Abstract
Biomimetic materials that mimic the extracellular matrix (ECM) provide a means to control cellular functions such as adhesion and growth, which are vital to successful engineering of tissue-incorporated biomaterials. Novel “ECM-like” biomimetic surfactant polymers consisting of a poly(vinyl amine) backbone with pendant cell-adhesive peptides derived from one of the heparin-binding domains of fibronectin were developed to improve endothelial cell adhesion and growth on vascular biomaterials. Heparin-binding peptide (HBP) sequences, alone and in combination with RGD peptides, were examined for their ability to promote human pulmonary artery endothelial cell (HPAEC) adhesion and growth (HBP1, WQPPRARI; HBP2, SPPRRARVT; HBP1:RGD; and HBP2:RGD) and compared with cell adhesion and growth on fibronectin and on negative control polymer surfaces in which alanines were substituted for the positively charged arginine residues in the two peptides. The results showed that HPAECs adhered and spread equally well on all HBP-containing polymers and the positive fibronectin control, showing similar stress fiber and focal adhesion formation. However, the HBP alone was unable to support long-term HPAEC growth and survival, showing a loss of focal adhesions and cytoskeletal disorganization by 24 h after seeding. With the addition of RGD, the surfaces behaved similarly or better than fibronectin. The negative control polymers showed little to no initial cell attachment, and the addition of soluble heparin to the medium reduced initial cell adhesion on both the HBP2 and HBP2:RGD surfaces. These results indicate that the HBP surfaces promote initial HPAEC adhesion and spreading, but not long-term survival.
INTRODUCTION
ONE GOAL IN THE DEVELOPMENT of materials designed to support the adhesion, growth, and function of anchorage-dependent cell types, such as endothelial cells, is to mimic the interaction between cell surface receptors and extracellular matrix (ECM) molecules. This interaction is vital in regulating in vivo cellular functions including adhesion, survival, proliferation, migration, and differentiation.1–8 The major focus in the literature has been on the integrin family of receptors, which interact with a wide variety of ECM proteins.9–11 A tripeptide (RGD) sequence, identified as the active integrin-binding domain of ECM proteins including fibronectin (FN), has been intensively studied, but other domains have been discovered that promote or enhance cell–ECM interaction.2–4,12–14
More recently, a family of four cell surface heparan sulfate proteoglycans (HSPGs), known as syndecans, has been identified as having a role in cell adhesion.15–17 Syndecans interact specifically with heparin-binding domains within ECM proteins. In addition, syndecans can interact with growth factors and act as coreceptors in the delivery of growth factors to their receptors. One of the major heparin-binding regions in FN encompasses domains FNIII12–14. FN–syndecan interactions, in particular between FN and syndecan-1 and syndecan-4, have been attributed to the peptide sequences WQPPRARI and SPPRRARVT, derived from FNIII14 and FNIII13, respectively. These interactions occur mainly through electrostatic interactions between the negatively charged HSPG and the cluster of positively charged amino acids within the FN-binding domains.18,19 A few studies have provided evidence of the involvement of syndecans in focal adhesion formation and cytoskeletal interactions.15,16,20,21
Understanding the specific nature of the cell surface receptor–ECM interactions provides a foundation for developing functional biomaterials designed to promote endothelial cell adhesion and growth. Many studies have focused on incorporating RGD into their biomaterial design,5,22–26 and a limited number have included peptides from heparin-binding domains on a biomaterial surface as a way to promote cell attachment.27,28 Verrecchio et al. designed heparin-binding peptides containing the consensus sequences (XBBXBX)n and (XBBBXXBB-X)n, where X indicates hydrophobic residues and B represents basic residues (either arginine or lysine), although they were not examined for their ability to support cell adhesion and growth.27 Renzania and Healy varied the ratio of RGD to heparin-binding peptide (FHRRIKA, found in bone sialoprotein) attached to amine-functionalized quartz surfaces.28 They showed that all the combination surfaces as well as RGD alone promoted focal adhesion and stress fiber formation of an osteoblast-like cell type. The RGD:HBP 1:1 and RGD:HBP 1:3 ratios supported the highest degree of cell spreading, although there was no significant difference in proliferation on any of the surfaces.28 Thus, surface modification with adhesive peptide sequences can be used to promote and enhance cell adhesion. Moreover, incorporation of components from a variety of adhesive domains (i.e., heparin-binding peptides and RGD) results in a surface that better mimics the ECM.
In the development of complex mimics of fibronectin, a clear understanding of how endothelial cells interact with individual adhesive domains is needed. On this basis, we have developed biomimetic polymer surfactants containing adhesive peptide ligands that allow for an effective, simple, dip-coated surface modification process. The surfactants consist of a flexible poly(vinyl amine) backbone with alkyl side chains to facilitate polymer attachment to a hydrophobic substrate through hydrophobic interactions, and pendant cell-adhesive peptides. In this article, we examine two different peptides derived from the heparin-binding domain of FN (GSSSGWQP-PRARI and GSWSGSPPRRARVT) in our surfactant polymer system (Fig. 1) for their ability to promote human endothelial cell adhesion, growth, focal adhesion formation, stress fiber formation, and cell survival. In addition, we examine endothelial cell behavior on surfaces containing both RGD and an HBP. Therefore, the information gained from the current study provides a foundation for the development of a biomimetic coatings with FN-derived functions.
FIG. 1.
