Abstract
Insulin-like growth factor binding protein 1 (IGFBP1) is a major secretory product of the decidualized endometrium. In this study, we investigated the role of two transcription factors, progesterone receptor (PGR) and a member of the forkhead box class O family of transcription factors, FOXO1A, in the regulation of the IGFBP1 gene in endometrial cells. Human endometrial fibroblasts (HuF) expressed FOXO1A, progesterone receptor A (PGRA) and progesterone receptor B (PGRB) proteins while the endometrial adenocarcinoma cell line, HEC-1B cells expressed only FOXO1A and no detectable PGR proteins. When FOXO1A expression was silenced using small interference RNA, IGFBP1 expression decreased in both HuF and HEC-1B cells. Using the chromatin immunoprecipitation technique, we demonstrated that liganded PGR was recruited to the IGFBP1 promoter region (−358 to −49). In addition, immunoprecipitation of HuF nuclear proteins with a PGR antibody followed by immunoblotting with anti-FOXO1A revealed that these two proteins interact in these cells. Reporter studies demonstrated that while liganded PGRA or PGRB increased a progesterone response element linked-reporter construct, pPRE/GRE.E1b.Luc, co-expression of FOXO1A inhibited the PGRB response in HuF and synergistically increased PGRA and PGRB response in HEC-1B. Furthermore, in HEC-1B cells, FOXO1A increased IGFBP1 promoter activity and co-expression of PGRA or PGRB further increased the promoter activity in a cooperative manner. In HuF, the response to FOXO1A and PGR was not additive but lower than the sum of individual responses. Thus, FOXO1A and PGR associate with one another and influence each other’s transactivating potential. The cell type dependent responses strongly implicate the involvement of other cofactors.
INTRODUCTION
Growth and differentiation of the human endometrium occurs in response to steroid hormones. During the secretory phase of the menstrual cycle, progesterone is involved in glandular differentiation and glycogenesis as well as stromal proliferation and decidualization [1]. During decidualization the fibroblast-like mesenchymal cells in the endometrium differentiate to decidual cells which are morphologically and biochemically different, expressing new proteins such as prolactin and insulin-like growth factor binding protein-1 (IGFBP1; 2). In culture, endometrial stromal cells exhibit a decidual phenotype when treated with progestins and this response is amplified with the addition of cAMP analogs [2, 3]. Although there are many studies that have defined the morphological and biochemical end points of a decidual cell, the sequence of cellular and molecular events associated with the transformation of a stromal fibroblast to a secretory decidual cell remains unclear.
There is abundant clinical and experimental evidence that support the importance of progesterone in the decidualization process. Progesterone’s effects are mediated through interaction with the progesterone receptor (PGR) [4]. The human PGR exists in two isoforms, PGRA and PGRB which are translated from individual mRNA species of a single gene under the control of distinct promoters [5]. PGRA lacks 164 amino acids from the N-terminus and has been shown to be functionally distinct from PGRB. PGRA and PGRB are both expressed in the endometrium [6]. IGFBP1, which is a major secretory protein of decidualized endometrial stromal cells, is activated by progesterone [7–9]. On the IGFBP1 promoter, three glucocorticoid receptor (NR3C1, also known as GR) response elements (GRE, Fig 1) have been identified and shown to be the sites responsible for the GR mediated increase in promoter activity [10]. GRE and PGR response elements (PGRE) share the same consensus sequence [11] and Gao et al [9] demonstrated that the GRE also serve as functional PGRE in endometrial stromal cells.
Figure 1.
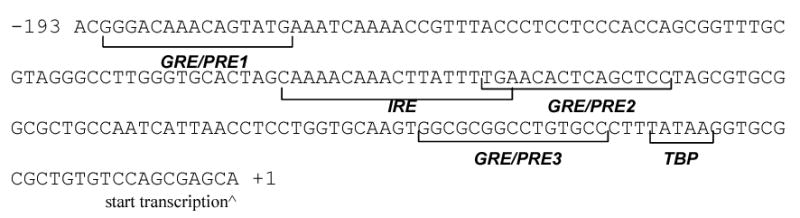
The IRE and GRE/PGRE in the human IGFBP1 proximal promoter. The proximal promoter region of the human IGFBP1 gene (Accession# M59316) contains one reported IRE that serves as a binding site for FOXO1A and 3 GRE/PGREs. The region −193 to +1 is shown. TBP: TATA binding protein.
Although progesterone is critical during decidualization, it has been shown to be a weak inducer of the decidual phenotype on its own. The addition of cAMP analogs significantly increase this process [reviewed in 2]. Microarray studies show that numerous genes are regulated during decidualization [12–14]. FOXO1A, a member of the FOXO sub-family of Forkhead/winged-helix family of transcription factors, was one of the earliest induced genes of decidualization [13]. In addition, Christian et al [15] have shown that cAMP agonists cause increased expression of FOXO1A in the human endometrium and targets FOXO1A to the nucleus. These data suggest that one way in which cAMP mediates its effects in the differentiation process is through the action of FOXO1A.
The influence of FOXO1A on the IGFBP1 gene has been studied in liver cells [16–18] and endometrial stromal cells [19]. FOXO1A binds to the insulin response element (IRE) located in the IGFBP1 proximal promoter region and activates promoter activity [16, 18]. Interestingly, contiguous to this sequence is a GRE/PGRE (Fig 1). FOXO1A has been shown to physically interact with other nuclear receptor proteins. It interacts with the liganded estrogen receptor alpha [20, 21] thyroid hormone receptor and retinoic acid receptor [21]. The ability of FOXO1A to associate with a liganded steroid receptor, the close proximity of the IRE and GRE/PGRE, and the ability of both PGR and FOXO1A to activate the IGFBP1 promoter, led us to investigate the potential interaction of FOXO1A with PGR. In this study, we investigated the importance of FOXO1A in IGFBP1 gene transcription, recruitment of PGR to the IGFBP1 gene and association of PGR with FOXO1A in whole cells. A novel mechanism of IGFBP1 regulation specifically by FOXO1A and PGR is shown. Parallel studies were done in HEC-1B cells as a comparison of cell specific responses.
