Abstract
Background
Sitosterolemia is a recessively inherited disorder that results from mutations in either ABCG5 or G8 proteins, with hyperabsorption of dietary sterols and decreased hepatic excretion of plant sterols and cholesterol. As a consequence of markedly elevated plasma and tissue sitosterol and campesterol levels, premature atherosclerosis develops.
Methods and Results
In this multicenter, double-blind, randomized, placebo-controlled study, we examined whether treatment with ezetimibe, an inhibitor of cholesterol absorption, reduces plant sterol levels in patients with sitosterolemia. After a 3-week placebo run-in, 37 patients were randomized to receive placebo (n=7) or ezetimibe 10 mg/d (n=30) for 8 weeks. Sitosterol concentrations decreased by 21% (P<0.001) in patients treated with ezetimibe compared with a nonsignificant 4% rise in those on placebo (between-group P<0.001). The reduction in sitosterol from baseline was progressive, with further decline observed at each subsequent biweekly visit. Campesterol also progressively declined, with a mean decrease after 8 weeks of 24% with ezetimibe and a mean increase of 3% with placebo treatment (between-group P<0.001). Reductions in plant sterol concentrations were similar irrespective of whether patients were undergoing concomitant treatment with resin or statin. Reductions in total sterols and apolipoprotein B were also observed. Ezetimibe was well tolerated, with no serious treatment-related adverse events or discontinuations due to adverse events being reported.
Conclusions
Ezetimibe produced significant and progressive reductions in plasma plant sterol concentrations in patients with sitosterolemia, consistent with the hypothesis that ezetimibe inhibits the intestinal absorption of plant sterols as well as cholesterol, leading to reductions in plasma concentrations.
Keywords: ezetimibe, sitosterolemia, cholesterol, coronary disease
Sitosterolemia is a rare, autosomal recessively inherited sterol storage disease in which markedly increased tissue and plasma plant sterol concentrations can lead to premature atherosclerosis and early cardiovascular death.1–4 Sitosterolemia is caused by complete mutations in either the ABCG5 or ABCG8 genes, located in a head-to-head organization on human chromosome 2p21.5–7 These genes encode ATP binding cassette (ABC) proteins that belong to the G family and may work together to pump sterols (cholesterol and plant sterols) from the brush border of enterocytes into the intestinal lumen and from the liver into bile.8 Normally, ≈500 mg of cholesterol and half as much plant sterols (predominately sitosterol and campesterol) are consumed in the diet each day.9,10 Approximately 50% of the cholesterol, <20% of campesterol, and <7% of sitosterol are absorbed.11–13 Despite their close structural similarity, plant sterols are recognized as distinct from cholesterol by the liver and are preferentially excreted into bile.11,14,15 Because of their lower intestinal absorption and preferential biliary excretion, plant sterols occur in very low concentrations in the plasma of normal individuals.16–18 In contrast, patients with sitosterolemia have markedly increased plant sterol absorption and diminished biliary excretion of these sterols.11,13,19–21 These 2 defects combine to lead to marked elevations of plant sterol plasma concentrations and consequent increases in total body stores and tissue levels.3,14,19,20 The clinical consequences of tissue plant sterol accumulation include premature atherosclerosis and coronary heart disease at a young age,1–4 tendon xanthomas similar to those observed in patients with homozygous familial hypercholesterolemia, hematologic sequelae including chronic hemolytic anemia and thrombocytopenia, and abnormal liver function tests.1,3,4,22
Available treatments for sitosterolemia include a diet restricted in cholesterol and plant sterols, bile salt-binding resins, ileal bypass surgery, or LDL immunoabsorption.3,4 Despite treatment, most patients continue to have markedly elevated concentrations of plant sterols and associated complications. Thus, current therapy is often inadequate to treat patients with sitosterolemia.
Ezetimibe is a novel inhibitor of intestinal cholesterol absorption in humans,23 shown to significantly lower plasma cholesterol and LDL cholesterol (LDL-C) concentrations in patients with hypercholesterolemia.24–26 Ezetimibe undergoes glucuronidation in the intestine and liver, and both the parent compound and its glucuronide localize to the brush border of the small intestine, where they block the absorption of dietary and biliary sources of cholesterol without affecting absorption of triglycerides, bile acids, or fat-soluble vitamins.27,28 In patients with mild to moderate hypercholesterolemia, ezetimibe reduces plasma concentrations of sitosterol and campesterol,23 most likely by reducing the absorption of these plant sterols. The purpose of the present study was to determine whether ezetimibe would also block the intestinal absorption of plant sterols and thus lead to lower plant sterol concentrations in patients with sitosterolemia.
