Abstract
Glycosaminoglycans (GAGs) are complex polysaccharides that participate in the regulation of physiological processes through the interactions with a wide variety of proteins. Acharan sulfate (AS), isolated from the giant African snail Achatina fulica, primarily consists of the repeating disaccharide structure α-D-N-acetylglucosaminyl (1→4) 2-sulfoiduronic acid. Exogenous AS was injected subcutaneously near the tumor tissue in C57BL/6 mice that had been implanted with Lewis lung carcinoma cells (LLCs). The location of AS in the tumor was assessed by staining of sectioned tissues with alcian blue and periodic acid–Schiff (PAS) reagent. In vitro assays indicated binding of cells to 50 μg/ml AS (or heparin) after a 5-h incubation. Immunofluorescence assays, using anti-AS antibody, detected AS at the cell surface. The outer-surface of LLCs were next biotinylated to identify the AS-binding proteins. Biotinylated cells were lysed, and the lysates were fractionated on the AS affinity column using a stepwise salt gradient (0, 0.1, 0.3, 0.5, 0.7, 1.0, and 2.0 M). The fractions were analyzed by SDS–PAGE with silver staining and western blotting. We focused on the proteins with high affinity for AS (eluting at 1 M NaCl) and detected only two bands by western blotting. ESI Q-TOF MS analysis of one of these bands, molecular weight ~110 kDa, showed it to be nucleolin. A phosphorylated form of nucleolin on the surface of cells acts as a cell surface receptor for a variety of ligands, including growth factors (i.e., basic fibroblast growth factor) and chemokines (i.e., midkine). These results show that nucleolin is one of several AS-binding proteins and suggest that AS might demonstrate its tumor growth inhibitory activity by binding the nucleolin receptor protein on the surface of cancer cells.
Keywords: AS-binding protein, biotinylation, Lewis lung carcinoma, nucleolin
Abbrevations: AS, acharan sulfate; BSA, bovine serum albumin; CAPS, 3-[cyclohexylamino]-1-propanesulfonic acid; DMEM, Dulbecco’s modified Eagle medium; D-PBS, Dulbecco’s phosphate buffered saline; EDTA, ethylenediamine tetraacetic acid; ELISA, enzyme-linked immunosorbent assay; ESI Q-TOF MS, electrospray ionization quadrupole timeof- flight mass spectrometry; FGF, fibroblast growth factor; FITC, fluorescein isothiocyanate; GAG, glycosaminoglycan; HRP, horseradish peroxidase; LLC, Lewis lung carcinoma; MS/MS, tandem mass spectrometry; MTT, methylthiazol-2-yl-2,5-diphenyltetrazolium bromide; PAS, periodic acid-Schiff; PVDF, polyvinylidene difluoride; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; VSV, vesicular stromatitis virus
Introduction
The roles of glycosaminoglycans (GAGs) on tumor growth have been reported for a number of years. In particular, heparin and heparan sulfate can inhibit or stimulate angio-genesis, tumor growth, and metastasis depending on the types of cancer and the animal model (Sasisekharan et al., 2002). Moreover, recent retrospective studies on human subjects treated with heparin and low-molecular-weight heparins show a decreased incidence of cancers (Borsig et al., 2001). GAGs, through their binding and regulation of a large number of ligands and receptors (Capila and Linhardt, 2002), are important mediators of normal and tumor cell behavior, such as proliferation, differentiation, migration, and adhesion. The specific structure of GAG chains and their binding proteins undoubtedly influence tumor cell proliferation, metastasis, and cancer progression (Folkman and Shing, 1992).
GAGs such as heparin, heparan sulfate, chondroitin sulfate, dermatan sulfate, and hyaluronic acid serve as key biological response modifiers by acting as (1) stabilizers, cofactors, and/or coreceptors for growth factors, cytokines, and chemokines; (2) regulators of enzyme activity; (3) signaling molecules in response to cellular damages, such as wound healing, infection, and tumorigenesis; and (4) targets for bacterial, viral, and parasitic virulence factors for attachment, invasion, and immune system (Schmidtchen et al., 2001).
A novel GAG, acharan sulfate (AS), isolated from the body of the giant African snail Achatina fulica, has a primary structure → 4)-2-acetamido-2-deoxy-α-D-glucopyranose( 1 → 4)-2-sulfo-α-L-idopyranosyluronic acid (1 → (GlcNAc-IdoA2SO3−) (Kim et al., 1996). It is related to the heparin and heparan sulfate families of GAGs but is distinctly different from all known members of these classes of GAGs. In previous studies, AS showed antiangiogenic activity in inflammation models (Ghosh et al., 2002), in vivo anticoagulant activity, antimitogenic activity on heparin-mediated basic fibroblast growth factor (FGF-2) (Wang et al., 1997), and immunomodulating action (Shim et al., 2002). AS showed no cytotoxicity (0–200 μg/ml) on tumor cells but rather inhibited tumor growth in vivo through an antiangiogeic mechanism (Lee et al., 2003). A cell binding and immunofluorescence assay showed that AS bound to cell surface molecules in a time- and concentration-dependent fashion. To answer the question of whether any specific binding proteins on the surface of cancer cells can be ascribed to the antitumor activity of AS, the cell surface proteins of Lewis lung carcinoma (LLC) cells were biotinylated, and the cell lysates were fractionated based on AS affinity chromatography. Characterization by mass spectrometry and western blotting suggested that nucleolin corresponded to the AS-binding protein, responsible for AS inhibition of tumor growth.
Results
Effects of AS on cancer cells
We measured the cytotoxicity of the purified AS by the methylthiazol-2-yl-2,5-diphenyltetrazolium bromide(MTT) assay on various cell lines. Incubation of cells for 24 or 48 h with AS at the concentrations of 0, 1, 10, 30, 50, 100, and 200 μg/ml, resulted in no cytotoxicity in any of the cell lines tested (data not shown).
