Abstract
Background
Panic disorder is a prevalent, often disabling condition among patients in the primary care setting. Although numerous studies have assessed the effectiveness of treatments for depression in primary care, few such studies have been conducted for panic disorder.
Objective
To implement and test the effectiveness of a combined pharmacotherapy and cognitive-behavioral intervention for panic disorder tailored to the primary care setting.
Design
Randomized, controlled study comparing intervention to treatment as usual.
Setting
Six primary care clinics associated with 3 university medical schools, serving an ethnically and socioeconomically diverse patient population.
Participants
Two hundred thirty-two primary care patients meeting DSM-IV criteria for panic disorder. Comorbid mental and physical disorders were permitted, provided these did not contraindicate the treatment to be provided and were not acutely life threatening.
Intervention
Patients were randomized to receive either treatment as usual or an intervention consisting of a combination of up to 6 sessions (across 12 weeks) of cognitive-behavioral therapy (CBT) modified for the primary care setting, with up to 6 follow-up telephone contacts during the next 9 months, and algorithm-based pharmacotherapy provided by the primary care physician with guidance from a psychiatrist. Behavioral health specialists, the majority inexperienced in CBT for panic disorder, were trained to deliver the CBT and coordinated overall care, including pharmacotherapy.
Main Outcomes Measures
Proportion of subjects remitted (no panic attacks in the past month, minimal anticipatory anxiety, and agoraphobia subscale score <10 on Fear Questionnaire) and responding (Anxiety Sensitivity Index score <20) and change over time in World Health Organization Disability Scale and short form 12 scores.
Results
The combined cognitive-behavioral and pharmacotherapeutic intervention resulted in sustained and gradually increasing improvement relative to treatment as usual, with significantly higher rates at all points of both the proportion of subjects remitted (3 months, 20% vs 12%; 12 months, 29% vs 16%) and responding (3 months, 46% vs 27%; 12 months, 63% vs 38%) and significantly greater improvements in World Health Organization Disability Scale (all points) and short form 12 mental health functioning (3 and 6 months) scores. These effects were obtained in spite of similar rates of delivery of guideline-concordant pharmacotherapy to the 2 groups.
Conclusion
Delivery of evidence-based CBT and medication using the collaborative care model and a CBT-naïve, midlevel behavioral health specialist is feasible and significantly more effective than usual care for primary care panic disorder.
OVER THE PAST DECADE, randomized studies have established the short-term 1–10 and longer-term3,5,10 effectiveness of interventions that support primary care physicians (PCPs) in delivering evidence-based treatments to patients with depression. However, few studies have tested models to improve outcomes for anxiety disorders in primary care, despite the fact that anxiety disorders are highly prevalent and disabling,11 costly,12,13 poorly recognized,14–18 and inadequately treated19–21 in this setting.
Panic disorder, one of the most disabling and costly anxiety disorders, commonly is seen in primary care where it frequently masquerades as physical illness22 and often prompts costly and sometimes unnecessary use of health care resources.23,24 Only 1 effectiveness study exists for the treatment of panic disorder in primary care settings.25 This study, using a collaborative care model and psychiatrists with proven anxiety-disorder expertise to assist primary care physicians in prescribing and managing medications for panic disorder, was both clinically and cost effective.26 However, similar to early primary care depression studies,27 immediate (3- to 6-month) effects of this intervention tended to fall off at 9 to 12 months, as treatment intensity decreased. That study did not provide any form of psychotherapy such as cognitive-behavioral therapy (CBT) for panic disorder. Cognitive-behavioral therapy is a modality with proven efficacy for panic disorder, is often preferred by primary care patients with anxiety,28 and has been demonstrated to increase durability of outcome in efficacy studies.29,30
This randomized, controlled trial, the Collaborative Care for Anxiety and Panic study, sought to determine the extent to which the benefits of evidence-based, specialist-delivered, panic-disorder interventions29 would generalize to primary care settings with nonspecialist therapists and more diverse patient populations. This intervention used therapists who were minimally or not at all trained in CBT to approximate outcomes that might be expected with relatively novice therapists when the treatment was introduced into primary care settings. These therapists were also used to promote evidence-based primary care–physician pharmacotherapy by relaying expert advice from psychiatrists who reviewed weekly therapist progress reports on patients’ clinical status and medication use. Combination treatment was provided because it is more effective for panic complicated by comorbid conditions30 and, in the maintenance phase, for uncomplicated panic.29 Patients with panic disorder in 3 West Coast primary care sites were randomly assigned to a combination of evidence-based medication treatment and CBT or to usual care. We hypothesized that the intervention: (1) would produce care that was more concordant with published treatment guidelines for panic disorder31; and (2) would result in greater and more sustained improvement in clinical symptoms and functioning.
METHODS
SETTING AND SUBJECTS
The settings for this study were university-affiliated primary care clinics in Seattle, Wash, San Diego, Calif, and Los Angeles, Calif. The Seattle and Los Angeles clinics were internal medicine clinics whereas San Diego also included family medicine clinics. Clinics were predominantly staffed by board-certified physicians with a minority of care (between 15%–30%) delivered by residents in training under attending supervision. Insurance was a mix of private (50%–80%) and public. Recruitment took place from March 2000 through March 2002.
Eligible subjects included patients who (1) were between 18 and 70 years of age, (2) met DSM-IV criteria for panic disorder with at least 1 panic attack in the prior week, (3) were English-speaking, (4) had access to a telephone, and (5) were “willing to accept” a combined treatment of antianxiety medication and CBT. Psychiatric and medical comorbidities were not reasons for exclusion, except those that were potentially life threatening (ie, suicidal ideation, terminal medical illness) or those expected to severely limit patient participation or adherence (eg, psychosis, current substance abuse, dementia, pregnancy). Patients receiving psychiatric disability benefits or those already seeing a psychiatrist or cognitive-behavioral therapist were excluded. Subjects were recruited in clinic waiting rooms on high-volume days using a validated 2-question panic disorder screener.32 Referrals from clinic physicians were also actively solicited. All patients who were positively screened or referred were administered a telephone diagnostic interview (the Composite International Diagnostic Interview [CIDI]33,34) by a research assistant to determine eligibility. The interviewer, blind to the randomization scheme, then gave eligible subjects’ names to a study coordinator who randomized subjects using alternating assignment, stratified within site by comorbid major depression and referral status (referred vs screened).35,36 Once randomized, and consistent with the effectiveness design, neither patients, therapists, nor PCPs were blind to assignment. The study was approved by the institutional review boards of all 3 universities (University of Washington, Seattle; University of California, Los Angeles; and University of California, San Diego).