Methods
Patients
Patients aged ≥10 years with a diagnosis of sitosterolemia who had plasma sitosterol levels >0.12 mmol/L (normal range <0.0239 mmol/L) despite current treatment were eligible to participate. Patients with poorly controlled diabetes or active liver or renal disease were excluded. Patients were expected to have been on a stable regimen of diet and/or medications for at least 4 weeks before the screening visit. If considered clinically appropriate, patients taking bile salt-binding resins had these agents discontinued before entry into the study because it was not known whether resins might lower ezetimibe concentrations. If it was not considered appropriate to modify the bile salt-binding medication that the patient was taking, patients could be enrolled while undergoing their current resin treatment. In these patients, ezetimibe was given at least 2 hours before or after bile acid–binding resins. Other treatments, including diet and statins, were continued but were expected to remain stable during the double-blind treatment period.
Study Design
This was a randomized, double-blind, placebo-controlled study conducted in 23 centers worldwide. After a 3-week single-blind placebo run-in period, patients were randomized (in a 4:1 ratio) to receive ezetimibe 10 mg/d or placebo. Given the relative rarity of this disorder, it was not expected that sufficient patients would be available to have an adequately powered study to assess between-group differences. Thus, the primary end point was percent change from baseline (defined as the average of the week −1 and the randomization visit) to end point (defined as the average of weeks 6 and 8) in sitosterol concentrations, and the 4:1 randomization ratio was used to ensure that sufficient patients would receive treatment with ezetimibe. More patients participated than expected, which provided sufficient power to assess both between- and within-group differences in plant sterols. Blood specimens collected with EDTA were obtained after an overnight fast at screening, at randomization, and after 2, 4, 6, and 8 weeks of treatment. The study protocol was approved by the institutional review boards at the participating sites, and all subjects or their legal guardians gave informed written consent.
Analytical Methods
All laboratory analyses other than those for sterols and genetic analyses were performed at Medical Research Laboratories, Highland Heights, Ky, or its subsidiary, Clinical Research Laboratories, Brussels, Belgium, according to published procedures.29–31 Sterols were analyzed by capillary gas-liquid chromatography (GLC).13,19 VLDL (density <1.006 g/mL) was isolated from plasma by preparative ultracentrifugation, and cholesterol was measured in the VLDL fraction and the infranatant by GLC. Plasma HDL cholesterol (HDL-C) was also measured by GLC after lipoproteins that contained apolipoprotein B had been precipitated with heparin-manganese. Cholesterol in the LDL fraction was taken as the difference between the cholesterol content of the infranatant and HDL fraction. The chromatographic quantification of lathosterol was performed by GLC-mass spectrometry-selected ion monitoring (GLC-MS-SIM).13 For mutational analyses, informed consent was obtained for the confirmation of known mutational analyses reported previously.6,7 In all cases, known mutations were confirmed by direct sequencing of polymerase chain reaction products.7
Achilles tendon thickness was measured by lateral foot radiography; x-ray films were centrally read by a radiologist blinded to treatment group assignment and to sequence (baseline or end of study). Radiographs were only available at baseline and end of study in 24 patients (6 placebo, 18 ezetimibe) because radiography was generally not performed in patients younger than 18 years of age.
Statistical Analysis
The primary efficacy variable was the within-group mean percent change in plasma sitosterol from baseline to end point. Key secondary variables were the within-group percent changes in plasma campesterol and in LDL-C. For all efficacy variables, percent change was assessed with summary statistics and 95% CIs. The assumption of normality was assessed by the Shapiro-Wilk statistic. Between-group differences were estimated with 95% CI. As prespecified before unblinding, a modified intention-to-treat analysis was performed without the single patient undergoing apheresis, who was excluded from the primary analysis given the distinctly different ongoing therapy; hence, the efficacy results are presented based on 29 patients treated with ezetimibe and 7 patients given placebo.