MATERIALS AND METHODS
Cell culture
Placental membrane was obtained at the term of pregnancy. Permission for using these human specimens was approved by the Human Subject Committee of our Institution in accordance with U.S. Department of Health regulations. Human endometrial fibroblasts were isolated from decidua parietalis dissected from the placental membranes after normal vaginal delivery at term, as previously described [22]. Decidualized uterine endometrium maintains a proliferating population of predecidual fibroblastic cells, which closely resemble stromal cells [23]. These cells were passaged as needed up to a maximum of 7 passages. Cells were maintained in RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented with sodium pyruvate, penicillin/streptomycin, and 10% fetal bovine serum (FBS, Mediatech, Herndon, VA) that was treated with dextran coated charcoal (Sigma) according to the manufacturer’s protocol to deplete FBS of steroids. At approximately 80% confluence, cells were treated with 36 nM estradiol-17β, 1 μM medroxyprogesterone acetate (MPA), and 0.1 mM dibutyryl cAMP ((Bu)2cAMP; Sigma, St Louis, MO) for 10–14 days, changing media every 2 days. Experiments were repeated using HuF isolated from different placenta. HEC-1B cells, a human endometrial adenocarcinoma cell line, were obtained from ATCC (Rockville, MD). Cells were maintained in MEM (Invitrogen) supplemented with sodium pyruvate, penicillin/streptomycin, and 10% FBS. The T47D cell line (gift from S. Bulun, Chicago, IL) was maintained in RPMI supplemented with sodium pyruvate, penicillin/streptomycin, and 10% FBS. T47D cells were serum-starved overnight and incubated with fresh RPMI and 100nM MPA for 2 h.
RT-PCR
Cells were lysed with TriReagent (Molecular Research Center, Cincinnati, OH). Total RNA was extracted using the protocol provided by the manufacturer. One microgram of total RNA was reverse transcribed and PCR amplification was performed using 2 μl of cDNA for 30 cycles with IGFBP1 primers [3], under the following conditions: 94 C for 1 min, 55 C for 1 min, 72 C for 1.5 min, and a final extension of 72 C for 10 min. PCR products were electrophoresed in a 1.0% agarose gel and DNA was visualized by ethidium bromide staining.
Western blot
Cells were lysed with RIPA buffer (150 mM NaCl, 1%IGEPAL (Sigma), 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0) with protease inhibitors (Sigma, St. Louis, MO) to recuperate whole cell proteins. Nuclear proteins were isolated from cells using the NE-PER Nuclear and Cytoplasmic Extraction kit (Pierce, Rockford, IL). Protein content was measured using the Micro BCA protein assay kit (Pierce). Freshly isolated proteins were run on a precast 7.5% acrylamide gel (BioRad), and transferred onto PVDF membrane. Membranes were blocked with 5% milk in Tris Buffered Saline with 0.1% Tween-20 (TBST) and then incubated with primary antibody to FOXO1A (FKHR #9462, Cell Signaling, Beverly, MA) or PGR (PGR antibody 1294, D. Edwards, 24), followed by incubation with secondary peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (Biorad, Hercules, CA). Protein complexes were detected with a chemiluminescent detection kit (Amersham Biosciences, Piscataway, NJ). Membranes were stripped with stripping buffer (Restore, Pierce) and re-blotted with a monoclonal antibody to beta actin (Sigma). For immunoprecipitation studies, 10 μg of a monoclonal PGR antibody (Santa Cruz, AB-52) or a purified mouse IgG (Vector Labs, Burlingame, CA) were added to 80 μg of fresh nuclear proteins in the presence of protease inhibitors). Protein-antibody complexes were rotated overnight at 4 C. Complexes were immunoprecipitated with Protein A Sepharose (CL-4B; Amersham Biosciences) which was washed and prepared in a 50% slurry with PBS. After gentle washing with PBS, Laemmli sample buffer (BioRad) with 2-mercaptoethanol (BioRad) was added to the complexes, boiled for 5 min, and run on a 7.5% acrylamide gel. All Western blots are representative of 3 independent experiments using cells from 3 different placentae.
Small interfering RNA
To silence FOXO1A gene expression, transfection of a small interfering RNA (siRNA) duplex was performed using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s protocol for siRNA. The FOXO siRNA was synthesized by Dharmacon (Lafayette, CO) and corresponded to nucleotides 961 to 979 of the human FOXO1A coding region (GAGCGTGCCCTACTTCAAG) as described by Potente et al [25]. The siRNA also targets the sequence for FOXO3. A non-related control siRNA that targeted the firefly luciferase protein (Dharmacon, catalog number D-001210-02-05) was used as a control. Cells were grown in 6-well plates until 50% confluence at which time they were transfected with FOXO siRNA or control siRNA with Lipofectamine 2000. For HEC-1B cells, after 72 h of transfection, cells were lysed with TriReagent for RT-PCR anlaysis or with RIPA buffer for protein analysis. HuF cells were transfected for 6 h after which time hormones (36 nM estradiol, 1 μM MPA) and 0.1 mM (Bu)2cAMP were added. Transfection continued for 48 h. Media was changed and treatment with hormones and (Bu)2cAMP continued for 3 additional days. Cells were lysed with TriReagent for RT-PCR or with RIPA buffer for protein analysis. Silencing of the FOXO1A gene was verified by Western blot analysis using FOXO1A antibody (Cell Signaling). IGFBP1 and GAPDH mRNAs were detected by RT-PCR.