INTERVENTION
The intervention (described in greater detail elsewhere [P.P.R., C.D.S., M.G.C., M.B.S., W.K., G.S., A. Means-Christensen, PhD, and A.B., unpublished data, July 2004]37) was based on the collaborative care model38 and used a behavioral health specialist to deliver CBT and coordinate care. We mostly recruited individuals with master-level or recent doctoral-level academic degrees and minimal or no CBT experience to perform the behavioral health specialist functions. The behavioral health specialist was trained to deliver a shortened version (6 sessions plus 6 brief follow-up telephone contacts) of evidence-based CBT, which targeted panic symptoms but also included modules to address depressive and social anxiety symptoms if they were prominent. Patients also received a video of preparatory information about panic disorder and its treatment and a revised and condensed version of a currently available patient workbook39,40 modified to include education about medications, their management, and possible synergies with CBT.
Primary care physicians managed subjects’ medication after receiving a 1-hour didactic on recognition and treatment of panic disorder along with a medication algorithm detailing medication types and dosing strategies. Specific recommendations for individual subjects were relayed as needed from a consulting psychiatrist to the PCP via the behavioral health specialist, who informed the psychiatrist about subject status through weekly meetings. Neither the behavioral health specialist nor the psychiatrist had access to whether the patient filled prescriptions and instead relied on patient self-report, which has been found in other primary care effectiveness studies to correlate well with pharmacy records.2,8 The medication algorithm41 began with dose titration of a selective serotonin reuptake inhibitor for at least 6 weeks, unless the subject had already failed trials of 2 selective serotonin reuptake inhibitors, in which case alternative antidepressants (eg, serotonin norepinephrine reuptake inhibitors, tricyclic antidepressants, nefazodone hydrochloride, mirtazapine) or adjunctive medications (eg, benzodiazepines) were tried first. Care was coordinated by the behavioral health specialist, using rapid systems of 2-way communication with the PCP (ie, telephone, fax, and e-mail).
Subjects were to complete the 6 CBT sessions within the first 3 months of the study. For subjects who were able to complete at least 3 sessions in person, subsequent sessions could be conducted over the telephone if preferred by the patient but had to be finished within 3 months. Six follow-up telephone booster sessions, each lasting from 15 to 30 minutes, were scheduled through the rest of the year at 6- to 12-week intervals to monitor clinical status, reinforce proper medication use and cognitive-behavioral skills, and make further medication recommendations if necessary.
USUAL CARE
Subjects in usual care received treatment as usual (typically pharmacotherapy) from their PCP, who received the results of the initial diagnostic telephone assessment so that eventual outcomes were not attributable to nonrecognition of panic disorder and associated disorders. Usual care subjects could also be referred or self-refer to mental health resources available to them in the community.
OUTCOME ASSESSMENT AND DEFINITION
Assessments were derived from telephone interviewer-administered questionnaires, queried by interviewers blind to subject intervention status, at baseline and every 3 months during the course of the study. The interview included portions of the CIDI,33 covering panic, generalized anxiety, social anxiety, and posttraumatic stress and major depressive disorders (baseline only); a battery of scales and individual items to dimensionally measure severity of symptoms, disability, and quality of life ; and questions to document type and amount of pharmacological and psychological treatments received, as well as use of medical and mental health services during this time.42 To measure clinical response, we used a composite measure of remission based on the concept of “high end-state functioning.”43 Using this measure, patients had to meet all 3 of the following criteria: no panic attacks in the past month44; minimal anticipatory anxiety about panic (0–1 on a 3-point scale)44; and an agoraphobia subscale score of 10 or less.45 To measure functional status and health-related quality of life, we used 5 items selected from the larger World Health Organization Disability Scale46 and mental and physical health–related quality of life using the Global Physical and Mental Health scales of the short form 12 (SF-12).47 The 5 World Health Organization items, not validated at the time, were suggested by the scale developer (B. Usten, MD, oral communication, July 1999) and, in our sample, had high internal consistency (α=.78) and correlated moderately (r=−0.48) to strongly (r=−0.61) with the emotional well-being and physical functional subscales, respectively, of the SF-12. The SF-12 reproduces short form 36 summary measures with an accuracy of more than 90% and has demonstrated good validity.47 We also measured severity of depression using the Center for Epidemiologic Studies–Depression Scale.48 As a secondary outcome measure, we categorized patients as responding or not, using as a criterion a score of less than 20 on the Anxiety Sensitivity Index (ASI),49 a scale that measures the cognitions that underlie panic-related somatization but is also sensitive to the frequency of recent panic attacks.50 This measure and criterion was used in the previous primary care panic study.25 We also used the ASI as a dimensional measure of outcome.
Finally, we measured intervention effects on quality of antianxiety pharmacotherapy and CBT. Pharmacotherapy was considered adequate when subjects reported, in the assessment of their service use, taking a guideline-concordant antipanic medication at a sufficient dose for at least 6 weeks.41 Cognitive-behavioral therapy was considered adequate when subjects reported attending a minimum of 3 specialty sessions and reported that their sessions contained a minimum of 4 of 7 key components considered characteristic of CBT.51
STATISTICAL ANALYSIS
Planned sample size calculations (n=320) were based on an estimated 20% to 30% response differential between intervention and usual care and an estimated 33% subject attrition across the study period. Although the enrolled sample size was smaller than this, subject attrition was less than anticipated.