Results
A total of 39 patients were screened, and 37 patients were randomized into the double-blind treatment period. Patient baseline characteristics are presented in Table 1. The 2 treatment groups were similar in age and body weight. However, the placebo group had a greater proportion of females than the ezetimibe treatment group. Most patients were undergoing medical therapies for sitosterolemia, which included bile salt-binding resins in 15 patients (10 of whom continued such therapy during the double-blind treatment period) and statins in 10 patients. In addition, 5 patients had had prior ileal bypass surgery to treat their disease. Most patients had had the diagnosis of sitosterolemia for many years at the time of recruitment, with an average duration since diagnosis of ≈15 years. Mutational analyses were performed in 29 subjects who gave informed consent; 28 of these were mutant for ABCG8, and 1 was mutant for ABCG5. This was expected, because almost all white sitosterolemic individuals are mutant for ABCG8, and all Japanese and Chinese patients are mutant for ABCG5.7 The present study cohort included only 1 Japanese proband. Clinically overt coronary artery disease was present in 12 patients (32.4%). Of note, 9 patients (24.3%) either had aortic stenosis (by examination and/or echocardiography) or a history of aortic valve replacement surgery. Low platelet counts (<100 000) were present in 11 patients (29.7%), and low hemoglobin was observed in 5 patients (13.5%), consistent with prior observations of hematologic complications in patients with sitosterolemia. Finally, abnormal liver enzyme levels at baseline were common, with 25 patients (67.6%) having elevations in 1 or more liver function tests; aspartate aminotransferase was elevated in 12 patients (32.4%), alanine aminotransferase in 14 patients (35.1%), and γ-glutamyl transferase in 5 patients (13.5%). Baseline lipid values are provided in Table 2. Plant sterol and lipid concentrations were generally similar between the 2 treatment groups and showed the expected dramatic elevations in both sitosterol and campesterol but relatively normal concentrations of cholesterol in the lipid fractions.
TABLE 1.
Baseline Characteristics of the Study Population
| Placebo (n=7) | Ezetimibe 10 mg/d (n=30) | |
|---|---|---|
| Age, y | ||
| Mean | 37.6 | 37.0 |
| Range | 13–57 | 9–72 |
| Age subgroup, n (%) | ||
| <18 y | 1 (14) | 4 (13) |
| ≥18 y | 6 (86) | 26 (87) |
| Gender, n (%) | ||
| Female | 6 (86) | 18 (60) |
| Male | 1 (14) | 12 (40) |
| Race, n (%) | ||
| White | 6 (86) | 27 (90) |
| Asian | 0 (0) | 1 (3) |
| Hispanic | 1 (14) | 2 (7) |
| Body weight, kg | ||
| Mean | 63.0 | 67.5 |
| Range | 38.9–90.5 | 37.2–103.6 |
| Time since diagnosis, y | ||
| Mean | 15.4 | 15.0 |
| Range | 6–32 | 0–32 |
| Prior treatment of sitosterolemia, n (%) | ||
| None | 0 (0) | 10 (33) |
| Statins | 2 (29) | 8 (27) |
| Bile salt-binding resins | 4 (57) | 11 (37) |
| Ileal bypass surgery | 2 (29) | 3 (10) |
| Apheresis | 0 (0) | 1 (3) |
TABLE 2.
Baseline and Percent Change From Baseline Lipid and Apolipoprotein Values
| Placebo |
Ezetimibe 10 mg/d |
||||||
|---|---|---|---|---|---|---|---|
| Sterol or Apolipoprotein | Baseline Mean, mmol/L | % Change (SE) | 95% CI for % Change | Baseline Mean, mmol/L | % Change (SE) | 95% CI for % Change | Between-Group Difference (95% CI) |
