Abstract
Matrix metalloproteinases (MMPs) are zinc-dependent proteases capable of degrading extracellular matrix components. The activity of these proteases is tightly regulated through the actions of the tissue inhibitors of metalloproteinases (TIMPs). Although the regulation of MMPs and TIMPs during physiological and pathological remodeling has been investigated in a number of systems, almost nothing is known about their role in skeletal muscle differentiation. To investigate the role of MMP-mediated proteolysis during myogenesis, the regulation of TIMP-2, MT1-MMP, and MMP-2 expression was investigated during differentiation of the mouse myoblastic C2C12 cell line. We show that this trio is upregulated coincident with myogenesis. The more diffuse spatial distribution of TIMP-2 relative to MT1-MMP and MMP-2 suggests that TIMP-2 may exert MMP-independent functions during myogenesis. Elucidating the regulation of these molecules during muscle differentiation in vitro may lead to a better understanding of their role in pathological processes in muscle tissue in vivo.
Keywords: extracellular matrix, matrix metalloproteinase, myogenesis, proteolysis, tissue inhibitor of metalloproteinase
ABBREVIATIONS: DIV, days in vitro; DMEM, Dulbecco’s modified Eagle’s medium; DPBS, Dulbecco’s phosphate buffered saline; ECM, extracellular matrix; FBS, fetal bovine serum; HRP, horseradish peroxidase; MMP, matrix metalloproteinase; RP, rapidly proliferating; TBS, Tris buffered saline; TBST, Tris buffered saline with Tween; TIMP, tissue inhibitor of metalloproteinase
INTRODUCTION
Matrix metalloproteinases (MMPs) are products of a multigene family of zinc-dependent neutral endopeptidases comprised of secreted and membrane-associated (membrane type, MT) members. The MMPs are recognized for their role in physiological as well as in pathological processes. MMPs release growth factors stored within the extracellular matrix (ECM) and process growth factor receptors, thereby regulating cell proliferation. In addition, MMPs degrade all ECM constituents, thereby facilitating cell migration and tissue remodeling. Because abnormal MMP function can lead to a broad range of pathological conditions, their activity must be tightly regulated 27,38. MMP activity is regulated at three points: gene expression, pro-enzyme activation, and enzyme inactivation. First, MMP expression is regulated at the transcriptional level. Expression is induced by cytokines, growth factors, chemical agents, and cell-cell or cell-matrix interactions. Enhanced MMP expression may be downregulated by suppressive factors such as retinoids and glucocorticoids. Second, MMPs undergo post-translational modification. MMPs are produced as inactive zymogens and must undergo activation to become proteolytically active. Some MMPs, particularly the MT-MMPs are activated during secretion via a furin-like convertase and thus are released as active proteases. Most MMPs are activated extracellularly by proteases (e.g., plasminogen, plasmin, or MMPs) or non-proteolytic agents (e.g., organomercurials, urea). Finally, MMP activity is inhibited by interaction with tissue inhibitor of metalloproteinases (TIMPs) in a 1:1 stoichiometric ratio. Inasmuch as humans possess 25 MMPs and only 4 TIMPs, the TIMPs form tight, but relatively low specificity interactions with MMPs. In addition to MMP inhibition, TIMP-2 acts as a mediator of proMMP-2 activation via the ternary proMMP-2/MT1-MMP/TIMP-2 complex 4. Evidence is mounting that TIMPs are multifactorial molecules that exert effects on cell growth and survival independent of their MMP inhibitory activity 2.
Although both MMPs and TIMPs are expressed in muscle, little is known about their role in development and function of skeletal muscle tissue 6,25. However, it is becoming clear that they have important physiological functions in the maintenance of the homeostasis of muscle fibers and of the ECM 6. The mouse myoblastic cell line C2 and its subclone C2C12 serve as a well-accepted model to investigate myogenesis in vitro 37. C2C12 myoblasts proliferate when maintained in the presence of a high concentration of serum (growth media). Upon serum deprivation, most cells terminally differentiate and form myotubes, others become quiescent, undifferentiated reserve cells which are similar to muscle satellite cells, and a subset of cells undergo apoptosis. The orderly expression of myogenic markers in C2C12 cells is similar to that observed for myogenesis in vivo, indicating that the factors regulating myogenesis in C2C12 cells are similar to those in vivo 30.