We conducted intent-to-treat analyses, where all randomized patients were included in the analysis whether they continued in the study. For the time-trend analysis we specified a (2-level) hierarchical model with random effects. The multilevel structure accounts for the nesting of the repeated measures within individuals as well as the variability across individuals. The repeated observations model (or level 1 model) was a piecewise linear growth model,52 which specifies a linear segment between baseline and the first 3 months’ follow-up (at which point the 6-session CBT ended) and then another linear segment for the subsequent 6, 9, and 12 months’ follow-ups. The 2 segments join at the first follow-up point (3 months). This model is intended to reflect a trend observed in previous effectiveness studies, where the greatest effect occurs during the acute study phase, mirroring the greater intensity of early intervention, and then effects remain stable, fall off, or increase at a much diminished rate. Time trends for patients within group were allowed to vary around the group-specific mean by the inclusion of patient-specific random effects for the intercept, the first slope, and the difference between the second and first slopes. We included site as a categorical predictor in the second level (or individual level) of the hierarchical models.
We adopted a Bayesian approach53,54 to fit this model to both continuous and dichotomous longitudinal responses using baseline and 3-, 6-, 9-, and 12-month follow-up data. We used previously described methods55,56 and WinBUGS57 software to implement the model. Although these more sophisticated models were felt to be more powerful than standard last observation carried forward analyses with repeated-measures analyses of variance, it should be noted that the results were not materially different when such approaches were used. Statistical tests were 2-tailed, with α set at .05.
RESULTS
ENROLLMENT AND SUBJECT CHARACTERISTICS
Figure 1 illustrates the flow of subject selection for the 2-year study period. Of the 618 patients with possible panic disorder who qualified for the CIDI, 168 (27.2%) refused to participate or could not be reached. There were no differences between those participating and those refusing to participate in demographics or screening characteristics. The 232 enrolled patients (61% identified by screen and 39% referred by their PCP) were randomized to either intervention (n=119) or care as usual (n=113) groups. Patients missing at least 1 follow-up interview were equally distributed between the treatment and usual care groups. Site was the only consistent predictor of nonresponse.
Figure 1.
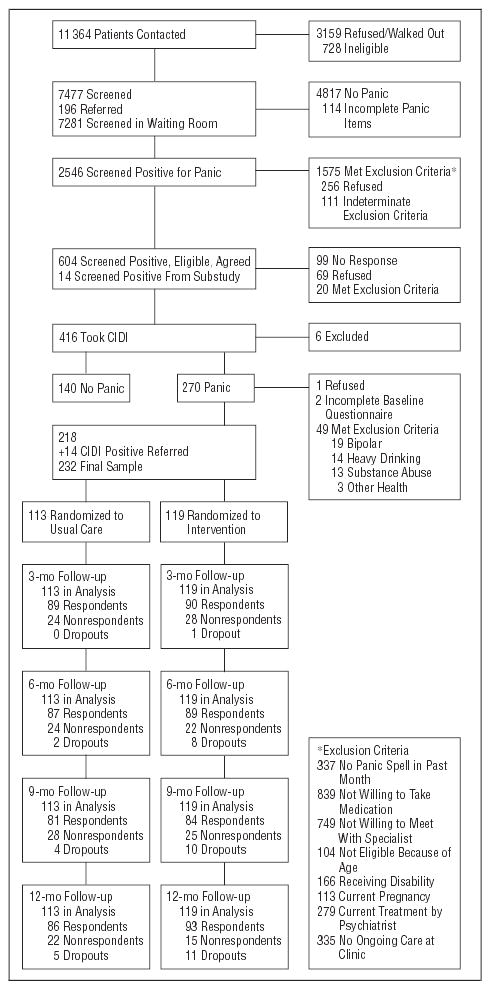
Flowchart of participants. CIDI indicates Composite International Diagnostic Interview.33,34
More than one third of subjects were of nonwhite ethnicity, with a wide range of ages, education, and income levels (Table 1). Almost two thirds had a comorbid medical condition, and more than 70% had at least 1 comorbid mood or anxiety disorder. At baseline, subjects reported low rates of guideline-concordant treatment with both medication and CBT in the prior 6 months (Table 2 presents baseline rates of quality of care prerandomization). Intervention and usual care groups were comparable at baseline on all measures.
Table 1.
Characteristics of Intervention and Usual Care Patients*
| Characteristic | Intervention Patients (n = 119) | Usual Care Patients (n = 113) | Total (N = 232) | Group TestPValue |
|---|---|---|---|---|
| Women | 68 | 66 | 67 | .78 |
| Mean age, y | 40.6 | 41.9 | 41.2 | .38 |
| Education | .27 | |||
| <High school | 5.9 | 8.0 | 6.9 | |
| 12 y | 13.4 | 20.4 | 16.8 | |
| >12 y | 80.7 | 71.7 | 76.3 | |
| Referred | 38.7 | 38.9 | 38.8 | .96 |
| Ethnicity | .28 | |||
| White | 67.2 | 63.7 | 65.5 | |
| Hispanic | 10.9 | 15.9 | 13.4 | |
| African American | 13.4 | 14.2 | 13.8 | |
| Other | 8.4 | 6.1 | 7.3 | |
| No. of chronic medical conditions | .77 | |||
| 0 | 35.3 | 32.7 | 34.1 | |
| 1 | 26.9 | 24.8 | 25.9 | |
| ≥2 | 37.8 | 42.5 | 40.1 | |
| Comorbid psychiatric conditions | ||||
| Social phobia | 41.2 | 38.0 | 39.7 | .63 |
| Posttraumatic stress disorder | 31.9 | 26.6 | 29.3 | .37 |
| Generalized anxiety disorder | 43.2 | 48.5 | 45.7 | .66 |
| Major depression | 53.8 | 57.5 | 55.6 | .57 |
| Agoraphobia (score >10 on agoraphobia subscale of FQ)45 | 38 | 41 | 39 | .63 |
| Mean full panic attack frequency | 1.57 | 1.50 | 1.53 | .53 |
| Mean limited symptom panic attack frequency | 3.35 | 2.47 | 2.92 | .11 |
| Mean anticipatory anxiety score (range, 0–4) | 1.84 | 1.79 | 1.81 | .77 |
| Mean emotional well-being score | 36.0 | 37.7 | 36.8 | .23 |
| Mean physical functioning score | 43.7 | 43.1 | 43.4 | .72 |
| Mean CES-D score48 | 27.9 | 26.8 | 27.4 | .56 |
| Mean Anxiety Sensitivity Index score49 | 34.4 | 32.3 | 33.4 | .20 |
| Mean FQ score45 | 34.2 | 32.3 | 33.3 | .46 |
| Received any mental health specialty care in past 3 mo | 24.4 | 23.2 | 23.8 | .84 |
| Received >2 counseling sessions with at least 3 CBT components | 4.2 | 2.7 | 3.5 | .53 |
| Received any appropriate antipanic prescriptions for ≥6 wks | 26.05 | 30.09 | 28.00 | .49 |
Abbreviations: CBT, cognitive-behavioral therapy; CES-D, Center for Epidemiologic Studies–Depression Scale; FQ, Fear Questionnaire.