| Sitosterol | 0.44 | 4.0 (5.3) | −6.9 to 14.8 | 0.50 | −21.0 (2.8) | −26.7 to −15.3 | 325.0 (−36.7 to −13.6) |
| Campesterol | 0.23 | 3.2 (5.5) | −7.9 to 14.3 | 0.27 | −24.3 (2.9) | −30.7 to −18.4 | −27.5 (−39.6 to −15.4) |
| Lathosterol | 0.012 | −7.2 (5.1) | −20.2 to 5.9 | 0.007 | 10.6 (6.1) | −1.9 to 23.2 | 17.8 (−7.9 to 43.4) |
| LDL-C* | 2.31 | 16.7 (19.7) | −31.6 to 64.9 | 2.47 | −13.6 (4.0) | −21.7 to −5.5 | NA |
| LDL sterols | 3.11 | 18.4 (7.7) | 2.8 to 34.0 | 3.39 | −14.9 (4.1) | −23.2 to −6.6 | −33.3 (−50.4 to −16.2) |
| HDL sterols | 1.27 | 5.5 (5.0) | −4.7 to 15.7 | 1.42 | 2.2 (2.6) | −3.2 to 7.6 | −3.3 (−14.4 to 7.8) |
| HDL-C (GC) | 0.86 | 8.3 (6.1) | −4.2 to 20.7 | 1.03 | 6.2 (3.2) | −0.4 to 12.8 | −2.1 (−15.6 to 11.5) |
| Cholesterol (GC) | 3.75 | 8.0 (6.1) | −11.3 to 27.3 | 4.35 | −4.8 (3.2) | −14.9 to 5.3 | −12.8 (−26.4 to 0.7) |
| Total sterols | 5.29 | 3.7 (4.8) | −6.2 to 13.5 | 5.62 | −8.7 (2.5) | −13.9 to −3.5 | −12.4 (−23.1 to −1.7) |
| Triglyceride* | 2.2 | −20.8 (6.9) | −37.6 to −4.0 | 1.66 | −2.1 (8.1) | −18.7 to 14.6 | NA |
| Apo A-I (mg/dL) | 146.7 | 0.6 (4.2) | −7.9 to 9.2 | 159.0 | 6.5 (2.2) | 2.0 to 11.0 | 5.9 (−3.4 to 15.2) |
| Apo B (mg/dL) | 128.2 | 3.1 (4.5) | −6.1 to 12.2 | 129.9 | −12.7 (2.4) | −17.5 to −7.9 | −15.8 (−25.8 to −5.8) |
| Lath:Chol ratio | 0.41 | −7.2 (11.2) | −30.1 to 15.8 | 0.18 | 10.6 (5.6) | −0.8 to 22.1 | 37.3 (1.0 to 73.7) |
Apo indicates apolipoprotein; GC, gas chromatography; and Lath:Chol, lathosterol:cholesterol.
LDL-C and triglyceride results presented as median, given nonnormal distribution of results, and hence, between-group differences are not provided.
Efficacy of Ezetimibe on Plant and Other Sterol Concentrations
Treatment with ezetimibe 10 mg/d resulted in a mean percent change in plasma sitosterol levels from baseline to end point of −21.0% (P<0.001) compared with a nonsignificant 4.0% increase in the placebo group (Table 2). The between-group difference in mean percent change in sitosterol was −25.0% (95% CI −36.7% to −13.2%; P<0.001). The reduction in plasma sitosterol during the double-blind period was progressive beginning at week 2, with greater reduction from baseline observed at each subsequent visit (Figure 1A). A high proportion of patients (27/29, 93%) had reductions in sitosterol with ezetimibe treatment; 25 (86%) of 29 patients had a ≥15% decrease and 10 (34%) of 29 had a ≥25% decrease from baseline in sitosterol concentrations. In contrast, only 1 (14%) of 7 patients in the placebo-treated group had a decrease of ≥15%, and none had a decrease of ≥25%.
Figure 1.
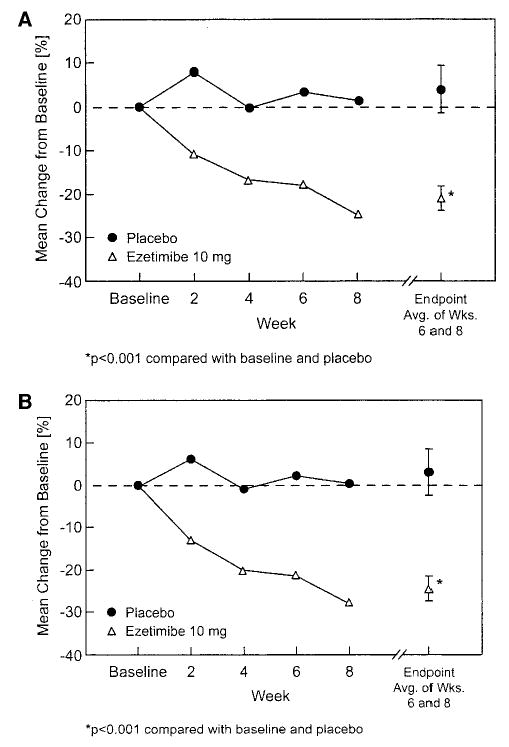
Mean percent change from baseline in plasma concentration of sitosterol (A) and campesterol (B) over time and at end point in 2 treatment groups. Avg. indicates average; Wks., weeks.
Results for the primary efficacy variable were examined in subgroups defined by patient baseline characteristics, including concomitant usage of bile salt-binding resins, statins, median baseline sitosterol, and gender. The results of the analyses indicate that the response to 10 mg of ezetimibe was consistent across these subgroups (Figure 2). There were too few patients under the age of 18 years to perform meaningful subgroup analysis by age; however, inspection of individual plasma sitosterol responses suggested that the effect of ezetimibe on sitosterol was similar in subjects under age 18 and those 18 years and older.
Figure 2.