Cell adhesion to AS
One milliliter of AS at concentrations 0–200 μg/ml was coated on each well of a 24-well plate and incubated for 0–7 h with LLCs. The extent of adhesion of LLCs to AS-coated plates was determined. Little adhesion of LLCs was detected when cells were incubated in wells for 0–3 h at all concentrations tested. When an AS concentration of at least 50 μg/ml was coated in a well and incubated with LLCs for 5 or 7 h, significant adhesion of LLCs was detected (see Figure 1). Heparin-coated wells were similarly prepared at 50 μg/ml, and again only wells incubated with LLCs for 5 or 7 h showed cell adhesion. In these experiments it was critical to use the proper number of cells (1.8 × 105) to obtain repeatable results (data not shown).
Fig. 1.
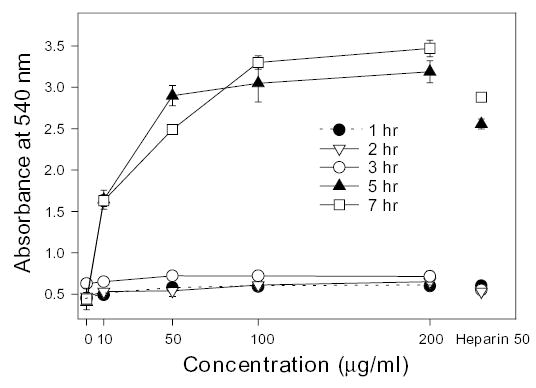
Adhesion of LLC cells to AS-coated plates. The five curves, corresponding to incubation time (1–7 h), show the adhesion of LLC cells to AS-coated 24-well plates as determined by measuring the absorbance at 540 nm as a function of AS concentration (0–200 μg/ml). A fixed concentration of heparin (50 μg/ml) coated to the wells of a plate was incubated for 1–7 h and they are shown on the right for comparison.
Localization of AS to tumor surface and cell surface
In a previous study, AS at a dose of 10 and 30 mg/kg showed a suppressive effect of tumor growth by 32.8% and 38.1% (Lee et al., 2003). In the current study, these tissues were removed and fixed for paraffin-embedded slides, and the slides were stained with alcian blue-periodic acid–Schiff (PAS) to confirm the localization of treated AS. Saline-treated tissues were not stained by alcian blue–PAS (along the outer layer as indicated by the arrow in Figure 2A), whereas AS-treated tissues showed blue spots around the tumor cell surfaces (as indicated by the arrow in Figure 2B). Because −COO− and −OSO3− groups of GAGs are stained by alcian blue-PAS, the blue portions of the slide represent acidic GAGs. We speculate that the suppression of tumor growth may result from interactions between exogenous AS and tumor cell surface as well as the inhibition of angiogenesis (Lee et al., 2003).
Fig. 2.
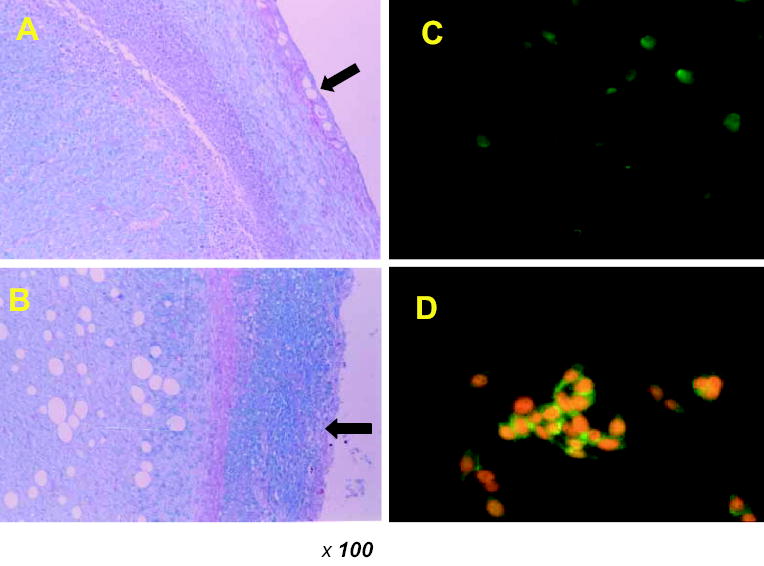
Detection of AS bound to LLC cells by alcian blue–PAS staining and immunofluoresence analysis. (A) Control group; (B) the AS-treated group. On the 21st day, tumor tissues were removed and the fixed tissues were prepared on paraffin-embedded slides. Light micrographs were taken at a magnification of 100×. Cultured LLC cells were incubated with (C) 0 and (D) 100 μg/ml AS for 5 h. After washing, the cells were incubated with anti-AS antibody and bound antibodies were detected using anti-mouse IgG-FITC antibodies.
We next carried out enzyme-linked immunosorbent assay (ELISA) with GAGs having similar structures, including AS, 2-de-O-sulfated AS, heparosan, and dermatan sulfate, to confirm the specificity of anti-AS antibody. This antibody specifically reacted to AS in a concentration-dependent manner (ten Dam et al., 2004). We further investigated the immunolocalization of AS adhering to tumor cell surface. After 5 h incubation of AS and LLCs, AS bound to cell surface was treated with anti-AS antibody and visualized by anti-mouse IgG- fluorescein isothiocyanate (FITC) conjugate. Staining with the anti-AS antibody was observed when AS was incubated at a concentration of 100 μg/ml (Figure 2D). LLC cells, which had not been treated with AS, were not stained and served as a negative control (Figure 2C). When cells were also incubated without the anti-AS antibody, no staining was observed. Permeabilization of cells with 0.1% Triton X-100 in 4% paraformaldehyde did not affect the results (data not shown).
