Abstract
To evaluate the pathophysiology of altered cortisol secretion in patients with primary adrenal hypercortisolism, cortisol secretion was investigated in 12 patients, seven with a unilateral adenoma and five with ACTH-independent macronodular adrenal hyperplasia compared with age- and gender-matched controls and with patients with pituitary-dependent hypercortisolism. Pulsatile secretion was increased 2-fold (P = 04), attributable to increased event frequency (P = 0.002). All patients showed a significant diurnal rhythm with a delay in phase shift of 3 h (P = 0.01). Approximate entropy ratio, a feedback-sensitive measure, was increased compared with controls (P = 0.00003) but similar to that of pituitary-dependent hypercortisolism (P = 0.77), denoting loss of autoregulation. Cortisol burst-mass tended to be smaller in patients with ACTH-independent macronodular adrenal hyperplasia than in unilateral adenoma (P = 0.06). In conclusion, increased cortisol secretion in patients with primary adrenal Cushing’s syndrome is caused by amplified pulsatile secretion via event frequency modulation. We speculate that partial preservation of secretory regularity and diurnal rhythmicity point to incomplete autonomy of these tumors.
Abbreviations: ACTH-R, ACTH receptor; AIMAH, ACTH-independent bilateral macronodular adrenal hyperplasia; ApEn, approximate entropy; HSD, honestly significantly different; PVN, paraventricular nucleus of the hypothalamus; SCN, suprachiasmatic nucleus
Primary adrenal cushing’s syndrome is characterized biochemically by increased 24-h cortisol synthesis, low or undetectable plasma ACTH concentrations, and a diminished diurnal rhythm. The primary adrenal form of Cushing’s syndrome is caused by either unilateral adrenal adenoma, exceptionally a cortisol-producing adrenal carcinoma, and rarely, bilateral pigmented micronodular hyperplasia or ACTH-independent bilateral macronodular adrenal hyperplasia (AIMAH). The hallmarks of the latter syndrome are bilateral nodular enlargement of the adrenal glands and clinical and biochemical signs of cortisol excess, associated with low or undetectable serum ACTH (1).
In patients with pituitary-dependent Cushing’s disease, ACTH and cortisol secretory activity has been studied in detail by sampling blood at 10-min intervals for 24 h. Hypercortisolism in this disease is characterized by increased basal and pulsatile secretion, as a result of increased secretory burst frequency and mean burst mass, and marked deterioration of secretory regularity (2). ACTH secretion displayed similar disruption but to a more marked extent (3, 4). Clinically, cortisol excess from primary adrenal causes or from pituitary-(ACTH)-dependent disease leads to the same detrimental catabolic state; however, there is no detailed knowledge of cortisol secretory abnormalities in the primary adrenal form. The pathogenetic mechanisms underlying the various clinical forms of hypercortisolism are different, but because the same end-organ is involved, i.e. the adrenal gland, we postulated some comparability of the secretory process. In particular, we tested the hypotheses that first, patients with adrenal Cushing’s syndrome display increased basal and pulsatile cortisol secretion, via increased burst frequency and burst mass, and more disorderly cortisol secretion patterns compared with age- and gender-matched controls. Second, we speculated that fundamental secretory differences between unilateral and bilateral adrenal pathology provide insights into distinct secretory pathophysiologies (5, 6).
Subjects and Methods
Twelve consecutive patients with primary adrenal Cushing’s syndrome, 12 patients with pituitary-dependent Cushing’s disease, and 12 healthy controls matched for gender and age were studied. The diagnosis of primary adrenal Cushing’s syndrome was established by elevated 24-h urinary excretion of free cortisol, subnormal or absent suppression of plasma cortisol concentrations after administration of 1 mg dexamethasone overnight, absent or subnormal suppression of urinary cortisol excretion during a low-dose dexamethasone test, and low or undetectable plasma ACTH concentrations. After establishing the biochemical diagnosis of ACTH-dependent Cushing’s syndrome, a computed tomography scan and/or magnetic resonance imaging scan of the adrenal glands was performed to identify the adrenal source of cortisol overproduction. All patients were operated with resection of the abnormal adrenal gland(s), resulting in complete resolution of Cushing’s syndrome. Histological diagnosis confirmed an adrenocortical adenoma in seven patients and bilateral macronodular hyperplasia in the remaining five patients (Table 1).
TABLE 1.
Clinical characteristics of 12 patients with primary adrenal Cushing’s syndrome
| Patient | Sex | Age (yr) | Diagnosis | Urinary cortisol excretion (nmol/24 h) | Size of adrenal gland(s) (CT/MRI) |
|---|---|---|---|---|---|
| 1 | F | 59 | UAA | 617 | 5 cm |
| 2 | F | 48 | UAA | 1017 | 2.8 cm |
| 3 | F | 43 | UAA | 300 | 3.5 cm |
| 4 | F | 21 | UAA | 2414 | 2.5 cm |
| 5 | F | 40 | UAA | 1677 | 2.0 cm |
| 6 | M | 58 | UAA | 490 | 4.8 cm |
| 7 | F | 25 | UAA | 1359 | 5.2 cm |
| 8 | M | 78 | AIMAH | 399 | Right, 3 cm; left, 2 cm |
| 9 | F | 41 | AIMAH | 1031 | Right, 2.5 cm; left, 3.4 cm |
| 10 | F | 48 | AIMAH | 641 | Right, 2.5 cm; left, 5 cm |
| 11 | F | 50 | AIMAH | 407 | Right, 2.8 cm; left, 2 cm |
| 12 | F | 45 | AIMAH | 429 | Right, 4.8 cm; left, 4.1 cm |
UAA, Unilateral adrenal adenoma; CT, computed tomography; MRI, magnetic resonance imaging; F, female; M, male. Normal upper limit for urinary free cortisol excretion is 220 nmol/24 h.