Of the 25 MMPs and 4 TIMPs, we chose to investigate the trio TIMP-2, MT1-MMP (MMP-14), and MMP-2 because these molecules are components of a ternary complex known to act in concert. Most notably, in this context TIMP-2 plays a role not only in MMP inhibition, but also MMP activation. The relative levels of TIMP-2 and MT1-MMP are critical, since low TIMP-2 levels are associated with MT1-MMP mediated activation of pro-MMP-2, and at higher TIMP-2 levels MT1-MMP function is blocked, preventing pro-MMP-2 activation 4. Previous studies have examined MMP-2 and MMP-9 expression in proliferating C2C12 myoblast 10 and differentiated myotubes 23. Here, we investigated the expression of TIMP-2, MT1-MMP and MMP-2 throughout the time course of C2C12 differentiation. Our results show that TIMP-2 expression is upregulated coincident with C2C12 differentiation. In contrast, MT1-MMP expression is downregulated with time in growth media but is upregulated with differentiation. MMP-2 expression is increased only at later stages of C2C12 differentiation. These data suggest that these three molecules play distinct roles in the differentiation of C2C12 cells.
MATERIALS AND METHODS
Antibodies
We used sheep anti-human TIMP-2 for immunocytochemistry (200X; Biogenesis Inc, Kingston, NH), rabbit anti-human TIMP-2 for western blot (1500X; AB801; Chemicon, Temecula, CA), rabbit anti-human MT1-MMP (RP1) and MMP-2 (RP3) (2500X for western blot, 500X for immunocytochemistry; TriplePointBiologics, Forest Grove, OR), goat anti-human actin (5000X; I19; Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-rat myogenin (50X; F5D; Santa Cruz), recombinant mouse myoD1 Ab-1 (10X; 5.8A, Lab Vision Corporation, Fremont, CA). Cy-2 (100X), Cy-3 (500X), and HRP-conjugated (3000X) antibodies were obtained from Jackson Immuno Research (West Grove, PA).
Cell culture
C2C12 cells (American Type Culture collection, CRL-1772) were maintained in growth medium (Dulbecco’s modified Eagle’s medium [DMEM], 10% fetal bovine serum [FBS], penicillin/streptomycin) with media replenished every 48 hours. For differentiation, cells were plated at a density of 2 x 105 cells per 60-mm dish and 2 x 104 cells per well of a 24-well plate. Cells were maintained on untreated tissue culture dishes for all phases of growth and differentiation. To determine the basal level of TIMP-2 expression in myoblasts, rapidly proliferating (RP) cells were harvested 24 hours after plating. After an additional 24 hours (T0), cells were switched to differentiation media (DMEM, 2% horse serum, penicillin/streptomycin). For serum-free differentiation, cells were grown in DMEM supplemented with N2 (Invitrogen; Carlsbad, CA) which contains transferrin and insulin required for myogenesis. Cells were harvested and conditioned media collected after an additional 1, 2, 3, 5, and 7 days in vitro (DIV).
Western Blot Analysis
Western blot analysis was performed as previously described 19 with minor modifications. Tissue was homogenized in Triton Lysis Buffer (20 mM Tris pH 7.4, 137 mM NaCl, 25 mM β-glycerolphosphate pH 7.4, 2 mM sodium pyrophosphate, 2 mM EDTA pH 7.4, 1% Triton X-100, 10% glycerol) containing a cocktail of protease inhibitors. Lysates were spun at 14,000 rpm for 15 minutes at 4 °C, and the supernatant used for western blot analysis. Crude protein homogenates (25 μg) or conditioned media (20 μl) were fractionated by SDS-PAGE and transferred to Immobilon-P membranes (Schleicher & Schuell; Keene, NH). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS; 10 mM Tris, pH 7.5, 150 mM NaCl), followed by overnight incubation at 4 °C with antibody diluted in TBS containing 0.4% Tween-20 (TBST) and 3% bovine serum albumin. After three washes with TBS, membranes were incubated with the appropriate secondary antibody coupled to horseradish peroxidase diluted in TBST with 5% nonfat dry milk. After washing as before, immunocomplexes were visualized by enhanced chemiluminescence according to manufacturer's instructions (PerkinElmer Life Sciences, Boston, MA).
Zymography
Conditioned media was electrophoresed through a 12% non-reducing SDS-PAGE gel containing 1% gelatin. After electrophoresis, the gel was washed once for 15 minutes and then overnight in wash buffer (2.5% Triton X-100, 50 mM Tris pH 7.5, and 5 mM CaCl2) to remove SDS. The gel was then rinsed three times in water, followed by 24 hours incubation at 37°C in incubation buffer (50 mM Tris pH 7.5 and 5 mM CaCl2). The gel was stained for 2 hours in Coomassie brilliant blue (0.05% Coomassie blue, 10% acetic acid, 30% isopropanol) and destained (10% isopropanol, 10% acetic acid).
