Abstract
The present study tests the hypothesis that estradiol (E2), compared with placebo (Pl), amplifies combined-secretagogue stimulation of GH secretion in premenopausal women studied at comparable IGF-I and testosterone concentrations. To this end, 13 women underwent GnRH agonist-induced gonadal down-regulation followed by graded transdermal add-back of E2 or Pl and randomly ordered iv infusions of saline or paired secretagogues on separate morning fasting. GH secretion was assessed by frequent blood sampling, immunochemiluminometry, and variable-waveform deconvolution analysis. Two-way ANOVA revealed that specific secretagogue combination (P < 0.001), E2 status (P = 0.012), and their interaction (P = 0.038) jointly determined GH secretory-burst mass. Compared with Pl, the E2-clamped milieu elevated mean fasting GH concentrations (P = 0.032), the mass of GH secreted in bursts (P = 0.037), and maximal stimulation by paired l-arginine/GH-releasing peptide (GHRP)-2 (P = 0.028). E2 also markedly accelerated the initial release of GH induced by GHRH/GHRP-2 (P < 0.001) and l-arginine/GHRH (P < 0.01). By linear regression analysis, E2 concentrations positively fore-cast 41% of intersubject variability in GH secretion stimulated by combined l-arginine/GHRP-2 (P = 0.018), whereas abdominal visceral-fat mass negatively predicted 49% of that due to l-arginine/GHRH (P = 0.012). These data indicate that pulsatile GH secretion in young women studied at constant IGF-I and testosterone concentrations is dictated 3-fold jointly by secretagogue pair, E2 availability, and intraabdominal adiposity. Moreover, the rapidity of GH release is controlled 2-fold jointly by E2 and GHRH.
Abbreviations: CV, Coefficient of variation2; E, estradiol; GHRP, GH-releasing peptide; Pl, placebo; SS, somatostatin; Te, testosterone
In epidemiological contexts, gonadal sex steroid hormones are dominant positive determinants of GH secretion in both young and older adults (1, 2). In interventional studies, supplementation with testosterone (Te) in hypoandrogenemic boys and men and female-to-male transsexual patients stimulates both GH and IGF-I production (3–9). The amplifying effect of Te on GH secretion is mediated in part via estradiol (E2) receptors because administration of an antiestrogen inhibits, whereas exposure to an antiandrogen stimulates, GH secretion (10–13). Conversely, nonaromatizable androgens do not augment GH or IGF-I production consistently (5, 6, 12–14). Supplementation of E2 transdermally and estrogen orally also drives pulsatile GH secretion but in the absence of a synthetic progestin lowers or does not affect IGF-I concentrations (5, 15–19). These mechanistic distinctions imply that valid dissection of the distinctive actions of E2 would require controlling Te availability concurrently (see below).
Sex steroids increase pulsatile and thereby total GH secretion by augmenting the mass of GH secreted in each burst (3, 7, 16, 17). GH secretory-burst mass in turn is determined by at least three key regulatory peptides, GHRH, GH-releasing peptide (GHRP/ghrelin) and somatostatin (SS) (1, 2, 20). Laboratory evidence for minimal three-peptide control derives from disruption of genes encoding GHRH, SS, and GHRP receptors and/or peptides (21–23). In the case of the GHRH receptor, rare sporadic mutations are recognized in the human that result in profound attenuation of somatic growth and GH secretory-burst mass (24, 25). Clinical studies using natural and synthetic agonists or antagonists of GHRH, GHRP, and SS further support significant roles of each signal (1, 2, 20). What is not so well understood is how the three peptides achieve interactive control of GH secretion.
Given this background, we hypothesized that valid examination of the mechanisms by which E2 stimulates pulsatile GH secretion would require depleting ovarian E2 and Te and then adding back a controlled amount of E2 at equivalent Te concentrations. A corollary objective was to maintain similar total IGF-I concentrations. The latter need arises because infusion of IGF-I suppresses and experimental reduction of IGF-I concentrations stimulates pulsatile GH secretion by approximately 2-fold (26, 27).
The basic notion of dual-secretagogue stimulation comes from modeling simulations (28–30) and two recent clinical studies (31, 32). The idea is that GHRH, SS, and ghrelin constitute a minimal set of regulators to pituitary somatotropes. Clamping any two inputs by maximal exogenous stimulation (using GHRH and GHRP-2) or putative withdrawal of endogenous inhibition (infusing l-arginine to limit SS outflow) allows indirect inferences about the third un-manipulated signal. By using the three separate pairwise combinations possible and observing the effect of E2 vs. placebo (Pl) on each pair, model-based simulations allow one to estimate which endogenous signals may be affected by E2. The pairs are complementary in that each is a distinct permutation that omits a different secretagogue of the three. The dual-secretagogue strategy is a powerful tool because data from the set of pairwise interventions are used to reach an inference on estrogen action.
Subjects and Methods
Subjects
Thirteen healthy premenopausal women completed the four study sessions (see below). Participants provided written, voluntary, witnessed informed consent approved by the Mayo Institutional Review Board. The protocol was reviewed by the U.S. Food and Drug Administration under an investigator-initiated new drug number. Exclusion criteria were acute or chronic systemic illness, pregnancy, lactation, significant weight change (≥3 kg in 1 month), body mass index 30 kg/m2 or greater, anemia, contraindications to E2 exposure, current psychiatric treatment, or recent substance abuse. Volunteers were free of known or suspected cardiac, cerebral, or peripheral arterial or venous thrombo-embolic disease, breast cancer, or untreated gallstones. None was receiving psycho- or neuroactive medications. Each subject had an unremarkable medical history and physical examination and normal screening laboratory tests of hepatic, renal, endocrine, metabolic, and hematologic function.
The mean (± sem) age was 27 ± 1.3 yr and body mass index 25 ± 1.2 kg/m2. There was no difference in these variables after randomization to E2 vs. Pl. Volunteers reported a normal menarchal and recent menstrual history. Women discontinued any oral contraceptives at least 1 month before study.
Statistical design
The study was a randomized parallel-cohort design (n = 8 women given E2; n = 5 administered Pl). The order of saline and secretagogue infusions was also randomized, Pl controlled, and patient blinded within cohort. Unequal numbers in the two cohorts reflect randomization using a simple binary sequence.
Estradiol clamp
Each volunteer received two consecutive im injections of depot leuprolide acetate (TAP Pharmaceuticals Inc., Deerfield, IL) 3.75 mg 3 wk apart. Leuprolide was started in the early follicular phase (within 7 d of menses onset) after establishing a negative blood pregnancy test. Beginning on the day of the second leuprolide injection (d 1), transdermal Pl or E2 (Estraderm Patch, Ciba Corp., Summit, NJ) was administered in sequential daily amounts of 0.05, 0.10, 0.15, and 0.20 mg with dose increments made every fourth day. The intent was to achieve a stepwise increase in E2 concentrations over a 2-wk interval, which would culminate in a late-follicular-phase value. For study purposes, the highest (0.2 mg) dose of E2 was continued for 7 d (d 15–21). Blood sampling and saline/secretagogue infusions were scheduled in random order on any 4 of the last 5 d of this time window. After the last sampling session, progesterone was administered (100 mg orally for 12 d) according to good standards of clinical practice.
Study paradigm
Volunteers were admitted to the General Clinical Research Center on the morning of study. To obviate food-related confounds, subjects were given a constant meal (turkey sandwich or vegetarian alternative) of 500 (±30) kcal containing 55% carbohydrate, 15% protein, and 30% fat at 2000 h. Participants remained fasting overnight until 1400 h the next day. On the morning of sampling and infusion(s), iv catheters were inserted in contralateral forearm veins at 0700 h. Blood was withdrawn for later assay of serum E2, Te, SHBG, LH, FSH, prolactin, and IGF-I concentrations. Samples (1.5 ml) for GH assay were collected in chilled plastic tubes containing EDTA. Separated plasma was frozen at −70 C within 30 min. Lunch was provided at 1400 h before discharge from the unit.
Infusion and sampling protocol
Infusions were performed on separate randomly ordered mornings fasting. The four protocols comprised iv delivery of saline (0800–1400 h) only; l-arginine 30 g over 30 min (1000–1030 h) followed by bolus GHRH (1 μg/kg, GRF, Serono, Norwalk, MA); l-arginine followed by bolus GHRP-2 (3 μg/kg); and combined GHRH and GHRP-2 at a constant rate of 1 μg/kg·h each (1000 to 1400 h). Blood was sampled every 10 min for 6 h concurrently (0800–1400 h). The foregoing peptide doses were chosen as maximally stimulatory in dose-response analyzes in women (31, 33).