Patients with pituitary-dependent Cushing’s disease were diagnosed by elevated 24-h urinary excretion of free cortisol, subnormal or absent suppression of plasma cortisol after administration of 1 mg dexamethasone overnight, absent or subnormal suppression of urinary cortisol excretion during a low-dose dexamethasone test, suppression of plasma cortisol by 190 nmol/liter or more during a 7-h iv infusion of dexamethasone at 1 mg/h (7), positive pituitary adenoma immunostaining for ACTH, and clinical cortisol dependency for several months after selective removal of the adenoma. Data on cortisol and ACTH secretory characteristics have been published before (2). Here the cortisol data are used for comparison with those of patients with primary adrenal cortisol excess.
Methods
Patients and control subjects were admitted to the hospital on the day of the study. An indwelling iv cannula was inserted in a forearm vein at least 60 min before the start of blood sampling. Blood samples were withdrawn at 10-min intervals for 24 h, starting at 0900 h. A slow infusion of 0.9% NaCl and heparin (1 U/ml) was used to keep the line open. The subjects were confined to their room and instructed not to sleep during the daytime. Meals were served at 0800, 1230, and 1730 h. Lights were turned off between 2200 and 2400 h. Plasma for cortisol measurement was collected, centrifuged at 4 C for 10 min, and stored at −20 C until later analysis. The study was approved by the ethical board of the Leiden University Medical Center, and informed written consent was obtained from all patients and control subjects.
Assays
Plasma cortisol concentrations were measured by RIA (Sorin Biomedica, Milan, Italy). The detection limit of the assay was 25 nmol/liter. The interassay variation varied from 2–4% at the concentrations obtained in this study.
Deconvolution analysis
A multiparameter deconvolution technique was used to estimate relevant measures of cortisol secretion from the 24-h serum cortisol concentration profiles, as described previously (8). Initial estimates of basal cortisol secretion rate were calculated with two component half-lives to approximate the lowest 5% of all plasma cortisol concentrations in the time series. Biexponential cortisol decay was defined by a rapid-phase half-life of 3.8 min, a slow-phase half-life determined analytically in each subject, and fractional (slow/total) decay amplitude of 0.67. The following four secretory and clearance measures of interest were estimated: 1) the number and locations of secretory events, 2) the amplitudes of secretory bursts, 3) the durations of randomly dispersed cortisol secretory bursts, and 4) the endogenous slow-component subject-specific plasma half-life of cortisol. It was assumed the cortisol distribution volume and half-lives were time and concentration invariant. The following parameters were calculated: secretory burst frequency, mean interburst interval, slow component of half-life, burst mass, basal secretion rate (time-invariant), pulsatile secretion rate, and their sum, viz. total secretion rate (9). Secretory pulse identification for cortisol required that the estimated secretory-burst amplitude exceeded zero by 95% joint statistical confidence intervals. Based upon cortisol model simulations, this statistical requirement affords 95% sensitivity and 93% specificity of cortisol pulse detection for 10-min data (10).
Cluster analysis
Cluster, a largely model-free computerized peak-detection algorithm, was used to identify statistically significant pulses in relation to dose-dependent measurement error in the cortisol concentration vs. time series (11). The 10-min samples were used to calculate cortisol burst frequency (number of significant burst/24 h), interpulse interval (time separating consecutive peak maxima), burst duration in minutes, height (maximal hormone concentration in a burst), area (burst mass), and increment (increase above nadir), along with interpulse valley mean and nadir concentrations. The variance model used in Cluster analysis was the between-replicate sd expressed as a power function of dose. Test cluster sizes were 2 × 1 in the moving nadir and peak with t = 2.0 as the significance level for both upstrokes and downstrokes in the data.
Approximate entropy
The univariate approximate entropy (ApEn) statistic was developed to quantify the degree of irregularity, or disorderliness, of a time series (12). High values of ApEn signify disruption of coordinate (interlinked) control of the secretory process and thus reflect degree of autonomy. Technically, ApEn quantifies the summed logarithmic likelihood that templates (of length m) of patterns in the data that are similar (within r) remain similar (within the same tolerance r) on the next incremental comparison and has been formally defined elsewhere (13). The ApEn calculation provides a single nonnegative number, which is an ensemble estimate of relative process randomness, wherein larger ApEn values denote greater irregularity, as observed for ACTH in Cushing’s disease, GH in acromegaly, and prolactin in prolactinomas (3, 14, 15). ApEn results are reported as the ratio of the absolute value to that of the mean of 1000 randomly shuffled data series. Ratio values that approach 1.0 thus denote mean empirical randomness. In addition, we applied ApEn to the serial interburst interval and burst-mass values from the deconvolution analysis. Thereby, we quantitate relative randomness of serial interburst interval and burst-mass values. For these measures, m = 1 and r = 85% are appropriate (16).
Nyctohemeral (24-h) rhythmicity
Diurnal variations in plasma cortisol concentrations were appraised by Cosinor analysis, as reported earlier (17). Ninety-five percent statistical confidence intervals were determined for the 24-h cosine amplitude (50% of the nadir-zenith difference), mesor (rhythmic mean), and acrophase (clock time of maximal value).
Statistical analysis
Results are expressed as the mean ± sem. Comparison between groups was done with one-way ANOVA, followed post hoc by Tukey’s honestly significantly different (HSD) test to contrast means. Derived measures (deconvolution and ApEn) were transformed logarithmically before analysis to limit dispersion of variance. In addition, linear regression was applied to evaluate the relation between relevant variables. The two forms of primary adrenal disease (unilateral vs. bilateral) were compared with the Kolmogorov-Smirnov test. Calculations were carried out with Systat (release 10, SPSS, Inc., Chicago, IL). Differences were considered significant for P < 0.05.
Results
The clinical characteristics of the 12 patients with primary adrenal Cushing’s syndrome are shown in Table 1. All patients met the biochemical criteria for primary adrenal Cushing’s syndrome. Radiological studies showed unilateral adrenal adenoma in seven patients and bilateral macronodular hyperplasia in five patients.
Cortisol secretion
Figure 1 illustrates the plasma cortisol concentration profiles in five patients with unilateral disease and in five patients with bilateral pathology. Pulsatile and total secretion was increased 2-fold compared with healthy controls and attributable to increased pulse frequency (28.8 ± 1.9 vs. 17.5 ± 0.9 bursts/24 h, P = 0.002; Fig. 2). Burst mass and half-life did not differ between the adrenal patient group and controls. In addition, no significant differences in cortisol secretion were present between primary adrenal hypercortisolism and pituitary-dependent hypercortisolism (Table 2). The fractional contribution of pulsatile secretion to total secretion was decreased in pituitary-dependent hypercortisolism but comparable in adrenal disease and healthy controls (Table 2).