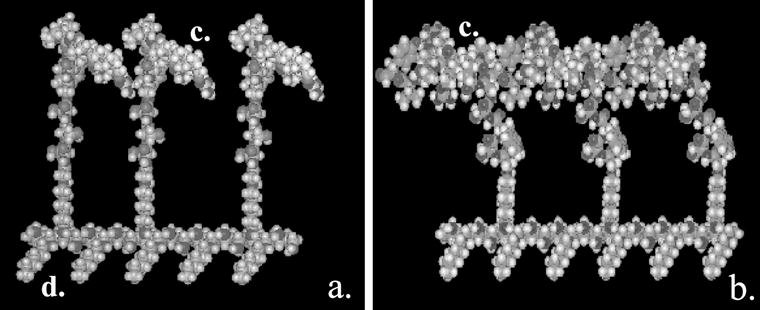
Molecular models of ECM-like surfactant polymers with individual cell-binding domains specific for heparan sulfate proteoglycans. (a) GSSSGWQPPRARI (HBP1), and (b) GSWSGSPPRRARVT (HBP2). Surfactant polymer consists of (c) pendant oligopeptides to facilitate cell adhesion and (d) hexanoyl side chains to facilitate spontaneous surface-induced self-assembly onto a hydrophobic substrate via hydrophobic interactions.
MATERIALS AND METHODS
Poly(vinyl amine) synthesis
All chemicals used for surfactant polymer synthesis were obtained from Sigma-Aldrich, Milwaukee, WI). Poly(vinyl amine) (PVAm; Mn = 6000) was synthesized as previously described.29 Briefly, N-vinyl formamide was polymerized in isopropanol for 4 h at 60°C, using AIBN (2,2′-azobisisobutyronitrile) as an initiator. The product was purified by gel-permeation chromatography (GPC) and then reacted with a 5 N NaOH solution for 6 h at 80°C, followed by neutralization with HCl. The sample was passed through an Amberlite-400 strong basic exchange column to obtain the final product.
Peptide synthesis
The heparin-binding peptides GSSSGWQPPRARI (HBP1) and GSWSGSPPRRARVT (HBP2); the negative control peptides GSSSGWQPPAAAI (xHBP1) and GSWSGSPPAAAAVT (xHBP2), and the integrin-binding peptide GSWSGRGDSPA (RGD) were synthesized by solid-phase peptide synthesis methods, using a PerSeptive Biosystems (model 9050) solid-phase peptide synthesizer (Applied Biosystems, Foster City, CA). For the negative control peptides, all positively charged arginine residues were changed to hydrophobic alanine residues. The hydrophilic GSSSG and GSWSG spacers present at the N terminus of the peptide were included to allow for elevation of the functional peptide sequences away from the PVAm backbone. The W residue was included for further surface characterization not addressed in this study. The peptides were purified by reversed phase high-pressure liquid chromatography, and characterized by 1H nuclear magnetic resonance (NMR) and mass spectroscopy. To facilitate peptide attachment to the PVAm backbone, peptide on the resin was first reacted with poly(ethylene oxide) (PEO) diacid, EDC (1-[3-(di-methylamino)propyl] 3-ethylcarbodiimide), and NHS (N-hydroxysuccinimide). The peptide–PEO was then cleaved from the solid support resin and attached to the PVAm backbone.
Biomimetic surfactant polymer synthesis
To create a surfactant polymer containing peptide side chains, HBP1–PEO, HBP2–PEO, xHBP1–PEO, xHBP2–PEO, or RGD–PEO was reacted with PVAm, using the same carbodiimide chemistry mentioned above. The solution was stirred for 3 h at 37°C, removed from the heat, and allowed to react overnight. Hexanoic acid, NHS, and EDC were then added to the reaction, which was again stirred for 3 h at 37°C, removed from heat, and allowed to react overnight. The peptide:hexanoyl ratio was controlled by the molar feed ratios, where two peptide–PEO were added for every three hexanoic acid. In the case of the HBP:RGD combination surfactant polymers, one HBP–PEO and one RGD–PEO were added for every three hexanoic acid. The product was purified by dialysis against water, followed by lyophilization. Spectra obtained from analysis by Fourier transform infrared spectroscopy (FTIR) were used to confirm successful attachment of the peptide. The final molar peptide:hexanoyl ratios were calculated by 1H NMR.
The surfactant polymers were dissolved in water and sterile filtered through 0.2-μm pore size nylon syringe filters. Each surfactant was then adsorbed on ethylene oxide-sterilized glass coverslips (15 mm in diameter, no. 2.; Fisher Scientific, Pittsburgh, PA) with a self-assembled monolayer of octadecyltrichlorosilane (OTS) for 24 h. The samples were removed from the solution and dried overnight. Reduced water contact angles were used to indicate successful adsorption of the surfactant onto the OTS surface.
Culture of human pulmonary artery endothelial cells
Human pulmonary artery endothelial cells (HPAECs) were harvested from nonatherosclerotic pulmonary arteries of heart transplant donors at the time of organ harvest on obtaining institutional review board-approved family consent.30,31 ECs (passages 2 to 10) were routinely subcultured via trypsinization and grown to confluence from a 1:3 split ratio in 25-cm2 flasks (Costar; Corning Life Sciences, New York, NY) coated with rabbit fibronectin (FN; 1 μg/cm2) in MCDB 131 medium (Sigma, St. Louis, MO) with 0.015% endothelial cell growth factor supplement (ECGS; Core Facilities Laboratory, Cell Biology Department, Cleveland Clinic Foundation, Cleveland, OH), 0.009% heparin (heparin activity, 16.3 U/mL; isolated from porcine mucosa, Sigma) and 10% fetal bovine serum (FBS; Sigma) (complete growth medium). The cobblestone morphology of the confluent cell layer in conjunction with anti-factor VIII immuno-fluorescence (American Diagnostica, Greenwich, CT) established the endothelial nature of the cells.