Characterization of AS-binding proteins using SDS–PAGE and western blotting
Recently the application of biotinylation to identify proteins on the cell surface has begun to replace the use of radio-isotopes (Cole et al., 1987). In our experiments, we used water-soluble sulfo-NHS-LC-biotin as a reagent to label LLC membrane proteins. Because biotin binds with a high affinity to streptavidin, biotinylated can be sensitively detected by enzymes or fluorescent tags conjugated to streptavidin. Cells that had been biotinylated at the surface were lysed with lysis buffer containing a 1% NP-40 detergent, and then the lysate was subjected to AS affinity column chromatography to identify AS-binding proteins. The bound proteins were eluted with a stepwise salt gradient (Figure 3A) as described in Materials and methods. The eluted fractions were collected, concentrated, and analyzed using 8% sodiumdodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and visualized by silver staining. Detection of biotinylated proteins on the cell surface by western blotting relied on horseradish peroxidase (HRP)-conjugated streptavidin and o-phenylenediamine (Figure 3B). Each lane in SDS–PAGE contained many protein bands visualized with silver staining, but western blotting showed a smaller number of bands that reacted to HRP-streptavidin. One prominent band of molecular weight ~110 kDa in the high-affinity fraction eluting at 1.0 M NaCl showed a strong response to HRP-streptavidin. Visualization of the blotted membrane with antinucleolin antibody immunologically confirmed the identified protein was nucleolin. Bands at 110 kDa in both 0.7 M and 1.0 M NaCl fractions were also reactive to antinucleolin antibody (Figure 3C). These results suggest that nucleolin is eluted from the immobilized AS column with 0.7 to 1.0 M NaCl.
Fig. 3.
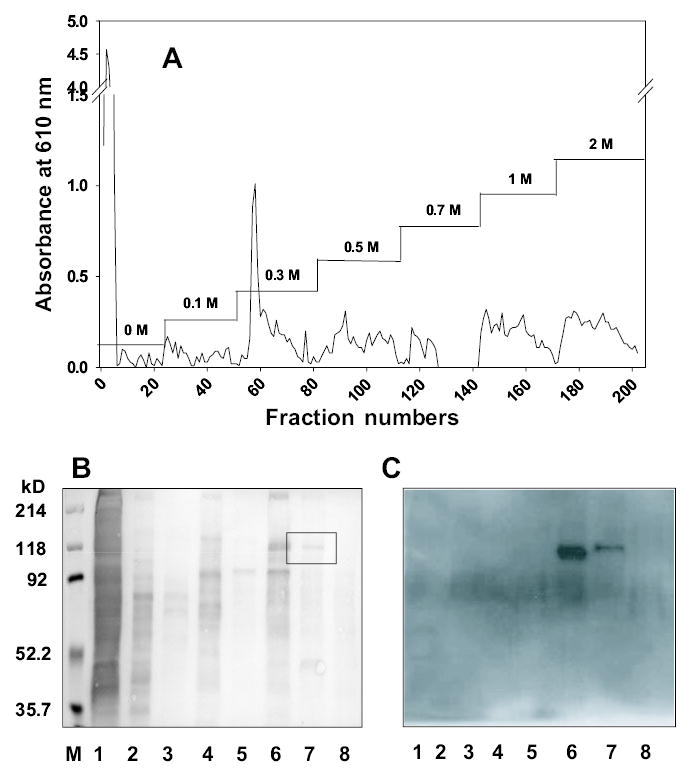
Purification of AS-binding proteins by AS affinity chromatography and western blotting of the collected fractions. (A) Fractionation of proteins from biotinylated cells that were lysed with lysis buffer containing 1% NP-40 detergent. The lysates were subjected to the AS affinity column, and the bound proteins were eluted by a stepwise salt gradient (0–2.0 M NaCl). Each fraction was collected and concentrated. The collections were subjected to electrophoresis on 8% SDS–PAGE and visualized by silver staining; the electroblotted membrane was visualized HRP-conjugated streptavidin (B). The box surrounds the faint band associated with nucleolin in lane 7. After running SDS–PAGE on the fractions obtained by affinity chromatography, the gel was transferred to the PVDF membrane and the membrane was visualized with antinucleolin antibody as described in Materials and methods. Two bands found in the fractions eluted with 0.7 M and 1.0 M NaCl were strongly reactive with antinucleolin antibody (C). The lanes in A and B correspond to M: molecular markers, 1: whole lysate; 2: 0 M NaCl fraction; 3: 0.1 M fraction; 4: 0.3 M fraction; 5: 0.5 M fraction; 6: 0.7 M fraction; 7: 1.0 M fraction; and 8: 2.0 M fraction.
Identification of AS-binding proteins to LLCs using ESI Q-TOF MS
The protein band eluting at a high (1.0 M) salt fraction corresponding to ~110 kDa was trypsinized on the SDS–PAGE gel and the resulting peptides were analyzed by electrospray ionization quadrupole time-of-flight mass spectrometry (ESI Q-TOF MS) using a nanospray high-performance liquid chromatography interfaced with tandem MS (MS/MS). MS data obtained on 5 peptidesmatched with a total 161 scores to murine nucleolin (accession number NM_010880.2 in NCBI database) (Figure 4A). The matched peptides are underscored in bold characters in the nucleolin sequence (Figure 4B). Individual ions with scores over 37 indicate more than 95% identity with a known protein. Murine nucleolin was reported to be composed of 707 amino acids with molecular weight 77 kDa, but it runs on SDS–PAGE with an apparent molecular mass of 100–110 kDa due to the high content of negatively charged amino acids at the amino-terminal domain (Lapeyre et al., 1987).
Fig. 4.
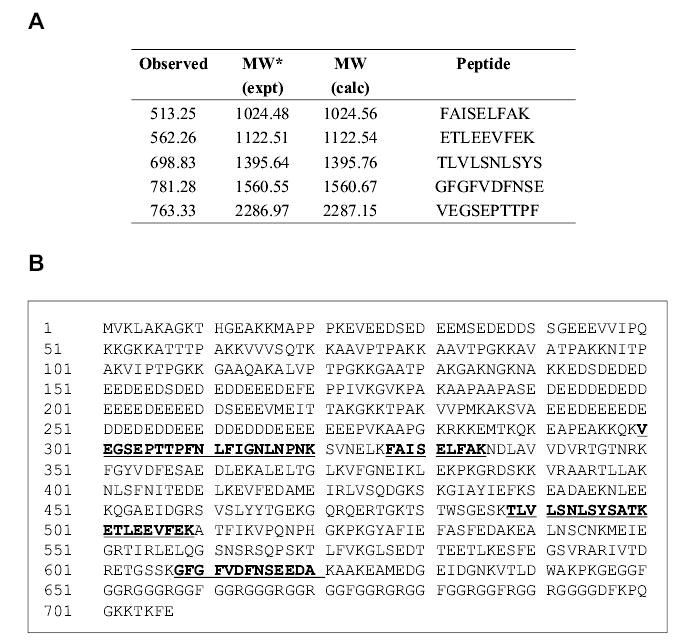
Comparison of the matched peptides within the nucleolin sequence. (A) MS analysis of the AS-binding protein; (B) tryptic matched peptides, which were detected either by MS determination or by MS/MS sequencing (in A) are underscored.