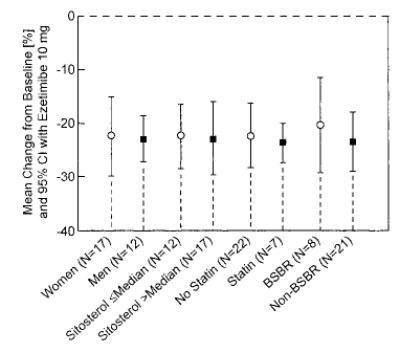
Point estimate and 95% CI response to ezetimibe 10 mg/d in sitosterol in various subgroups of population, defined by baseline characteristics. For baseline sitosterol subgroup, median sitosterol concentration used was based on all randomized subjects; 18 subjects (6 placebo and 12 ezetimibe) had plasma sitosterol levels at or below median at baseline, and 18 subjects (1 placebo and 17 ezetimibe) had baseline sitosterol levels above median. BSBR indicates bile salt-binding resin.
Plasma campesterol was reduced by 24.3% (P<0.001) in the 29 patients treated with ezetimibe compared with a nonsignificant 3.2% rise in the 7 subjects given placebo. This resulted in a between-group difference of 27.5% (P<0.001; Table 2). As with plasma sitosterol, plasma campesterol decreased progressively over the 8-week treatment period (Figure 1B).
Table 2 provides mean changes from baseline in lipids and apoproteins. For LDL-C measured by GLC, the median percent change was −13.6% for patients treated with ezetimibe compared with a 16.7% rise for those given placebo. This difference was not statistically significant. LDL-C was relatively low at baseline, with a median value of 2.43 mmol/L. Patients with baseline LDL-C above the median level had a median change of −17.5% compared with 1.5% in patients whose LDL-C at baseline was lower than the median value. Total sterols (including cholesterol and plant sterols) and apolipoprotein B were reduced in the ezetimibe group (−8.7% and −12.7%, respectively) and increased slightly in patients on placebo (3.7% and 3.1%, respectively). The between-group differences (−12.4% and −15.8%, respectively) were significant (P<0.05 for both). Both total sterols and apolipoprotein B were decreased at week 2 and remained generally stable throughout the study. No significant effects of ezetimibe on HDL sterols, HDL-C, apolipoprotein A-I, or triglycerides were observed. In the ezetimibe-treated group, plasma lathosterol (a cholesterol precursor) and the ratio of lathosterol to cholesterol increased progressively, which indicates enhanced biosynthesis. Levels in the placebo group did not change. After 8 weeks, the between-group difference in the ratio of lathosterol to cholesterol was 37.3% (P=0.044).
Achilles Tendon Radiography
At baseline, the mean Achilles tendon thickness, measured by radiography, was 15.7 mm in the placebo group (n=6) and 18.2 mm in the ezetimibe group (n=18). After 8 weeks of treatment, Achilles tendon thickness decreased slightly in the ezetimibe-treated group (−0.6%), and modestly increased in the placebo-treated group (8%) for a significant (P=0.013) between-group difference of −8.6% (95% CI −15.1% to −2.0%).
Safety and Tolerability
Ezetimibe was generally well tolerated, and all patients completed the double-blind treatment period. Overall, 21 (70%) of 30 ezetimibe-treated patients and 2 (40%) of 7 placebo-treated subjects reported 1 or more adverse experiences. The most commonly reported adverse experiences were in the gastrointestinal system in the ezetimibe-treated group, with occurrences in 12 patients (40%). Six of these events were considered by the investigator to be possibly or probably related to the study drug. The gastrointestinal adverse events reported were generally mild and of short duration and included mild abdominal pain, diarrhea, loose and frequent bowel movements, nausea, and toothache.
Safety laboratory studies remained essentially stable during the treatment period. Platelet count increased slightly in the ezetimibe-treated group by a mean of 17 800 (P<0.05) but also rose in the placebo-treated group by a mean of 26 700 (between-group P=NS). Hematocrit rose by 1.1% in the ezetimibe-treated group and decreased by 0.2% in the placebo-treated group. Renal and liver function tests remained essentially unchanged during the double-blind treatment period; no patient receiving ezetimibe experienced elevations in aspartate aminotransferase or alanine aminotransferase of >3-fold or in creatine kinase >10-fold the upper limit of normal.