Chromatin Immunoprecipitation (ChIP)
Formaldehyde (Fisher, Fairlawn, NJ) was added directly into cell culture medium to a final concentration of 1%. Fixation proceeded at room temperature for 10 min and was stopped by addition of glycine to a final concentration of 0.125 M. Dishes were rinsed with cold PBS. Cells were removed by scraping, collected by centrifugation, and washed in cold PBS. Cells were incubated on ice for 10 min in cell lysis buffer [5 mM PIPES, pH 8.0, 85 mM KCl, 0.5% NP-40, and protease inhibitors]. Nuclei were released and collected by microcentrifugation at 4000rpm for 10min at 4C. Pellets were resuspended in nuclear lysis buffer [1% SDS, 10 mM EDTA, 50 mM Tris (pH 8.1), and protease inhibitors], and incubated on ice for 10 min. Samples were sonicated on ice to an average length of 300 to 600 bp and then microfuged at 14000 rpm for 10 min. Samples were diluted 5-fold in ChIP dilution buffer [0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.1, 167 mM NaCl and protease inhibitors] and the chromatin solution was precleared by incubation with salmon sperm DNA/Protein A agarose (Upstate, Lake Placid, NY) for 1 h at 4 C. Each sample was divided into 2 aliquots of 500 μl. Each aliquot was mixed with either 5 μg PGR antibody (Santa Cruz, AB-52) or purified mouse IgG (Vector Labs) as a negative control. Incubation occurred overnight at 4 C on a rotator. 40 μl of protein A agarose was added and incubated for 1 h at 4 C with rotation. Immunoprecipitates were collected by centrifugation at 1000 rpm for 1 min. Pellets were washed twice with low salt buffer [0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 150 mM NaCl], once with high salt buffer [0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 500 mM NaCl], four times with LiCl buffer [0.25 M LiCl, 1% IGEPAL-CA630, 1% deoxycholic acid (sodium salt), 1 mM EDTA, 10 mM Tris, pH 8.1] and twice with TE Buffer [10 mM Tris-HCl, 1 mM EDTA, pH 8.0]. Elution of immune complexes was carried out by addition of 250 μl of elution buffer [1%SDS, 0.1 M NaHCO3] and rotation for 15 min at room temperature. Elution was repeated a second time. After addition of 5 M NaCl, samples were reverse cross-linked by incubating at 65 C for 4 h. Proteins were removed by addition of proteinase K for 1 h at 45 C. DNA was extracted with phenol:chloroform followed by precipitation with 0.1 volume of 3 mM sodium acetate and 2.5 volumes of ethanol. Pellets were collected by microcentrifugation, resuspended in 50 μl of water, and analyzed by PCR. PCR reactions contained 2 μl of DNA, standard PCR reagents, and 50 pmoles of each primer: IGFBP1 (Left, 5′-GAG ACG CTT TGC AGG AGA (−358 to −341); Right, 5′-TTG CAC CAG GAG GTT AAT GA (−49 to −68)). Negative control primers which encompass a sequence distal (−910 to −626) to the putative binding sites were chosen to monitor specificity of binding (Left, 5’ CTCCCTGATCACAGCTCTCC (−910 to −891); Right, 5’ TCTGGAGGGGCAGTTAAGAA (−626 to −645). After 33 cycles of amplification, PCR products were run on a 1 % agarose gel and visualized by ethidium bromide staining.
Reporter Gene Constructs and Expression Vectors
The pHbp1-358.Luc corresponds to the −358 to +75 region relative to the transcription start site of the IGFBP1 gene inserted into the pGL3-basic reporter plasmid (Promega, Madison, WI) as described previously [19]. The pPRE/GRE.E1b.Luc was a generous gift from MJ Tsai and B O’Malley (Houston, TX) and constructed as previously described [26]. The human FOXO1A expression vector used in these studies is the mutant form where the three consensus PKB phosphorylation sites, Thr-24, Ser-256, Ser-319 were mutated to alanines creating a constitutively active form [16]. This expression vector was given to us by TG Unterman (Chicago, IL; 16). The PGRA and PGRB cDNAs were gifts from P Chambon (Strasbourg, France). All sequences were verified by dideoxy sequencing.
Cell transfection and reporter gene studies
Transient transfection of HuF and HEC-1B cells, grown in 12-well plates, was performed using Lipofectamine 2000 (Invitrogen). Cells were transiently transfected in DMEM with 1 μg/well of the firefly luciferase reporter plasmid with or without 0.5 μg/well FOXO1A and/or 0.5 μg/well PGRA or PGRB expression vectors, along with a beta-galactosidase reporter plasmid (pCMV SPORT, Promega) used as an internal control for normalization. After 4 h, the media were changed to RPMI-1640 with 2% stripped FBS with 100nM MPA. Cells were incubated for an additional 48 h. Cell extracts were harvested and luciferase activity was measured with the luciferase reagent kit (Promega). To assess the internal standard activity, β -galactosidase activity was measured with the β-Galactosidase Enzyme Assay kit (Promega). Normalized Relative Luciferase Units (RLU) were calculated as Firefly luciferase units/β -galactosidase units. Fold induction was calculated as normalized RLU of expression vector divided by normalized RLU of the control (basal activity with no expression vectors). Data are presented as the mean ± SEM of three or more independent experiments, each performed in triplicate.
Statistical Analysis
Multigroup comparisons were done by one-way analysis of variance (ANOVA I), followed by the Newman-Keules test as post hoc analysis. Differences were considered significant when P < 0.05.