Fig. 1.
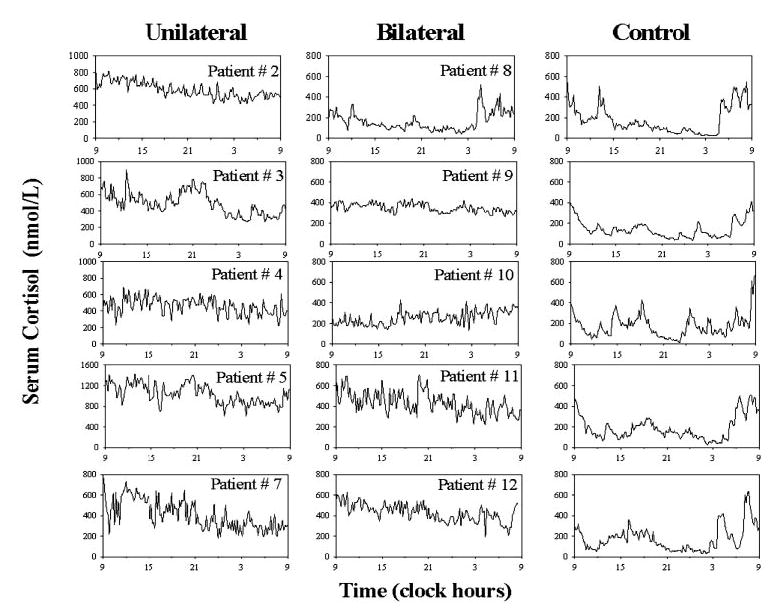
Cortisol concentration profiles, obtained by 10-min blood sampling for 24 h. Data are from patients with unilateral adenoma (left), AIMAH (middle), and controls (right).
Fig. 2.
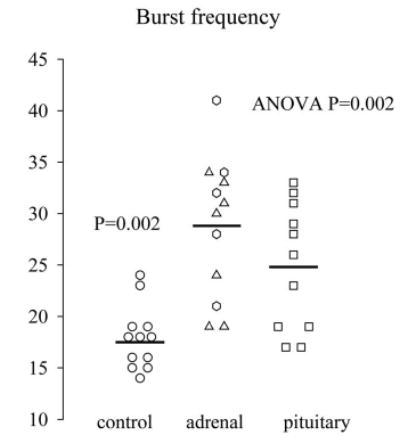
Burst frequency (number of significant pulses/24 h) estimated by multiparameter deconvolution analysis (Subjects and Methods). In primary adrenal hypercortisolism, mean frequency (events/24 h) was 29, in pituitary-dependent hypercortisolism 25, and in controls 18.
TABLE 2.
Deconvolution of the plasma cortisol concentration profiles
| Primary adrenal Cushing’s syndrome | Pituitary Cushing’s disease | Controls | ANOVA | |
|---|---|---|---|---|
| Half-life (min) | 65.4 ± 2.8 | 60.9 ± 2.9 | 62.0 ± 1.5 | 0.73 |
| Secretory-burst half duration (min) | 10.6 ± 2.3a | 7.3 ± 2.0 | 12.5 ± 1.2 | 0.05 |
| Mean inter-burst interval (min) | 53 ± 4b | 63 ± 7 | 81 ± 4 | 0.001 |
| Burst mass (nmol/liter) | 260 ± 38 | 260 ± 39 | 214 ± 18 | 0.89 |
| Basal secretion (nmol/liter · 24 h) | 1610 ± 620 | 710 ± 270 | 360 ± 60 | 0.43 |
| Pulsatile secretion (nmol/liter · 24 h) | 7550 ± 1270c | 6390 ± 1240 | 3720 ± 240 | 0.01 |
| Total secretion (nmol/liter · 24 h) | 9160 ± 1615d | 7780 ± 1160 | 4125 ± 240 | 0.004 |
| Percentage pulsatile secretion | 85 ± 4 | 77 ± 5.0e | 91 ± 1.0 | 0.048 |
Results are expressed as the mean ± sem. Comparison between groups was done with one-way ANOVA, followed post hoc by Tukey’s HSD test to contrast means. Derived measures were transformed logarithmically before analysis to limit dispersion of variance.
P values of primary adrenal Cushing’s syndrome vs. controls:
0.002;
0.001;
0.04;
0.005.
P = 0.038 vs. controls. No significant differences were found between pituitary-dependent and primary-adrenal hypercortisolism.
Complementary to the deconvolution analysis, the plasma cortisol profiles were analyzed by Cluster as a model-independent approach (Fig. 3). In patients with the primary adrenal form of Cushing’s syndrome, the increased cortisol secretion, reflected by the integrated area, was caused by increased burst frequency and increased valley (nadir) concentration. Secretory burst duration in patients was shorter and the pulse height increased compared with healthy controls resulting in no net increase of mean burst area. No significant differences in any of these parameters were present between patients with pituitary-dependent and primary-adrenal hypercortisolism.
Fig. 3.