Hormone assays
Plasma GH concentrations were measured in duplicate by automated ultrasensitive double-monoclonal immunoenzymatic, magnetic particle-capture chemiluminescence assay using 22-kDa recombinant human GH as assay standard (Sanofi Diagnostics Pasteur Access, Chaska, MN). All 148 samples from any given subject were analyzed together. Sensitivity is 0.010 μg/liter, defined as 3 sds above the zero-dose assay tube. Interassay coefficients of variation (CVs) were 7.9 and 6.3%, respectively, at GH concentrations of 3.4 and 12 μg/liter; and intraassay CVs were 4.9% at 1.12 μg/liter and 4.5% at 20 μg/liter. No values fell less than 0.020 μg/liter. Molar cross-reactivity with a 20-kDa GH or GHBP is less than 5%. Serum LH and FSH concentrations were quantitated by automated chemiluminescence assay (ACS 180, Bayer, Norwood, MA), using as standards the first and second international reference preparations, respectively. Procedural sensitivities for LH and FSH are 0.20 and 0.25 IU/liter, respectively. Intraassay CVs for LH were 4.7, 3.5, and 3.8%, and interassay CVs were 5.8, 3.7, and 4.7% at 4.4, 18, and 39 IU/liter, respectively. For FSH measurements, intraassay CVs were 5.6, 4.3, and 3.5% and interassay CVs 6, 4, and 2.8% at 4.6, 25 and 62 IU/liter, respectively. E2 and Te were quantitated by automated competitive chemiluminescent immunoassay (ACS Corning, Bayer, Tarrytown, NY). For E2, sensitivity was 8 pg/ml; intraassay CVs were 4.1% at 173 pg/ml and 5.6% at 30 pg/ml; and interassay CVs were 4% at 261 pg/ml and 7% at 71 pg/ml (multiply by 3.67 for picomoles per liter). For Te, mean intra- and interassay CVs were 6.8 and 8.3% and assay sensitivity was 8 ng/dl (multiply by 0.0347 for nanomoles per liter). SHBG and total IGF-I concentrations were measured by immunoradiometric assay without and with extraction, respectively (Diagnostic Systems Laboratories, Webster, TX). For IGF-I, interassay CVs were 9% at 64 μg/liter and 6.2% at 157 μg/liter; and intraassay CVs were 3.4% at 9.4, 3% at 55, and 1.5% at 264 μg/liter.
Visceral fat mass
Intraabdominal visceral fat mass was estimated within 2 wk of infusions by single-slice abdominal computed tomography scan at L5, as described (32).
Deconvolution analyses of basal (nonpulsatile) and GHRH-stimulated burst-like GH secretion
Earlier deconvolution methods in some cases yield nonunique estimates of basal hormone secretion and elimination rates (34). To address this technical impasse, basal and pulsatile GH secretions were estimated simultaneously via a new maximum-likelihood methodology. The basic assumptions are that observed concentration peaks reflect the mass contained in a flexible secretory-burst waveform (three-parameter generalized gamma probability density); combined diffusion, advection, and irreversible elimination proceed via biexponential kinetics; and the solution is statistically conditioned on a priori estimates of pulse-onset times, as previously described (35–38). The present implementation of this model is reviewed briefly elsewhere (39). The principal analytical outcomes are cohort-defined basal and individually attributed pulsatile GH secretion during saline infusion (micrograms per liter per 6 h); the summed mass of GH secreted in bursts after stimulation by iv saline or combined secretagogues (micrograms per liter per 4 h); and the apparent waveform or shape of the underlying GH secretory burst, defined by the modal time in minutes to attain maximal secretion within the reconstructed burst.
Statistical confidence intervals were determined by bootstrap analysis of the residuals in the case of basal secretion and the mode of secretory-burst latency and from the (second derivative of the) maximum-likelihood estimate of secretory-burst mass, as described elsewhere (Appendix of Ref. 39).
Interpulse-interval times were modeled as a two-parameter Weibull distribution rather than a one-parameter Poisson distribution (35, 36). The Weibull function allows for variable dispersion of interpulse-interval values about the statistical mean (37). For example, the Poisson distribution fixes interpulse variability at a CV of 100% (sd/mean × 100%), whereas the Weibull function includes a term (gamma) that permits lesser variability than 100% (gamma > 1.0) independently of the probabilistic mean frequency (lambda).
Statistical comparisons
Two-way ANOVA in a two- by four-factor repeated-measures design was applied to compare the logarithms of the mass of GH secreted during E2 vs. Pl administration (two factors) and after saline and paired-secretagogue infusions (four factors). Post hoc contrasts were made by Tukey’s honestly significantly different test at experiment-wise P < 0.05 (40). Fasting hormone concentrations were first averaged across the four sessions in each individual and then compared via an unpaired Student’s t test. Linear regression analysis was used to examine the relationship between GH secretory-burst mass and E2 concentrations or intraabdominal visceral fat mass (computed tomography cross-sectional area) in the combined cohorts (41).
Data are presented as the arithmetic mean ± sem.
Results
Administration of E2, compared with Pl, caused breast tenderness, headache, nausea, mild pedal edema, or a sense of abdominal bloating in several volunteers. Secretagogue infusions were associated with a brief sensation of facial warmth, flushing, or metallic taste in one third of subjects.
Table 1 summarizes mean fasting hormone concentrations in the two study cohorts. E2 concentrations were 7.8-fold higher (P < 0.001), prolactin concentrations 1.9-fold higher (P = 0.016), and SHBG concentrations no different in women receiving E2, compared with Pl. LH and Te concentrations were reduced to equivalent values in both cohorts [absolute maxima in all subjects 1.1 IU/liter and 22 ng/dl (0.76 nmol/liter), respectively]. FSH concentrations were 4.8-fold lower after E2 than Pl administration. Total IGF-I concentrations did not differ by estrogen status.
TABLE 1.
Hormone concentrations attained during an exogenous estradiol clamp
| Estrogenic status
|
|||
|---|---|---|---|
| Hormone | Placebo (n = 5) | Estradiol (n = 8) | P |
| Estradiol (pg/ml)a | 18 ± 4.1 | 141 ± 11 | <0.001 |
| SHBG (nmol/liter) | 45 ± 8.0 | 64 ± 5.5 | <0.068 |
| Molar estradiol/SHBG ratio (pmol/nmol) | 1.5 ± 0.18 | 8.1 ± 0.63 | <0.001 |
| IGF-I (μg/liter) | 249 ± 20 | 308 ± 40 | NS |
| LH (IU/liter) | 0.37 ± 0.38 | 0.51 ± 0.10 | NS |
| FSH (IU/liter) | 4.3 ± 0.83 | 0.89 ± 0.17 | <0.001 |
| Prolactin (μg/liter) | 5.3 ± 1.0 | 10 ± 1.2 | 0.016 |
| Testosterone (ng/dl)b | 17 ± 4.7 | 19 ± 2.1 | NS |
| Molar testosterone/SHBG ratio (nmol/nmol) | 0.016 ± 0.005 | 0.012 ± 0.003 | NS |
Data are the mean ± sem. NS denotes P > 0.05 by unpaired parametric comparison.
To convert to picomoles per liter, multiply by 3.67.
To convert to nanomoles per liter, multiply by 0.0347.
Figure 1 depicts mean (± sem) GH concentration time series in the four study conditions in premenopausal women given E2 or Pl. Deconvolution analysis was applied to the 6-h saline infusion session to examine the basis for elevated mean GH concentrations in E2-supplemented subjects. As shown in Fig. 2, volunteers given E2, compared with Pl, maintained significantly higher mean GH concentrations (2.4-fold, P = 0.032), which was due to greater total (2.1-fold, P = 0.038) and pulsatile (2.2-fold, P = 0.037) but not basal GH secretion. In contradistinction, E2 did not influence the mean intersecretory-burst interval, defined as the reciprocal of lambda, or the variability, defined by gamma of intersecretory-burst intervals (Fig. 3). Estimates of the former were 56 ± 12 (E2) vs. 55 ± 10 (Pl) min and the latter 1.3 (E2) vs. 1.5 (Pl). Note that gamma approaching unity denotes large variability of interpulse-waiting times.
Fig. 1.