Initial cell adhesion
Control glass coverslips were coated with FN for 1 h before EC seeding. Cells were plated in either complete growth medium or basal MCDB 131 containing bovine serum albumin (BSA, 2 mg/mL) (serum-free media) on the surfactant-modified coverslips. For all initial adhesion studies, HPAECs were seeded at a density of 15,000 cells/cm2, and allowed to adhere for 3 h at 37°C. To examine the effect of soluble heparin on HPAEC adhesion to the HBP-containing surfaces, cells were plated in either complete growth medium or complete growth medium containing heparin at 15 mg/mL (heparin activity, 2715 U/mL). Coverlsips were examined by phase-contrast microscopy, and images of adherent cells were obtained for the different surfaces. Coverslips were rinsed with fresh medium to remove nonadherent cells, and cell numbers were quantified by MTS assay (Promega, Madison, WI). Cells were incubated for 2 h with MTS reagent [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt], and aliquots of medium with MTS were taken in triplicate and transferred to a 96-well plate. The absorbance was measured at 490 nm and the value obtained converted to a cell number, using a standard curve obtained by plating known densities of cells on FN. The MTS assay provides an estimate of actual cell numbers, as the MTS is a metabolic assay and metabolic activity may not remain constant with all culture conditions such as confluence or differentiation state.
Cell growth
HPAEC growth was examined over time on the various surfaces, from an initial seeded density of 15,000 cells/cm2. Phase-contrast images of the same coverslips were acquired at set time intervals over a 48-h time period. Corresponding coverslips were rinsed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde (PFA; Sigma) for fluorescent staining, or cells were incubated with MTS reagent and used to examine proliferation over time via an MTS assay.
Fluorescent staining of HPAECs
Organization of the actin cytoskeleton and focal adhesion complex was examined by fluorescence microscopy. HPAECs were rinsed with PBS and fixed with 4% PFA for 20 min. HPAECs were then rinsed three times with Tris-buffered saline (TBS) and submerged in HEPES blocking buffer (10 mM HEPES, 150 mM NaCl, and BSA [1 μg/mL]), where they were stored at 4°C overnight. They were rinsed again three times with TBS, and permeabilized with 0.2% Triton X-100 in TBS for 20 min. HPAECs were again rinsed with TBS and then incubated for 1 h with a 1:100 dilution of primary murine monoclonal antibodies against human vinculin (clone hVIN-1; Sigma) in TBS containing 4% fetal bovine serum. HPAECs were washed in TBS after incubation and then stained with a biotinylated goat anti-mouse IgG secondary antibody for 45 min. HPAECs were rinsed with TBS and then stained with a mixture of Alexafluor 568 phalloidin (Molecular Probes, Eugene, OR) for actin stress fibers and streptavidin–Alexafluor 488 (Molecular Probes) for the focal adhesion protein vinculin. Fluorescently stained HPAECs were examined by scanning confocal microscopy (MRC 600; Bio-Rad, Hercules, CA) and at least five images were obtained at different positions for each time point.
Statistical analysis
Statistical analysis was performed in Excel 97 (Microsoft, Redmond, WA). A Student t test was performed to compare differences in cell adhesion and growth on the various surfaces. Significance was considered a p value less than 0.05. All experiments were performed in triplicate.
RESULTS
A series of novel biomimetic surfactant polymers containing either pendant oligopeptides from the heparin-binding domain of fibronectin only, or a combination of one of the HBPs and RGD, were examined for their ability to support cell adhesion, growth, focal adhesions, and stress fiber formation. Table 1 shows the ratio of hydrophilic peptide to hydrophobic side chain in the HBP-containing polymers as determined by 1H NMR, as well as water contact angles of the surfactant adsorbed on OTS. Typical contact angles for the OTS self-assembled monolayer range from 107 to 110°, which drops drastically after surfactant polymer adsorption.
Table 1 .
Ligand Ratios and Water Contact Angles for Heparin-Binding Domain Surfactant Polymersa
| Surfactant polymer | Molar feed ratios (HBP: RGD: HEX) | Water contact angle |
|---|---|---|
| HBP1 | 2:0:3 | 46 ± 16 |
| HBP2 | 2:0:3 | 67 ± 80 |
| xHBP1 | 2:0:3 | 17 ± 50 |
| xHBP2 | 2:0:3 | 49 ± 12 |
| HBP1:RGD | 1:1:3 | 69 ± 19 |
| HBP2:RGD | 1:1:3 | 52 ± 23 |
| RGD | 0:2:3 | 68 ± 24 |
Abbreviations: HBP, heparin-binding peptide; HEX, hexanoic acid.
Water contact angle for uncoated OTS was 107–110°.
OTS, octadecyltrichlorosilane self-assembled monolayer on glass.
Surfactant polymers containing HBP1 only, HBP2 only, HBP1:RGD (1:1), or HBP2:RGD (1:1) adsorbed on OTS-, or FN-coated glass were examined for their ability to promote HPAEC adhesion, growth, stress fiber, and focal adhesion formation. Figure 2 shows phase-contrast images of HPAEC adhesion and growth with time on the surfaces. Initially (3 h after seeding), cells adhered equally well to HBP1 (Fig. 2D), HBP2 (Fig. 2G), HBP1:RGD (Fig. 2J), HBP2:RGD (Fig. 2M), and the positive FN control surface (Fig. 2A). By 24 h, HPAECs on FN and the two HBP:RGD combination surfaces (Fig. 2B, K, and N) approach confluence, demonstrating the characteristic cobblestone morphology. However, HPAECs on HBP1 (Fig. 2E) have acquired a more spindled morphology and do not cover a significant portion of the surface. HPAECs on the HBP2 surface (Fig. 2H) also appear spindled, with even lower surface coverage. By 48 h, HPAECs on FN and the combination surfaces (Fig. 2C, L, and O) appear healthy and cover a large portion of the surface, whereas HPAECs on HBP1 (Fig. 2F) cover slightly more area than they did at 24 h but still appear spindled, and most cells on the HBP2 surface have detached and died (Fig. 2I).
FIG. 2.