Discussion
In sitosterolemic individuals, plant sterol absorption is increased and hepatic removal is slowed.11,13,14,19–21 The net effect leads to a progressive accumulation of plant sterols in virtually every tissue except brain, where the blood-brain barrier prevents the uptake of circulating plant sterols (as well as cholesterol).1 Increased cholesterol absorption at the high-normal level is also noted in patients with sitosterolemia.12–19 As a result of plant sterol accumulation, individuals with sitosterolemia are at high risk of atherosclerosis and coronary heart disease–related morbidity and mortality.1–4
Treatment of patients with sitosterolemia has only been partially effective; bile salt-binding resins lower plant sterols but do not lead to normalization, and plasma sterol levels often do not decline when patients are treated with statin drugs that inhibit HMG-CoA reductase activity.3,32,33 Indeed, activities of many of the enzymes along the cholesterol biosynthesis pathway are reduced in liver biopsy samples from sitosterolemic individuals, which perhaps accounts for the lack of efficacy of the statin drugs.34 There are no prior, adequately controlled clinical trials of medications for the treatment of sitosterolemia; hence, the extent of efficacy with other agents is not known. Low-sterol diets are difficult for patients to maintain and are often unsuccessful in substantially lowering plant sterol levels; paradoxical increases in plasma plant sterol levels have even been observed.33 Thus, a significant need exists for new, effective treatments for sitosterolemia.
The results of the present study demonstrated that treatment with ezetimibe produced significant reductions from baseline in sitosterol and campesterol, the predominant plant sterols, as well as reductions in total sterols and apolipoprotein B concentrations. Plant sterols declined even if the subjects were also receiving treatment with bile salt-binding resins or statins, and ezetimibe was similarly effective in both men and women with the disease and in patients with higher or lower sitosterol concentrations at baseline. Plant sterol levels declined progressively, although they remained significantly above the normal range and likely did not reach plateau in the present study. LDL-C reduction with ezetimibe did not achieve statistical significance relative to placebo; however, baseline LDL-C levels were low, and it appeared that individuals with higher baseline LDL-C concentrations had more pronounced reductions. Whether this indicates greater efficacy in patients with elevated baseline LDL-C or a regression to the mean phenomenon can only be assessed with additional study in this patient population. Ezetimibe was well tolerated, with no discontinuations related to adverse experiences. Although more adverse experiences were observed in the active-treatment group, the number of patients in the placebo group was quite small, which limits any meaningful conclusions. Nonetheless, the majority of adverse experiences were mild, transient, and resolved despite continued treatment.
Ezetimibe has been shown to block the intestinal absorption of both cholesterol27 and phytosterols (Harry Davis, PhD, Schering-Plough Research Institute, unpublished observation, 2000); however, the exact mechanism of action has not been defined. The fact that ezetimibe blocks the uptake of cholesterol and plant sterols suggests that they may be absorbed through a common mechanism. Ezetimibe does not appear to affect the expression of the ABCG5 or ABCG8 genes directly.35 The effect of ezetimibe on the function of the ABCG5/G8 proteins cannot be assessed because assays to test for the putative activity of these proteins are not available.
An important observation was that ezetimibe reduced plasma total cholesterol levels rapidly, with essentially the full reduction observed by the second week of double-blind treatment. Cholesterol synthesis increased, as evidenced by the 35% rise in plasma ratio of lathosterol to cholesterol (Table 2). The rise in cholesterol synthesis may explain the failure to see a further decline in total cholesterol. Because plant sterols cannot be synthesized by the human body, progressive decline in these sterols may reflect continued tissue depletion.
Whether the complications of sitosterolemia, including atherosclerosis, hemolysis, and low platelet counts, may be reversed with ezetimibe treatment remains to be seen. Although small increases in platelet counts and hematocrit were observed in the ezetimibe-treated group, a rise in platelets was also observed in patients given placebo. It is interesting that Achilles tendon thickness by radiography, used to assess change in Achilles tendon xanthoma, decreased in the ezetimibe-treated group relative to the placebo group after 8 weeks of treatment. Whether such a change can be substantiated with more dramatic reductions in thickness with long-term treatment must await further study. Nonetheless, this may be an indication that tissue stores of plant sterols were affected by ezetimibe treatment. A trial of long-term treatment is planned to determine whether ezetimibe will lower plant sterol levels further, leading to evidence of diminution in tissue stores and a reduction in cardiovascular disease.
Acknowledgments
This study was sponsored by Merck Research Laboratories on behalf of Merck/Schering-Plough Pharmaceuticals, North Wales, Pa. We thank Anja Kerksiek, Sylvia Winnen, Bibiana Pcolinsky, and Barbara Rouse for excellent technical assistance and Laura O’Grady and Geraldine Macdonell for excellent work in the coordination of this multicenter study.