Values are expressed as percentages unless otherwise indicated.
Table 2.
Adjusted Means for Quality Measures
|
Proportion |
||||
|---|---|---|---|---|
| Intervention Patients | Usual Care Patients | Difference (95% CI*) | Test Value | |
| Received Appropriate Antipanic Pharmacotherapy for ≥6 Wk | ||||
| Baseline | 0.26 | 0.30 | −0.04 (−0.11 to 0.03) | P = .26 |
| 3 mo | 0.44 | 0.40 | 0.04 (−0.03 to 0.12) | P = .28 |
| 6 mo | 0.42 | 0.39 | 0.03 (−0.03 to 0.08) | P = .35 |
| 9 mo | 0.41 | 0.39 | 0.02 (−0.04 to 0.08) | P = .50 |
| 12 mo | 0.41 | 0.39 | 0.02 (−0.05 to 0.09) | P = .61 |
| All months (n = 126; 52% of sample) | 0.31 | 0.24 | 0.07 | ; P = .34 |
| Received ≥3 Sessions Counseling Plus at Least 4 of 7 CBT Techniques | ||||
| Baseline | 0.05 | 0.03 | 0.02 (−0.20 to 0.06) | P = .40 |
| 3 mo | 0.63 | 0.14 | 0.48 (0.38 to 0.58) | P<.001 |
| 6 mo | 0.17 | 0.09 | 0.07 (0.02 to 0.13) | P = .005 |
| 9 mo | 0.08 | 0.10 | −0.02 (−0.06 to 0.02) | P = .42 |
| 12 mo | 0.06 | 0.11 | −0.05 (−0.10 to −0.01) | P = .02 |
| Received Any Antipanic Pharmacotherapy | ||||
| Baseline | 0.45 | 0.48 | 0.03 (−0.10 to 0.03) | P = .32 |
| 3 mo | 0.62 | 0.56 | 0.06 (−0.01 to 0.06) | P = .11 |
| 6 mo | 0.59 | 0.55 | 0.04 (−0.01 to 0.09) | P = .14 |
| 9 mo | 0.56 | 0.53 | 0.02 (−0.03 to 0.08) | P = .39 |
| 12 mo | 0.54 | 0.52 | 0.01 (−0.06 to 0.09) | P = .67 |
| Received Any Counseling | ||||
| Baseline | 0.25 | 0.23 | 0.02 (−0.06 to 0.10) | P = .60 |
| 3 mo | 0.70 | 0.34 | 0.36 (0.27 to 0.45) | P<.001 |
| 6 mo | 0.39 | 0.32 | 0.07 (0.00 to 0.13) | P = .05 |
| 9 mo | 0.24 | 0.33 | −0.09 (−0.15 to −0.03) | P = .004 |
| 12 mo | 0.18 | 0.34 | −0.16 (−0.24 to −0.10) | P<.001 |
Abbreviations: CBT, cognitive-behavioral therapy; CI, confidence interval.
Ninety-five percent CI is the equal-tails credible interval (based on posterior density estimates).
INTERVENTION PARTICIPATION AND FIDELITY
Of the 119 subjects assigned to the intervention arm, 14 (11.8%) had no CBT sessions, 24 (20.1%) had 1 to 3 sessions, and 81 (68.1%) had 4 to 6 sessions. Of 513 CBT sessions, 3.5% were on the telephone; 12.6% of patients had at least 1 CBT session by telephone. During the 9-month follow-up, 75 subjects (63%) received follow-up telephone calls, with the modal number being 5 (range, 1–6). Using methods previously used by the large multicenter collaborative panic efficacy study,29 expert CBT master-level or newly graduated doctoral-level psychologists independently rated 63 separate behavioral health specialist sessions, randomly selected across the 6 CBT sessions, for adherence to content (rated 1–7), overall competency (rated 0–8) to deliver the treatment, and session length. Average adherence was 4.1 (SD 0.74) and average competency was 4.4 (SD 1.9), indicating adequate adherence and competency in these newly trained behavioral health specialists. Session lengths ranged from 45 to 60 minutes. There were no cross-site differences in the behavioral health specialist ratings of adherence and competency nor did the medication recommendations provided by study psychiatrists differ across sites, based on an independent analysis of concordance across pairs of sites for case descriptions for 1 of every 6 intervention patients. Usual care patients whose PCP had a patient in the intervention (n=77) were no more likely to receive guideline-concordant pharmacotherapy than usual care patients whose physicians had no patients (n=36) in the intervention ( ; P>.30 at all points); this suggests that “spillover” of the intervention to treatment as usual patients was unlikely to have occurred.
PROCESS OF CARE
Table 2 depicts the proportion of subjects in intervention and usual care groups who received guideline-concordant pharmacotherapy and CBT at each of the 3-month assessment points during the 12-month study. The differences in pharmacotherapy did not reach statistical significance at any point, with proportions increasing from baseline in both groups. In contrast, as would be expected given that CBT was part of the intervention, there was a marked difference in CBT received. Significantly more subjects in the intervention group received, as planned, CBT of high quality during the first 3 months of the study (63%) than did usual care subjects (14%). As expected, this difference dropped off substantially after 3 months, once the provision of CBT had ended.