Immunocytochemistry
Cells were fixed with 2% paraformaldehyde for 20 minutes at room temperature and then washed three times for 10 minutes with Dulbecco’s phosphate buffered saline (DPBS). Cells were incubated in blocking media (DMEM, 5% FBS, 0.1% glycine, 0.1% lysine) plus 0.2% Triton X-100 for 1 hour at room temperature prior to primary antibody incubation, diluted in blocking buffer, at 4 °C overnight. After washing three times for 10 minutes with DPBS, the cells were incubated in secondary antibody, diluted in blocking buffer, for 1 hour at room temperature. After washing as before, cells were examined using a Nikon inverted epifluorescence microscope (Micro Video Instruments, Avon, MA) and digital images captured with a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI). Figures were prepared using Adobe Photoshop (Adobe Systems Inc., San Jose, CA) with minor adjustment to color contrast.
For live labeling, cells were incubated in primary antibody at 37 °C for 10 minutes. Cells were washed twice for 5 minutes with DPBS, then incubated with secondary antibody, diluted in PBS with 5% FBS, for 1 hour at room temperature. The cells were fixed with 4% formaldehyde (Polysciences Inc., Warrington, PA) for 10 minutes and washed three times for 10 minutes with DPBS prior to examination.
Transfection
Full length Timp-2 cDNA was generated by reverse transcription polymerase chain reaction from adult rat brain. The primers used were: forward 5’ ATTTAGAATTCATGGGCGCCGCGGCCCGC 3’; reverse 5’ GTCTGCTCGAGCGGGTCCTCGATGTCAAG 3’. The PCR product was cloned into the EcoRI and XhoI sites in the pIRES-hrGFP-1a vector (Stratagene, La Jolla, CA) and sequenced to verify a complete open reading frame. Twenty-four hours after plating, cells were transfected with 800 ng DNA (vector only or TIMP-2) using Lipofectamine 2000 (Invitrogen). After overnight incubation, fresh growth medium was added and cells were analyzed for myoD and myogenin expression 72 hours after transfection.
RESULTS
The regulation of TIMP-2, MT1-MMP, and MMP-2 expression during differentiation was determined by western blot analysis using whole C2C12 cell lysates (Fig. 1). TIMP-2 is barely detected in proliferating myoblasts (RP, collected 24 hours after plating). In contrast, MT1-MMP is abundantly expressed and MMP-2 moderately expressed in myoblasts. Growth in media containing 10% fetal calf serum for an additional 24 hours has little effect on either TIMP-2 or MMP-2 expression. In sharp contrast, MT1-MMP expression is decreased. Upon switching to differentiation media containing 2% horse serum, TIMP-2 expression steadily increases. The expression of both MT1-MMP and MMP-2 is largely unchanged during myoblast migration (1 DIV) and fusion (2 DIV). However, expression slowly increases coincident with the appearance of differentiated myotubes. The most pronounced increase in MT1-MMP and MMP-2 expression occurs at 7 DIV at which point all three molecules are expressed at comparable levels.
Figure 1. TIMP-2, MT1-MMP, and MMP-2 are differentially regulated coincident with C2C12 differentiation.
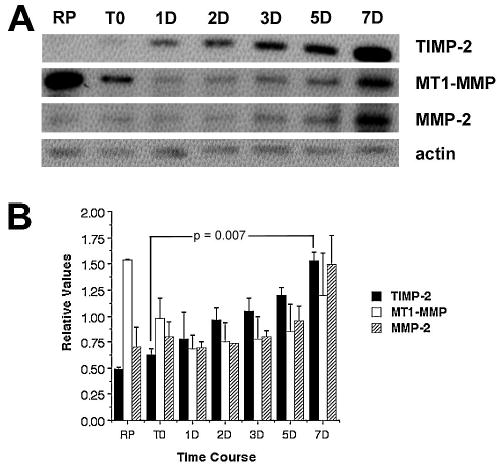
Western blot analysis with 25 μg protein of C2C12 whole cell lysates. A) Protein expression was examined in rapidly proliferating myoblasts (RP), prior to addition of differentiation media (T0), and after 1, 2, 3, 5, and 7 days in differentiation media. TIMP-2 expression is up-regulated coincident with C2C12 differentiation. In contrast, MT1-MMP expression is down-regulated with time in growth media (RP to T0) and is further decreased upon serum reduction, but is up-regulated with differentiation. MMP-2 expression is increased only at later stages of C2C12 differentiation. B) Densitometric analysis of TIMP-2, MT1-MMP, and MMP-2 expression normalized to actin at each time point. Data are representative of two independent experiments and presented as mean ± standard deviation.