Appendix
Coinvestigators of the Multicenter Sitosterolemia Study Group
Canada, David Mymin, MD (Winnipeg, Manitoba), and Jiri Frohlich, MD (Vancouver, BC); Finland, Tatu Miettinen, MD (HYKS, Helsinki); France, Eric Bruckert, MD (Paris); Germany, Klaus von Bergmann, MD; Dieter Lütjohann, PhD; and Thomas Sudhop, MD (Bonn), and Ulrich Beil, MD (Hamburg); Norway, Leiv Ose, MD (Oslo); Sweden, Ingamar Bjorkhem, MD (Huddinge); Switzerland, Zeno Stanga, MD (Bern); South Africa, Adrian David Marais, MD (Observatory); Frederick Johan Raal, MD (Parktown); The Netherlands, Anton F. Stalenhoef, MD (Nijmegen); and United States, Robert D. Shamburek, MD (Maryland); William E. Connor, MD (Oregon); Charles Herring, MD (North Carolina); Peter H. Jones, MD (Texas); John Kane, MD, PhD (California); Peter O. Kwiterovich, MD (Maryland); Robert S. Lees, MD (Massachusetts); Timothy O’Brien, MD (Minnesota); Shailesh B. Patel, MD, FRCP (South Carolina); Gerald Salen, MD (New Jersey); Allen Weiss, MD (Florida); Joseph L. Witztum, MD (California); Charles Grant, MD (Minnesota); Holmes Morton, MD (Pennsylvania); Alvin Graber, MD (Indiana); and Brian Henry, MD (South Carolina).
References
- 1.Salen G, Horak I, Rothkopf M, et al. Lethal atherosclerosis associated with abnormal plasma and tissue sterol composition in sitosterolemia with xanthomatosis. J Lipid Res. 1985;26:1126–1133. [PubMed] [Google Scholar]
- 2.Kolovou G, Voudris V, Drogari E, et al. Coronary bypass grafts in a young girl with sitosterolemia. Eur Heart J. 1996;17:965–966. doi: 10.1093/oxfordjournals.eurheartj.a014983. [DOI] [PubMed] [Google Scholar]
- 3.Salen G, Shefer S, Nguyen LB, et al. Sitosterolemia. J Lipid Res. 1992;33:945–955. [PubMed] [Google Scholar]
- 4.Björkhem I, Muri-Boberg K, Leitersdorf E. Inborn errors in bile acid biosynthesis and storage of sterols other than cholesterol. In: Scriver C, Beaudet A, Sly W, et al, eds. The Metabolic Bases of Inherited Disease. New York, NY: McGraw Hill; 2001:2961–2988.
- 5.Berge KE, Tian H, Graf GA, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 6.Lee MH, Lu K, Hazard S, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu K, Lee M-H, Hazard S. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am J Hum Genet. 2001;69:278–290. doi: 10.1086/321294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klett EL, Patel S. Genetic defenses against noncholesterol sterols. Curr Opin Lipidol. 2003;14:341–345. doi: 10.1097/01.mol.0000083763.66245.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weihrauch J, Gardner J. Sterol content of foods of plant origin. J Am Diet Assoc. 1978;73:39–47. [PubMed] [Google Scholar]
- 10.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- 11.Salen G, Ahrens EH, Jr, Grundy SM. Metabolism of beta-sitosterol in man. J Clin Invest. 1970;49:952–967. doi: 10.1172/JCI106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinemann T, Axtmann G, von Bergmann K. Comparison of intestinal absorption of cholesterol with different plant sterols in man. Eur J Clin Invest. 1993;23:827–831. doi: 10.1111/j.1365-2362.1993.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 13.Lütjohann D, Bjorkhem I, Beil UF, et al. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: effect of sitostanol treatment. J Lipid Res. 1995;36:1763–1773. [PubMed] [Google Scholar]
- 14.Bhattacharyya AK, Connor WE, Lin DS, et al. Sluggish sitosterol turnover and hepatic failure to excrete sitosterol into bile cause expansion of body pool of sitosterol in patients with sitosterolemia and xanthomatosis. Arterioscler Thromb. 1991;11:1287–1294. doi: 10.1161/01.atv.11.5.1287. [DOI] [PubMed] [Google Scholar]
- 15.Sudhop T, Sahin Y, Lindenthal B, et al. Comparison of the hepatic clearances of campesterol, sitosterol, and cholesterol in healthy subjects suggests that efflux transporters controlling intestinal sterol absorption also regulate biliary sterol secretion. Gut. 2002;51:860–863. doi: 10.1136/gut.51.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellies M, Ishikawa T, Gartside P, et al. Effects of varying maternal dietary cholesterol and phytosterol in lactating women and their infants. Am J Clin Nutr. 1978;31:1347–1354. doi: 10.1093/ajcn/31.8.1347. [DOI] [PubMed] [Google Scholar]