Discussion
GAGs constitute a considerable fraction of glycoconjugates found on cellular membranes and in the extracellular matrix of virtually all mammalian tissues. Their ability to bind proteins (Capila and Linhardt, 2002) play important roles of cellular response in development, homeostasis, and disease. Hyaluronic acid (hyaluronan) (Herrera-Gayol and Jothy, 2001) exerts its biological effects through the binding with cell surface receptors, CD44 (Ahrens et al., 2001) and the receptor for hyaluronan-mediated motility. These interactions work on motility and angiogenesis of endothelial cells (Savani et al., 2001) and as mediators of human malignant mesothelioma cells (Asplund and Heldin, 1994), astrocytomas cells (Akiyama et al., 2001), and breast cancer cells (Assmann et al., 1998). Heparin (Lever and Page, 2002) and heparan sulfate (Sasisekharan et al., 2002) play a central role in regulating biological processes and molecules through the interactions with many proteins, including growth factors, adhesion molecules, cytokines, and extracellular matrix proteins. As an example, heparan sulfate binds to a wide variety of signaling molecules related to tumor development, such as fibroblast growth factor (FGF), vascular endothelial growth factor, transforming growth factor β and platelet-derived growth factor (Sasisekharan et al., 2002). For example, FGF-2 is a potent mitogen that stimulates proliferation, migration, and differentiation of cells as a potent angiogenic factor abundant in normal and malignant cells. FGF-2 has a strong affinity for heparin and heparin-like cell surface receptors that possess intrinsic tyrosine kinase activity (Wang et al., 1997).
The structure of AS is composed of IdoA2SO3− GlcNAc and structurally resembles the heparin/heparan sulfate family of GAGs based on its alternating α1 → 4 linkage and its IdoA2SO3− residue. It is also similar to hyaluronan in the aspect of containing a GlcNAc residue and having a relatively low charge density. IdoA is a uniquely conformationally flexible saccharide residue and appears to play a key role in binding of IdoA containing GAGs to a variety of proteins in cells (Westergren-Thorsson et al., 1991). Although AS is obtained from an invertebrate, its diverse pharmacological activities in mammalian systems may result from its interactions with a wide variety of proteins under physiological conditions. AS shows no cytotoxicity toward various cell types and in vivo, whereas it inhibits the growth of tumor-induced LLC-bearing C57BL/6 mice.
Cell-binding and immunofluorescence assays demonstrate that AS adheres to cell surface proteins in a dose- and time-dependent fashion. Biotinylation of tumor cell surface and AS affinity fractionation of cell lysates demonstrate that although many proteins interact with AS as detected on SDS–PAGE with silver staining, only two biotinylated proteins (~52 and 110 kDa) were detected in the high (1.0 M) salt fraction by western blotting. ESI Q-TOF MS demonstrated that the larger one of these two proteins was nucleolin. The use of antinucleolin antibody confirmed its identity.
Nucleolin was first described by Orrick et al. (1973), and the same protein was then identified from Chinese hamster ovary cells and several other eukaryotic cells, including human, rat, mouse, and chicken. Nucleolin has been described as a major nuclear protein, having an apparent molecular mass of 100–110 kDa as determined by SDS– PAGE and a calculated molecular mass of 76–77 kDa as predicted by the amino acid sequence (Lapeyre et al., 1987). The difference between the calculated and observed molecular masses is most likely due to posttranslational modifications and a high content of negatively charged amino acids in the N-terminal part of nucleolin (Harms et al., 2001). The functions of nucleolin have been studied according to the location of the nucleus, cytoplasm, and cell surface of several cell lines (Srivastava and Pollard, 1999).
Although nucleolin plays an important role in ribosome biogenesis in the nucleolus, it also has been involved as the adhesion receptor L-selectin, which is expressed on leucocytes and hemotopoietic progenitor cells (Harms et al., 2001). It specifically binds apo B and apo E containing lipoprotein to the surface of the HepG2 cells (Semenkovich et al., 1990) and serves as a substrate for an ecto-protein kinase on the cell surface of HeLa cells (Jordan et al., 1994). The neurite-promoting IKVAV site of laminin-1 binds to nucleolin on the cell surface and has been found to promote the differentiation of primary neurons and a variety of neural cell lines (Kleinman et al., 1991). Recently, nucleolin has been shown to interact with the amino-terminal domain of hepatitis δ antigens and modulate hepatitis δ virus replications (Lee et al., 1998). Nucleolin is also associated with the actin cytoskeleton (Hovanessian et al., 2000) and related to inhibit HIV infection by the cytokine midkine (Callebaut et al., 2001). It is very meaningful that nucleolin exists on the cell surface and binds to midkine. Midkine is a 13-kDa heparin-binding growth/differentiation factor structurally unrelated to FGFs and is rich in basic amino acids. Midkine has been reported to promote angiogenesis (Choudhuri et al., 1997), neurite outgrowth, survival of neurons, fibrinolysis and cell growth (Muramatsu et al., 1993), and migration (Maeda et al., 1999) and is overexpressed in a number of human carcinomas, such as colon, breast, lung, bladder, and neuroblastoma. This enhanced expression of midkine implies that it is beneficial to tumor growth and GAG synthesis of endothelial cells (Sumi et al., 2002).