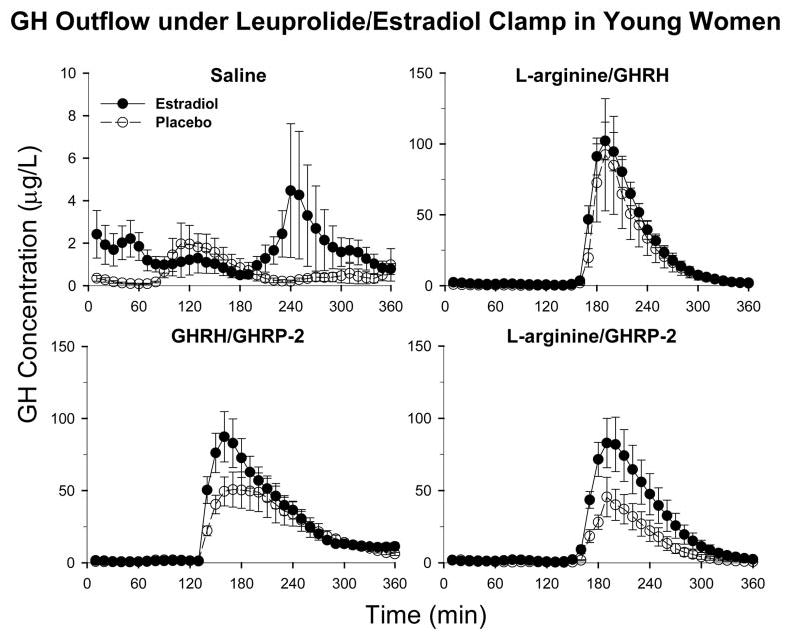
Cohort mean (± sem) GH concentration time series in pre-menopausal women receiving Pl (n = 5, open circles) or E2 (n = 8, closed circles) in an E2- and Te-depleted milieu imposed by leuprolide administration. On d 17–21 of the experimental sex steroid clamp, volunteers underwent blood sampling every 10 min for 6 h while fasting. The indicated secretagogue pairs were infused after baseline sampling (see Subjects and Methods). Data are the mean ± sem.
Fig. 2.
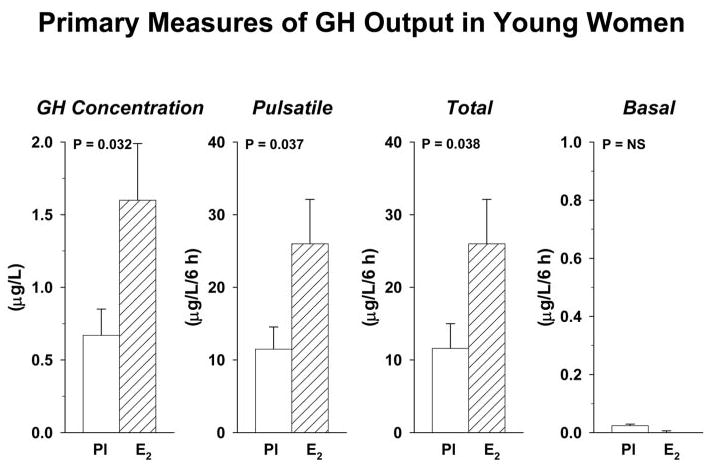
Impact of E2 repletion on GH secretion in premenopausal women monitored during ovarian suppression. E2 addback in premenopausal women with controlled IGF-I and Te concentrations elevated fasting 6-h mean GH concentrations (micrograms per liter) and both pulsatile (burst-like) and total (basal plus pulsatile) GH secretion (micrograms per liter per 6 h). P values denote unpaired statistical contrasts between responses to E2 and Pl. Data are the mean ± sem (n = 5 placebo, n = 8 estradiol).
Fig. 3.
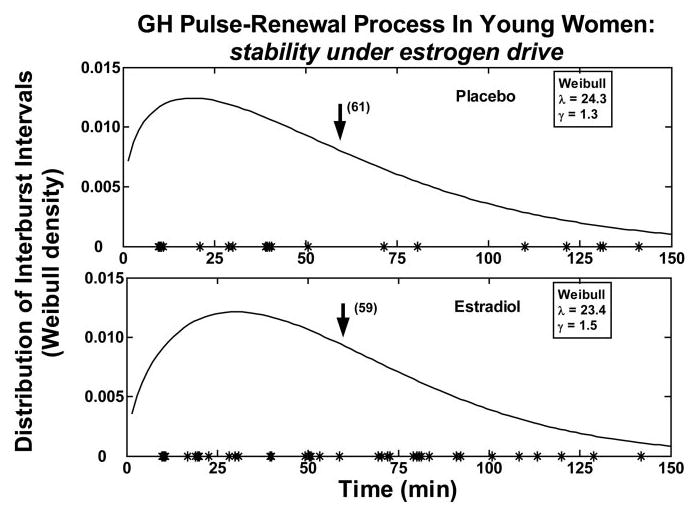
Probability distribution of GH intersecretory-burst intervals determined after saline infusion in a midphysiological (E2) and postmenopausal (Pl) estrogenic milieu. Values on the y-axis give the expectation of observing any given GH interpulse-interval length (x-axis). The mathematical terms lambda and gamma stated within the two boxes denote, respectively, mean pulse frequency (number of bursts per 24 h) and the relative variability of interpulse intervals (gamma > 1.0 signifies lesser variability than that of a Poisson model, wherein the CV is 100% definitionally) (see Subjects and Methods). Asterisks on the axes mark the onset of significant pulses.
Figure 4 presents the summed mass of GH secreted in pulses after infusion of saline or combined stimuli. Two-way ANOVA with repeated measures revealed significant interventional effects of secretagogue type (P < 0.001), E2 supplementation (P = 0.012), and their interaction (P = 0.038). Each secretagogue combination stimulated GH secretion more than saline (P < 0.005). Relative efficacy of the three secretagogue pairs after Pl was GHRH/GHRP-2 = l-arginine/GHRH > l-arginine/GHRP-2 (P < 0.05), and after E2, l-arginine/GHRP-2 = GHRH/GHRP-2 = l-arginine/GHRH. Post hoc comparisons indicated that GH secretion in E2-treated women exceeded that in Pl-treated subjects after infusion of saline by 2.3-fold (P = 0.041) and after l-arginine/GHRP-2 by 2.2-fold (P = 0.028). E2 did not alter responses to L-arginine/GHRH or GHRH/GHRP-2.
Fig. 4.
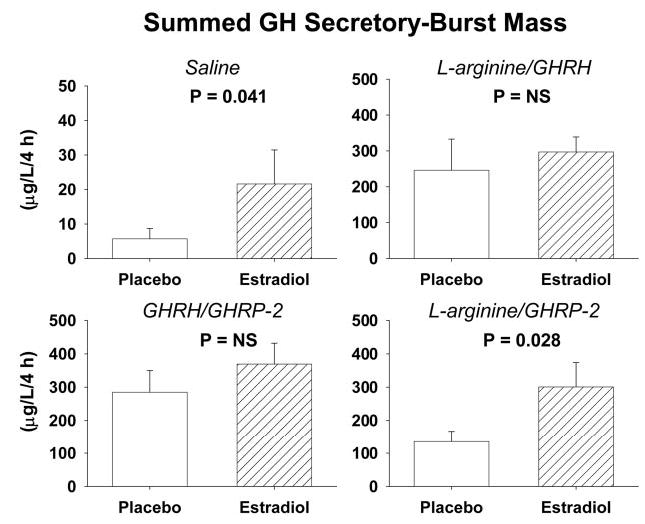
Fasting (saline) and dual secretagogue-stimulated GH secretory-burst mass (micrograms per liter per 4 h) in premenopausal individuals after gonadal down-regulation and replacement with E2 or Pl. L-Arginine was infused before bolus iv injection of a maximally effective dose of GHRH (1 μg/kg) or GHRP-2 (3 μg/kg). GHRH and GHRP-2 were infused together continuously iv for 4 h (1 μg/kg·h each). Data are presented as noted in the legend of Fig. 2.
Figure 5 illustrates GH concentration time courses stimulated by saline vs. the three secretagogue pairs in one woman after administration of Pl vs. E2. Separate curves are given for measured and deconvolution-estimated GH peaks, thus illustrating relative goodness of fit. Asterisks are used to denote objectively identified burst-onset times before and after each stimulus. Secretagogues typically evoked a volley of GH secretory bursts.
Fig. 5.