- 17.Miettinen TA, Farkkila M, Vuoristo M, et al. Serum cholestanol, cholesterol precursors, and plant sterols during placebo-controlled treatment of primary biliary cirrhosis with ursodeoxycholic acid or colchicine. Hepatology. 1995;21:1261–1268. [PubMed] [Google Scholar]
- 18.Nguyen TT, Dale LC, von Bergmann K, et al. Cholesterol-lowering effect of stanol ester in a US population of mildly hypercholesterolemic men and women: a randomized controlled trial. Mayo Clin Proc. 1999;74:1198–1206. doi: 10.4065/74.12.1198. [DOI] [PubMed] [Google Scholar]
- 19.Salen G, Shore V, Tint GS, et al. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J Lipid Res. 1989;30:1319–1330. [PubMed] [Google Scholar]
- 20.Salen G, Tint GS, Shefer S, et al. Increased sitosterol absorption is offset by rapid elimination to prevent accumulation in heterozygotes with sitosterolemia. Arterioscler Thromb. 1992;12:563–568. doi: 10.1161/01.atv.12.5.563. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen T. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: a case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur J Clin Invest. 1980;10:27–35. doi: 10.1111/j.1365-2362.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 22.Belamarich PF, Deckelbaum RJ, Starc TJ, et al. Response to diet and cholestyramine in a patient with sitosterolemia. Pediatrics. 1990;86:977–981. [PubMed] [Google Scholar]
- 23.Sudhop T, Lütjohann D, Kodal A, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 24.Bays HE, Moore PB, Drehobl MA, et al. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin Ther. 2001;23:1209–1230. doi: 10.1016/s0149-2918(01)80102-8. [DOI] [PubMed] [Google Scholar]
- 25.Dujovne CA, Ettinger MP, McNeer JF, et al. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1084–1091. doi: 10.1016/s0002-9149(02)02798-4. [DOI] [PubMed] [Google Scholar]
- 26.Gagne C, Bays HE, Weiss SR, et al. for the Ezetimibe Study Group. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1092–1097. doi: 10.1016/s0002-9149(02)02774-1. [DOI] [PubMed] [Google Scholar]
- 27.van Heek M, Farley C, Compton DS, et al. Ezetimibe selectively inhibits intestinal cholesterol absorption in rodents in the presence and absence of exocrine pancreatic function. Br J Pharmacol. 2001;134:409–417. doi: 10.1038/sj.bjp.0704260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knopp R, Bays H, Manion C. Effect of ezetimibe on serum concentrations of lipid-soluble vitamins. Atheroscler Suppl. 2001;2:90. [Google Scholar]
- 29.Steiner P, Freidel J, Bremmer W, et al. Standardization of micromethods for plasma cholesterol, triglyceride and HDL-cholesterol with the clinics’ methodology. J Clin Chem. 1981;19:850–851. [Google Scholar]
- 30.Warnick G, Albers J. A comprehensive evaluation of the heparin manganese precipitation procedure for estimating high-density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 31.Lipid Research Clinics Program. Manual of Laboratory Operations: Lipid and Lipoprotein Analysis. Washington, DC: US Department of Health, Education and Welfare; 1982. NIH Publication no. 75–628.
- 32.Nguyen LB, Salen G, Shefer S, et al. Unexpected failure of bile acid malabsorption to stimulate cholesterol synthesis in sitosterolemia with xanthomatosis: comparison with lovastatin. Arteriosclerosis. 1990;10:289–297. doi: 10.1161/01.atv.10.2.289. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen LB, Cobb M, Shefer S, et al. Regulation of cholesterol biosynthesis in sitosterolemia: effects of lovastatin, cholestyramine, and dietary sterol restriction. J Lipid Res. 1991;32:1941–1948. [PubMed] [Google Scholar]
- 34.Patel SB, Honda A, Salen G. Sitosterolemia: exclusion of genes involved in reduced cholesterol biosynthesis. J Lipid Res. 1998;39:1055–1061. [PubMed] [Google Scholar]
- 35.Repa JJ, Dietschy JM, Turley SD. Inhibition of cholesterol absorption by SCH 58053 in the mouse is not mediated via changes in the expression of mRNA for ABCA1, ABCG5, or ABCG8 in the enterocyte. J Lipid Res. 2002;43:1864–1874. doi: 10.1194/jlr.m200144-jlr200. [DOI] [PubMed] [Google Scholar]