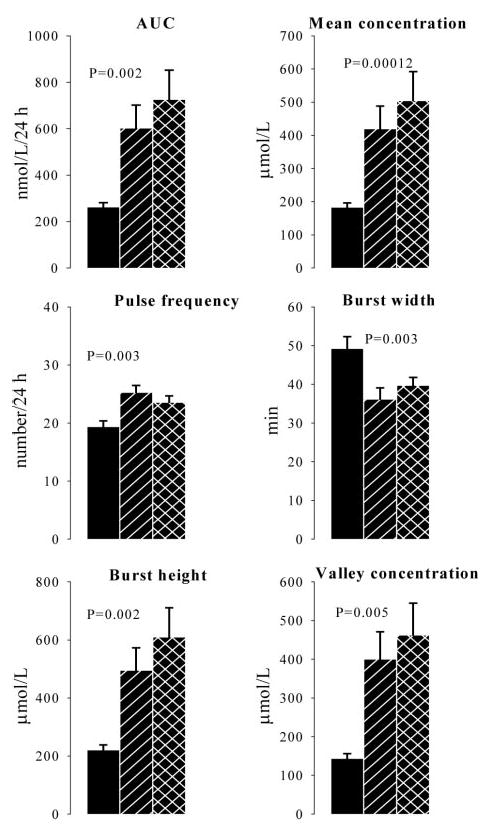
Derived parameters of cortisol output based on Cluster analysis. Solid bars represent control subjects, slashed bars patients with primary-adrenal hypercortisolism, and crossed bars patients with pituitary-dependent hypercortisolism. P values indicate significance of contrast between patients with primary-adrenal hypercortisolism and healthy subjects.
Nyctohemeral variation
Cosinor analysis showed a significant diurnal rhythm in all patients with primary adrenal Cushing’s syndrome and in pituitary-dependent hypercortisolism. The mesor (mean) was increased in primary adrenal Cushing’s syndrome compared with controls but similar in the two forms of hypercortisolism. The amplitudes in the three groups were similar (Table 3). Of note was that the acrophase in primary adrenal Cushing’s syndrome was approximately 3 h delayed compared with controls and pituitary-dependent hypercortisolism (Table 3).
TABLE 3.
Cosinor analysis of the 24-h serum cortisol concentration series
| Primary adrenal | Pituitary disease | Control | P value (primary adrenal vs. pituitary Cushing’s disease) | P value (primary adrenal Cushing’s syndrome vs. controls) | |
|---|---|---|---|---|---|
| Mesor (nmol/liter)a | 390 ± 67 | 406 ± 63 | 136 ± 9 | 0.57 | 0.0009 |
| Amplitude (nmol/liter)b | 83 ± 10.4 | 81 ± 18.5 | 79 ± 10.8 | 0.93 | 0.64 |
| Ratio amplitude/mean | 0.28 ± 0.06 | 0.21 ± 0.04 | 0.57 ± 0.04 | 0.65 | 0.0006 |
| Acrophase (clock hours ± min)c | 1346 ± 72 | 1020 ± 91 | 1025 ± 34 | 0.01 | 0.01 |
Data are shown as mean ± sem. Comparison between groups was done with one-way ANOVA, followed post hoc by Tukey’s HSD test to contrast means. Derived measures were transformed logarithmically before analysis to limit dispersion of variance.
Mean value about which the 24-h rhythm varies.
50% of the nadir-to-zenith difference in cortisol concentration.
Time of maximum value.
Approximate entropy
The secretory process regularity of cortisol was disrupted in primary adrenal Cushing’s syndrome compared with healthy controls, with an increased ApEn ratio (0.793 ± 0.047 vs. 0.553 ± 0.025, P = 0.00003) but similar to that in pituitary-dependent hypercortisolism (0.826 ± 0.029, P = 0.77) (Fig. 4). Both ratios are less than unity by more than 10 sd values, thus denoting measurable orderliness and regulated feedback. A unit ApEn defines empirically mean random, or apparent complete loss of integrative control. We further quantitated the regularity of the burst mass and interval, estimated by deconvolution of the concentration-time series. Neither burst mass nor burst interval regularity differed significantly between the three investigated groups (ANOVA P = 0.38 and P = 0.40, respectively). Thus, subordinate secretion rather than the pulse-renewal process is strongly disrupted in cortisol excess of adrenal and pituitary origin.
Fig. 4.
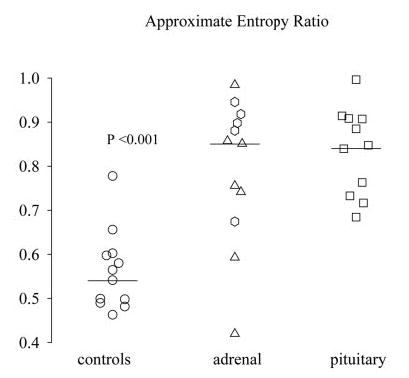
ApEn (feedback-sensitive regularity), expressed as ratio of observed to random series, in patients with pituitary-dependent hypercortisolism, patients with primary-adrenal hypercortisolism, and healthy subjects. Patients with unilateral adenoma are represented by triangles and patients with bilateral nodular hyperplasia by diamonds. The P value reflects the difference in mean ApEn ratio between primary-adrenal hypercortisolism and controls. ApEn ratios were similar in the different forms of hypercortisolism.
Comparisons between unilateral vs. bilateral nodular disease
The mean cortisol mass released per burst tended to be decreased in patients with AIMAH (179 ± 35 nmol/liter vs. 317 ± 51 nmol/liter, P = 0.06). However, basal, pulsatile and total cortisol secretions were similar in both groups (Table 4).
TABLE 4.
Deconvolution of plasma cortisol profiles of patients with unilateral and bilateral adrenal adenomas
| Unilateral (7) | Bilateral (5) | P value | |
|---|---|---|---|
| Basal secretory rate (nmol/liter·min) | 1.004 ± 0.502 | 1.278 ± 0.829 | 0.78 |
| Half-life (min) | 65.6 ± 1.4 | 65.2 ± 6.9 | 0.95 |
| Secretory-burst half duration (min) | 14.0 ± 3.5 | 8.8 ± 0.4 | 0.06 |
| No. of secretory bursts/24 h | 27.1 ± 2.4 | 31.2 ± 3.3 | 0.35 |
| Mean burst interval (min) | 55 ± 6 | 48 ± 5 | 0.38 |
| Burst mass (nmol/liter) | 317 ± 51 | 179 ± 35 | 0.06 |
| Secretory burst amplitude (nmol/liter·min) | 32.5 ± 7.6 | 34.1 ± 8.1 | 0.88 |
| Basal secretion (nmol/liter·24 h) | 1,445 ± 720 | 1,840 ± 1,190 | 0.78 |
| Pulsatile secretion | 8,980 ± 1,860 | 5,555 ± 1,280 | 0.16 |
| Total secretion | 10,425 ± 2,380 | 7,400 ± 1,990 | 0.35 |
Data are shown as mean ± sem. Comparison between groups was done with the two-tailed Student’s t test.