Previously, we reported that AS suppresses tumor growth by the inhibition of angiogenesis. Very recently, it has been reported that nucleolin on the cell surface is a marker of endothelial cells in angiogenic blood vessels (Christian et al., 2003) and participates in both the binding and endocytosis of lactoferrin in target cells (Legrand et al., 2004). From these previous reports, we speculate that nucleolin is one of the proteins on the cell surface that binds AS and its complex might block the growth of cells induced by midkine. Furthermore, we consider that AS and nucleolin can be translocated into cytoplasm by endocytosis and trigger the intracellular communications. A better understanding of these interactions is likely a key in solving the inhibitory mechanism of AS on the tumor growth.
Materials and methods
Materials
Sodium bicarbonate, ethylenediamine tetra-acetic acid (EDTA), HEPES, Dulbecco’s modified Eagle mediun (DMEM), Dulbecco’s phosphate buffered saline (D-PBS), DEAE-Sepharose Fast Flow, alcian blue 8 GX, basic fuchsin, periodic acid, citric acid, phenylmethylsulfonyl fluoride, MTT reagent, 3-[cyclohexylamino]-1-propanesulfonic acid (CAPS), o-phenylenediamine, TRIZMA Base, NP-40, Triton X-100, Tween 20, and protease inhibitor cocktails were purchased from Sigma (St. Louis, MO). Hydrophobic polyvinylidene difluoride (PVDF) membranes were obtained from Amersham Bioscience (Uppsala, Sweden). Poly L-lysine-coated cover glasses (25 mm diameter) were from Iwaki (Tokyo). Anti-mouse IgG (whole molecule)-FITC conjugate and anti-vesicular stromatitis virus (VSV) glycoprotein clone P5D4 were purchased from Sigma-Aldrich. Antinucleolin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). ImmunoPure streptavidin-HRP conjugate, EZ-Link sulfo-NHS-LC-biotin, and EDC/DADPA (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/diaminodipropylamine) were from Pierce (Rockford, IL). Anti-AS antibody tagged with VSV glycoprotein was prepared by phage display technique (ten Dam et al., 2004).
Preparation of AS
AS was isolated from the soft body of the giant African snail by proteolysis of defatted tissue and purified by fractional precipitation and ion-exchange chromatography as previously described (Jeong et al., 2001; Kim et al., 1996).
Measurement of solid tumors induced by LLC in C57BL/6 mice
All animal experiments were carried out in the specific pathogenic-free barrier zone of Clinical Research Institute, Seoul National University Hospital, in accordance with the procedure outlined in the Guide for the Care and Use of Laboratory Animals. Five-week-old, specific pathogen-free male C57BL/6NTac mice were purchased from Samtako Bio (Osan, Korea). LLCs were adjusted to 1 × 106 cells/0.1 ml cold PBS (Lee et al., 2003), and 0.1 ml cell suspension was injected to C57BL/6 mice subcutaneously. After tumor volume was at least 75–90 mm3, mice were randomized and received the samples (saline and AS) subcutaneously around the tumor mass once daily for 24 days (Lee et al., 2003). During the administration period, tumor volumes were measured in two dimensions by caliper every 3 days. Animals were sacrificed by cervical dislocation, and the tumor tissues were fixed in a 40% formaldehyde solution over 24 h.
Alcian blue–PAS staining of tumor tissue sections
The fixed tissues were embedded in paraffin and sectioned with thickness of 5 μm on glass slides. The slides were dried at room temperature overnight, and paraffin sections were stained with alcian blue (pH 2.5)–PAS (Jeong et al., 2001).
Cell culture
Mouse LLC cells were purchased from American Type Culture Collection (Rockville, MD). A549 (human lung adenocarcinoma cell), KM 1214 (human colon carcinoma cell), and Caki-1 (human kidney carcinoma cell) were obtained from KCLB (Korea Cell Line Bank, Seoul). These cell lines were cultured in DMEM containing 10% fetal bovine serum at 37°C in a humidified, 5% CO2 atmosphere.
Cytotoxicity assay
Cytotoxicity of AS was measured by the MTT method. Briefly, cultured LLC, A549, Caki-1, and KM 1214 cells were trypsinized and spread on 96-well plates at a density of 3 × 104 cells/well. After incubation for 24 h, the various concentrations of diluted AS (0–200 μg/ml) were treated to the culture plate. The plates were incubated at 37°C for 24 h in 5% CO2. Cell viability was determined based on mitochondrial conversion of MTT to formazan. The amount of MTT reduced to formazan is indicative of the number of viable cells (Pumphrey et al., 2002). Each well was supplemented with 200 μl 0.5 mg/ml MTT solution for 3 h. The solution was carefully removed from each well and 200 μl dimethyl sulfoxide was added. The plates were gently agitated until the formazan (precipitate) was dissolved. The extent was measured by absorbance at 540 nm using a microplate reader.
Cell binding assay
In this binding assay, the 24-well (1.85 cm diameter) plates were coated with 0–200 μg AS in 1 ml of a coating solution and dried overnight at 37°C or room temperature. These plates were blocked with 3% bovine serum albumin (BSA) in D-PBS for 1 h at 37°C and then washed twice with 0.1% BSA in D-PBS (Engbring et al., 2002). The cells, cultured in DMEM containing 0.1% BSA (starvation medium) to remove traces of serum were added to each well and incubated at 37°C for varying times up to 7 h. Each well was washed with 0.1% BSA in D-PBS, and the unbound cells were removed from the plate with trypsin-EDTA. The adherent cells were stained for 10 min with 0.2% crystal violet and washed twice with D-PBS. The cells were lysed with 250 μl 10% SDS, and the absorbance of the solubilized crystal violet was measured at 540 nm. Each assay was performed in triplicate at least four times.