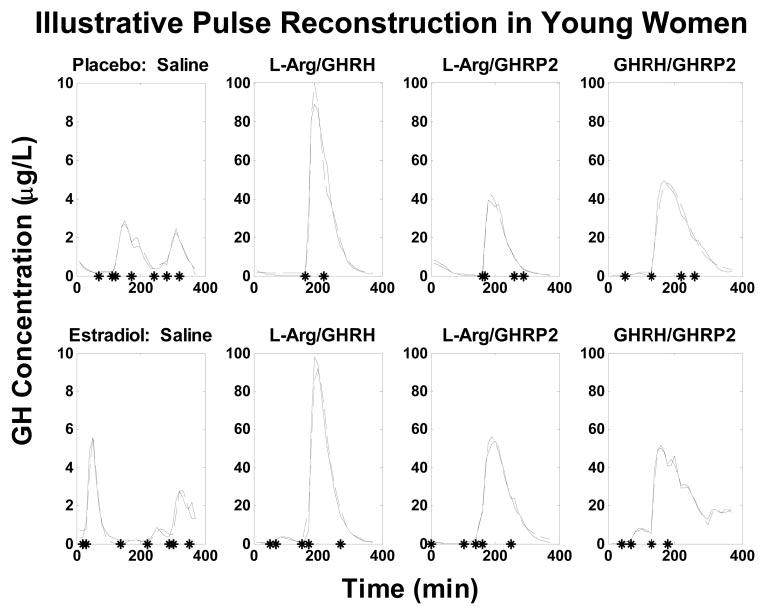
Illustrative time courses of measured (solid lines) and deconvolution-estimated (interrupted lines) GH concentrations in individual women given either Pl (upper plots) or E2 (lower plots). Asterisks on the x-axis mark the onset of significant pulses.
Figure 6 depicts intervention-specific analytical estimates of the (unit-area normalized) GH secretory-burst waveform in the two cohorts. The waveform is the calculated time-evolution (shape) of hormone secretion within a discrete burst. The mathematical function describing the waveform is statistically independent of secretory-burst mass (see Subjects and Methods). The analytical end point of waveform shape is the modal time delay in minutes required to reach maximal GH secretion after onset of the burst. The mode was estimated from the frequency distribution of 100 bootstrap calculations. The corresponding histograms are shown below each set of waveforms (Fig. 6). In women receiving Pl, l-arginine/GHRP-2 infusion abbreviated by 1.7-fold (P < 0.001), whereas GHRH/GHRP-2 infusion prolonged by 1.3-fold (P < 0.01), the time to maximal GH secretion, compared with either saline or l-arginine/GHRH. In women receiving E2, each of the three secretagogue pairs accelerated initial GH release by about 1.8-fold, compared with saline; viz., mean modal time latency 33 ± 0.51 min for saline vs. an absolute range of 17–19 min for the three secretagogue pairs (pooled sem ± 0.31 min, each P < 0.001). E2, compared with Pl replacement, accelerated the attainment of maximal GH secretion after infusion of GHRH/GHRP-2 by 1.8-fold (P < 0.001) and after l-arginine/GHRH by 1.2-fold (P < 0.01). In contrast, E2 extended the latency to maximal GH secretion after infusion of l-arginine/GHRP-2 by 1.3-fold (P < 0.05). Thus, both E2 status and secretagogue combination determine the apparent waveform of GH secretory bursts.
Fig. 6.
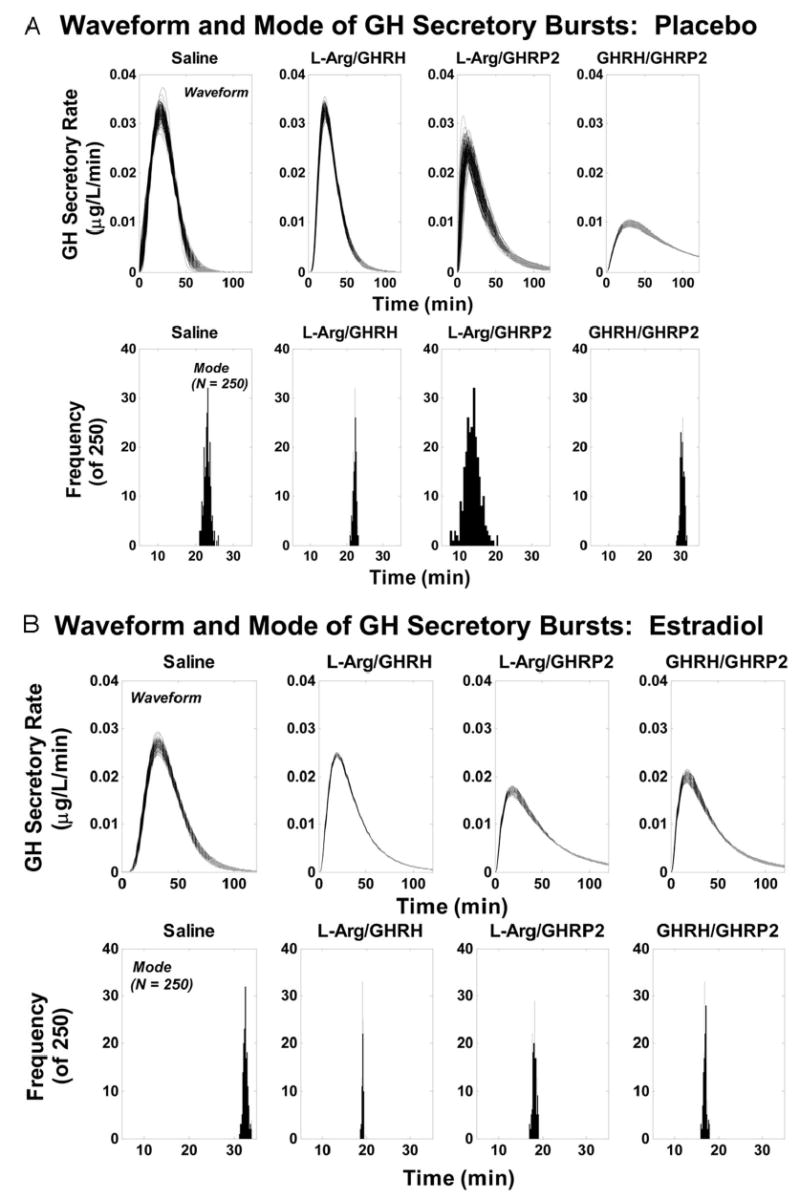
Analytically reconstructed GH secretory-burst waveforms (shapes) in individual premenopausal volunteers given Pl or E2 transdermally (A and B, respectively) after ovarian down-regulation. Secretagogue pairs comprised iv saline, l-arginine/GHRH, l-arginine/GHRP-2, or GHRH/GHRP-2. The waveform is the (unit area-normalized) time course of GH secretion within a discrete burst (upper plots). The end point is the modal time delay to achieve maximal GH release (lower plots). Burst shape is independent of the mass of GH released in the pulse (see data in Fig. 4). Arg, Arginine.
Linear regression analysis was applied to data obtained in the combined cohorts (n = 13 women). By univariate regression analysis, E2 concentration positively determined l-arginine/GHRP-2-stimulated GH secretory-burst mass (R2 = 0.41; P = 0.018; Fig. 7A); and, abdominal visceral-fat area negatively determined l-arginine/GHRH-stimulated GH secretion (R2 = 0.49; P = 0.012; Fig. 7B). Bivariate regression analyses corroborated the foregoing univariate outcomes, as defined by significant partial correlation coefficients for either E2 concentration or fat mass.
Fig. 7.