CLINICAL, FUNCTIONAL, AND QUALITY OF LIFE OUTCOMES
Using the most conservative measure of panic disorder outcome (ie, high end-state functioning or remission), the proportion of subjects with zero panic attacks and minimal anticipatory anxiety and phobic avoidance was significantly greater in the intervention group at all points (Figure 2) (Table 3). At 12 months, 29% of intervention patients had remitted, compared with 16% in the usual care group. The longitudinal models also showed robust intervention effects across time for the primary functional status and mental health–related quality of life outcome measures, as well as for depressive symptoms (Table 3). In contrast, the physical health component of the SF-12 changed little in either group. Response using the measure of “core” panic cognitions and symptoms, the ASI, was also significantly greater whether analyzed as a dichotomous measure of response (66% vs 38% at 12 months) (Figure 3) or as a continuous measure (Table 3). The effects sizes for significantly different outcomes ranged from small to medium (0.23–0.51) (Table 3) and were largest for the ASI and smallest for dichotomously determined remission rates. Response to the intervention did not vary by the presence or absence of agoraphobia (agoraphobia subscale45 score <10) nor by the presence or absence of current major depression (determined by the CIDI).
Figure 2.
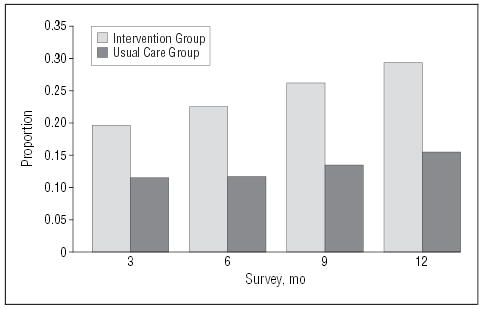
Remission rates. Remission means no panic attacks, minimal anticipatory anxiety, and Fear Questionnaire agoraphobia score45 less than 10.
Table 3.
Adjusted Means for Panic Outcomes
|
Proportion |
|||||
|---|---|---|---|---|---|
| Intervention Patients | Usual Care Patients | Difference (95% CI*) | PValue/Effect Size | ||
| High End-State Functioning43 | |||||
| 3 mo | 0.20 | 0.12 | 0.08 (0.002 to 0.16) | .04/0.23 | |
| 6 mo | 0.22 | 0.12 | 0.10 (0.06 to 0.16) | <.001/0.29 | |
| 9 mo | 0.26 | 0.14 | 0.12 (0.07 to 0.19) | <.001/0.32 | |
| 12 mo | 0.29 | 0.16 | 0.13 (0.06 to 0.21) | <.001/0.34 | |
| Anxiety Sensitivity Index Score <2049 | |||||
| 3 mo | 0.46 | 0.27 | 0.19 (0.11 to 0.27) | <.001/0.40 | |
| 6 mo | 0.53 | 0.31 | 0.22 (0.16 to 0.28) | <.001/0.48 | |
| 9 mo | 0.58 | 0.34 | 0.24 (0.19 to 0.30) | <.001/0.47 | |
| 12 mo | 0.63 | 0.38 | 0.24 (0.17 to 0.32) | <.001/0.51 | |
| Anxiety Sensitivity Index Score49 | |||||
| 3 mo | 23.11 | 28.94 | −5.83 (−9.20 to −2.37) | <.001/0.44 | |
| 6 mo | 21.63 | 27.73 | −6.10 (−9.30 to −2.86) | <.001/0.45 | |
| 9 mo | 20.16 | 26.52 | −6.37 (−9.84 to −2.88) | <.001/0.44 | |
| 12 mo | 18.68 | 25.32 | −6.64 (−10.73 to −2.58) | <.001/0.43 | |
| World Health Organization Disability Score46 | |||||
| 3 mo | 10.07 | 11.39 | −1.32 (−2.46 to −0.14) | .02/0.29 | |
| 6 mo | 9.93 | 11.37 | −1.44 (−2.54 to −0.33) | .01/0.31 | |
| 9 mo | 9.78 | 11.35 | −1.57 (−2.72 to −0.42) | .007/0.33 | |
| 12 mo | 9.63 | 11.33 | −1.70 (−3.00 to −0.40) | .01/0.34 | |
| Mental Health Composite Score (SF-12)47 | |||||
| 3 mo | 43.58 | 39.83 | 3.75 (1.02 to 6.68) | .01/0.33 | |
| 6 mo | 43.76 | 40.69 | 3.08 (0.65 to 5.60) | .01/0.27 | |
| 9 mo | 43.94 | 41.54 | 2.40 (−0.19 to 5.00) | .07/0.21 | |
| 12 mo | 44.12 | 42.40 | 1.73 (1.40 to 4.82) | .28/0.14 | |
| Physical Health Composite Score (SF-12)47 | |||||
| 3 mo | 45.40 | 43.94 | 1.46 (−1.58 to 4.52) | .35/0.12 | |
| 6 mo | 45.13 | 43.67 | 1.46 (−1.46 to 4.41) | .32/0.12 | |
| 9 mo | 44.87 | 43.41 | 1.46 (−1.51 to 4.47) | .33/0.12 | |
| 12 mo | 44.61 | 43.14 | 1.47 (−1.81 to 4.74) | .37/0.12 | |
| Depression Score (CES-D)48 | |||||
| 3 mo | 19.27 | 23.30 | −4.04 (−7.49 to −0.42) | .02/0.29 | |
| 6 mo | 19.06 | 23.00 | −3.94 (−7.12 to −0.64) | .01/0.29 | |
| 9 mo | 18.85 | 22.70 | −3.85 (−7.18 to −0.47) | .02/0.27 | |
| 12 mo | 18.64 | 22.39 | −3.75 (−7.51 to 0.07) | .05/0.26 | |
Abbreviations: CES-D, Center for Epidemiologic Studies–Depression Scale; CI, confidence interval, SF-12, short form 12.
Ninety-five percent CI is the equal-tails credible interval (based on posterior density estimates).
Figure 3.
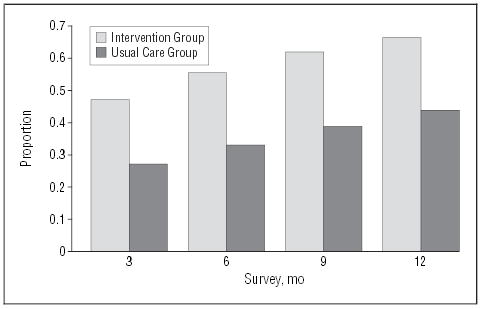
Response rates. Response means score of less than 20 on the Anxiety Sensitivity Index.