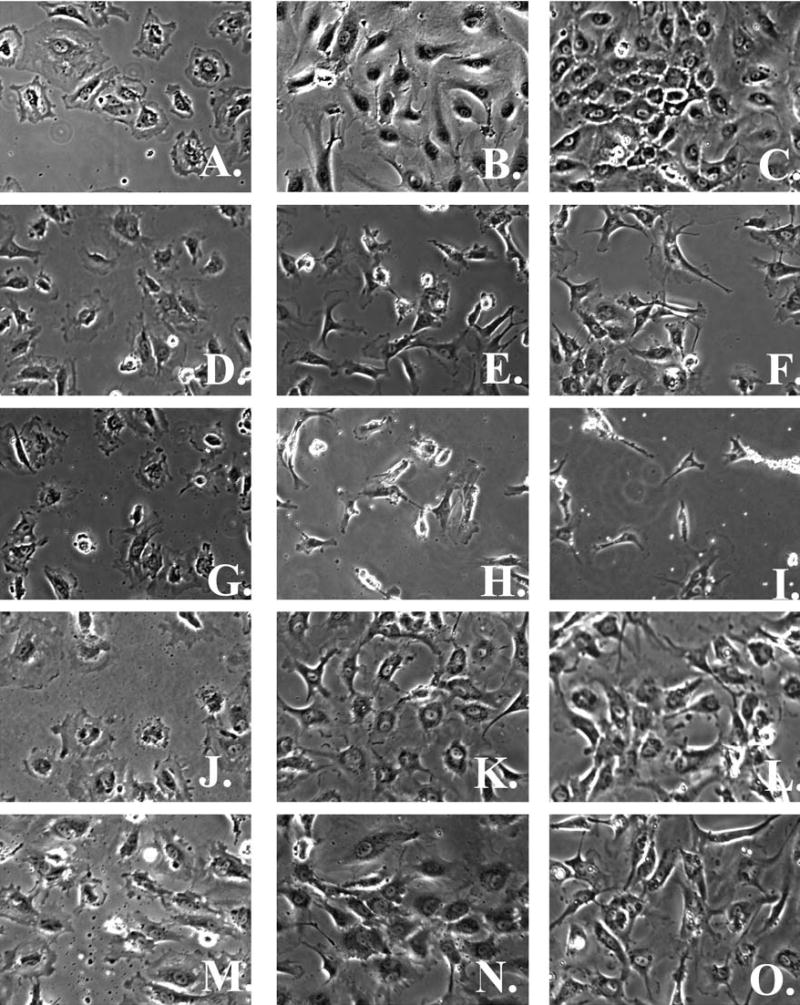
Phase-contrast images of human pulmonary artery endothelial cells (HPAECs) on fibronectin (FN) (A–C), HBP1 (D–F), HBP2 (G–I), HBP1:RGD (J–L), and HBP2:RGD (M–O) at 3 h (A, D, G, J, and M), 24 h (B, E, H, K, and N), and 48 h (C, F, I, L, and O) after seeding at 15,000 cells/cm2 (original magnification, ×10).
Examination of cell density over time (Fig. 3) shows a steady increase in cell number on the FN surface, a slight decrease followed by a slower increase in cell number on the HBP1 surface, and a decrease in the number of cells present on the HBP2 surface over a 48-h period. Cell density on the HBP:RGD combination surfaces was similar to that on FN. Examination of cytoskeletal and focal adhesion formation after 3 h on the various surfaces (Fig. 4A, D, G, J, and M) shows stress fibers with modest focal adhesions for HPAECs on FN (Fig. 4A), whereas HPAECs on any of the HBP-containing surfaces—HBP1 (Fig. 4D), HBP2 (Fig. 4G), HBP1:RGD (Fig. 4J), and HBP2:RGD (Fig. 4M)—demonstrate similar stress fiber formation and more focal adhesions. By 6 h after seeding (Figs. 4B, E, H, K, and N), all surfaces support HPAEC adhesion and growth, showing good stress fiber and focal adhesion formation. The inability of HBP alone to support long-term HPAEC survival is demonstrated by the loss of focal adhesions and the disorganized cytoskeleton that develops, as seen in Fig. 4F and I, acquired 48 h after EC seeding, whereas cells on the combination surfaces appear healthy and maintain their focal adhesions and stress fibers (Fig. 4L and O).
FIG. 3.
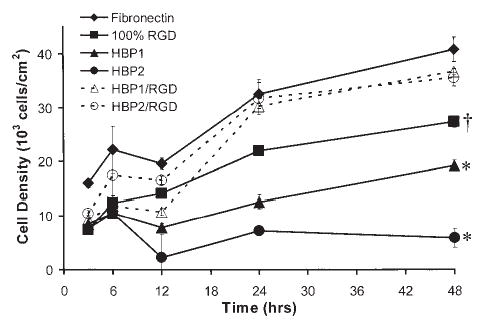
Changes in HPAEC density over time on fibronectin (FN), HBP1, HBP2, 100% RGD, HBP1:RGD, and HBP2:RGD as determined by MTS assay. HPAECs fail to survive on HBP2 over the 48-h time period, and growth on HBP1 is poor, whereas HPAECs on FN reach confluence (~40,000 cells/cm2). HPAECs were seeded at a density of 15,000 cells/cm2 and grown in complete growth medium. *Significantly (p < 0.05) lower cell density at 48 h compared with FN, HBP1:RGD, HBP2:RGD, and 100% RGD. †Significantly (p < 0.05) lower cell density at 48 h compared with FN, HBP1:RGD, and HBP2:RGD. For data concerning 100% RGD see Murugesan et al.8
FIG. 4.
