin J D, Lu J R, Antos C L, Markham B, Richardson J, Robbins J, Grant S R, Olson E N. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molkentin J D. Circ Res. 2000;87:731–738. doi: 10.1161/01.res.87.9.731. [DOI] [PubMed] [Google Scholar]
- 8.Rothermel B A, McKinsey T A, Vega R B, Nicol R L, Mammen P, Yang J, Antos C L, Shelton J M, Bassel-Duby R, Olson E N, Williams R S. Proc Natl Acad Sci USA. 2001;98:3328–3333. doi: 10.1073/pnas.041614798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Windt L J, Lim H W, Bueno O F, Liang Q, Delling U, Braz J C, Glascock B J, Kimball T F, del Monte F, Hajjar R J, Molkentin J D. Proc Natl Acad Sci USA. 2001;98:3322–3327. doi: 10.1073/pnas.031371998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou Y, Hiroi Y, Uozumi H, Takimoto E, Toko H, Zhu W, Kudoh S, Mizukami M, Shimoyama M, Shibasaki F, et al. Circulation. 2001;104:97–101. doi: 10.1161/01.cir.104.1.97. [DOI] [PubMed] [Google Scholar]
- 11.Lammeli U K. Nature (London) 1970;227:680–685. [Google Scholar]
- 12.Jones W K, Grupp I L, Doetschman T, Grupp G, Osinska H, Hewett T E, Boivin G, Gulick J, Ng W A, Robbins J. J Clin Invest. 1996;98:1906–1917. doi: 10.1172/JCI118992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bueno O F, De Windt L J, Tymitz K M, Witt S A, Kimball T R, Klevitsky R, Hewett T E, Jones S P, Lefer D J, Peng C-F, et al. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichihara S, Senbonmatsu T, Price E, Jr, Ichiki T, Gaffney F A, Inagami T. Circulation. 2001;104:346–351. doi: 10.1161/01.cir.104.3.346. [DOI] [PubMed] [Google Scholar]
- 15.Lim H W, De Windt L J, Steinberg L, Taigen T, Witt S A, Kimball T R, Molkentin J D. Circulation. 2000;101:2431–2437. doi: 10.1161/01.cir.101.20.2431. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Kutschke W, Richardson K E, Karimi M, Hill J A. Circulation. 2001;104:1657–1663. doi: 10.1161/hc3901.095766. [DOI] [PubMed] [Google Scholar]
- 17.Deng L, Huang B, Qin D, Ganguly K, El-Sherif N. J Cardiovasc Electrophysiol. 2001;12:1055–1061. doi: 10.1046/j.1540-8167.2001.01055.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Nolan B, Kutschke W, Hill J A. J Biol Chem. 2001;276:17706–17711. doi: 10.1074/jbc.M100544200. [DOI] [PubMed] [Google Scholar]
- 19.Shimoyama M, Hayashi D, Zou Y, Takimoto E, Mizukami M, Monzen K, Yazaki Y, Nagai R, Komuro I. J Cardiol. 2001;37:114–118. [PubMed] [Google Scholar]
- 20.Sakata Y, Masuyama T, Yamamoto K, Nishikawa N, Yamamoto H, Kondo H, Ono K, Otsu K, Kuzuya T, Miwa T, et al. Circulation. 2000;102:2269–2275. doi: 10.1161/01.cir.102.18.2269. [DOI] [PubMed] [Google Scholar]
- 21.Fatkin D, McConnell B K, Mudd J O, Semsarian C, Moskowitz I G, Schoen F J, Giewat M, Seidman C E, Seidman J G. J Clin Invest. 2000;106:1351–1359. doi: 10.1172/JCI11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ventura H O, Malik F S, Mehra M R, Stapleton D D, Smart F W. Curr Opin Cardiol. 1997;12:375–381. [PubMed] [Google Scholar]
- 23.Rothermel B, Vega R B, Yang J, Wu H, Bassel-Duby R, Williams R S. J Biol Chem. 2000;275:8719–8725. doi: 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- 24.Fuentes J J, Genesca L, Kingsbury T J, Cunningham K W, Perez-Riba M, Estivill X, de la Luna S. Hum Mol Genet. 2000;9:1681–1690. doi: 10.1093/hmg/9.11.1681. [DOI] [PubMed] [Google Scholar]
- 25.Youn H D, Sun L, Prywes R, Liu J O. Science. 1999;286:790–793. doi: 10.1126/science.286.5440.790. [DOI] [PubMed] [Google Scholar]
- 26.De Windt L J, Lim H W, Haq S, Force T, Molkentin J D. J Biol Chem. 2000;275:13571–13579. doi: 10.1074/jbc.275.18.13571. [DOI] [PubMed] [Google Scholar]
- 27.Murat A, Pellieux C, Brunner H R, Pedrazzini T. J Biol Chem. 2000;275:40867–40873. doi: 10.1074/jbc.M008071200. [DOI] [PubMed] [Google Scholar]
- 28.Werlen G, Jacinto E, Xia Y, Karin M. EMBO J. 1998;17:3101–3111. doi: 10.1093/emboj/17.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberg S F. J Mol Cell Cardiol. 2000;32:1381–1384. doi: 10.1006/jmcc.2000.1202. [DOI] [PubMed] [Google Scholar]
- 30.Dolmetsch R E, Lewis R S, Goodnow C C, Healy J I. Nature (London) 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 31.Lorenz J N, Robbins J. Am J Physiol. 1997;272:H1137–H1146. doi: 10.1152/ajpheart.1997.272.3.H1137. [DOI] [PubMed] [Google Scholar]