Because of the wide range of number of specialty visits attended, we examined remission and response rates at the 3-month point for those intervention subjects with outcomes data at this point (n=90) who also received at least 4 CBT components and attended at least 6 sessions (n=38) vs those who received at least 4 CBT components but only attended 3 to 5 sessions (n=25). Although not significantly different because of limited power, there was a clear dose-response trend with rates of response (56% vs 40%; ; P>.20) and remission (29% vs 15%; ; P>.20) higher in those with 6 sessions compared with those with 3 to 5 sessions. Similarly, we divided intervention patients meeting our liberal definition of receiving “appropriate” medication into those who took medication for the entire 3 months (n=28) and those who took medication for between 6 and 11 weeks (n=14). There were similar dose-response trends for remission (21% vs 0%; ; P =.06) and response (35% vs 29%; ; P>.20), though once again, these were not significant because of limited power.
COMMENT
Our intervention for panic disorder, a combination of CBT and antianxiety medication delivered by a behavioral health specialist in liaison with PCPs and with the assistance of a psychiatric consultant, resulted in substantially better outcomes than did usual care. Significantly more subjects receiving the intervention had responded and remitted at each of the 4 assessment periods over the year, and changes in disability were also significant and persistent. The large effects on ASI scores are particularly noteworthy given that they are both reflective of recent panic and predictive of future symptom status.49,58 Even though reliance on a self-report measure of core cognitions associated with panic disorder is subject to responder biases, the longevity of observed effects on the ASI is unlikely to be fully attributable to such biases. The relatively robust intervention effects are also noteworthy given the high rate of depression in these subjects and the previously observed refractoriness to treatment of comorbid panic and depression.35 These results are more likely to be generalizable than a previous study of primary care panic disorder25 because they were observed across 3 distinct geographical sites, included a larger more ethnically heterogeneous group of primary care patients, required that patients pay for their medication, and used relatively inexperienced therapists and/or care managers to deliver CBT and coordinate medication management instead of highly trained expert psychiatrists.
The poor quality of care for primary care panic disorder observed in all subjects at baseline is consistent with the results of previously published studies in both panic disorder59 and depression.2,5 The intervention, contrary to expectation, did not result in superior provision of guideline-concordant pharmacotherapy, with rates increasing in both groups. Interestingly, this is consistent with recently published findings in primary care patients with depression.60 Reasons for this failure are unclear but are, according to our analyses, not a by-product of physicians of usual care patients implementing what they learned in the care of Collaborative Care for Anxiety and Panic study patients (ie, a spillover effect).61 It is possible that the non-medical background of the behavioral health specialist or the competing demands of both delivering CBT and trying to maximize medication use may have led to less than optimal focus on or achievement of quality medication. It is also possible that patients were less motivated to pay for and maximize their use of medications when CBT was already improving their symptoms and was being provided free of charge. Future interventions will need to explore these and other possibilities to develop solutions to improve the quality of pharmacotherapy provided to patients. The absence of an intervention effect on antianxiety pharmacotherapy quality, taken together with the substantial body of data supporting the efficacy of CBT in panic disorder as well as our data indicating a dose-response trend wherein more specialty visits with CBT components were associated with better outcomes, suggests that the improved outcomes for the intervention group may be attributed primarily to the CBT component of the intervention. Our findings stand in contrast to those of a recent review of “counseling” studies in primary care, which suggested that improvements with these mostly unstructured therapies were modest and only persisted in the short-term.62 The structured and skills-oriented nature of CBT may account for the greater longevity of our effects, consistent with results from many efficacy studies of CBT for panic disorder conducted in specialized clinical research settings,29,63 even though the CBT “dose” achieved in this effectiveness study was lower than in typical efficacy studies (eg, 72% of subjects completed the entire course of CBT in the Barlow et al29 efficacy study, while only about 40% of our subjects did). Notably, the reason for this discrepancy is because, unlike efficacy studies, we did not exclude patients who failed to attend a minimum number of sessions. Our results suggest that in a real-world setting serving primary care patients with multiple medical and psychiatric comorbidities, where treatment is less carefully controlled, CBT is still capable of exerting a significantly beneficial effect, although more work needs to be done to optimize adherence to the full course of treatment that produced optimal results. Our study was not intended, however, to test the effectiveness of CBT alone, and many patients were taking antianxiety medications, even if at less than optimal doses or durations. Thus, the outcomes achieved in this study cannot definitively be attributed to CBT alone. Nonetheless, the possibility that concomitant medication may not be necessary for some patients and that CBT alone tailored for the primary care setting might be an efficacious treatment for panic disorder should be systematically tested.
This study has a number of limitations. First, all care was delivered in university settings ostensibly limiting generalizability of both efficacy and cost estimates (though many of these settings served low-income, ethnically diverse, and disadvantaged populations). Second, CBT was provided free of charge, making it unlikely that this kind of program could be sustained and disseminated without added funds. Third, the multiple treatment elements make it impossible to determine the exact contribution of each element (ie, CBT and/or antianxiety pharmacotherapy). Fourth, master-level and/or newly graduated doctoral-level behavioral health specialists are not likely to be available to smaller primary care practices, although they are now being used by midsized community health practices through a program funded by the Bureau of Primary Care, Health Resources and Services Administration, Washington, DC. Furthermore, there is no reason to believe that nursing staff cannot be trained to implement the collaborative care model of treatment for anxiety disorders since this has been successfully done for depression.5
Although the aim of this study was to deliver and test a treatment for panic disorder, we learned that a narrow focus on this single disorder might be inadequate in the primary care setting. There were many patients (approximately 70%) with other anxiety disorders and/or major depression. These findings suggest a need to develop interventions that can better address the wide range of mood and anxiety disorders in these patients. We also learned that many patients did not adhere to the entire CBT program, even though it was brief and delivered with considerable flexibility of scheduling. This finding suggests the need for qualitative research to elucidate the reasons for nonadherence in these patients. A major goal of future work in this area should be to develop, implement, and disseminate approaches to treatment of anxiety disorders that are maximally acceptable to patients, physicians, and payers. The latter will be particularly important in ensuring the sustainability of such programs in the primary care setting.