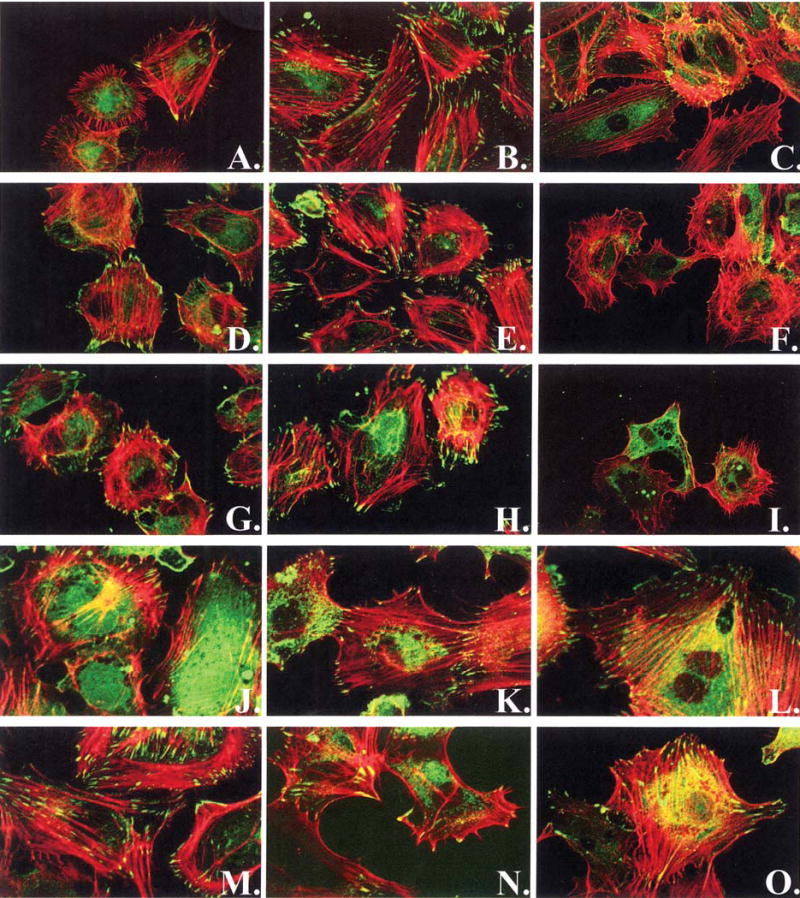
Focal adhesion and stress fiber formation on FN (A–C), HBP1 (D–F), HBP2 (G–I), HBP1:RGD (J–L), and HBP2:RGD (M–O) at 3 h (A, D, G, J, and M), 6 h (B, E, H, K, and N), and 48 h (C, F, I, L, and O). HPAECs are stained for actin stress fibers (red), and the focal adhesion protein vinculin (green). Images were obtained by scanning confocal microscopy (original magnification, ×60).
Assessment of adherent cell density 3 h after seeding (Fig. 5) in both complete growth medium and serum-free medium on the three surfaces showed significantly more cells present on FN than on HBP1, HBP2, and HBP1:RGD surfaces in complete growth medium, but similar numbers in serum-free medium for these surfaces. Interestingly, the HBP2:RGD surface displayed cell adhesion similar to that on FN in the presence of serum in the medium and significantly more adherent cells than on FN in the absence of serum in the medium. In addition, when the positive arginine (R) residues in the HBPs were converted to alanine (A) (xHBP1 and xHBP2), HPAEC adhesion was significantly reduced on both HBP1 and HBP2 in the presence of serum, and almost completely abolished in the absence of serum.
FIG. 5.
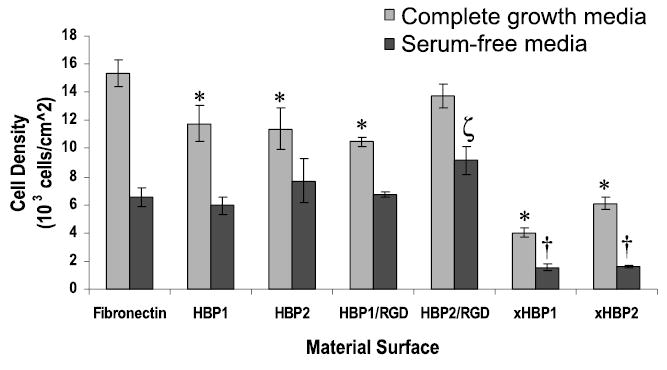
Number of adherent HPAECs on fibronectin (FN), HBP1, HBP2, xHBP1 (arginine replaced by alanine in HBP1 sequence), and xHBP2 (arginine replace by alanine in HBP2 sequence) surfaces in complete growth medium, which has a 10% serum content, and serum-free medium 3 h after seeding. HPAECs were seeded at a density of 15,000 cells/cm2. *Significantly (p < 0.05) lower cell density compared with the FN positive control in complete growth medium. †Significantly (p < 0.05) lower cell density compared with the FN positive control in serum-free medum. ζSignificantly (p < 0.05) higher cell density compared with the FN positive control in serum-free medium.
To examine the specificity of the EC–HBP interaction, soluble heparin (15 mg/mL) was added to the medium to compete with HSPG binding to the HBPs. Interestingly, the number of adherent HPAECs 3 h after seeding was significantly reduced (but not completely inhibited) on the HBP2 and HBP2:RGD surfaces, but not on any of the other surfaces (Fig. 6).
FIG. 6.
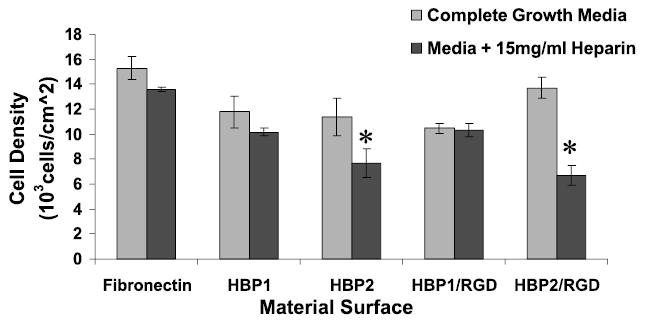
Number of adherent HPAECs on FN, HBP1, and HBP2 surfaces in complete growth medium and complete growth medium plus heparin (15 mg/mL) 3 h after seeding. HPAECs were seeded at a density of 15,000 cells/cm2. *Significantly (p < 0.05) lower cell density compared with the same surface in complete growth medium.