To determine the localization of these molecules in C2C12 cells, immunocytochemistry was performed (Fig. 2). The level of immunolabeling for each molecule in Triton-permeabilized cells was consistent with the western blot results (Fig. 2A). TIMP-2 was most abundantly expressed, and expression increased throughout myotube maturation. MT1-MMP expression was greatest in rapidly proliferating myoblasts and mature myotubes. MMP-2 expression was principally expressed only in well-differentiated myotubes. Preabsorption of antibodies reduced immunolabeling to levels comparable to secondary antibody alone controls. To verify terminal differentiation, cells at 3 DIV were immunolabeled with myoD (Fig. 2Bb, e, h). All three molecules were expressed in multinucleated myoD-positive myotubes (Fig. 2Bc, f, i). Similar results were obtained with myogenin. Examination at higher magnification revealed that TIMP localization differed from the other two molecules. TIMP-2 was diffusely localized throughout the cell (Fig. 2Ba). In contrast, MT1-MMP (Fig. 2Bd) and MMP-2 (Fig. 2Bg) showed punctate localization consistent with the membrane association of MT1-MMP and MMP-2’s interaction with it. The more diffuse expression of TIMP-2 suggested interaction with other molecules.
Figure 2. TIMP-2, MMP-2, and MT1-MMP are differentially localized in the myoblasts as they differentiate into myotubes.
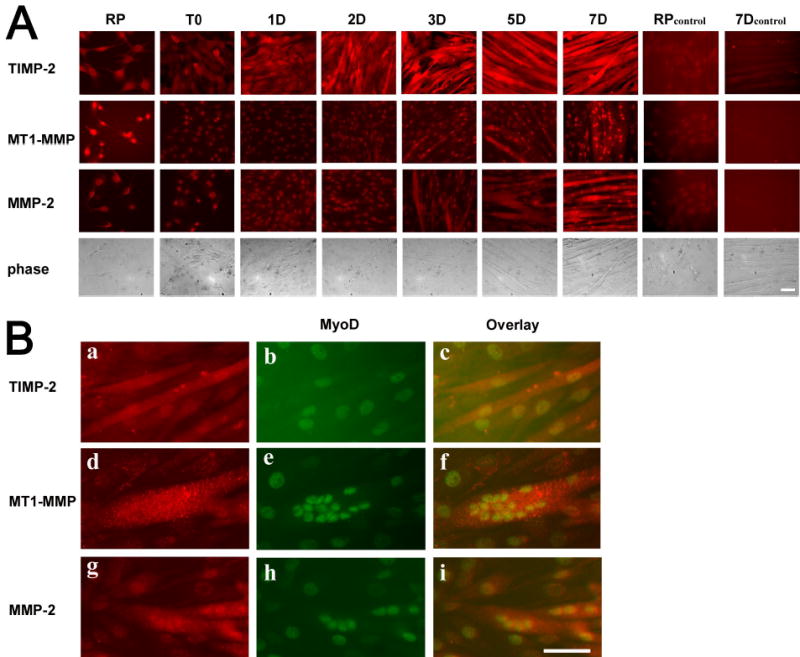
Immunocytochemistry of Triton permeabilized C2C12 cells. A) Cells were cultured as described in Fig 1. As a control, myoblasts (RP) and differentiated myotubes (7D) were stained with secondary antibody alone. Note that the nuclear staining detected is due to the sheep (TIMP-2) and rabbit (MT1-MMP and MMP-2) secondary antibody staining alone. B) After 3 days in differentiation media, TIMP-2 (a) is diffusely localized throughout the differentiated myotube. In contrast, MT1-MMP (d) and MMP-2 (g) labeling show punctate localization consistent with membrane bound localization. MyoD (b, e, and h) labeling demonstrates differentiated myotubes. Scale bar = 50 μm.
To examine more closely the cell surface expression of TIMP-2, live label immunocytochemistry was performed at 7 DIV when TIMP-2 expression was maximal. No surface labeling was detected at 7 DIV (Fig. 3A) or any other time point examined. To determine whether the absence of cell surface expression was due to the lack of TIMP-2 secretion from C2C12 cells, TIMP-2 expression in conditioned media were examined by western blot analysis (Fig. 3B). Because serum contains endogenous MMPs and TIMPs, conditioned media was collected from cells grown under serum-free conditions in addition to cells grown in 2% horse serum. MMP-2 was detected in both 2% horse serum and serum-free conditioned media, confirming previous reports that MMP-2 is secreted by C2C12 cells 10,23. Furthermore, zymography demonstrated that MMP-2 was present in its active form (Fig. 3C). In contrast, TIMP-2 was not detected in serum-free conditioned media. The TIMP-2 detected in conditioned media of cells grown in 2% horse serum likely represents that present endogenously in serum. If TIMP-2 was secreted from C2C12 cells, it was below detectable levels and explains why cell surface TIMP-2 expression on myotubes was not detected.
Figure 3. Unlike MMP-2, TIMP-2 is not secreted from C2C12 cells at detectable levels.