Cosinor analysis of plasma cortisol concentration-time series in unilateral adenomas disclosed a 2-fold increase in amplitude over values in AIMAH (Table 5).
TABLE 5.
Cosinor analysis of plasma cortisol profiles in patients with primary unilateral and bilateral adrenal hypercortisolism
| Unilateral (7) | Bilateral (5) | P value | |
|---|---|---|---|
| Mean (nmol/liter)a | 454 ± 106 | 302 ± 52 | 0.23 |
| Amplitude (nmol/liter)b | 106 ± 10 | 51 ± 7.7 | 0.004 |
| Ratio amplitude/mean | 0.33 ± 0.08 | 0.20 ± 0.07 | 0.29 |
| Acrophase (clock hours ± min)c | 1450 ± 59 | 1218 ± 154 | 0.68 |
Data are shown as mean ± sem. Difference between the groups were calculated with Kruskal-Wallis test.
Mean value about which the 24-h rhythm varies.
50% of the nadir-to-zenith difference in cortisol concentration.
Time of maximum value.
The regularity of the cortisol secretion process was equally disrupted for unilateral adenoma compared with hyperplasia (ApEn ratio 0.74 ± 0.07 vs. 0.86 ± 0.05, P = 0.19) (Fig. 2). Mean urinary 24-h cortisol secretion was slightly but not significantly higher in patients with a unilateral adenoma compared with bilateral adrenal enlargement (mean, 1056 ± 311 vs. 581 ± 121 nmol/24 h, P = 0.25).
Discussion
The present comprehensive analysis of 24-h cortisol secretory activity in consecutive patients with primary adrenal Cushing’s disease shows that hypercortisolism in this setting is caused by 2-fold increased pulsatile cortisol secretion. Augmented pulsatile secretion was primarily a result of increased burst frequency. In addition, the regularity of the cortisol secretory process was decreased in patients. All patients had a significant diurnal rhythm but showed a 3-h delay in time of maximal serum concentrations. Unilateral adenoma and bilateral macronodular hyperplasia behaved similarly.
In contrast to pituitary-dependent hypercortisolism where adrenal secretion is driven by tumor ACTH output, the basic abnormality in primary hypercortisolism is by definition located in the adrenal gland(s). Nevertheless, cortisol secretory patterns were very similar. Pulsatile cortisol secretion in the primary adrenal form was enhanced predominantly via increased burst frequency and not, in contradistinction with the pituitary-dependent form (and other pituitary adenomas, including prolactinoma and somatotropinomas), via amplitude and frequency modulation (2, 14, 15).
From a clinical perspective, the underlying cause of primary adrenal Cushing’s syndrome, e.g. unilateral adenoma vs. AIMAH, usually cannot be established from the presence of specific signs or symptoms, and the present results demonstrate that the serum cortisol profile also does not add to the differential diagnosis. In both circumstances, signs of cortisol excess dominate the clinical picture. There is increasing evidence that pathologically excessive adrenocortical steroidogenesis may be mediated, at least in some cases, by non-ACTH circulating hormones for which their respective (functional) receptors are expressed in the adrenal tumors. Thus, several studies observed aberrant stimulation of cortisol secretion in response to gastric inhibitory peptide, exogenous arginine vasopressin, catecholamines, LH/β-human chorionic gonadotropin, serotonin receptor agonists, angiotensin II, and leptin in AIMAH and, rarely, in unilateral adrenal adenomas (1). For instance, in a recent study, aberrant receptors for gastric inhibitory peptide were found in four of eight AIMAH, but only one of 16 unilateral adenoma patients (6). In addition, the pathogenesis of primary adrenal Cushing’s syndrome may include persistent expression of the ACTH receptor (ACTH-R) on adrenocortical adenoma cells, with suppression of ACTH-R on neighboring nonneoplastic cells (18). Indeed, a close linear correlation between P450scc mRNA, the rate-limiting step in adrenal steroidogenesis, and ACTH-R mRNA has been found in (benign) adrenal adenoma and may explain the rise in serum cortisol after ACTH administration (18–20). However, it does not explain the pulsatile cortisol secretion as we observed here, because until now no activating mutation of the receptor was described in adenomas (21).
Because of the fundamentally different pathogeneses of the two forms of hypercortisolism (monoclonal vs. polyclonal), we did not expect the secretion characteristics, estimated by two independent techniques in a limited number of patients, to be comparable. In fact, differences were minor and limited to the magnitude of cortisol secretory-burst mass (22). A recent prospective study in 21 patients with unilateral adrenal incidentaloma with subclinical autonomous cortisol hypersecretion demonstrated aberrant adrenal sensitivity to multiple ligands in vivo (23) and supports the emerging notion that functional differences between uninodular and bilateral adrenal adenoma might be less pronounced than has been assumed in the past.
The adrenal gland is a complex organ, richly innervated (both cortex and medulla) by splanchnic nerves and by an intrinsic peptidergic system. Interactions occur between chromaffin cells and cortical cells, especially in the many dispersed islets of cortical cells in the medulla and islets of chromaffin cells in the cortex. In addition, the peptidergic system in conjunction with sympathetic neuronal input supervises steroid (cortisol) output in (patho)physiology (24–26). Neuropeptides apparently involved in paracrine actions include vasoactive intestinal peptide, galanin, vasopressin, neuropeptide Y, pituitary adenylate cyclase-activating polypeptide, atrial natriuretic peptide, enkephalin, orexin, CRH, ghrelin, and agouti-related protein (10, 27–32). Loss or partial loss of steroidogenic control by paracrine mechanisms may be relevant to the increased cortisol pulse frequency in adrenal adenoma and hyperplasia.