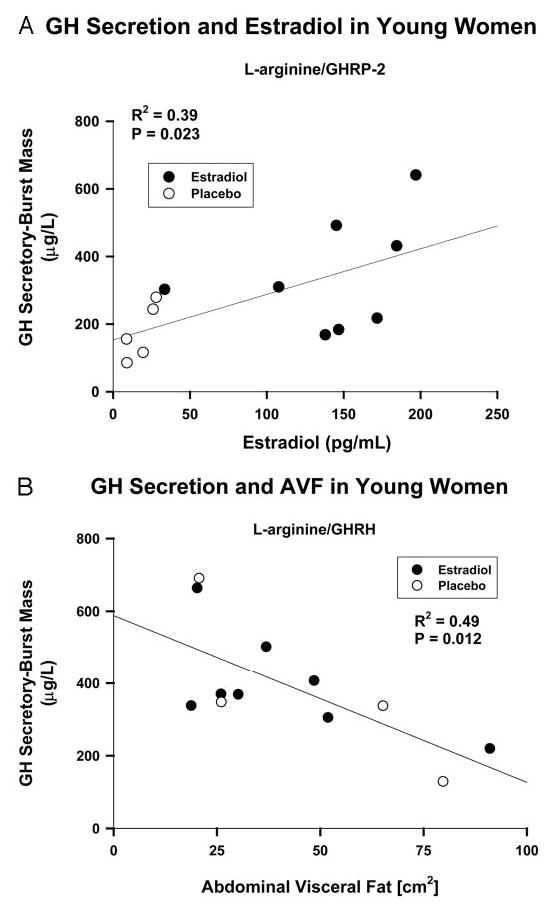
Linear regression analyses of the relationships between l-arginine/GHRP-2 (A) and l-arginine/GHRH (B)-stimulated GH secretory-burst mass (y-axis) and E2 concentrations or abdominal visceral-fat cross-sectional area (x-axis), respectively. Data are from the combined premenopausal cohorts (n = 13 subjects). The square of the correlation coefficient (R2) is a measure of the fraction of the total variation in GH secretory-burst mass that is explained by the independent variable. To convert E2 concentrations to picomoles per liter, multiply by 3.67. AVF, Abdominal visceral fat.
Discussion
The present investigation appraises the mechanisms by which E2 modulates combined peptidyl regulation of burst-like GH secretion in healthy young women. To this end, we implemented a tripartite experimental paradigm comprising gonadal-axis down-regulation with a GnRH agonist to deplete ovarian-derived E2 and Te; controlled transdermal add-back of E2 vs. Pl to mimic late-follicular-phase vs. postmenopausal E2 concentrations; and stimulation of GH secretion by saline and specific secretagogue pairs. Statistical analyses demonstrated that a physiological, compared with low E2, concentration selectively amplifies the mass of GH secreted per burst in the fasting state, augments the amount of GH secreted during combined l-arginine/GHRP-2 drive, and accelerates initial GH release induced by GHRH/GHRP-2. The foregoing outcomes were selective, inasmuch as E2 repletion did not influence total IGF-I or Te concentrations, GH secretory-burst frequency, basal GH secretion, or responses to l-arginine/GHRH and GHRH/GHRP-2. Regression analyses disclosed that E2 concentrations positively predict 41% of the GH-response variability to l-arginine/GHRP-2, whereas abdominal visceral-fat mass negatively determines 49% of the response variability to l-arginine/GHRH infusion. Based on a three-peptide feedback model of GH control, a plausible unifying inference is that E2 facilitates hypothalamopituitary stimulation by GHRP, whereas relative visceral adiposity inhibits pituitary stimulation by GHRH.
The E2-predominant vs. E2-withdrawn milieu enhanced saline-infused and l-arginine/GHRP-2-stimulated burst-like GH secretion in the face of equivalent mean concentrations of total IGF-I and Te. Comparable IGF-I and Te concentrations are pertinent, in that IGF-I inhibits and Te stimulates pulsatile GH secretion (1). In the first regard, experimental reduction of total IGF-I concentrations by 34% in healthy men and women doubles pulsatile GH secretion (42). Conversely, elevation of IGF-I concentrations into the late-pubertal range by constant iv infusion of this peptide suppresses GH secretion by more than 65% (39, 43). Given similar total IGF-I concentrations in the two interventional groups studied here, a plausible inference is that E2 can augment spontaneous and l-arginine/GHRP-2-stimulated GH secretion by central effects on the hypothalamopituitary unit rather than exclusively by depleting total serum IGF-I concentrations. In corollary, similar Te concentrations in the two cohorts would point to sex steroid selectivity of E2 action. The lack of estrogenic augmentation of responses to l-arginine/GHRH or GHRH/GHRP-2 corroborates the selectivity of the control pathways affected by E2.
The precise mechanism mediating estrogenic facilitation of GH secretion in response to sequential l-arginine/GHRP-2 infusion is not known. In principle, E2 could enhance the efficacy of GHRP-2, potentiate feed-forward by GHRH, and/or attenuate negative feedback by GH/IGF-I. Among these theoretical considerations, clinical investigations indicate that E2 supplementation in postmenopausal women augments stimulation by GHRP-2 and submaximal drive by GHRH, reduces submaximal inhibition by somatostatin-14, attenuates negative feedback by recombinant human GH on the GHRP-2 stimulus, and augments the suppressive effect of recombinant human IGF-I on basal GH secretion (33, 39, 44–46). According to such outcomes, an ensemble of mechanisms could explicate E2’s enhancement of the combined l-arginine/GHRP-2 stimulus.
Formalizing the primary interactions among GHRH, GHRP, and SS in a simplified biomathematical model provides one avenue to parse observed GH responses to a set of stimuli (28–30, 47, 48). Ensemble-based simulations can thereby aid intuitive interpretations of more complex outcomes, here driven by three distinct paired stimuli. The requirement was to explain the selectivity of E2’s potentiation of a combined l-arginine/GHRP-2 stimulus. Analytical modeling was consistent most parsimoniously with estrogenic enhancement of the central actions of GHRP (Fig. 8). Central effects of GHRP are believed to comprise both antagonism of SSergic inhibition of somatotrope cells and arcuate-nucleus neurons and stimulation of hypothalamic GHRH and pituitary GH release (33, 44, 49–53). Laboratory data are consistent with a unifying notion that E2 may up-regulate receptor-dependent actions of GHRP. For example, gene transcripts encoding the pituitary GHRP receptor are 2-to 10-fold more abundant in the adult female than male rodent pituitary, and E2 induces transcription of the human GHRP receptor gene in vitro (23, 54, 55).
Fig. 8.
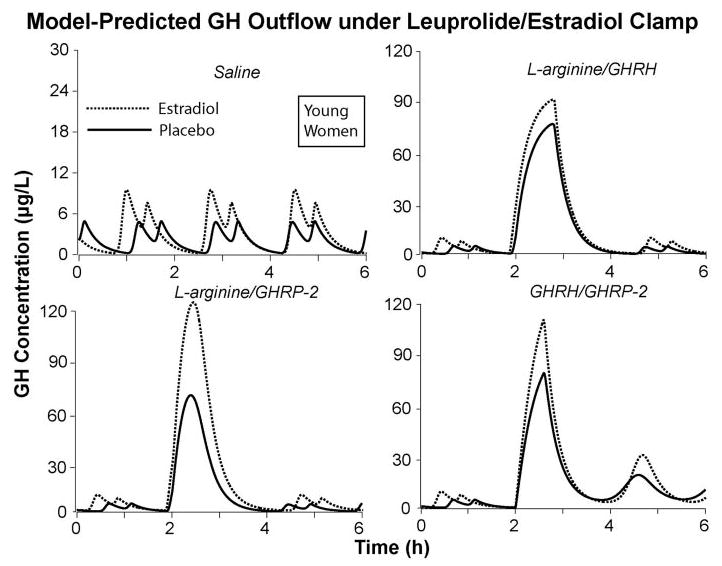
Model-assisted predictions of the impact of E2 vs. Pl on dual-secretagogue action in young women, based on a simplified four-peptide (GHRH, SS, GHRP, and GH feedback) ensemble of hypothalamopituitary interactions (see Discussion).
Compared with placebo, E2 did not potentiate combined stimulation by GHRH/GHRP-2. This outcome could reflect lesser statistical power due to greater variability of GH responses in this particular intervention (31). The present results using l-arginine/GHRH corroborate an earlier analysis, showing that E2 does not enhance maximal GHRH action monitored during putative SS withdrawal (33).
From a technical vantage, we implemented a novel variable-waveform biexponential deconvolution technique to reconstruct GH secretion rates (35–38). Specifically, the new analytical platform was developed to permit potentially asymmetric (rather than exclusively Gaussian) secretory bursts and ensure valid discrimination among simultaneous and highly correlated contributions to a hormone-concentration profile. Technically, the earlier methodologies could not guarantee a unique secretion solution for any given hormone profile (34) because of strong interdependencies among estimates of rapid and slow elimination kinetics; basal secretion; and secretory-burst mass, number, and location (37). The present mathematical structure for the first time allows direct statistical verification of maximum-likelihood estimates, as validated empirically by in vivo experiments in the horse and sheep (38, 56). The resultant methodology predicts that physiological compared with low concentrations of E2 determine not only the mass of GH secretory bursts but also their inferred waveform (Fig. 6). In particular, E2 accelerates the initial rate of GH release stimulated by GHRH/GHRP-2 and l-arginine/GHRH, and conversely, prolongs the latency to maximal GH secretion induced by combined l-arginine/GHRP-2. A plausible hypothesis to account for these outcomes is that E2 can either facilitate or retard exocytotic release of presynthesized GH stores, depending on the nature of the two-peptide stimulus. Although the molecular mechanisms that mediate such inferred interactions at the somatotrope level are not known, simultaneous stimulation with E2 and GHRH is the shared intervention in this and an earlier finding of rapid GH release (39).