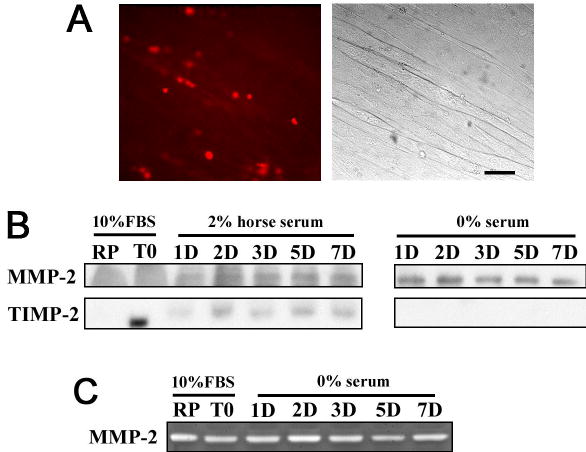
A) Live label immunocytochemistry to detect cell surface TIMP-2 expression at 7 DIV. Although there appears to be ECM associated TIMP-2 expression, close examination of phase contrast micrographs reveals that the staining does not correspond to myotubes. Scale bar = 50 μm. B) Conditioned media was collected from cells grown in growth media (10% FBS) and differentiation media (2% horse serum and serum-free). Western blot analysis (20 μl unconcentrated media) demonstrates that MMP-2, but not TIMP-2 is secreted from C2C12 cells. MMP-2 is detected in both 2% horse serum and serum-free media. In contrast, TIMP-2 is detected only in media containing 2% horse serum. This likely represents the endogenous presence of TIMP-2 in serum. C) Zymography of conditioned media demonstrates that MMP-2 possesses proteolytic activity.
Since MMP-2 and MMP-9 are expressed by human satellite cells 13 and ADAM-12 is expressed in C2C12 reserve cells5, we sought to determine whether MMP-2, MT1-MMP or TIMP-2 were expressed in reserve cells. Several satellite cells markers were tested; however, these markers were either also expressed in myotubes (e.g., M-cadherin and VCAM-1) or were not present at detectable levels (e.g., p130). Therefore, we cannot exclude the possibility that MMP-2, MT1-MMP or TIMP-2 are also expressed by reserve cells.
To elucidate the role of TIMP-2 in differentiation, TIMP-2 was overexpressed in myoblasts (Fig. 4). The adrenal medullary pheochromocytoma cell line, PC12, can be induced to differentiation into neurons with a sympathetic-like phenotype in the presence of nerve growth factor 12. Overexpression of TIMP-2 in proliferating PC12 cells (maintained in 15% serum) induced cell cycle arrest and neuronal differentiation in the absence of nerve growth factor, indicating that TIMP-2 alone was sufficient to induce differentiation (Perez-Martinez and Jaworski, unpublished observation). In contrast to PC12 cells, where TIMP-2 was able to override the mitogenic signals present in serum and promote differentiation, TIMP-2 was unable to induce differentiation of myoblasts maintained in 10% serum. However, TIMP-2 overexpression in cells maintained in 2% horse serum did influence differentiation. Cells were transfected either with TIMP-2 (Fig. 4a, b) or vector alone (Fig. 4c, d) and the expression of MyoD and myogenin was examined immunocytochemically 3 days after transfection. Untransfected cells served as a control (Fig. 4e–h). As expected for cells maintained in differentiation media, vector transfected cells express both myoD, a marker of differentiation commitment, and myogenin, a marker of terminal differentiation. The lack of myogenin expression by TIMP-2 transfected cells and the presence of myogenin in vector and untransfected cells suggested that TIMP-2 plays a role in keeping cells in an undifferentiated state.
Figure 4. TIMP-2 might have a role in delaying differentiation of the myoblasts into myotubes.
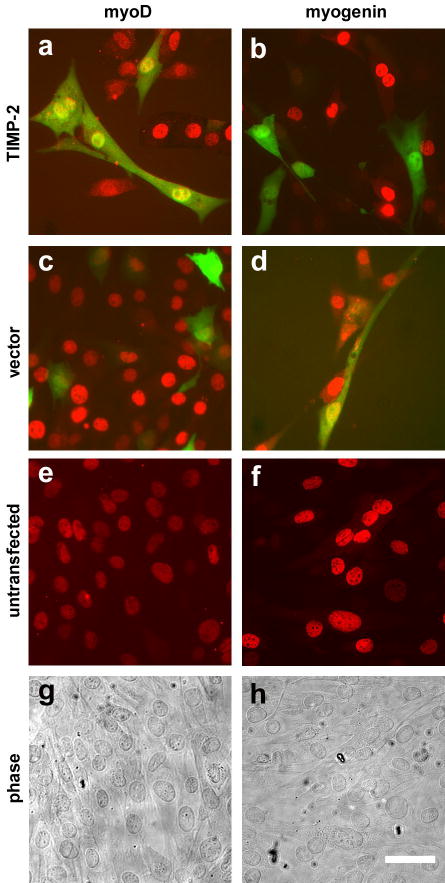
To determine the role of TIMP-2 in myogenesis, C2C12 myoblasts were transfected with TIMP-2 in differentiation media (DMEM, 2% horse serum). Three days after transfection, cells were analyzed immunocytochemically for MyoD, a marker for myogenic commitment, and myogenin, a marker for differentiated myotubes. In contrast to vector transfected GFP-positive cells which express MyoD (c) and myogenin (d), TIMP-2 transfected GFP-positive cells only express MyoD (a). Myogenin expression was not detected in any TIMP-2 transfected GFP- positive cell (b). Untransfected cells served as a control (e, f). Phase contrast micrographs (g, h) are of untransfected cells. Since myogenin marks a more differentiated phase of myotube this suggests that TIMP-2 over-expression has a role in delaying differentiation. Scale bar = 50 μm.