Decreased secretory regularity is observed in somatotropinomas and prolactinomas and also in parathyroid hyperplasia of renal failure. Thus, inferred erosion of negative feedback control may be a hallmark of endocrine tumors. In our patients, cortisol secretion regularity was clearly decreased but nevertheless highly significantly (>10 sd) different from purely random. These observations and others in tumor states indicate that benign glandular tumors are still under measurable influence of controlling hormonal signals. Indeed, treatment of acromegalic patients with octreotide partially restores GH secretion regularity, similar to the effect of somatostatin in healthy individuals (33, 34). If aberrant receptors in bilateral nodular hyperplasia maintain responsiveness to the corresponding agonists, this pathway would impose partial (albeit abnormal) regularity of timing and mass of cortisol secretory events. In cortical adenomas, regularity might be enforced by paracrine effects of (peptidergic) neurons, which are found in these tumors (35). A potential negative feedback signal to steroid secretion contributing to regularity might be increased concentrations of leptin associated with hypercortisolism, which appears to suppress corticosteroid secretion by normal and adenomatous adrenal tissue (36–39).
A significant diurnal rhythm persisted in all the patients studied here, albeit with a phase delay of approximately 3 h. These observations indicate that ACTH, which is markedly suppressed, is not an absolute prerequisite for cortisol rhythmicity. For the maintenance of biological rhythms, the suprachiasmatic nucleus (SCN) is essential. The axis between the SCN and the paraventricular nucleus of the hypothalamus (PVN) is crucial for the organization and synchronization of the neuroendocrine and autonomic nervous system with the time of day (40). The SCN-neuroendocrine PVN axis governs timely hormonal secretion, while at the same time the SCN-autonomic PVN axis finely tunes receptor-mediated actions of the corresponding hormones. Essential for the latter concept in case of the adrenal gland was the demonstration of an anatomical and functional multisynaptic pathway between the adrenal gland and the SCN (41). Depending on circadian timing, a light signal decreased corticosterone in rats or increased cortisol in the human (41, 42). Other reports also pointed to the functional significance of the autonomic system for glucocorticoid secretion. Adrenal innervation modulates sensitivity to ACTH stimulation in several species, including dog, calf, and sheep, and sectioning of the splanchnic nerves decreases whereas stimulation enhances steroidogenic responsiveness (43, 44). In other experiments, nerve stimulation increased steroid release independently of ACTH, probably via local release of neurotransmitters (45). Moreover, men with spinal cord injuries manifest impaired adrenal stimulation by ACTH (46). Other basic studies relate adrenal innervation to the normal diurnal variation in cortisol secretion (47). Collectively, these experimental findings in animals and clinical data in the human suggest that autonomic neuronal input via the SCN may contribute to the (modified) diurnal cortisol rhythm observed in human adrenal tumors in the absence of the ACTH oscillatory signal.
The only other type of adrenal adenomas studied in a comparable way is the aldosteronoma. In 10 patients with proven primary aldosteronism, basal and pulsatile secretion was greatly amplified, but in contrast to cortisol-producing adenomas, pulsatile steroid secretion was enhanced by increased pulse mass rather than increased pulse frequency (48). Interestingly, all tumors had a significant diurnal secretory rhythm but without phase shifting of the acrophase, observed here in cortisol-producing adenomas. Similar to the present findings, aldosterone-secreting adenomas had decreased secretory regularity. The contrasts in secretion characteristics suggest that different control mechanisms operate in the adrenal tumors originating from different steroidogenic cell types.
In conclusion, increased cortisol secretion in patients with primary adrenal Cushing’s syndrome is caused by an amplified frequency of discrete secretory events with significant but not complete loss of secretory regularity and preservation of a (modified) diurnal rhythm. These collective features suggest that intra- and/or extra-adrenal regulatory signals and attendant communication are key features allowing persistence and preservation of cortisol secretion characteristics and the diurnal rhythm, albeit clearly modified, in primary hypercorticism.
References
- 1.Lacroix A, Ndiaye N, Tremblay J, Hamet P. Ectopic and abnormal hormone receptors in adrenal Cushing’s syndrome. Endocr Rev. 2001;22:75–110. doi: 10.1210/edrv.22.1.0420. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berg G, Frölich M, Veldhuis JD, Roelfsema F. Combined amplification of the pulsatile and basal modes of adrenocorticotropin and cortisol secretion in patients with Cushing’s disease: evidence for decreased responsiveness of the adrenal glands. J Clin Endocrinol Metab. 1995;80:3750–3757. doi: 10.1210/jcem.80.12.8530629. [DOI] [PubMed] [Google Scholar]
- 3.Van den Berg G, Pincus SM, Veldhuis JD, Frölich M, Roelfsema F. Greater disorderliness of ACTH and cortisol release accompanies pituitary-dependent Cushing’s disease. Eur J Endocrinol. 1997;136:394–400. doi: 10.1530/eje.0.1360394. [DOI] [PubMed] [Google Scholar]
- 4.Roelfsema F, Pincus SM, Veldhuis JD. Patients with Cushing’s disease secrete adrenocorticotropin and cortisol jointly more asynchronously than healthy subjects. J Clin Endocrinol Metab. 1998;83:688–692. doi: 10.1210/jcem.83.2.4570. [DOI] [PubMed] [Google Scholar]