RESULTS
Expression of IGFBP1, PGR, and FOXO1A in endometrial cells
Human endometrial fibroblasts (HuF) were isolated from decidua parietalis dissected from the placental membranes after normal vaginal delivery at term [22]. HuF are predecidual fibroblastic cells, closely resembling endometrial stromal cells, which exhibit a decidual phenotype in culture when treated with estradiol, progestin and (Bu)2cAMP for 10–14 days [22, 23]. Treatment of HuF with estradiol, MPA and (Bu)2cAMP for 10 days (HuF +/+) induced expression of IGFBP1 mRNA where none was detected in the non-treated cells (HuF −/−; Fig 2A). HEC-1B cells which are an epithelial cell line from an endometrial adenocarcinoma endogenously expressed IGFBP1 (Fig 2A). FOXO1A protein was present in nuclear extracts of both untreated (−/−) and treated (+/+) HuF, as well as HEC-1B as demonstrated by Western blot analysis (Fig 2B). FOXO1A protein levels in HuF (+/+) were higher than that of HuF (−/−) (Fig 2B). The FOXO1A band for HEC1B migrated at a higher molecular weight than that of HuF. This product could be a post-translationally modified form of FOXO1A which remains to be determined. Both PGRA and PGRB were expressed in HuF nuclear extracts (Fig 2C). The level of expression was lower in HuF (+/+) indicative of downregulation of receptor expression by the long term hormone and (Bu)2cAMP treatment. Furthermore, PGRA and PGRB in HuF (+/+) were of higher molecular weight as displayed by their up-shift on the SDS/PAGE gel. This up-shift is similar to that seen in whole cell extracts of T47D cells treated with MPA (Fig 2C) which reflects increased phosphorylation at multiple serine residues [27]. HEC-1B cells did not express detectable levels of PGRA or PGRB protein in nuclear protein lysates.
Figure 2.
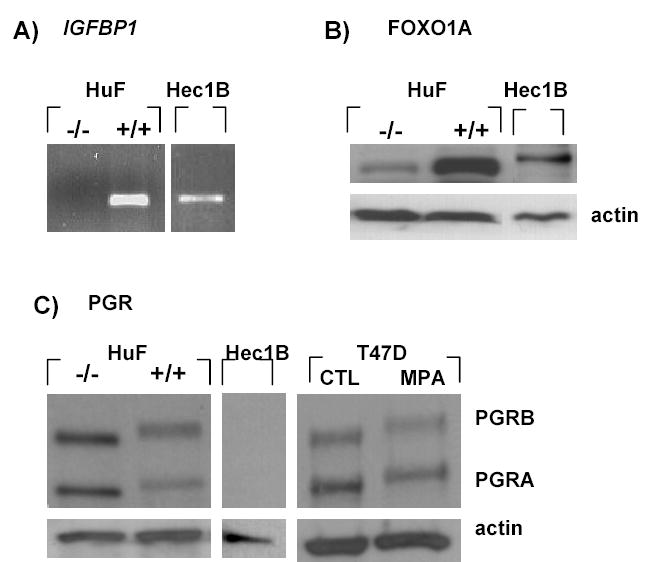
Expression of IGFBP1, FOXO1A and PGR in endometrial cells. HuF were treated without (−/−) or with (+/+) estradiol, MPA and (Bu)2cAMP for 10 days. HEC-1B cells were grown to confluence with no treatment. A) IGFBP1 mRNA was measured by RT-PCR, B) FOXO1A protein and C) PGR protein were measured in nuclear extracts of HuF (−/−), HuF (+/+), and HEC-1B by Western blot. As a positive control for PGR hyperphosphorylation, T47D cells were treated with MPA for 2 h and total protein was analyzed by Western blot. Data are representative of 3 independent experiments. Three placentae were used for the HuF experiments. CTL: control
Silencing FOXO1A decreases IGFBP1 gene expression
Although FOXO1A is upregulated during decidualization [13, 15, 19] and increases IGFBP1 promoter activity [16, 19], its physiological role in the regulation of IGFBP1 in endometrial cells is unclear. Small interfering RNA specific to FOXO1A [25] was transfected into HuF or HEC-1B cells. Transfection with the FOXO1A siRNA decreased total FOXO1A protein expression in both HEC-1B (Fig 3A) and HuF (Fig 3B) compared to the cells transfected with the control oligo. As a result of FOXO1A silencing, endogenous expression of IGFBP1 mRNA in HEC-1B cells, significantly decreased (Fig 3A). GAPDH expression was not affected with FOXO1A siRNA. In HuF, treatment with hormones and (Bu)2cAMP for 5 days induced IGFBP1 mRNA expression as expected, with the control siRNA (Fig 3B). When FOXO1A was silenced, the expression of IGFBP1 mRNA in response to the hormones significantly decreased. We show here for the first time, the critical role of FOXO1A in the induction of IGFBP1.
Figure 3.