DISCUSSION
ECM degradation must be exquisitely regulated to ensure appropriate morphogenetic remodeling during myogenesis. Although a role for MMPs in myoblast fusion has long been proposed 9, only recently have the responsible proteases been identified. This study was undertaken to characterize the members of the ternary TIMP-2/MT1-MMP/MMP-2 complex during C2C12 myogenesis.
Previous studies have demonstrated that C2C12 myoblasts secrete MMP-2 in a constitutive manner 10,21,23. Our results confirm these observations in that MMP-2 expression was largely unchanged throughout C2C12 differentiation and suggests that MMP-2 does not play a significant role in myoblast migration and/or fusion. However, MMP-2 overexpression increases C2C12 migration 10. Since MMP-2 was present in the active form and MT1-MMP mRNA was detected 10, it was assumed that C2C12 cells also express MT1-MMP.
Here, we demonstrate that C2C12 cells express protein for MT1-MMP as well as its inhibitor, TIMP-2. MT1-MMP is best characterized for its role in migration and tumor invasion17. The downregulation of MT1-MMP with time in growth media (RP to T0) suggests it mediates pericellular proteolysis of rapidly proliferating cells. As cells approach confluence, when less ECM remodeling is required, MT1-MMP expression then declines. Unlike MMP-2, MT1-MMP expression slowly increases during C2C12 differentiation; therefore, MT1-MMP may play a role in myoblast migration and/or fusion. It was recently demonstrated that overexpression of MMP-2 or MT1-MMP alone in C2C12 cells was associated with decreased myotube formation, whereas overexpression of both augmented myotube formation 26. This suggests that MMP-2 and MT1-MMP play complementary, but not overlapping roles in myogenesis.
The regulation of TIMP-2 protein during C2C12 differentiation observed here is similar to that reported for TIMP-1 mRNA 23 and that recently reported for TIMP-2 by DNA microarray 34. The increased expression of TIMP-1 and TIMP-2 during differentiation suggests that these molecules participate in all phases of myogenic differentiation. If TIMP-2 promotes myogenesis, one would expect to observe enhanced myotube formation when overexpressed. However, TIMP-2 overexpression in growth media containing 10% serum had no effect on differentiation, and overexpression in differentiation media (2% horse serum) was associated with decreased myogenin expression in transfected cells. TIMP-2 can either inhibit 24 or promote14 cell growth. Inasmuch as the growth-inhibiting effect of TIMP-2 only occurs when TIMP-2 is present prior to, not subsequent to or in conjunction with, mitogenic stimulation, it has been suggested that TIMP-2 could serve as a cell cycle regulator to prevent inappropriate mitogenic signaling 15.
Proliferating C2C12 cells express MyoD and Myf-5, transcription factors that activate skeletal muscle-specific genes 3,33. Upon serum deprivation, C2C12 cells undergo cell cycle arrest and express myogenin, a transcription factor associated with terminal differentiation 1. Since TIMP-2 was overexpressed in the presence of 2% horse serum, it is possible that TIMP-2 acts in concert with myoD to allow commitment to a myogenic phenotype, but not permit terminal differentiation due to the presence of serum. It is interesting to note the presence of multiple E-box consensus sequences, binding sites for myogenic transcription factors, in the mouse TIMP-2 promoter (unpublished observation). The role of TIMP-2 in myogenesis is not skeletal muscle-specific. Recently it was demonstrated that the expression of TIMP-2, MMP-2 and MT1-MMP (MMP-14) are upregulated coincident with the appearance of differentiated cardiac myocytes 39.
Although recognized for their role in MMP inhibition, there is increasing evidence that some TIMP functions are independent of MMP inhibition 11,20,31. The MMP-independent antiproliferative effects of TIMP-2 on microvascular endothelial cells are mediated by TIMP-2’s interaction with α3β1 integrin 31. In addition, TIMP-2 induces PC12 cell cycle arrest and neuronal differentiation independent of MMP inhibition via α3β1 integrin (Perez-Martinez and Jaworski, unpublished observation). Given the role of β1 integrin in myognesis 28, it will be interesting to determine whether TIMP-2’s role in myogenesis is similarly MMP-independent and mediated by integrins.