ELISA
To test the stability of an anti-AS antibody, the stock solutions of several GAGs (AS, dermatan sulfate, heparosan, and de-O-sulfated AS) were diluted to proper concentrations (0–100 μg/100 μl) in the coating solution. Aliquots of 100 μl diluted solutions were added to a 96-well plate and incubated to dry overnight at 4°C (Alban and Gastpar, 2001). The unbound GAGs were discarded by washing with PBS–0.05% Tween 20 (PBST) three times. The plate was necessarily washed with PBST three times for 10 min every step. After blocking by 2% BSA-PBST, the plate was incubated with 100 μl anti-AS antibody tagged with the His-VSV diluted 1:10 overnight at 4°C or at 37°C for 2 h. Then, 100 μl of mouse anti-VSV antibody (clone P5D4) was added to each well and incubated at 37°C for 1 h. After washing, 100 μl anti-mouse IgG conjugated with FITC diluted by 1:100 was added to the plate and incubated at 37°C for 1 h. After last washing, 100 μl of PBST was added to the plate for fluorescence detection. The fluorescence was determined at excitation 490 nm and emission 525 nm.
Immunofluorescence staining for cultured cells
LLC cells were grown on glass coverslips in DMEM containing 10% fetal bovine serum for 24 h. After incubation, AS was added to the cells for 5 h. Each well was washed with PBS and fixed in 4% paraformaldehyde or 0.1% Triton X-100 in 4% PFA for 10 min at room temperature (Goicoechea et al., 2000). To quench autofluorescence, the cells were blocked with 2% BSA-PBS for 1 h at 37°C, followed by 30 min incubation in 0.02 M glycine (Asplund and Heldin, 1994). Then the cells were incubated with the primary antibody-anti-AS antibody tagged with His-VSV (1:10) overnight at 4°C. After washing with 2% BSA-PBS three times, the cells were incubated with mouse anti-VSV antibody (clone P5D4) at 37°C for 1 h and washed three times. Then anti-mouse IgG-FITC conjugate (1:100 or 1:64) and propidium iodide were treated to the coverslips at 37°C for 1 h. After washing with 0.1% Triton X-100 in PBS and PBST three times, respectively, the coverslips were mounted in an antifading agent. The samples were visualized by fluorescence microscopy.
Biotinylation of cell surface proteins
LLC cells grown in culture dishes were washed with ice-cold PBS (without added serum or protein). Each 1.0 ml of cell suspension (107 cells) was added to 0.5 mg/ml EZ-Link sulfo-NHS-LC-biotin in PBS (pH 7.4) (Goicoechea et al., 2000), and the cells were incubated at 4°C for 45 min. The biotinylation reaction was terminated by addition of 50 mM Tris–HCl (pH 7.5). Then the cells were washed in PBS and lysed in lysis buffer (50 mM Tris–HCl, pH 7.4, 1 mM EDTA, 150 mM NaCl, 1% NP 40, 1 mM phenylmethylsulfonyl fluoride, and a mixture of protease inhibitors). These lysates were centrifuged at 20,000 × g at 4°C for 30 min. The supernatant was stored at −80°C until being used for affinity chromatography.
Preparation of AS affinity column
A diaminodipropylamine column was extensively washed by water and conjugation buffer, pH 4.7 (0.9% NaCl in 0.1 M 2-[N-morpholino] ethanesulfonic acid), respectively, five times. After washing, 250 mg of AS in 25 ml conjugation buffer was mixed with the gel. Then 750 mg EDC dissolved in conjugation buffer was added to the gel-AS slurry, and they were mixed by shaking for 3 h at room temperature. The resulting affinity column was washed with conjugation buffer containing 1.0 M NaCl. The total amount of the immobilized AS was quantified by carbazole assay (Bitter and Muir, 1962).
Purification of biotinylated surface proteins
Solubilized biotinylated surface proteins from LLC cells were purified on the AS affinity column. The column was incubated with the supernatant (cell lysate) and washed with the running buffer (50 mM Tris–HCl, 1 mM EDTA). The column was eluted sequentially with 25 ml of the running buffer containing 0, 0.1, 0.3, 0.5, 0.7, 1.0, and 2.0 M NaCl. Each fraction was concentrated by ultrafiltration (molecular weight cutoff 10,000).
SDS–PAGE and western blotting
Each fraction was separated by SDS–PAGE on 8% and 10% gels. After electrophoresis, gels were stained with either Coomassie blue or 0.1% silver staining solution. Alternatively, gels were electrotransferred to the PVDF membranes at 0.25 A at 4°C for 16–17 h in CAPS buffer. The membranes were then blocked for 1.5 h at room temperature with 3% BSA in TBS buffer containing 0.1% Tween 20 (TBST). The blots were incubated with HRP-conjugated streptavidin (1:10,000 dilution) for 1.5 h at room temperature. The membranes were washed four times with TBST for 30 min. For visualization, HRP was reacted to o-phenylenediamine in a phosphate-citrate buffer (pH 5.0). To confirm more specific bands, the electrotransblotted membranes were blocked with 5% skim milk in TBST overnight at 4°C. Blots were incubated with mouse antinucleolin antibody–conjugated HRP (at a 1:50 dilution) for 1.5 h at room temperature. After washing, the membranes were detected with ECL plus reagent.
ESI Q-TOF MS
The each gel spot was reduced by dithiothreitol, alkylated with iodoacetic acid, and digested with trypsin. The resulting peptides were dissolved in 0.1% acetic acid for MS analysis (Wu et al., 2002). To identify proteins by ESI Q-TOF MS, all MS/MS experiments for peptide sequencing were performed using a nano-LC/MS system consisting of an Ultimate HPLC system (LC Packings, Netherlands) and a Q-TOF2 mass spectrometer (Micro-mass, UK) equipped with a nano-ESI source. Each sample (10 μl) was loaded by the autosampler (FAMOS, LC Packings) onto a C18 trap column (ID 300 μm, length 5 mm, particle size 5 μm; LC Packings) for desalting and concentration at a flow rate 30 μl/min. Then the peptides trapped were back-flushed and separated on a C18 nano-column (ID 75 μm, length 150 mm, particle size 5 μm; LC Packings). The gradient used was 0% acetonitrile for 10 min, followed by 0% to 50% acetonitrile over 80 min and 50% acetonitrile for 10 min at a flow rate 150 nl/min. In the nano-ESI source, the end of the capillary tubing from the nano-LC column was connected to pico-tip silica tubing (ID 5 μm; New Objectives, USA). The applied voltage to liquid junction to produce an electrospray was 1.5–2.0 kV, and cone voltage was set at 30 V. Argon was introduced as a collision gas at a pressure of 10 psi. MS/MS spectra were acquired in data-dependant MS/MS mode, for which collision energy was increased to 25, 30, and 35 eV. For protein identification, MS/MS spectra were searched by MASCOT (Matrix Science, UK) or manually sequenced by Masslynx software 3.5 (Micro-mass, UK). Proteins containing at least one significant peptide (≥ individual score) were selected from database search results.