Effect of FOXO1A silencing on IGFBP1 expression. A) HEC-1B cells were transfected with Control (CTL) or FOXO1A (FX) siRNA for 72 h. FOXO1A and actin proteins from whole cell extracts were measured by Western blot while IGFBP1 and GAPDH mRNAs were measured by RT-PCR. B) HuF were transfected with Control (CTL) or FOXO1A (FX) siRNA for 48 h. Cells were treated for 5 days without (−/−) or with (+/+) estradiol, MPA and (Bu)2cAMP. FOXO1A and actin proteins from whole cell extracts were detected by Western blot while IGFBP1 and GAPDH mRNAs were measured by RT-PCR. Data are representative of 3 independent experiments. Three placentae were used for the HuF experiments
Recruitment of PGR to IGFBP1 gene
Ligand-activated progesterone receptor mediates many of the progestational effects in endometrium. In the proximal region of the IGFBP1 promoter, three GRE have been identified [10] (Fig 1). Gao et al. [9] have shown that GREs are also functional PGREs in endometrial cells and that PGR activates the IGFBP1 promoter through at least two of the three PGREs (PGRE1 and PGRE2). To date, binding of PGR to the IGFBP1 promoter region in a physiological setting has not been shown. We demonstrate here by the ChIP technique, recruitment of PGR to the IGFBP1 proximal promoter region (Fig 4). HuF were treated for 14 days without (−/−) or with (+/+) hormones and (Bu)2cAMP. Cells were fixed, nuclear proteins isolated, DNA sonicated and complexes immunoprecipitated using a monoclonal PGR antibody or purified mouse IgG. PCR analysis of the immunoprecipitated complexes showed an amplification of the −358 to −49 region (relative to the transcription start site) of the IGFBP1 promoter for HuF +/+ (Fig 4). No bands were detected with IgG. Another region of the IGFBP1 promoter corresponding to −910 to −626, which is distal to the putative binding sites, was amplified to determine specificity of the ChIP assay (Fig 4). While positive bands were detected in the input samples (total DNA), no bands were observed for the IgG or PGR antibody immunoprecipitated samples. Thus, ligand activated PGR was recruited to the −358 to −49 region of the IGFBP1 gene.
Figure 4.
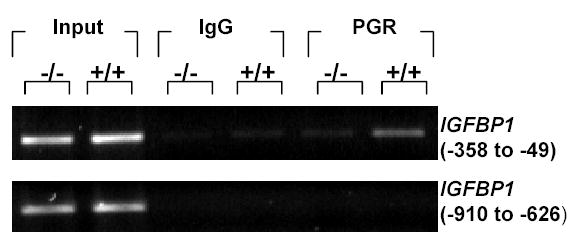
Recruitment of PGR to the IGFBP1 promoter region. HuF were treated without (−/−) or with (+/+) estradiol, MPA and (Bu)2cAMP for 14days and ChIP assay was done using mouse IgG or PGR antibody. The −358 to −49 region on the IGFBP1 promoter was amplified by PCR. A region distal to the putative binding sites (−910 to −626) of the IGFBP1 promoter was also amplified to confirm specificity of recruitment. Data are representative of 3 independent experiments using 3 placentae.
FOXO1A interacts with PGR
We investigated whether PGR associates with FOXO1A in HuF. HuF were treated without (−/−) or with (+/+) hormones and (Bu)2cAMP for 14 days. Nuclear proteins were isolated and immunoprecipitated using a monoclonal PGR antibody or purified mouse IgG as a control. Complexes were then analyzed by Western blot using anti-FOXO1A. When HuF nuclear proteins were immunoprecipitated with a PGR antibody, FOXO1A was detected in this complex (Fig 5). FOXO1A was present in both HuF (−/−) and HuF (+/+) cells. Furthermore, higher levels of FOXO1A were detected in HuF (+/+) compared to HuF (−/−). No bands were observed with IgG. These results clearly demonstrate that FOXO1A interacts with PGR in HuF. Whether this interaction is direct or indirect through the association of other cofactors, remains to be investigated.
Figure 5.

Interaction of PGR and FOXO1A. HuF were treated without (−/−) or with (+/+) estradiol, MPA and (Bu)2cAMP for 14 days. Nuclear proteins were isolated and immunoprecipitated with mouse IgG or PGR antibody. Immunoprecipitated complexes were immunoblotted with FOXO1A antibody. Data are representative of 3 independent experiments using 3 placentae.
FOXO1A and PGR regulate promoter activity
To determine whether the interaction of FOXO1A and PGR influence transactivation function, FOXO1A and PGRA or PGRB receptor were co-expressed with either a progesterone response element linked-reporter construct, pPRE/GRE.E1b.Luc [26] or the previously reported IGFBP1 promoter pHbp1-358.Luc [19]. In all of the transfection studies, the human FOXO1A expression vector is the mutant form where the three consensus PKB phosphorylation sites (Thr-24, Ser-256, Ser-319) were mutated to alanines creating a constitutively active form, thus eliminating possible regulation of translocation by hormones or (Bu)2cAMP. In the absence of MPA, there was no activation of pPRE/GRE.E1b.Luc in response to PGRA, PGRB or FOXO1A. (results not shown). In the presence of MPA, both PGRA and PGRB increased pPRE/GRE.E1b.Luc activity with PGRB being the stronger transactivator of this promoter in HuF and HEC-1B cells (Fig 6). In HuF, when FOXO1A was co-expressed with PGRB, the activity of pPRE/GRE.E1b.Luc was significantly lower than that of PGRB alone (P<0.05; Fig 6A). There was no effect of FOXO1A on PGRA transactivation of pPRE/GRE.E1b.Luc. In HEC-1B cells, ligand-activated PGRA and PGRB increased pPRE/GRE.E1b.Luc as expected (Fig 6B). FOXO1A alone did not increase pPRE/GRE.E1b.Luc activity but when co-expressed with liganded PGRA or PGRB, the promoter activity increased significantly to levels that were higher than that of PGR alone, showing an enhancement of PGR-dependent transcription by FOXO1A (P<0.05).
Figure 6.

Influence of FOXO1A on PGR transactivation of pPRE/GRE.E1b.Luc. PGRA or PGRB was co-transfected with FOXO1A and the reporter pPRE/GRE.E1b.Luc in (A) HuF or (B) HEC-1B cells. After 4 h of transfection, cells were treated with 100 nM MPA. Luciferase activity was measured after 48 h. Data are represented as the mean + SEM of 5 (placentae for HuF) or 3 (HEC-1B) independent experiments. Differences were considered statistically significant when P < 0.05 and represented by letters a-d.