The disruption of MMP balance is a major characteristic of many pathological processes affecting muscle. MMP-2 and MMP-9 expression is increased in inflammatory myopathies including sporadic inclusion-body myositis, polymyositis, and dermatomyositis 8,22,29. No change in TIMP mRNA expression was detected in these myopathies 22. The expression of MMPs on non-necrotic muscle fibers in these myopathies may facilitate lymphocyte adhesion and enhance T-cell-mediated cytotoxicity. However, MMP expression is also increased in non-inflammatory myopathies. Increased MMP-7 activity has been detected in serum of Duchenne muscular dystrophic (DMD) patients 32. In addition, increased MMP-2, TIMP-1 and TIMP-2 mRNA, as well as MMP-2 activity, is present in DMD muscle 35. Taken together, these data suggest that dysregulation of MMP-9/TIMP-1 or MMP-2/TIMP-2 expression may contribute to muscle pathogenesis. Muscle phenotypes have not been reported in MT1-MMP 16 or MMP-2 18 knockout mice. It will be interesting to examine TIMP-2 knockout mice 7,36 to determine whether they posses deficits in myogenesis.
In the present report, we showed that TIMP-2, MT1-MMP and MMP-2 are differentially regulated during C2C12 differentiation, suggesting that each may subserve a distinct function during myogenesis. Further studies are needed to elucidate the mechanism by which these molecules regulate myogenesis during development to determine whether similar mechanisms underlie their activities in myopathies. If TIMP’s role in myogenesis is MMP-independent it may open new avenues of therapies for myopathies.
Acknowledgments
This work was supported by Grant NS045225 co-funded by NINDS and NCRR to DMJ. Densitometric analysis was performed in the VT Cancer Center DNA Analysis Facility and was supported, in part, by grant P30CA22435 from the NCI.
References
- 1.Andres V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 3.Braun T, Buschhausen-Denker G, Bober E, Tannich E, Arnold HH. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. Embo J. 1989;8:701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, van Westrum SS, et al. The TIMP2 membrane type 1 metalloproteinase "receptor" regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998;273:871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Zhao Z, Gruszczynska-Biegala J, Zolkiewska A. Role of metalloprotease disintegrin ADAM12 in determination of quiescent reserve cells during myogenic differentiation in vitro. Mol Cell Biol. 2003;23:6725–6738. doi: 10.1128/MCB.23.19.6725-6738.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeli E, Moas M, Reznick AZ, Coleman R. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve. 2004;29:191–197. doi: 10.1002/mus.10529. [DOI] [PubMed] [Google Scholar]
- 7.Caterina JJ, Yamada S, Caterina NC, Longenecker G, Holmback K, Shi J, et al. Inactivating mutation of the mouse tissue inhibitor of metalloproteinases-2(Timp-2) gene alters proMMP-2 activation. J Biol Chem. 2000;275:26416–26422. doi: 10.1074/jbc.M001271200. [DOI] [PubMed] [Google Scholar]
- 8.Choi YC, Dalakas MC. Expression of matrix metalloproteinases in the muscle of patients with inflammatory myopathies. Neurology. 2000;54:65–71. doi: 10.1212/wnl.54.1.65. [DOI] [PubMed] [Google Scholar]
- 9.Couch CB, Strittmatter WJ. Rat myoblast fusion requires metalloendoprotease activity. Cell. 1983;32:257–265. doi: 10.1016/0092-8674(83)90516-0. [DOI] [PubMed] [Google Scholar]
- 10.El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res. 2000;258:279–287. doi: 10.1006/excr.2000.4962. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez CA, Butterfield C, Jackson G, Moses MA. Structural and functional uncoupling of the enzymatic and angiogenic inhibitory activities of tissue inhibitor of metalloproteinase-2 (TIMP-2): loop 6 is a novel angiogenesis inhibitor. J Biol Chem. 2003;278:40989–40995. doi: 10.1074/jbc.M306176200. [DOI] [PubMed] [Google Scholar]
- 12.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerin CW, Holland PC. Synthesis and secretion of matrix-degrading metalloproteases by human skeletal muscle satellite cells. Dev Dyn. 1995;202:91–99. doi: 10.1002/aja.1002020109. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa T, Yamashita K, Ohuchi E, Shinagawa A. Cell growth-promoting activity of tissue inhibitor of metalloproteinases-2 (TIMP-2) J Cell Sci. 1994;107:2373–2379. doi: 10.1242/jcs.107.9.2373. [DOI] [PubMed] [Google Scholar]