Acknowledgments
We thank the following clinics for their participation in the study: Roosevelt GIMC Clinic and Adult Medicine Clinic, Harborview Medical Center, University of Washington, Seattle; Family Health Center and Les Kelly Family Health Center, University of California, Los Angeles; Valley Internal Medicine Group, Los Angeles; Family and Preventive Medicine Group and La Jolla Internal Medicine Group, University of California, San Diego. We would also like to acknowledge the helpful support of clinic directors and staff: Michelle Bholat, MD, MPH, David Dugdale, MD, Daniel Lessler, MD, Burt Liebross, MD, Bob Maurer, PhD, Martin Schulman, MD, Janine Parker, MD, and Jennifer Wu, MD. Adrienne Means-Christensen, PhD, Erin Michelson, BA, Holly Hazlett-Stevens, PhD, and Bobby Verdugo, PhD, coordinated our research efforts. Denise Chavira, PhD; Leslie Zeigenhorn, PhD; Jonathan Bricker, PhD; Jonathan Kantor, PhD; Erin Dunn, PhD; Bonnie Zucker, PhD, Tyler Story, MA, and Barbara Paris, MA, served as behavioral health specialists. Bernadette Benjamin, MS, provided database management and programming support. Finally, we would like to acknowledge the contributions of the Data Safety Monitoring Board (Mark Sullivan, MD; Emmett Keeler, PhD; and Dennis Munjack, MD), the Scientific Advisory Board (Ken Wells, MD; Richard Veith, MD; and Lewis Judd, MD), the Primary Care Advisory Board (Elizabeth Lin, MD, and Joseph Lieberman, MD), and the Ethnic Advisory Board (Jeanne Miranda, PhD; Peter Guarnaccia, PhD; Maja Jackson-Triche, MD; and David Takeuchi, PhD).
Footnotes
Funding/Support: This study was supported by grants MH57858 and MH065324 (Dr Roy-Byrne), MH57835 and MH64122 (Dr Stein), and MH58915-03 (Dr Craske) from the National Institutes of Health, Bethesda, Md.
References
- 1.Katon W, von Korff M, Lin EH, Walker E, Simon GE, Bush T, Robinson P, Russo J. Collaborative management to achieve treatment guidelines: impact on depression in primary care. JAMA. 1995;273:1026–1031. [PubMed] [Google Scholar]
- 2.Katon W, Robinson P, Von Korff M, Lin E, Bush T, Ludman E, Simon G, Walker E. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry. 1996;53:924–932. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- 3.Wells KB, Sherbourne C, Schoenbaum M, Duan N, Meredith L, Unutzer J, Miranda J, Carney MF, Rubenstein LV. Impact of disseminating quality improvement programs for depression in managed primary care: a randomized controlled trial. JAMA. 2000;283:212–220. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]
- 4.Simon GE, VonKorff M, Rutter C, Wagner E. Randomized trial of monitoring, feedback, and management of care by telephone to improve treatment of depression in primary care. BMJ. 2000;320:550–554. doi: 10.1136/bmj.320.7234.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unutzer J, Katon K, Callahan CM, Williams JW, Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C. IMPACT Investigators. Improving mood-promoting access to collaborative treatment: collaborative care management of late-life depression in the primary care setting. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 6.Rost K, Nutting P, Smith J, Werner J, Duan N. Improving depression outcomes in community primary care practice: a randomized trial of the quEST intervention. Quality Enhancement by Strategic Teaming. J Gen Intern Med. 2001;16:143–149. doi: 10.1111/j.1525-1497.2001.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulberg HC, Block MR, Madonia MJ, Scott CP, Rodriguez E, Imber SD, Perel J, Lave J, Houck PR, Coulehan JL. Treating major depression in primary care practice: eight-month clinical outcomes. Arch Gen Psychiatry. 1996;53:913–919. doi: 10.1001/archpsyc.1996.01830100061008. [DOI] [PubMed] [Google Scholar]
- 8.Katon W, Von Korff M, Lin E, Simon G, Walker E, Unutzer J, Bush T, Russo J, Ludman E. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry. 1999;56:1109–1115. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- 9.Katzelnick DJ, Simon GE, Pearson SD, Simon G, Walker E, Unutzer J, Bush T, Russo J, Ludman E. Randomized trial of a depression management program in high utilizers of medical care. Arch Fam Med. 2000;9:345–351. doi: 10.1001/archfami.9.4.345. [DOI] [PubMed] [Google Scholar]
- 10.Rost K, Nutting P, Smith JL, Elliott CE, Dickinson M. Managing depression as a chronic disease: a randomized trial of ongoing treatment in primary care. BMJ. 2002;325:934. doi: 10.1136/bmj.325.7370.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendlowicz MV, Stein MB. Quality of life in individuals with anxiety disorders. Am J Psychiatry. 2000;157:669–682. doi: 10.1176/appi.ajp.157.5.669. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, Ballenger JC, Fyer AJ. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- 13.Walker EA, Katon W, Russo J, Ciechanowski P, Newman E, Wagner AW. Health care costs associated with posttraumatic stress disorder symptoms in women. Arch Gen Psychiatry. 2003;60:369–374. doi: 10.1001/archpsyc.60.4.369. [DOI] [PubMed] [Google Scholar]
- 14.Katon W. Panic Disorder in the Medical Setting Washington, DC: Washington DC Superintendent of Documents, US Government Press; 1989. NIMH DHHS publication 89–1629.