- 5.Beuschlein F, Reincke M, Karl M, Travis WD, Jaursch-Hancke C, Abdelhamid S, Chrousos GP, Allolio B. Clonal composition of human adrenocortical neoplasms. Cancer Res. 1994;54:4927–4932. [PubMed] [Google Scholar]
- 6.Groussin L, Perlemoine K, Contesse V, Lefebvre H, Tabarin A, Thieblot P, Schlienger JL, Luton JP, Bertagna X, Bertherat J. The ectopic expression of the gastric inhibitory polypeptide receptor is frequent in adrenocorticotropin-independent bilateral macronodular adrenal hyperplasia, but rare in unilateral tumors. J Clin Endocrinol Metab. 2002;87:1980–1985. doi: 10.1210/jcem.87.5.8458. [DOI] [PubMed] [Google Scholar]
- 7.Biemond P, de Jong FH, Lamberts SW. Continuous dexamethasone infusion for seven hours in patients with the Cushing syndrome: a superior differential diagnostic test. Ann Intern Med. 1990;112:738–742. doi: 10.7326/0003-4819-112-10-738. [DOI] [PubMed] [Google Scholar]
- 8.Johnson ML, Veldhuis JD. Evolution of deconvolution analysis as a hormone pulse detection method. Methods Neurosci. 1995;28:1–24. doi: 10.1016/S0076-6879(04)84004-7. [DOI] [PubMed] [Google Scholar]
- 9.Veldhuis JD, Carlson ML, Johnson ML. The pituitary gland secretes in bursts: appraising the nature of glandular secretory impulses by simultaneous multiple-parameter deconvolution of plasma hormone concentrations. Proc Natl Acad Sci USA. 1987;84:7686–7890. doi: 10.1073/pnas.84.21.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci USA. 2001;98:4028–4033. doi: 10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 12.Veldhuis JD, Pincus SM. Orderliness of hormone release patterns: a complementary measure to conventional pulsatile and circadian analyses. Eur J Endocrinol. 1998;138:358–362. doi: 10.1530/eje.0.1380358. [DOI] [PubMed] [Google Scholar]
- 13.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartman ML, Pincus SM, Johnson ML, Matthews DH, Faunt LM, Vance ML, Thorner MO, Veldhuis JD. Enhanced basal and disorderly growth hormone secretion distinguish acromegalic from normal pulsatile growth hormone release. J Clin Invest. 1994;94:1277–1288. doi: 10.1172/JCI117446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groote Veldman R, van den Berg G, Pincus SM, Frölich M, Veldhuis JD, Roelfsema F. Increased episodic release and disorderliness of prolactin secretion in both micro- and macroprolactinomas. Eur J Endocrinol. 1999;140:192–200. doi: 10.1530/eje.0.1400192. [DOI] [PubMed] [Google Scholar]
- 16.Pincus SM, Hartman ML, Roelfsema F, Thorner MO, Veldhuis JD. Hormone pulsatility discrimination via coarse and short time sampling. Am J Physiol Endocrinol Metab. 1999;277:E948–E957. doi: 10.1152/ajpendo.1999.277.5.E948. [DOI] [PubMed] [Google Scholar]
- 17.Veldhuis JD, Iranmanesh A, Johnson ML, Lizarralde G. Twenty-four-hour rhythms in plasma concentrations of adenohypophyseal hormones are generated by distinct amplitude and/or frequency modulation of underlying pituitary secretory bursts. J Clin Endocrinol Metab. 1990;71:1616–1623. doi: 10.1210/jcem-71-6-1616. [DOI] [PubMed] [Google Scholar]
- 18.Imai T, Sarkar D, Shibata A, Funahashi H, Morita-Matsuyama T, Kikumori T, Ohmori S, Seo H. Expression of adrenocorticotropin receptor gene in adrenocortical adenomas from patients with Cushing syndrome: possible contribution for the autonomous production of cortisol. Ann Surg. 2001;234:85–91. doi: 10.1097/00000658-200107000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reincke M, Beuschlein F, Latronico AC, Arlt W, Chrousos GP, Allolio B. Expression of adrenocorticotrophic hormone receptor mRNA in human adrenocortical neoplasms: correlation with P450scc expression. Clin Endocrinol (Oxf) 1997;46:619–626. doi: 10.1046/j.1365-2265.1997.1991009.x. [DOI] [PubMed] [Google Scholar]
- 20.Bertagna C, Orth DN. Clinical and laboratory findings and results of therapy in 58 patients with adrenocortical tumors admitted to a single medical center (1951–1978) Am J Med. 1981;71:855–875. doi: 10.1016/0002-9343(81)90384-3. [DOI] [PubMed] [Google Scholar]
- 21.Latronico AC, Reincke M, Mendonca BB, Arai K, Mora P, Allolio B, Wajchenberg BL, Chrousos GP, Tsigos C. No evidence for oncogenic mutations in the adrenocorticotropin receptor gene in human adrenocortical neoplasms. J Clin Endocrinol Metab. 1995;80:875–877. doi: 10.1210/jcem.80.3.7883845. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Cano SJ, de Miguel M, Blanes A, Tashjian R, Galera H, Wolfe HJ. Clonality as expression of distinctive cell kinetics patterns in nodular hyperplasias and adenomas of the adrenal cortex. Am J Pathol. 2000;156:311–319. doi: 10.1016/S0002-9440(10)64732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reznik Y, Lefebvre H, Rohmer V, Charbonnel B, Tabarin A, Rodien P, Lecomte P, Bardet S, Coffin C, Mahoudeau J REHOS Study Group . Aberrant adrenal sensitivity to multiple ligands in unilateral incidentaloma with subclinical autonomous cortisol hypersecretion: a prospective clinical study. Clin Endocrinol (Oxf) 2004;61:311–319. doi: 10.1111/j.1365-2265.2004.02048.x. [DOI] [PubMed] [Google Scholar]
- 24.Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19:101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- 25.Toth IE, Hinson JP. Neuropeptides in the adrenal gland: distribution, localization of receptors, and effects on steroid hormone synthesis. Endocr Res. 1995;21:39–51. doi: 10.3109/07435809509030419. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Johansson H, Kjellman M, Grimelius L. Neuroendocrine differentiation and nerves in human adrenal cortex and cortical lesions. APMIS. 1998;106:807–817. doi: 10.1111/j.1699-0463.1998.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 27.Dhillo WS, Small CJ, Gardiner JV, Bewick GA, Whitworth EJ, Jethwa PH, Seal LJ, Ghatei MA, Hinson JP, Bloom SR. Agouti-related protein has an inhibitory paracrine role in the rat adrenal gland. Biochem Biophys Res Commun. 2003;301:102–107. doi: 10.1016/s0006-291x(02)02991-1. [DOI] [PubMed] [Google Scholar]