- 15.Hoegy SE, Oh HR, Corcoran ML, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 (TIMP-2) suppresses TKR-growth factor signaling independent of metalloproteinase inhibition. J Biol Chem. 2001;276:3203–3214. doi: 10.1074/jbc.M008157200. [DOI] [PubMed] [Google Scholar]
- 16.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 17.Holmbeck K, Bianco P, Yamada S, Birkedal-Hansen H. MT1-MMP: a tethered collagenase. J Cell Physiol. 2004;200:11–19. doi: 10.1002/jcp.20065. [DOI] [PubMed] [Google Scholar]
- 18.Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- 19.Jaworski DM, Fager N. Regulation of tissue inhibitor of metalloproteinase-3 (Timp-3) mRNA expression during rat CNS development. J Neurosci Res. 2000;61:396–408. doi: 10.1002/1097-4547(20000815)61:4<396::AID-JNR6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002;21:2245–2252. doi: 10.1038/sj.onc.1205291. [DOI] [PubMed] [Google Scholar]
- 21.Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdiere-Sahuque M, et al. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol. 1999;205:158–170. doi: 10.1006/dbio.1998.9107. [DOI] [PubMed] [Google Scholar]
- 22.Kieseier BC, Schneider C, Clements JM, Gearing AJ, Gold R, Toyka KV, et al. Expression of specific matrix metalloproteinases in inflammatory myopathies. Brain. 2001;124:341–351. doi: 10.1093/brain/124.2.341. [DOI] [PubMed] [Google Scholar]
- 23.Lewis MP, Tippett HL, Sinanan AC, Morgan MJ, Hunt NP. Gelatinase-B (matrix metalloproteinase-9; MMP-9) secretion is involved in the migratory phase of human and murine muscle cell cultures. J Muscle Res Cell Motil. 2000;21:223–233. doi: 10.1023/a:1005670507906. [DOI] [PubMed] [Google Scholar]
- 24.Murphy AN, Unsworth EJ, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J Cell Physiol. 1993;157:351–358. doi: 10.1002/jcp.1041570219. [DOI] [PubMed] [Google Scholar]
- 25.Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR. Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett. 2004;563:129–134. doi: 10.1016/S0014-5793(04)00281-9. [DOI] [PubMed] [Google Scholar]
- 26.Oh J, Takahashi R, Adachi E, Kondo S, Kuratomi S, Noma A, et al. Mutations in two matrix metalloproteinase genes, MMP-2 and MT1-MMP, are synthetic lethal in mice. Oncogene. 2004;23:5041–5048. doi: 10.1038/sj.onc.1207688. [DOI] [PubMed] [Google Scholar]
- 27.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 28.Rohwedel J, Guan K, Zuschratter W, Jin S, Ahnert-Hilger G, Furst D, et al. Loss of beta1 integrin function results in a retardation of myogenic, but an acceleration of neuronal, differentiation of embryonic stem cells in vitro. Dev Biol. 1998;201:167–184. doi: 10.1006/dbio.1998.9002. [DOI] [PubMed] [Google Scholar]
- 29.Schoser BG, Blottner D, Stuerenburg HJ. Matrix metalloproteinases in inflammatory myopathies: enhanced immunoreactivity near atrophic myofibers. Acta Neurol Scand. 2002;105:309–313. doi: 10.1034/j.1600-0404.2002.1o104.x. [DOI] [PubMed] [Google Scholar]
- 30.Seale P, Rudnicki MA. A new look at the origin, function, and "stem-cell" status of muscle satellite cells. Dev Biol. 2000;218:115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- 31.Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 32.Sohar I, Laszlo A, Gaal K, Mechler F. Cysteine and metalloproteinase activities in serum of Duchenne muscular dystrophic genotypes. Biol Chem Hoppe Seyler. 1988;369(Suppl):277–279. [PubMed] [Google Scholar]
- 33.Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, Lassar AB. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- 34.Tomczak KK, Marinescu VD, Ramoni MF, Sanoudou D, Montanaro F, Han M, et al. Expression profiling and identification of novel genes involved in myogenic differentiation. FASEB J. 2004;18:403–405. doi: 10.1096/fj.03-0568fje. [DOI] [PubMed] [Google Scholar]
- 35.von Moers A, Zwirner A, Reinhold A, Bruckmann O, van Landeghem F, Stoltenburg-Didinger G, et al. Increased mRNA expression of tissue inhibitors of metalloproteinase-1 and -2 in Duchenne muscular dystrophy. Acta Neuropathol (Berl) 2004 doi: 10.1007/s00401-004-0941-0. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 38.Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young DA, Gavrilov S, Pennington CJ, Nuttall RK, Edwards DR, Kitsis RN, et al. Expression of metalloproteinases and inhibitors in the differentiation of P19CL6 cells into cardiac myocytes. Biochem Biophys Res Commun. 2004;322:759–765. doi: 10.1016/j.bbrc.2004.07.178. [DOI] [PubMed] [Google Scholar]