Acknowledgments
This work was supported by the KOSEF grant RO1-1999-2-209-010-5 (to Y.S.K.) and Korea-Japan Joint Project F01-2002-000-20017-0. We thank the Korea Basic Science Center for ESI Q-TOF MS service.
References
- Ahrens T, Assmann V, Fieber C, Termeer C, Herrlich P, Hofmann M, Simon JC. CD44 is the principal mediator of hyaluronicacid-induced melanoma cell proliferation. J Invest Dermatol. 2001;116:93–101. doi: 10.1046/j.1523-1747.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- Akiyama Y, Jung S, Salhia B, Lee S, Hubbard S, Taylor M, Mainprize T, Akaishi K, van Furth W, Rutka JT. Hyaluronate receptors mediating glioma cell migration and proliferation. J Neurooncol. 2001;53:115–127. doi: 10.1023/a:1012297132047. [DOI] [PubMed] [Google Scholar]
- Alban S, Gastpar R. Development of SPC-ELISA: a new assay principle for the study of sulfated polysaccharide-protein interactions. J Biomol Screen. 2001;6:393–400. doi: 10.1177/108705710100600605. [DOI] [PubMed] [Google Scholar]
- Asplund T, Heldin P. Hyaluronan receptors are expressed on human malignant mesothelioma cells but not on normal mesothelial cells. Cancer Res. 1994;54:4516–4523. [PubMed] [Google Scholar]
- Assmann V, Marshall JF, Fieber C, Hofmann M, Hart IR. The human hyaluronan receptor RHAMM is expressed as an intracellular protein in breast cancer cells. J Cell Sci. 1998;111:1685–1694. doi: 10.1242/jcs.111.12.1685. [DOI] [PubMed] [Google Scholar]
- Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA. 2001;98:3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut C, Nisole S, Briand JP, Krust B, Hovanessian AG. Inhibition of HIV infection by the cytokine midkine. Virology. 2001;281:248–264. doi: 10.1006/viro.2000.0767. [DOI] [PubMed] [Google Scholar]
- Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Choudhuri R, Zhang HT, Donnini S, Ziche M, Bicknell R. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res. 1997;57:1814–1819. [PubMed] [Google Scholar]
- Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol. 2003;163:871–878. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SR, Ashman LK, Ey PL. Biotinylation: an alternative to radioiodination for the identification of cell surface antigens in immunoprecipitates. Mol Immunol. 1987;24:699–705. doi: 10.1016/0161-5890(87)90051-4. [DOI] [PubMed] [Google Scholar]
- Engbring JA, Hoffman MP, Karmand AJ, Kleinman HK. The B16F10 cell receptor for a metastasis-promoting site on laminin-1 is a heparan sulfate/chondroitin sulfate-containing proteoglycan. Cancer Res. 2002;62:3549–3554. [PubMed] [Google Scholar]
- Folkman J, Shing Y. Control of angiogenesis by heparin and other sulfated polysaccharides. Adv Exp Med Biol. 1992;313:355–364. doi: 10.1007/978-1-4899-2444-5_34. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Hirasawa N, Lee YS, Kim YS, Shin KH, Ryu N, Ohuchi K. Inhibition by acharan sulphate of angiogenesis in experimental inflammation models. Br J Pharmacol. 2002;137:441–448. doi: 10.1038/sj.bjp.0704886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea S, Orr AW, Pallero MA, Eggleton P, Murphy-Ullrich JE. Thrombospondin mediates focal adhesion disassembly through interactions with cell surface calreticulin. J Biol Chem. 2000;275:36358–36368. doi: 10.1074/jbc.M005951200. [DOI] [PubMed] [Google Scholar]
- Harms G, Kraft R, Grelle G, Volz B, Dernedde J, Tauber R. Identification of nucleolin as a new L-selectin ligand. Biochem J. 2001;360:531–538. doi: 10.1042/0264-6021:3600531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Gayol A, Jothy S. Effects of hyaluronan on the invasive properties of human breast cancer cells in vitro. Int J Exp Pathol. 2001;82:193–200. doi: 10.1046/j.1365-2613.2001.iep0082-0193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian AG, Puvion-Dutilleul F, Nisole S, Svab J, Perret E, Deng JS, Krust B. The cell-surface-expressed nucleolin is associated with the actin cytoskeleton. Exp Cell Res. 2000;261:312–328. doi: 10.1006/excr.2000.5071. [DOI] [PubMed] [Google Scholar]
- Jeong J, Toida T, Muneta Y, Kosiishi I, Imanari T, Linhardt RJ, Choi HS, Wu SJ, Kim YS. Localization and characterization of acharan sulfate in the body of the giant African snail Achatina fulica. Comp Biochem Physiol B. 2001;130:513–519. doi: 10.1016/s1096-4959(01)00468-7. [DOI] [PubMed] [Google Scholar]
- Jordan P, Heid H, Kinzel V, Kubler D. Major cell surfacelocated protein substrates of an ecto-protein kinase are homologs of known nuclear proteins. Biochemistry. 1994;33:14696–14706. doi: 10.1021/bi00253a007. [DOI] [PubMed] [Google Scholar]
- Kim YS, Jo YY, Chang IM, Toida T, Park Y, Linhardt RJ. A new glycosaminoglycan from the giant African snail Achatina fulica. J Biol Chem. 1996;271:11750–11755. doi: 10.1074/jbc.271.20.11750. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Weeks BS, Cannon FB, Sweeney TM, Sephel GC, Clement B, Zain M, Olson MO, Jucker M, Burrous BA. Identification of a 110-kDa nonintegrin cell surface lamininbinding protein which recognizes an A chain neurite-promoting peptide. Arch Biochem Biophys. 1991;290:320–325. doi: 10.1016/0003-9861(91)90547-v. [DOI] [PubMed] [Google Scholar]