The pHbp1-358.Luc reporter (−358 to +75 in pGL3-basic) was co-transfected with PGRA/PGRB and/or FOXO1A. In both HuF and HEC-1B cells, FOXO1A significantly increased activity of pHbp1-358.Luc (Fig 7A,B). Liganded PGRA alone did not significantly induce promoter activity in either HuF or HEC-1B cells and liganded PGRB alone was a relatively weak inducer of this promoter. In HEC-1B cells, addition of FOXO1A with PGRA or PGRB increased promoter activity to levels significantly higher than that of FOXO1A alone (P<0.05; Fig 7B). Furthermore, this increase was higher than the sum of the individual responses, suggesting a cooperative increase. In HuF, the combination of FOXO1A and PGRA or PGRB, did not increase IGFBP1 promoter activity to levels beyond that of FOXO1A alone (Fig 7A). The levels were lower or the same as for FOXO1A indicating that the combinatorial effect was not additive, suggesting an interaction between the two transcription factors.
Figure 7.

Influence of PGR and FOXO1A on IGFBP1 promoter. A) HuF and (B) HEC-1B cells were transfected with PGRA or PGRB and/or FOXO1A with the IGFBP1 promoter construct, pHbp1-358.Luc. After 4 h of transfection, cells were treated with 100 nM MPA. Luciferase activity was measured after 48 h. Data are represented as the mean + SEM of 6 (placentae for HuF) or 3 (HEC-1B) independent experiments. Differences were considered statistically significant when P < 0.05 and represented by letters a-d.
DISCUSSION
IGFBP1 is a major secretory product of the decidualized endometrium. The molecular mechanisms in inducing such decidua-specific genes are complex given the plethora of genes that are regulated during the decidualization process [12–14]. It is generally accepted that PGR is a critical molecule during this process. PGRA knockout mice have impaired decidualization of stromal cells in response to traumal stimulation [28]. However, it appears that not all decidua-specific genes are under primary control of activated PGR. This is supported by the fact that in vivo, expression of decidua-specific genes is not apparent until ten days after the initial rise of progesterone [29]. Also, we previously demonstrated that stromal cells from the baboon endometrium, when treated with cycloheximide along with estradiol, MPA and (Bu)2cAMP, did not express IGFBP1 whereas hormone treated cells without cycloheximide did express IGFBP1 [30]. This indicates that the progestin effect via PGRs did not directly activate IGFBP1 gene transcription and that de novo protein synthesis was required for the expression of IGFBP1. Thus, other proteins are essential in inducing the expression of IGFBP1 in endometrial stromal cells. In this report, we clearly demonstrate the importance of the FOXO family of transcription factors in inducing IGFBP1 expression. In HuF, silencing FOXO1A inhibited induction of IGFBP1 despite 5 days of hormone and (Bu)2cAMP treatment. Potente et al [25] showed that silencing FOXO gene expression in HUVEC decreased p27Kip1 protein levels, another gene target of FOXO1A, and increased proliferation. We show here that FOXO1A may be one target of cAMP action and that it is critical in inducing IGFBP1 in both HEC-1B cells and HuF. Whether FOXO1A silencing affects other markers of decidualization is under investigation.
Studies have shown that PGR can transactivate the IGFBP1 promoter in endometrial cells [16]. Until now, it has been unclear whether PGR directly binds to chromatin, specifically on the IGFBP1 gene. Here we demonstrate for the first time, the recruitment of PGR to the proximal promoter region (−358 to −49) of the IGFBP1 gene, using the ChIP technique. PGR is recruited to the IGFBP1 promoter region when cells have been treated with hormones and (Bu)2cAMP, indicating that it is the liganded PGR that is recruited to the promoter. Within this region (−358 to −49) are three PGREs that have been identified (Fig 1).
We have shown in this study that nuclear FOXO1A and PGR proteins interact with each other in situ both in HuF (−/−) and HuF (+/+). Other studies have shown FOXO1A to physically interact with other transcription factors, albeit in vitro. FOXO1A interacts with liganded estrogen receptor [20], CCAAT/enhancer-binding protein beta [15], thyroid hormone receptor and retinoic acid receptor [21], HOXA5 [31], HOXA10 [19] and peroxisome proliferative activated receptor gamma, coactivator 1 alpha [32]. Schuur et al [20] reported that there was no interaction between FOXO1A and PGR proteins. In their study, interaction was analyzed in vitro using recombinant proteins and the GST-pulldown method. This technique excludes all other molecules that are present within the cells which may be involved in the transcriptional complex. In our study, the interaction of FOXO1A and PGR is analyzed in vivo. Other factors that may be involved in this complex remain to be identified.
In studies that demonstrate interaction of FOXO1A with other transcription factors, transactivating function is either repressed or stimulated. Here, we show that FOXO1A activates (in HEC-1B) or inhibits (in HuF) PGR transactivation of the PRE-linked reporter, pPRE/GRE.E1b.Luc. Zhao et al [21] reported a decrease in PRE/GRE-TATA-Luc reporter activity with FOXO1A and PGR in COS-1 cells. This is in contrast to the response in HEC-1B in which FOXO1A increased both PGRA and PGRB action. The different responses to FOXO1A and PGR in HEC-1B compared to HuF strongly implicate the involvement of different cofactors in regulating this interaction. HEC-1B cells are an epithelial cell line and thus the repertoire of cofactors may be quite different from that of a stromal cell line. In addition, since HEC-1B cells do not express endogenous PGR, cofactors of PGR may also be absent or if they are present, may not associate with overexpressed PGR as they do in HuF. In HuF, FOXO1A inhibited PGRB transactivation of pPRE/GRE.E1b.Luc by FOXO1A but not PGRA. Interestingly, it has been reported that PGRA is the major isoform in decidualized endometrial stromal cells [33,34]. We can speculate that during decidualization, FOXO1A would not have a significant effect on PGR action since PGRA is the major isoform expressed but that PGRA would regulate FOXO1A action. Thus, the inhibiting effect of PGR to FOXO1A activity may be one way in which the decidualization process is controlled and relatively slow, occurring over days of hormone exposure.