Deconvolution analysis disclosed comparable mean values and variability of GH interburst intervals and similar basal GH secretion rates in the two estrogenic extremes. The inference of a stable GH secretory burst-renewal process under E2 drive would agree with the reported uniformity of GH pulse frequency at different stages of puberty, across the menstrual cycle, and in men and women (3, 7, 17, 57–59). Equivalent basal GH secretion in a low and high E2 milieu indicates that E2 is not a primary determinant of nonpulsatile GH secretion in the human (1, 39).
The daily GH secretion rate varies inversely with estimated abdominal visceral-fat mass in middle-aged men and women (60). The current analyses offer further mechanistic insights into the basis of this relationship by demonstrating a strongly negative association (R2 = 0.49) between the stimulatory effect of l-arginine/GHRH on GH release and visceral-fat mass in young women. The selectivity of this outcome would suggest that relative intraabdominal adiposity in some manner attenuates GHRH efficacy in a putatively SS-withdrawn context. In contradistinction, E2 positively determines the stimulatory impact of l-arginine/GHRP-2. The different outcomes point to signal specificity of GH regulation.
The measured distribution volume of GH does not differ significantly among young women and men; pre-, mid-, and postpubertal boys; and postmenopausal women receiving estradiol and placebo (46, 61, 62). This inference is important on analytical grounds because GH secretion is quantitated as the mass of hormone (micrograms) released per unit time per unit distribution volume (liters). Thus, the effects of E2 on GH concentrations should reflect changes in GH secretion.
Certain caveats should be considered. First, the present analyses do not address the effects of prolonged dual-secretagogue stimulation under E2-clamped conditions. This question arises because continuous sc GHRP-2 infusion for 1 month stimulates GH secretion by 2-fold more in older estrogen-replaced women than comparably aged men (63). Second, the accompanying inferences in 13 subjects will be important to corroborate in a larger group of volunteers. In addition, although l-arginine inhibits SS release in the experimental animal and inferentially in the human (1), whether this amino acid acts via additional pathways is not known.
In summary, the present study uses an investigational paradigm designed to contrast the influence of follicular-phase and postmenopausal E2 concentrations on dual-secretagogue-stimulated GH secretion at controlled total IGF-I and Te concentrations in healthy young women. In this sex steroid-clamped paradigm, E2, compared with Pl, doubles burst-like GH release driven by l-arginine/GHRP-2 and accelerates the attainment of maximal GH secretion induced by GHRH/GHRP-2. By regression analyses, E2 concentrations determine two fifths of the variability of l-arginine/GHRP-2 feed-forward, whereas abdominal visceral-fat mass predicts half of the variability associated with l-arginine/GHRH stimulation. According to a minimal analytical model of reciprocal interactions among GHRH, GHRP, and SS, the foregoing outcomes may be unified under the most frugal hypothesis that E2 potentiates the combined hypothalamopituitary actions of GHRP.
Acknowledgments
We thank Kris Nunez and Kandace Bradford for excellent support of manuscript preparation and data analysis; the Mayo Immunochemical Laboratory for assay assistance; and the Mayo research nursing staff for conduct of the protocol.
Footnotes
This work was supported in part by the General Clinical Research Center Grant MO1 RR00585 to the Mayo Clinic and Foundation from the National Center for Research Resources (Rockville, MD) and Grants K25 HD01474 and R01 NIA AG 14799 from the National Institutes of Health (Bethesda, MD).
References
- 1.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 2.Veldhuis JD, Bowers CY. Three-peptide control of pulsatile and entropic feedback-sensitive modes of growth hormone secretion: modulation by estrogen and aromatizable androgen. J Pediatr Endocrinol Metab. 2003;16(Suppl 3):587–605. [PubMed] [Google Scholar]
- 3.Mauras N, Blizzard RM, Link K, Johnson ML, Rogol AD, Veldhuis JD. Augmentation of growth hormone secretion during puberty: evidence for a pulse amplitude-modulated phenomenon. J Clin Endocrinol Metab. 1987;64:596–601. doi: 10.1210/jcem-64-3-596. [DOI] [PubMed] [Google Scholar]
- 4.Giustina A, Scalvini T, Tassi C, Desenzani P, Poiesi C, Wehrenberg WB, Rogol A, Veldhuis JD. Maturation of the regulation of growth hormone secretion in young males with hypogonadotropic hypogonadism pharmacologically exposed to progressive increments in serum testosterone. J Clin Endocrinol Metab. 1997;82:1210–1219. doi: 10.1210/jcem.82.4.3871. [DOI] [PubMed] [Google Scholar]
- 5.Veldhuis JD, Metzger DL, Martha Jr PM, Mauras N, Kerrigan JR, Keenan B, Rogol AD, Pincus SM. Estrogen and testosterone, but not a non-aromatizable androgen, direct network integration of the hypothalamosomatotrope (growth hormone)-insulin-like growth factor I axis in the human: evidence from pubertal pathophysiology and sex-steroid hormone replacement. J Clin Endocrinol Metab. 1997;82:3414–3420. doi: 10.1210/jcem.82.10.4317. [DOI] [PubMed] [Google Scholar]
- 6.Fryburg DA, Weltman A, Jahn LA, Weltman JY, Samolijik E, Veldhuis JD. Short-term modulation of the androgen milieu alters pulsatile but not exercise or GHRH-stimulated GH secretion in healthy men. J Clin Endocrinol Metab. 1997;82:3710–3719. doi: 10.1210/jcem.82.11.4379. [DOI] [PubMed] [Google Scholar]
- 7.Gentili A, Mulligan T, Godschalk M, Clore J, Patrie J, Iranmanesh A, Veldhuis JD. Unequal impact of short-term testosterone repletion on the somatotropic axis of young and older men. J Clin Endocrinol Metab. 2002;87:825–834. doi: 10.1210/jcem.87.2.8222. [DOI] [PubMed] [Google Scholar]
- 8.van Kesteren P, Lips P, Deville W, Popp-Snijders C, Asscheman H, Megens J, Gooren L. The effect of one-year cross-sex hormonal treatment on bone metabolism and serum insulin-like growth factor-1 in transsexuals. J Clin Endocrinol Metab. 1996;81:2227–2232. doi: 10.1210/jcem.81.6.8964856. [DOI] [PubMed] [Google Scholar]
- 9.Veldhuis JD, Evans WS, Iranmanesh A, Weltman AL, Bowers CY. Short-term testosterone supplementation relieves growth hormone autonegative feedback in men. J Clin Endocrinol Metab. 2004;89:1285–1290. doi: 10.1210/jc.2003-031017. [DOI] [PubMed] [Google Scholar]
- 10.Metzger DL, Kerrigan JR. Androgen receptor blockade with flutamide enhances growth hormone secretion in late pubertal males: evidence for independent actions of estrogen and androgen. J Clin Endocrinol Metab. 1993;76:1147–1152. doi: 10.1210/jcem.76.5.8496305. [DOI] [PubMed] [Google Scholar]
- 11.Metzger DL, Kerrigan JR. Estrogen receptor blockade with tamoxifen diminishes growth hormone secretion in boys: evidence for a stimulatory role of endogenous estrogens during male adolescence. J Clin Endocrinol Metab. 1994;79:513–518. doi: 10.1210/jcem.79.2.8045971. [DOI] [PubMed] [Google Scholar]
- 12.Weissberger AJ, Ho KKY. Activation of the somatotropic axis by testosterone in adult males: evidence for the role of aromatization. J Clin Endocrinol Metab. 1993;1407:1412. doi: 10.1210/jcem.76.6.8501143. [DOI] [PubMed] [Google Scholar]