DISCUSSION
In the initial phase of cell surface interactions after seeding, the HBP surfaces demonstrated results like those of RGDS-containing surfactant polymer, as reported previously.8 Both HBP1 and HBP2 promoted EC adhesion in the presence or absence of serum, indicating that some specific interaction occurred between the ECs and the HBPs. Furthermore, in peptide sequences where the arginine was converted to an alanine, cell adhesion was significantly reduced in the presence of serum, and almost abolished in the absence of serum, further indicating a specific receptor–ligand interaction. Moreover, when surfaces contained both the RGD and either of the HBPs, cellular adhesion and growth were similar and in some cases superior to the FN control. Although we have demonstrated a definite specific interaction with our RGD surfaces in previous studies,8 the ability of the HBPs to promote a specific interaction remains largely unknown.
Heparan sulfate contains negatively charged sulfate groups, whereas the heparin-binding domains of proteins contain a large number of positively charged amino acids (arginine, lysine, and histidine at a ratio of ~3:2:1). Published studies indicate that the numbers and placement of these amino acids in the binding sequence dictate their ability to interact with heparan sulfate.2,32 Moreover, serine and glycine, which have small side chains that provide minimal steric hindrance and allow for increased chain flexibility, are present in larger amounts compared with other amino acids in heparin-binding domains. Their location between basic residues should allow for optimal electrostatic interaction with the anionic sulfate groups of heparan sulfate.32 Our HBPs contain the basic arginine residues needed for heparin binding in conjunction with a flexible N terminus consisting of glycines and serines, thereby allowing the possibility of at least a non-specific electrostatic interaction with any negatively charged species present on the cell surface, including heparin sulfate proteoglycan. With respect to promoting cellular adhesion, the syndecans (cell surface HSPGs), and in particular syndecan-4, have been implicated as the receptors responsible for interaction between cells and the heparin-binding domains of ECM proteins. Syndecan-4 acts as a coreceptor with the integrins and localizes to focal adhesion sites, where it plays both a structural and functional role in cellular adhesion.21,33–35 More recently, it has been shown that although syndecan-4 is not necessary for focal adhesion formation, it is sufficient, by itself, to facilitate focal adhesion formation.36 Both of the HBP surfaces are able to promote focal adhesion formation even in the absence of serum proteins. Although our results indicate some specificity, more research is necessary to fully determine the possible role of the syndecans in this interaction.
In an attempt to further investigate the role of charge in cellular adhesion to the HBP surfaces, soluble heparin was added before initial cell attachment. The negatively charged heparin should bind to the positively charged HBPs, thereby hindering the interaction between the cells and the surface. The heparin used in this study was of low molecular weight (MW ~3000 g/mol), derived from a porcine source. As expected, soluble heparin had little to no effect on adhesion to FN. Although FN contains heparin-binding domains that may interact with the soluble heparin, it also contains a wide variety of other cell-binding domains, including the RGD sequence, which promote EC adhesion by other mechanisms.
Interestingly, although there was a significant decrease in adhesion to HBP2 with the addition of heparin, no significant difference was observed with respect to cell adhesion to HBP1. The same was true for the combination surface containing HBP2:RGD, but not the surface containing HBP1:RGD. HBP1 is the WQPPRARI sequence found in FNIII14. This sequence has been shown to interact specifically a variety of cell surface receptors.37,38 Thus, heparin may be able to block some but not all of the possible interactions that may take place between HBP1 and the cell surface and therefore have a lesser effect on cellular adhesion to the HBP1-containing surfaces. In addition, the HBP1 sequence contains fewer arginine residues, and thus has a reduced ionic interaction with the negatively charged heparin compared with the HBP2 surface. This reduced interaction may explain the ineffectiveness of soluble heparin in inhibiting adhesion to HBP1.
The HBP surfaces, unlike the positive FN control, showed poor results with respect to long-term cell growth and survival. Initially, ECs were able to adhere, form focal adhesions, and proliferate. However, after about 12 h, these functions deteriorated, particularly on the HBP2 surface, where cell numbers decreased. Although cell numbers increased slightly by 48 h on the HBP1 surface, the ECs appeared unhealthy and did not achieve confluence. A number of reports have implicated cell surface HSPG, or HSPG shed from the cell surface, in the reduced cell proliferation and the induction of apoptosis for a variety of cell types, although this has not yet been demonstrated for ECs.39–41 With time, ECs on the HBP surfaces lose their focal adhesions and stress fiber development. This may be a major factor in the initiation of cell death that occurs on these surfaces. ECs on the RGD combination polymer surfaces maintain focal adhesions and stress fiber development and continue to proliferate to confluence. The results obtained from the combination surfaces indicate that the RGD sequence is necessary for long-term survival, which is likely due to the initiation of integrin signaling.1,3,4
FN represents an ideal surface for promoting EC adhesion, growth, and survival because of the presence of a variety of cell-binding domains including RGD and heparin-binding domains that have been shown to interact specifically with cell surface syndecans. Evidence exists of “cross-talk” between different cell surface receptors and, therefore, when binding to both integrins and HSPG occurs, they are able to colocalize to the focal adhesion sites and act cooperatively in generating focal adhesions and stress fibers, and in promoting cell survival as seen on the FN surfaces.36 Therefore, synthesizing a surface that contains both RGD and heparin-binding domains should provide a more desirable biomimetic material for promoting cell adhesion, growth, and function than those containing only the individual components. The combination RGD:HBP surfaces show cell adhesion and growth similar to and in some cases better than on FN. In the case of the HBP-only surfaces, the results obtained here for EC–HBP interactions are somewhat different from those obtained by Rezania et al.22,28 They showed that proliferation of osteoblasts on their HBP polymers was no different than on RGD polymers or polymers with both RGD and HBP in combination. However, the HBP sequence (derived from bone sialoprotein) and the cell types used in their study were different from those examined here. Moreover, a report in which WQP-PRARI and SPPRRARVT sequences were adsorbed to tissue culture surfaces showed that human umbilical vein endothelial cells could adhere to and spread on these peptides, but long-term cell growth and survival was not examined.42
The use of small cell-binding peptides to create a cell reactive surface is advantageous for a variety of reasons. First, they are easily synthesized and reproducible. In addition, covalent coupling of these peptides to material surfaces and surface coatings can be done simply and in a controlled fashion. Third, their small size allows them to be included with other peptides and functional components that can impart desirable surface properties for the selected application. These properties, in conjunction with the fact that specific peptide sequences can promote cellular adhesion and growth, make peptide modification of biomaterial surfaces an ideal method of creating EC-adhesive surfaces. Our biomimetic surfaces provide a system that is easily modified to alter peptide composition and density. By incorporating peptides derived from the heparin-binding domains of FN, we have demonstrated the ability to engineer endothelial cell-adhesive surfaces using graft polymer architecture. Further elaboration of the surfactant polymer system should provide opportunities to determine quantitative relationships between RGD and HBP peptide densities on the polymer and EC responses.