- Lapeyre B, Bourbon H, Amalric F. Nucleolin, the major nucleolar protein of growing eukaryotic cells: an unusual protein structure revealed by the nucleotide sequence. Proc Natl Acad Sci USA. 1987;84(6):1472–1476. doi: 10.1073/pnas.84.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Chang SC, Chen CJ, Chang MF. The nucleolin binding activity of hepatitis delta antigen is associated with nucleolus targeting. J Biol Chem. 1998;273:7650–7656. doi: 10.1074/jbc.273.13.7650. [DOI] [PubMed] [Google Scholar]
- Lee YS, Yang HO, Shin KH, Choi HS, Jung SH, Kim YM, Oh DK, Linhardt RJ, Kim YS. Suppression of tumor growth by a new glycosaminoglycan isolated from the African giant snail Achatina fulica. Eur J Pharmacol. 2003;465:191–198. doi: 10.1016/s0014-2999(03)01458-4. [DOI] [PubMed] [Google Scholar]
- Legrand D, Vigie K, Said EA, Elass E, Masson M, Slomianny MC, Carpentier M, Briand JP, Mazurier J, Hovanessian AG. Surface nucleolin participates in both the binding and endocytosis of lactoferrin in target cells. Eur J Biochem. 2004;271:303–317. doi: 10.1046/j.1432-1033.2003.03929.x. [DOI] [PubMed] [Google Scholar]
- Lever R, Page CP. Novel drug development opportunities for heparin. Nat Rev Drug Discov. 2002;1:140–148. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M. A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J Biol Chem. 1999;274:12474–12479. doi: 10.1074/jbc.274.18.12474. [DOI] [PubMed] [Google Scholar]
- Muramatsu H, Shirahama H, Yonezawa S, Maruta H, Muramatsu T. Midkine, a retinoic acid-inducible growth/differentiation factor: immunochemical evidence for the function and distribution. Dev Biol. 1993;159:392–402. doi: 10.1006/dbio.1993.1250. [DOI] [PubMed] [Google Scholar]
- Orrick LR, Olson MO, Busch H. Comparison of nucleolar proteins of normal rat liver and Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA. 1973;70:1316–1320. doi: 10.1073/pnas.70.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumphrey CY, Theus AM, Li S, Parrish RS, Sanderson RD. Neoglycans, carbodiimide-modified glycosaminoglycans: a new class of anticancer agents that inhibit cancer cell proliferation and induce apoptosis. Cancer Res. 2002;62:3722–3728. [PubMed] [Google Scholar]
- Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat Rev Cancer. 2002;2:521–528. doi: 10.1038/nrc842. [DOI] [PubMed] [Google Scholar]
- Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem. 2001;276:36770–36778. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- Schmidtchen A, Frick IM, Bjorck L. Dermatan sulphate is released by proteinases of common pathogenic bacteria and inactivates antibacterial alpha-defensin. Mol Microbiol. 2001;39:708–713. doi: 10.1046/j.1365-2958.2001.02251.x. [DOI] [PubMed] [Google Scholar]
- Semenkovich CF, Ostlund RE, Jr, Olson MO, Yang JW. A protein partially expressed on the surface of HepG2 cells that binds lipoproteins specifically is nucleolin. Biochemistry. 1990;29:9708–9713. doi: 10.1021/bi00493a028. [DOI] [PubMed] [Google Scholar]
- Shim JY, Lee YS, Jung SH, Choi HS, Shin KH, Kim YS. Pharmacological activities of a new glycosaminoglycan, acharan sulfate isolated from the giant African snail Achatina fulica. Arch Pharm Res. 2002;25:889–894. doi: 10.1007/BF02977010. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Pollard HB. Molecular dissection of nucleolin’s role in growth and cell proliferation: new insights. FASEB J. 1999;13:1911–1922. [PubMed] [Google Scholar]
- Sumi Y, Muramatsu H, Takei Y, Hata K, Ueda M, Muramatsu T. Midkine, a heparin-binding growth factor, promotes growth and glycosaminoglycan synthesis of endothelial cells through its action on smooth muscle cells in an artificial blood vessel model. J Cell Sci. 2002;115:2659–2667. doi: 10.1242/jcs.115.13.2659. [DOI] [PubMed] [Google Scholar]
- ten Dam GB, van de Westerlo EM, Smesters TF, Willemse M, van Muijen GN, Merry CL, Gallagher JT, Kim YS, van Kuppevelt TH. Detection of 2-O-sulfated iduronate and N-acetylglucoamine units in heparan sulfate by an antibody selected against acharan sulfate (IdoA2S-GlcNAc)n. J Biol Chem. 2004;279:38346–38352. doi: 10.1074/jbc.M404166200. [DOI] [PubMed] [Google Scholar]
- Wang H, Toida T, Kim YS, Capila I, Hileman RE, Bernfield M, Linhardt RJ. Glycosaminoglycans can influence fibroblast growth factor-2 mitogenicity without significant growth factor binding. Biochem Biophys Res Commun. 1997;235:369–373. doi: 10.1006/bbrc.1997.6789. [DOI] [PubMed] [Google Scholar]
- Westergren-Thorsson G, Onnervik PO, Fransson LA, Malmstrom A. Proliferation of cultured fibroblasts is inhibited by L-iduronate-containing glycosaminoglycans. J Cell Physiol. 1991;147:523–530. doi: 10.1002/jcp.1041470319. [DOI] [PubMed] [Google Scholar]
- Wu SL, Amato H, Biringer R, Choudhary G, Shieh P, Hancock WS. Targeted proteomics of low-level proteins in human plasma by LC/MSn: using human growth hormone as a model system. J Proteome Res. 2002;1:459–465. doi: 10.1021/pr025537l. [DOI] [PubMed] [Google Scholar]