- 13.Devesa J, Lois N, Arce V, Diaz MJ, Lima L, Tresguerres JA. The role of sexual steroids in the modulation of growth hormone (GH) secretion in humans. J Steroid Biochem Mol Biol. 1991;40:165–173. doi: 10.1016/0960-0760(91)90179-9. [DOI] [PubMed] [Google Scholar]
- 14.Keenan BS, Richards GE, Ponder SW, Dallas JS, Nagamani M, Smith ER. Androgen-stimulated pubertal growth: the effects of testosterone and dihydrotestosterone on growth hormone and insulin-like growth factor-I in the treatment of short stature and delayed puberty. J Clin Endocrinol Metab. 1993;76:996–1001. doi: 10.1210/jcem.76.4.8473416. [DOI] [PubMed] [Google Scholar]
- 15.Frantz AG, Rabkin MT. Effects of estrogen and sex difference on secretion of human growth hormone. J Clin Endocrinol Metab. 1965;25:1470–1480. doi: 10.1210/jcem-25-11-1470. [DOI] [PubMed] [Google Scholar]
- 16.Mauras N, Rogol AD, Veldhuis JD. Increased hGH production rate after low-dose estrogen therapy in prepubertal girls with Turner’s syndrome. Pediatr Res. 1990;28:626–630. doi: 10.1203/00006450-199012000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Shah N, Evans WS, Veldhuis JD. Actions of estrogen on the pulsatile, nyctohemeral, and entropic modes of growth hormone secretion. Am J Physiol. 1999;276:R1351–R1358. doi: 10.1152/ajpregu.1999.276.5.R1351. [DOI] [PubMed] [Google Scholar]
- 18.Friend KE, Hartman ML, Pezzoli SS, Clasey JL, Thorner MO. Both oral and transdermal estrogen increase growth hormone release in postmenopausal women—a clinical research center study. J Clin Endocrinol Metab. 1996;81:2250–2256. doi: 10.1210/jcem.81.6.8964860. [DOI] [PubMed] [Google Scholar]
- 19.Lissett CA, Shalet SM. The impact of dose and route of estrogen administration on the somatotropic axis in normal women. J Clin Endocrinol Metab. 2003;88:4668–4672. doi: 10.1210/jc.2003-022036. [DOI] [PubMed] [Google Scholar]
- 20.Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Mauras N, Bowers CY. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26:114–148. doi: 10.1210/er.2003-0038. [DOI] [PubMed] [Google Scholar]
- 21.Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest. 2001;107:1571–1580. doi: 10.1172/JCI11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng H, Bailey A, Jiang MH, Honda K, Chen HY, Trumbauer ME, van der Ploeg LH, Schaeffer JM, Leng G, Smith RG. Somatostatin receptor subtype 2 knockout mice are refractory to growth hormone-negative feedback on arcuate neurons. Mol Endocrinol. 1997;11:1709–1717. doi: 10.1210/mend.11.11.0016. [DOI] [PubMed] [Google Scholar]
- 23.Shuto Y, Shibasaki T, Otagiri A, Kuriyama H, Ohata H, Tamura H, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest. 2002;109:1429–1436. doi: 10.1172/JCI13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann G, Maheshwari H. The Dwarfs of Sindh: severe growth hormone (GH) deficiency caused by a mutation in the GH-releasing hormone receptor gene. Acta Paediatr Suppl. 1997;432:33–38. doi: 10.1111/j.1651-2227.1997.tb18366.x. [DOI] [PubMed] [Google Scholar]
- 25.Roelfsema F, Biermasz NR, Veldman RG, Veldhuis JD, Frolich M, Stokvis-Brantsma WH, Wit J-M. Growth hormone (GH) secretion in patients with an inactivating defect of the GH-releasing hormone (GHRH) receptor is pulsatile: evidence for a role for non-GHRH inputs into the generation of GH pulses. J Clin Endocrinol Metab. 2000;86:2459–2464. doi: 10.1210/jcem.86.6.7536. [DOI] [PubMed] [Google Scholar]
- 26.Veldhuis JD, Bidlingmaier M, Anderson SM, Evans WS, Wu Z, Strassburger CJ. Impact of experimental blockade of peripheral growth hormone (GH) receptors on the kinetics of endogenous and exogenous GH removal in healthy women and men. J Clin Endocrinol Metab. 2002;87:5737–5745. doi: 10.1210/jc.2001-011885. [DOI] [PubMed] [Google Scholar]
- 27.Keenan DM, Veldhuis JD. Cortisol feedback state governs adrenocorticotropin secretory-burst shape, frequency and mass in a dual-waveform construct: time-of-day dependent regulation. Am J Physiol. 2003;285:R950–R961. doi: 10.1152/ajpregu.00299.2003. [DOI] [PubMed] [Google Scholar]
- 28.Farhy LS, Veldhuis JD. Joint pituitary-hypothalamic and intrahypothalamic autofeedback construct of pulsatile growth hormone secretion. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1240–R1249. doi: 10.1152/ajpregu.00086.2003. [DOI] [PubMed] [Google Scholar]
- 29.Farhy LS, Veldhuis JD. Putative GH pulse renewal: periventricular somatostatinergic control of an arcuate-nuclear somatostatin and GH-releasing hormone oscillator. Am J Physiol. 2004;286:R1030–R1042. doi: 10.1152/ajpregu.00473.2003. [DOI] [PubMed] [Google Scholar]
- 30.Farhy LS, Veldhuis JD, Hypothalamo-pituitary mechanisms mediating the effects of ghrelin on the somatotrope axis: a deterministic model. Program of the 86th Annual Meeting of The Endocrine Society, New Orleans, LA, 2004 (Abstract 850703)
- 31.Veldhuis JD, Evans WS, Bowers CY. Impact of estradiol supplementation on dual peptidyl drive of growth-hormone secretion in postmenopausal women. J Clin Endocrinol Metab. 2002;87:859–866. doi: 10.1210/jcem.87.2.8251. [DOI] [PubMed] [Google Scholar]
- 32.Erickson D, Keenan DM, Mielke K, Bradford K, Bowers CY, Miles JM, Veldhuis JD. Dual secretagogue drive of burst-like growth hormone secretion in postmenopausal compared with premenopausal women studied under an experimental estradiol clamp. J Clin Endocrinol Metab. 2004;89:4746–4754. doi: 10.1210/jc.2004-0424. [DOI] [PubMed] [Google Scholar]
- 33.Veldhuis JD, Evans WS, Bowers CY. Estradiol supplementation enhances submaximal feed-forward drive of growth hormone (GH) secretion by recombinant human GH-releasing hormone-1,44-amide in a putatively somatostatin-withdrawn milieu. J Clin Endocrinol Metab. 2003;88:5484–5489. doi: 10.1210/jc.2003-030410. [DOI] [PubMed] [Google Scholar]
- 34.Veldhuis JD, Evans WS, Johnson ML. Complicating effects of highly correlated model variables on nonlinear least-squares estimates of unique parameter values and their statistical confidence intervals: estimating basal secretion and neurohormone half-life by deconvolution analysis. Methods Neurosci. 1995;28:130–138. [Google Scholar]
- 35.Keenan DM, Veldhuis JD. Stochastic model of admixed basal and pulsatile hormone secretion as modulated by a deterministic oscillator. Am J Physiol. 1997;273:R1182–R1192. doi: 10.1152/ajpregu.1997.273.3.R1182. [DOI] [PubMed] [Google Scholar]
- 36.Keenan DM, Veldhuis JD, Yang R. Joint recovery of pulsatile and basal hormone secretion by stochastic nonlinear random-effects analysis. Am J Physiol. 1998;275:R1939–R1949. doi: 10.1152/ajpregu.1998.275.6.R1939. [DOI] [PubMed] [Google Scholar]
- 37.Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD. Physiological control of pituitary hormone secretory-burst mass, frequency and waveform: a statistical formulation and analysis. Am J Physiol. 2003;285:R664–R673. doi: 10.1152/ajpregu.00195.2003. [DOI] [PubMed] [Google Scholar]
- 38.Keenan DM, Alexander SL, Irvine CHG, Clarke IJ, Canny BJ, Scott CJ, Tilbrook AJ, Turner AI, Veldhuis JD. Reconstruction of in vivo time-evolving neuroendocrine dose-response properties unveils admixed deterministic and stochastic elements in interglandular signaling. Proc Natl Acad Sci USA. 2004;101:6740–6745. doi: 10.1073/pnas.0300619101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veldhuis JD, Anderson SM, Kok P, Iranmanesh A, Frystyk J, Orskov H, Keenan DM. Estradiol supplementation modulates growth hormone (GH) secretory-burst waveform and recombinant human insulin-like growth factor-I-enforced suppression of endogenously driven GH release in post-menopausal women. J Clin Endocrinol Metab. 2004;89:1312–1318. doi: 10.1210/jc.2003-031482. [DOI] [PubMed] [Google Scholar]