The nature of FOXO1A interaction with PGR as well as the mechanism by which the two transcription factors influence transactivation is unknown. Moilanen et al [35] reported the function of one nuclear protein, SNURF, which is able to activate steroid receptor-dependent transcription by forming a functional link between nuclear receptors and other transcription factors, ie, SP1 [36]. Alternatively, interaction of FOXO1A and PGR could sequester either of these factors and restrict availability for transactivation function. A detailed study of this interaction as well as interaction with other cofactors is currently under investigation.
In summary, we have shown that FOXO1A is critical in the induction of IGFBP1, FOXO1A interacts with PGR, liganded PGR is recruited to the IGFBP1 promoter and that FOXO1A and PGR regulate each others’ transactivation function. The physiologic consequence of this interaction depends on the cell type. We provide here a novel mechanism by which PGR and FOXO1A regulate one decidua-specific gene, IGFBP1, in endometrial cells.
Acknowledgments
We are grateful to Terry Unterman for his insightful suggestions and comments during the preparation of this manuscript. We thank MJ Tsai and B O’Malley, P Chambon, S Bulun, D Edwards and T Unterman for donating the PRE reporter construct, PGRA/PGRB expression vectors, T47D cells, PGR monoclonal antibody, and the FOXO1A expression vector respectively. We acknowledge the National Institutes of Health (HD044715) and the Friends of Prentice of Northwestern Memorial Foundation for their grant support.
Footnotes
These studies were supported by grant HD044715 from the National Institutes of Health and a grant from the Friends of Prentice.
References
- 1.Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18:502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- 2.Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178:357–372. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- 3.Kim JJ, Jaffe RC, Fazleabas AT. Comparative studies on the in vitro decidualization process in baboons and humans. Biol Reprod. 1998;59:160–168. doi: 10.1095/biolreprod59.1.160. [DOI] [PubMed] [Google Scholar]
- 4.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 5.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conneely OM, Lydon JP. Progesterone receptors in reproduction: functional impact of the A and B isoforms. Steroids. 2000;65:571–577. doi: 10.1016/s0039-128x(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 7.Tseng L, Gao JG, Chen R, Zhu HH, Mazella J, Powell DR. Effect of progestin, antiprogestin, and relaxin on the accumulation of prolactin and insulin-like growth factor binding protein-1 messenger ribonucleic acid in human endometrial stromal cells. Biol Reprod. 1992;47:441–450. doi: 10.1095/biolreprod47.3.441. [DOI] [PubMed] [Google Scholar]
- 8.Gao J, Mazella J, Tseng L. Activation of human insulin-like growth factor binding protein-1 gene promoter by a distal regulatory sequence in a human endometrial adenocarcinoma cell line. Mol Endocrinol. 1995;9:1405–1412. doi: 10.1210/mend.9.10.8544848. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Mazella J, Suwanichkul A, Powell DR, Tseng L. Activation of the insulin-like growth factor binding protein-1 promoter by progesterone receptor in decidualized human endometrial stromal cells. Mol Cell Endocrinol. 1999;153:11–17. doi: 10.1016/s0303-7207(99)00096-9. [DOI] [PubMed] [Google Scholar]
- 10.Goswami R, Lacson R, Yang E, Sam R, Unterman T. Functional analysis of glucocorticoid and insulin response sequences in the rat insulin-like growth factor- binding protein-1 promoter. Endocrinology. 1994;134:736–743. doi: 10.1210/endo.134.2.7507835. [DOI] [PubMed] [Google Scholar]
- 11.Evans RM. The steroid and thyroid receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popovici R, Kao L, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology. 2000;141:3510–3513. doi: 10.1210/endo.141.9.7789. [DOI] [PubMed] [Google Scholar]
- 13.Brar AK, Handwerger S, Kessler CA, Aronow BJ. Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics. 2001;7:135–148. doi: 10.1152/physiolgenomics.00061.2001. [DOI] [PubMed] [Google Scholar]
- 14.Tierney EP, Tulac S, Huang ST, Giudice LC. Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiol Genomics. 2003;16:47–66. doi: 10.1152/physiolgenomics.00066.2003. [DOI] [PubMed] [Google Scholar]
- 15.Christian M, Zhang X, Schneider-Merck T, Unterman TG, Gellersen B, White JO, Brosens JJ. Cyclic AMP-induced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer-binding protein beta in differentiating human endometrial stromal cells. J Biol Chem. 2002;277:20825–20832. doi: 10.1074/jbc.M201018200. [DOI] [PubMed] [Google Scholar]
- 16.Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 17.Cichy SB, Uddin S, Daniilkovich A, Guo S, Klippel A, Unterman TG. Protein kinase B/Akt mediates effects of insulin on hepatic insulin-like growth factor-binding protein-1 gene expression through a conserved insulin response sequence. J Biol Chem. 1998;273:6482–6487. doi: 10.1074/jbc.273.11.6482. [DOI] [PubMed] [Google Scholar]