Acknowledgments
The authors acknowledge financial support by National Institutes of Health grant EB-00206 and the equipment facilities provided by the Center for Cardiovascular Biomaterials.
References
- 1.Aplin AE, Howe AK, Juliano RL. Cell adhesion molecules, signal transduction and cell growth. Curr Opin Cell Biol. 1999;11:737. doi: 10.1016/s0955-0674(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 2.Busby TF, Argraves WS, Brew SA, Pechik I, Gilliland GL, Ingham KC. Heparin binding by fibronectin module III-13 involves six discontinuous basic residues brought together to form a cationic cradle. J Biol Chem. 1995;270:18558. doi: 10.1074/jbc.270.31.18558. [DOI] [PubMed] [Google Scholar]
- 3.Garcia AJ, Boettiger D. Integrin–fibronectin inter-actions at the cell–material interface: Initial integrin binding and signaling. Biomaterials. 1999;20:2427. doi: 10.1016/s0142-9612(99)00170-2. [DOI] [PubMed] [Google Scholar]
- 4.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 5.Kouvroukoglou S, Dee KC, Bizios R, McIntire LV, Zygourakis K. Endothelial cell migration on surfaces modified with immobilized adhesive peptides. Biomaterials. 2000;21:1725. doi: 10.1016/s0142-9612(99)00205-7. [DOI] [PubMed] [Google Scholar]
- 6.Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 7.Mann BK, West JL. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60:86. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- 8.Murugesan GM, Ruegsegger MA, Kligman F, Marchant RE, Kottke-Marchant K. Integrin-dependent interaction of human vascular endothelial cells on biomimetic peptide surfactant polymers. Cell Commun Adhes. 2002;9:59. doi: 10.1080/15419060214148. [DOI] [PubMed] [Google Scholar]
- 9.Hillis GS, Flapan AD. Cell adhesion molecules in cardiovascular disease: A clinical perspective. Heart. 1998;79:429. doi: 10.1136/hrt.79.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massia SP, Stark J. Immobilized RGD peptides on surface-grafted dextran promote biospecific cell attachment. J Biomed Mater Res. 2001;56:390. doi: 10.1002/1097-4636(20010905)56:3<390::aid-jbm1108>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Culp, L.A., O’Conner, K.L., Lechner, R. Extracellular matrix adhesion: Biological, molecular, and pathogenic mechanisms. In: Bittar, E.E., and Bittar, N., eds. Principles of Medical Biology: Membranes and Cell Signaling, Vol. 7B. Greenwich, CT: JAI Press, 1997.
- 12.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 13.Redick SD, Settles DL, Briscoe G, Erickson HP. Defining fibronectin’s cell adhesion synergy site by site-directed mutagenesis. J Cell Biol. 2000;149:521. doi: 10.1083/jcb.149.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant RP, Spitzfaden C, Altroff H, Campbell ID, Mardon HJ. Structural requirements for biological activity of the ninth and tenth FIII domains of human fibronectin. J Biol Chem. 1997;272:6159. doi: 10.1074/jbc.272.10.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods A, Couchman JR. Syndecans: Synergistic activators of cell adhesion. Trends Cell Biol. 1998;8:189. doi: 10.1016/s0962-8924(98)01244-6. [DOI] [PubMed] [Google Scholar]
- 16.Bernfield M, Kokenyesi R, Kato M, et al. A family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 17.Elenius K, Jalkanen M. Function of the syndecans: A family of cell surface proteoglycans. J Cell Sci. 1994;107:2975. doi: 10.1242/jcs.107.11.2975. [DOI] [PubMed] [Google Scholar]
- 18.Ingham KC, Brew SA, Migliorini M. An unusual heparin-binding peptide from the carboxy-terminal hep-2 region of fibronectin. Arch Biochem Biophys. 1994;314:242. doi: 10.1006/abbi.1994.1436. [DOI] [PubMed] [Google Scholar]
- 19.Mooradian DL, McCarthy JB, Skubitz AP, Cameron JD, Furcht LT. Characterization of FN-C/H-V, a novel synthetic peptide from fibronectin that promotes rabbit corneal epithelial cell adhesion, spreading, and motility. Invest Ophthalmol Vis Sci. 1993;34:153. [PubMed] [Google Scholar]
- 20.Couchman JR. Syndecans and cell adhesion. J Biol Chem. 2000;275:24233. doi: 10.1074/jbc.R000001200. [DOI] [PubMed] [Google Scholar]
- 21.Woods A, Couchman JR. Syndecan-4 and focal adhesion function. Curr Opin Cell Biol. 2001;13:578. doi: 10.1016/s0955-0674(00)00254-4. [DOI] [PubMed] [Google Scholar]