- 40.Kuehl RO 1994 Split-plot designs. Statistical principles of research design and analysis. Belmont, CA: Duxbury Press; 473–498
- 41.Fisher LD, van Belle G 1996 Descriptive statistics. Biostatistics: a methodology for the health sciences. New York: John Wiley, Sons; 58–74
- 42.Veldhuis JD, Bidlingmaier M, Anderson SM, Wu Z, Strassburger CJ. Lowering total plasma insulin-like growth factor I concentrations by way of a novel, potent, and selective growth hormone (GH) receptor antagonist, pegvisomant (B2036-peg), augments the amplitude of GH secretory bursts and elevates basal/nonpulsatile GH release in healthy women and men. J Clin Endocrinol Metab. 2001;86:3304–3310. doi: 10.1210/jcem.86.7.7656. [DOI] [PubMed] [Google Scholar]
- 43.Jaffe CA, Ocampo-Lim B, Guo W, Krueger K, Sugahara I, DeMott-Friberg R, Bermann M, Barkan AL. Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J Clin Invest. 1998;102:153–164. doi: 10.1172/JCI2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson SM, Shah N, Evans WS, Patrie JT, Bowers CY, Veldhuis JD. Short-term estradiol supplementation augments growth hormone (GH) secretory responsiveness to dose-varying GH-releasing peptide infusions in healthy postmenopausal women. J Clin Endocrinol Metab. 2001;86:551–560. doi: 10.1210/jcem.86.2.7240. [DOI] [PubMed] [Google Scholar]
- 45.Bray MJ, Vick TM, Shah N, Anderson SM, Rice LW, Iranmanesh A, Evans WS, Veldhuis JD. Short-term estradiol replacement in postmenopausal women selectively mutes somatostatin’s dose-dependent inhibition of fasting growth hormone secretion. J Clin Endocrinol Metab. 2001;86:3143–3149. doi: 10.1210/jcem.86.7.7647. [DOI] [PubMed] [Google Scholar]
- 46.Anderson SM, Wideman L, Patrie JT, Weltman A, Bowers CY, Veldhuis JD. Estradiol supplementation selectively relieves GH’s autonegative feedback on GH-releasing peptide-2-stimulated GH secretion. J Clin Endocrinol Metab. 2001;86:5904–5911. doi: 10.1210/jcem.86.12.8076. [DOI] [PubMed] [Google Scholar]
- 47.Farhy LS, Straume M, Johnson ML, Kovatchev BP, Veldhuis JD. A construct of interactive feedback control of the GH axis in the male. Am J Physiol. 2001;281:R38–R51. doi: 10.1152/ajpregu.2001.281.1.R38. [DOI] [PubMed] [Google Scholar]
- 48.Farhy LS, Straume M, Johnson ML, Kovatchev B, Veldhuis JD. Unequal autonegative feedback by GH models the sexual dimorphism in GH secretory dynamics. Am J Physiol. 2002;282:R753–R764. doi: 10.1152/ajpregu.00407.2001. [DOI] [PubMed] [Google Scholar]
- 49.Dickson SL, Viltart O, Bailey AR, Leng G. Attenuation of the growth hormone secretagogue induction of Fos protein in the rat arcuate nucleus by central somatostatin action. Neuroendocrinology. 1997;66:188–194. doi: 10.1159/000127237. [DOI] [PubMed] [Google Scholar]
- 50.Di Vito L, Broglio F, Benso A, Gottero C, Prodam F, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Ghigo E, Arvat E. The GH-releasing effect of ghrelin, a natural GH secretagogue, is only blunted by the infusion of exogenous somatostatin in humans. Clin Endocrinol (Oxf) 2002;56:643–648. doi: 10.1046/j.1365-2265.2002.01530.x. [DOI] [PubMed] [Google Scholar]
- 51.Iranmanesh A, Bowers CY, Veldhuis JD. Activation of somatostatin-receptor subtype-2/-5 suppresses the mass, frequency, and irregularity of growth hormone (GH)-releasing peptide-2-stimulated GH secretion in men. J Clin Endocrinol Metab. 2004;89:4581–4587. doi: 10.1210/jc.2004-0205. [DOI] [PubMed] [Google Scholar]
- 52.Guillaume V, Magnan E, Cataldi M, Dutour A, Sauze N, Renard M, Razafindraibe H, Conte-Devolx B, Deghenghi R, Lenaerts V. Growth hormone (GH)-releasing hormone secretion is stimulated by a new GH-releasing hexapeptide in sheep. Endocrinology. 1994;135:1073–1076. doi: 10.1210/endo.135.3.7915227. [DOI] [PubMed] [Google Scholar]
- 53.Fletcher TP, Thomas GB, Clarke IJ. Growth hormone-releasing and somatostatin concentrations in the hypophysial portal blood of conscious sheep during the infusion of growth hormone-releasing peptide-6. Domest Anim Endocrinol. 1996;13:251–258. doi: 10.1016/0739-7240(96)00017-3. [DOI] [PubMed] [Google Scholar]
- 54.Horikawa R, Tachibana T, Katsumata N, Ishikawa H, Tanaka T. Regulation of pituitary growth hormone-secretagogue and growth hormone-releasing hormone receptor RNA expression in young Dwarf rats. Endocr J. 2000;47(Suppl):S53–S56. doi: 10.1507/endocrj.47.supplmarch_s53. [DOI] [PubMed] [Google Scholar]
- 55.Kamegai J, Wakabayashi I, Kineman RD, Frohman LA. Growth hormone-releasing hormone receptor (GHRH-R) and growth hormone secretagogue receptor (GHS-R) mRNA levels during postnatal development in male and female rats. J Neuroendocrinol. 1999;11:299–306. doi: 10.1046/j.1365-2826.1999.00330.x. [DOI] [PubMed] [Google Scholar]
- 56.Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci USA. 2001;98:4028–4033. doi: 10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martha Jr PM, Goorman KM, Blizzard RM, Rogol AD, Veldhuis JD. Endogenous growth hormone secretion and clearance rates in normal boys as determined by deconvolution analysis: relationship to age, pubertal status and body mass. J Clin Endocrinol Metab. 1992;74:336–344. doi: 10.1210/jcem.74.2.1730812. [DOI] [PubMed] [Google Scholar]
- 58.van den Berg G, Veldhuis JD, Frolich M, Roelfsema F. An amplitude-specific divergence in the pulsatile mode of GH secretion underlies the gender difference in mean GH concentrations in men and premenopausal women. J Clin Endocrinol Metab. 1996;81:2460–2466. doi: 10.1210/jcem.81.7.8675561. [DOI] [PubMed] [Google Scholar]
- 59.Veldhuis JD, Roemmich JN, Rogol AD. Gender and sexual maturation-dependent contrasts in the neuroregulation of growth hormone secretion in prepubertal and late adolescent males and females—a general clinical research center-based study. J Clin Endocrinol Metab. 2000;85:2385–2394. doi: 10.1210/jcem.85.7.6697. [DOI] [PubMed] [Google Scholar]
- 60.Vahl N, Jorgensen JO, Skjaerback C, Veldhuis JD, Orskov H, Christiansen J. Abdominal adiposity rather than age and sex predicts the mass and patterned regularity of growth hormone secretion in mid-life healthy adults. Am J Physiol. 1997;272:E1108–E1116. doi: 10.1152/ajpendo.1997.272.6.E1108. [DOI] [PubMed] [Google Scholar]
- 61.Shah N, Aloi J, Evans WS, Veldhuis JD. Time-mode of growth hormone (GH) entry into the bloodstream and steady-state plasma GH concentrations rather than sex, estradiol, or menstrual-cycle stage primarily determine the GH elimination rate in healthy young women and men. J Clin Endocrinol Metab. 1999;84:2862–2869. doi: 10.1210/jcem.84.8.5881. [DOI] [PubMed] [Google Scholar]
- 62.Richmond E, Rogol AD, Basdemir D, Veldhuis OL, Clarke W, Bowers CY, Veldhuis JD. Accelerated escape from GH autonegative feedback in midpuberty in males: evidence for time-delimited GH-induced somatostatinergic outflow in adolescent boys. J Clin Endocrinol Metab. 2002;87:3837–3844. doi: 10.1210/jcem.87.8.8770. [DOI] [PubMed] [Google Scholar]
- 63.Bowers CY, Granda R, Mohan S, Kuipers J, Baylink D, Veldhuis JD. Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. J Clin Endocrinol Metab. 2004;89:2290–2300. doi: 10.1210/jc.2003-031799. [DOI] [PubMed] [Google Scholar]