- 18.Durham SK, Suwanichkul A, Scheimann AO, Yee D, Jackson JG, Barr FG, Powell DR. FKHR binds to the insulin response element in the insulin-like growth factor binding protein-1 promoter. Endocrinology. 1999;140:3140–3146. doi: 10.1210/endo.140.7.6856. [DOI] [PubMed] [Google Scholar]
- 19.Kim JJ, Taylor HS, Akbas GE, Foucher I, Trembleau A, Jaffe RC, Fazleabas AT, Unterman TG. Regulation of insulin-like growth factor binding protein-1 promoter activity by FKHR and HOXA10 in primate endometrial cells. Biol Reprod. 2003;68:24–30. doi: 10.1095/biolreprod.102.009316. [DOI] [PubMed] [Google Scholar]
- 20.Schuur ER, Loktev AV, Sharma M, Sun Z, Roth RA, Weigel RJ. Ligand-dependent interaction of estrogen receptor-alpha with members of the forkhead transcription factor family. J Biol Chem. 2001;276:33554–33560. doi: 10.1074/jbc.M105555200. [DOI] [PubMed] [Google Scholar]
- 21.Zhao HH, Herrera RE, Coronado-Heinsohn E, Yang MC, Ludes-Meyers JH, Seybold-Tilson KJ, Nawaz Z, Yee D, Barr FG, Diab SG, Brown PH, Fuqua SA, Osborne CK. Forkhead homologue in rhabdomyosarcoma functions as a bifunctional nuclear receptor-interacting protein with both coactivator and corepressor functions. J Biol Chem. 2001;276:27907–27912. doi: 10.1074/jbc.M104278200. [DOI] [PubMed] [Google Scholar]
- 22.Strakova Z, Srisuparp S, Fazleabas AT. Interleukin-1beta induces the expression of insulin-like growth factor binding protein-1 during decidualization in the primate. Endocrinology. 2000;141:4664–4670. doi: 10.1210/endo.141.12.7810. [DOI] [PubMed] [Google Scholar]
- 23.Richards RA, Brar AK, Frank GR, Hartman SM, Jikihara H. Fibroblast cells from term human decidua closely resemble endometrial stromal cells: induction of prolactin and insulin-like growth factor binding protein-1 expression. Biol Reprod. 1995;52:609–615. doi: 10.1095/biolreprod52.3.609. [DOI] [PubMed] [Google Scholar]
- 24.Clemm DL, Sherman L, Boonyaratanakornkit V, Schrader WT, Weigel NL, Edwards DP. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol Endocrinol. 2000;14:52–65. doi: 10.1210/mend.14.1.0413. [DOI] [PubMed] [Google Scholar]
- 25.Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic Acid-induced Inhibition of FOXO Factors Promotes Endothelial Proliferation by Down-Regulating p27Kip1. J Biol Chem. 2003;278:29619–29625. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- 26.Nawaz Z, Lonard DM, Smith CL, Lev-Lehman E, Tsai SY, Tsai MJ, O’Malley BW. The Angelman syndrome-associated protein, E6-AP is a coactivator for the nuclear hormone receptor superfamily. Mol Cell Biol. 1999;19:1182–1189. doi: 10.1128/mcb.19.2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheridan PL, Evans RM, Horwitz KB. Phosphotryptic Peptide Analysis of Human Progesterone Receptors. New phosphorylated sites formed in nuclei after hormone treatment. J Biol Chem. 1989;264:6520–6528. [PubMed] [Google Scholar]
- 28.Biserka Mulac-Jericevic, Robert A. Mullinax, Francesco J. DeMayo. Subgroup of Reproductive Functions of Progesterone Mediated by Progesterone Receptor–B Isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 29.de Ziegler D, Fanchin R, de Moustier B, Bulletti C. The hormonal control of endometrial receptivity: estrogen (E2) and progesterone. Journal of Reproductive Immunology. 1998;39:149–166. doi: 10.1016/s0165-0378(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 30.Kim JJ, Jaffe RC, Fazleabas AT. Insulin-Like Growth Factor Binding Protein-1 Expression in Baboon Endometrial Stromal Cells: Regulation by Filamentous Actin and Requirement for de Novo Protein Synthesis. Endocrinology. 1999;140:997–1004. doi: 10.1210/endo.140.2.6474. [DOI] [PubMed] [Google Scholar]
- 31.Foucher I, Volovitch M, Frain M, Kim JJ, Souberbielle JC, Gan L, Unterman TG, Prochiantz A, Trembleau A. Hoxa5 overexpression correlates with IGFBP1 upregulation and postnatal dwarfism: evidence for an interaction between Hoxa5 and Forkhead box transcription factors. Development. 2002;129:4065–4074. doi: 10.1242/dev.129.17.4065. [DOI] [PubMed] [Google Scholar]
- 32.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1A–PGC-1a interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 33.Tseng L, Zhu HH. Biol Reprod. 1999;57:1360–1366. doi: 10.1095/biolreprod57.6.1360. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Critchley HO, Kelly RW, Shen D, Baird DT. Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol Hum Reprod. 1998;4:407–412. doi: 10.1093/molehr/4.4.407. [DOI] [PubMed] [Google Scholar]
- 35.Moilanen AM, Poukka H, Karvonen U, Hakli M, Janne OA, Palvimo JJ. Identification of a novel RING finger protein as a coregulator in steroid receptor-mediated gene transcription. Mol Cell Biol. 1998;18:5128–5139. doi: 10.1128/mcb.18.9.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poukka H, Aarnisalo P, Santti H, Janne OA, Palvimo JJ. Coregulator small nuclear RING finger protein (SNURF) enhances Sp1- and steroid receptor-mediated transcription by different mechanisms. J Biol Chem. 2000;275:571–579. doi: 10.1074/jbc.275.1.571. [DOI] [PubMed] [Google Scholar]