- 22.Rezania A, Healy KE. The effect of peptide surface density on mineralization of a matrix deposited by osteogenic cells. J Biomed Mater Res. 2000;52:595. doi: 10.1002/1097-4636(20001215)52:4<595::aid-jbm3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Quirk RA, Chan WC, Davies MC, Tendler SJ, Shakesheff KM. Poly(l-lysine)-GRGDS as a biomimetic surface modifier for poly(lactic acid) Biomaterials. 2001;22:865. doi: 10.1016/s0142-9612(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 24.Massia SP, Stark J. Immobilized RGD peptides on surface-grafted dextran promote biospecific cell attachment. J Biomed Mater Res. 2001;56:390. doi: 10.1002/1097-4636(20010905)56:3<390::aid-jbm1108>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.McMillan R, Meeks B, Bensebaa F, Deslandes Y, Sheardown H. Cell adhesion peptide modification of gold-coated polyurethanes for vascular endothelial cell adhesion. J Biomed Mater Res. 2001;54:272. doi: 10.1002/1097-4636(200102)54:2<272::aid-jbm15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Wilke MS, Vespa J, Skubitz AP, Furcht LT, Mc-Carthy JB. Human keratinocytes adhere to and spread on synthetic peptide FN-C/H-V derived from fibronectin. J Invest Dermatol. 1993;101:43. doi: 10.1111/1523-1747.ep12358823. [DOI] [PubMed] [Google Scholar]
- 27.Verrecchio A, Germann MW, Schick BP, Kung B, Twardowski T, San Antonio JD. Design of peptides with high affinities for heparin and endothelial cell proteoglycans. J Biol Chem. 2000;275:7701. doi: 10.1074/jbc.275.11.7701. [DOI] [PubMed] [Google Scholar]
- 28.Rezania A, Healy KE. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol Prog. 1999;15:19. doi: 10.1021/bp980083b. [DOI] [PubMed] [Google Scholar]
- 29.Qiu YZ, Zhang TH, Ruegsegger MA, Marchant RE. Novel nonionic oligosaccharide surfactant polymers derived from poly(vinylamine) with pendant dextran and hexanoyl groups. Macromolecules. 1998;31:165. [Google Scholar]
- 30.Sagnella S, Kwok J, Marchant RE, Kottke-Marchant K. Shear-induced platelet activation and adhesion on human pulmonary artery endothelial cells seeded onto hydrophilic polymers. J Biomed Mater Res. 2001;57:419. doi: 10.1002/1097-4636(20011205)57:3<419::aid-jbm1185>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 31.Kottke-Marchant K, Veenstra AA, Marchant RE. Human endothelial cell growth and coagulant function varies with respect to interfacial properties of polymeric substrates. J Biomed Mater Res. 1996;30:209. doi: 10.1002/(SICI)1097-4636(199602)30:2<209::AID-JBM11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 32.Caldwell EE, Nadkarni VD, Fromm JR, Linhardt RJ, Weiler JM. Importance of specific amino acids in protein binding sites for heparin and heparan sulfate. Int J Biochem Cell Biol. 1996;28:203. doi: 10.1016/1357-2725(95)00123-9. [DOI] [PubMed] [Google Scholar]
- 33.Woods A, McCarthy JB, Furcht LT, Couchman JR. A synthetic peptide from the COOH-terminal heparin-binding domain of fibronectin promotes focal adhesion formation. Mol Biol Cell. 1993;4:605. doi: 10.1091/mbc.4.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods A, Longley RL, Tumova S, Couchman JR, Klass CM. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts: Control of extracellular matrix assembly by syndecan-2 proteoglycan. Arch Biochem Biophys. 2000;374:66. doi: 10.1006/abbi.1999.1607. [DOI] [PubMed] [Google Scholar]
- 35.Bernfield M, Gotte M, Woo Park P, Reizes O, Fitzgerald M, Linececum J, Zako M. Function of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 36.Echtermeyer F, Baciu PC, Saoncella S, et al. Syndecan-4 core protein is sufficient for the assembly of focal adhesions and actin stress fibers. J Cell Sci. 1999;112:3433. doi: 10.1242/jcs.112.20.3433. [DOI] [PubMed] [Google Scholar]
- 37.Mostafavi-Pour Z, Askari JA, Whittard JD, Humphries MJ. Identification of a novel heparin-binding site in the alternatively spliced IIICS region of fibronectin: Roles of integrins and proteoglycans in cell adhesion to fibronectin splice variants. Matrix Biol. 2001;20:63. doi: 10.1016/s0945-053x(00)00131-1. [DOI] [PubMed] [Google Scholar]
- 38.Sharma A, Askari JA, Humphries MJ, Jones EY, Stuart DI. Crystal structure of a heparin- and integrin-binding segment of human fibronectin. EMBO J. 1999;18:1468. doi: 10.1093/emboj/18.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhodapkar MV, Sanderson RD. Syndecan-1 (CD 138) in myeloma and lymphoid malignancies: A multi-functional regulator of cell behavior within the tumor microenvironment. Leuk Lymphoma. 1999;34:35. doi: 10.3109/10428199909083378. [DOI] [PubMed] [Google Scholar]
- 40.Khoury J, Langleben D. Heparin-like molecules inhibit pulmonary vascular pericyte proliferation in vitro. Am J Physiol Lung Cell Mol Physiol. 2000;279:L252. doi: 10.1152/ajplung.2000.279.2.L252. [DOI] [PubMed] [Google Scholar]
- 41.Ishiguro K, Kojima T, Taguchi O, Saito H, Muramatsu T, Kadomatsu K. Syndecan-4 expression is associated with follicular atresia in mouse ovary. Histochem Cell Biol. 1999;112:25. doi: 10.1007/s004180050388. [DOI] [PubMed] [Google Scholar]
- 42.Huebsch JC, McCarthy JB, Diglio CA, Mooradian DL. Endothelial cell interactions with synthetic peptides from the carboxyl-terminal heparin-binding domains of fibronectin. Circ Res. 1995;77:43. doi: 10.1161/01.res.77.1.43. [DOI] [PubMed] [Google Scholar]