- 28.Andreis PG, Malendowicz LK, Frejter M, Neri G, Spinazzi R, Rossi GP, Nussdorfer GG. Ghrelin and growth hormone secretagogue receptor are expressed in the rat adrenal cortex: evidence that ghrelin stimulates the growth but not the secretory activity of adrenal cells. FEBS Lett. 2003;536:173–179. doi: 10.1016/s0014-5793(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 29.Mazzocchi G, Malendowicz LK, Gottardo L, Aragona F, Nussdorfer GG. Orexin A stimulates cortisol secretion from human adrenal cortical cells through activation of the adenylate-dependent signalling cascade. J Clin Endocrinol Metab. 2001;86:778–782. doi: 10.1210/jcem.86.2.7233. [DOI] [PubMed] [Google Scholar]
- 30.Randeva HS, Karteris E, Grammatopoulos D, Hillhouse EW. Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis. J Clin Endocrinol Metab. 2001;86:4808–4813. doi: 10.1210/jcem.86.10.7921. [DOI] [PubMed] [Google Scholar]
- 31.Rossi GP, Andreis PG, Colonna S, Albertin G, Aragona F, Belloni AS, Nussdorfer GG. Endothelin-1[1–31]: a novel autocrine-paracrine regulator of human adrenal cortex secretion and growth. J Clin Endocrinol Metab. 2002;87:322–328. doi: 10.1210/jcem.87.1.8134. [DOI] [PubMed] [Google Scholar]
- 32.Willenberg HS, Bornstein SR, Hiroi N, Path G, Goretzki PE, Scherbaum WA, Chrousos GP. Effects of a novel corticotrophin-releasing-hormone receptor type I antagonist on human adrenal function. Mol Psychiatry. 2000;5:137–141. doi: 10.1038/sj.mp.4000720. [DOI] [PubMed] [Google Scholar]
- 33.Biermasz NR, Pereira AM, Frölich M, Romijn JA, Veldhuis JD, Roelfsema F. Octreotide represses secretory-burst mass and nonpulsatile secretion but does not restore event frequency or orderly secretion in acromegaly. Am J Physiol Endocrinol Metab. 2004;286:E25–E30. doi: 10.1152/ajpendo.00230.2003. [DOI] [PubMed] [Google Scholar]
- 34.Fahry LS, Veldhuis JD. Joint pituitary-hypothalamic and intrahypothalamic autofeedback construct of pulsatile growth hormone secretion. Am J Physiol Integr Comp Physiol. 2003;285:R1240–R1249. doi: 10.1152/ajpregu.00086.2003. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Johansson H, Grimelius L. Innervation of human adrenal gland and adrenal cortical lesions. Virchows Arch. 1999;435:580–589. doi: 10.1007/s004280050444. [DOI] [PubMed] [Google Scholar]
- 36.Bornstein SR, Uhlmann K, Haidan A, Ehrhart-Bornstein M, Scherbaum WA. Evidence for a novel peripheral action of leptin as a metabolic signal to the adrenal gland: leptin inhibits cortisol release directly. Diabetes. 1997;46:1235–1238. doi: 10.2337/diab.46.7.1235. [DOI] [PubMed] [Google Scholar]
- 37.Pralong FP, Roduit R, Waeber G, Castillo E, Mosimann F, Thoreus B, Gaillard RC. Leptin inhibits directly glucocorticoid secretion by normal human and rat adrenal gland. Endocrinology. 1998;139:4264–4268. doi: 10.1210/endo.139.10.6254. [DOI] [PubMed] [Google Scholar]
- 38.Groote Veldman R, Frölich M, Pincus SM, Veldhuis JD, Roelfsema F. Hyperleptinemia in women with Cushing’s disease is driven by high-amplitude pulsatile, but orderly and eurhythmic, leptin secretion. Eur J Endocrinol. 2001;144:21–27. doi: 10.1530/eje.0.1440021. [DOI] [PubMed] [Google Scholar]
- 39.Szucs N, Varga I, Jakab C, Patocs A, Glaz E, Toth M, Kiss R, Racz K. Leptin inhibits cortisol and corticosterone secretion in pathologic human adrenal cortical cells. Pituitary. 2001;4:71–77. doi: 10.1023/a:1012990928218. [DOI] [PubMed] [Google Scholar]
- 40.Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J Endocrinol. 2003;177:17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- 41.Buijs RM, Wortel J, van Heerikhuize JJ, Feenstra MGP, Ter Horst GJ, Herms J, Romijn J, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 42.Scheer FAJL, Buijs RM. Light affects morning salivary cortisol in humans. J Clin Endocrinol Metab. 1999;84:3395–3398. doi: 10.1210/jcem.84.9.6102. [DOI] [PubMed] [Google Scholar]
- 43.Edwards AV, Jones CT, Bloom SR. Reduced adrenal cortical sensitivity to ACTH in lambs with cut splanchnic nerves. J Endocrinol. 1986;110:81–85. doi: 10.1677/joe.0.1100081. [DOI] [PubMed] [Google Scholar]
- 44.Edwards AV, Jones CT. The effect of splanchnic nerve stimulation on adrenal cortical activity in conscious calves. J Physiol. 1987;382:385–396. doi: 10.1113/jphysiol.1987.sp016373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA, Pfeiffer EF, Holst JJ. Effects of splanchnic nerve stimulation on the adrenal cortex may be mediated by chromaffin in a paracrine manner. Endocrinology. 1990;127:900–906. doi: 10.1210/endo-127-2-900. [DOI] [PubMed] [Google Scholar]
- 46.Wang YH, Huang TS. Impaired adrenal reserve in men with spinal cord injury: results of low- and high-dose adrenocorticotropin stimulation tests. Arch Phys Med Rehabil. 1999;80:863–866. doi: 10.1016/s0003-9993(99)90075-8. [DOI] [PubMed] [Google Scholar]
- 47.Dijkstra I, Binnekade R, Tilders FJH. Diurnal variation in resting levels of corticosterone is not mediated by variation in adrenal responsiveness to adrenocorticotropin but involves splanchnic nerve integrity. Endocrinology. 1996;137:540–547. doi: 10.1210/endo.137.2.8593800. [DOI] [PubMed] [Google Scholar]
- 48.Siragy HM, Vieweg WVR, Pincus S, Veldhuis JD. Increased disorderliness and amplified basal and pulsatile aldosterone secretion in patients with primary aldosteronism. J Clin Endocrinol Metab. 1995;80:28–33. doi: 10.1210/jcem.80.1.7829626. [DOI] [PubMed] [Google Scholar]