- 15.Barsky AJ, Delamater BA, Orav JE. Panic disorder patients and their medical care. Psychosomatics. 1999;40:50–56. doi: 10.1016/S0033-3182(99)71271-5. [DOI] [PubMed] [Google Scholar]
- 16.Fifer SK, Mathias SD, Patrick DL, Mazonson PD, Lubeck DP, Buesching DP. Untreated anxiety among adult primary care patients in a health maintenance organization. Arch Gen Psychiatry. 1994;51:740–750. doi: 10.1001/archpsyc.1994.03950090072010. [DOI] [PubMed] [Google Scholar]
- 17.Stein MB, McQuaid JR, Laffaye C, McCahill E. Social phobia in the primary care medical setting. J Fam Pract. 1999;48:514–519. [PubMed] [Google Scholar]
- 18.Harman JS, Schulberg HC, Mulsant BH, Reynolds CF., III The effect of patient and visit characteristics on diagnosis of depression in primary care. J Fam Pract. 2001;50:1068. [PubMed] [Google Scholar]
- 19.Young AS, Klap R, Sherbourne CD, Wells KB. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry. 2001;58:55–61. doi: 10.1001/archpsyc.58.1.55. [DOI] [PubMed] [Google Scholar]
- 20.Stein MB, Sherbourne CD, Craske MG, Means-Christenson A, Bystritsky A, Roy-Byrne PP. Quality of care for primary care patients with anxiety disorders. Am J Psychiatry. 2004;161:2230–2237. doi: 10.1176/appi.ajp.161.12.2230. [DOI] [PubMed] [Google Scholar]
- 21.Katzelnick DJ, Kobak KA, DeLeire T, Henk HJ, Greist JH, Davidson JR, Schneier FR, Stein MB, Helstad CP. Impact of generalized social anxiety disorder in managed care. Am J Psychiatry. 2001;158:1999–2007. doi: 10.1176/appi.ajp.158.12.1999. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan DV. Current concepts in psychiatry: panic attacks and phobias. N Engl J Med. 1982;307:156–158. doi: 10.1056/NEJM198207153070304. [DOI] [PubMed] [Google Scholar]
- 23.Roy-Byrne PP, Stein MB, Russo J, Mercier E, Thomas R, McQuaid J, Katon WJ, Craske MG, Bystritsky A, Sherbourne CD. Panic disorder in the primary care setting: comorbidity, disability, service utilization, and treatment. J Clin Psychiatry. 1999;60:492–499. doi: 10.4088/jcp.v60n0713. [DOI] [PubMed] [Google Scholar]
- 24.Roy-Byrne PP, Clary CM, Miceli RJ, Colucci SV, Xu Y, Grudzinski AN. The effect of SSRI treatment of panic disorder on emergency room and laboratory resource utilization. J Clin Psychiatry. 2001;62:678–682. doi: 10.4088/jcp.v62n0903. [DOI] [PubMed] [Google Scholar]
- 25.Roy-Byrne P, Katon W, Cowley D, Russo J. A randomized effectiveness trial of collaborative care for patients with panic disorder in primary care. Arch Gen Psychiatry. 2001;58:869–876. doi: 10.1001/archpsyc.58.9.869. [DOI] [PubMed] [Google Scholar]
- 26.Katon W, Roy-Byrne P, Russo J, Cowley D. Cost-effectiveness and cost offset of a collaborative care intervention for primary care patients with panic disorder. Arch Gen Psychiatry. 2002;59:1098–1104. doi: 10.1001/archpsyc.59.12.1098. [DOI] [PubMed] [Google Scholar]
- 27.Lin EH, Simon GE, Katon WJ, Russo JE, Von Korff M, Bush TM, Ludman EJ, Walker EA. Can enhanced acute-phase treatment of depression improve long-term outcomes? a report of randomized trials in primary care. Am J Psychiatry. 1999;156:643–645. doi: 10.1176/ajp.156.4.643. [DOI] [PubMed] [Google Scholar]
- 28.Hazlett-Stevens H, Craske MG, Roy-Byrne PP, Sherbourne CD, Stein MB, Bystritsky A. Predictors of willingness to consider medication and psychosocial treatment of panic disorder in a primary care sample. Gen Hosp Psychiatry. 2002;24:316–321. doi: 10.1016/s0163-8343(02)00204-9. [DOI] [PubMed] [Google Scholar]
- 29.Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283:2529–2536. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- 30.Telch M, Lucas R. Combined pharmacological and psychological treatment of panic disorder: current status and future directions. In: Wolfe BE, Maser JD, eds. Treatment of Panic Disorder: A Consensus Development Conference Washington, DC: American Psychiatric Press; 1994:177–197.
- 31.Practice guideline for the treatment of patients with panic disorder. Work Group on Panic Disorder. Am J Psychiatry. 1998;155(suppl):1–34. [PubMed] [Google Scholar]
- 32.Stein MB, Roy-Byrne PP, McQuaid JR, Laffaye C, Russo J, McCahill ME, Katon W, Craske M, Bystritsky A, Sherbourne CD. Development of a brief diagnostic screen for panic disorder in primary care. Psychosom Med. 1999;61:359–364. doi: 10.1097/00006842-199905000-00016. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Composite International Diagnostic Interview (CIDI) 2.1 Geneva, Switzerland: United Nations; 1997.
- 34.Means-Christensen A, Sherbourne C, Roy-Byrne P, Craske MG, Bystritsky A, Stein MB. The Composite International Diagnostic Interview (CIDI-Auto): problems and remedies for diagnosing panic disorder and social phobia. Int J Methods Psychiatr Res. 2003;12:167–181. doi: 10.1002/mpr.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy-Byrne PP, Stang P, Wittchen HU, Ustun B, Walters EE, Kessler RC. Lifetime panic-depression comorbidity in the National Comorbidity Survey: association with symptoms, impairment, course and help-seeking. Br J Psychiatry. 2000;176:229–235. doi: 10.1192/bjp.176.3.229. [DOI] [PubMed] [Google Scholar]
- 36.Roy-Byrne PP, Katon W, Cowley DS, Russo JE, Cohen E, Michelson E, Parrot T. Panic disorder in primary care: biopsychosocial differences between recognized and unrecognized patients. Gen Hosp Psychiatry. 2000;22:405–411. doi: 10.1016/s0163-8343(00)00101-8. [DOI] [PubMed] [Google Scholar]
- 37.Roy-Byrne P, Sherbourne CD, Craske MG, Stein MB, Katon W, Sullivan G, Means-Christensen A, Bystritsky A. Moving treatment research from clinical trials to the real world: the design of a first-generation effectiveness study for panic disorder. Psychiatr Serv. 2003;54:327–332. doi: 10.1176/appi.ps.54.3.327. [DOI] [PubMed] [Google Scholar]
- 38.Katon W, Von Korff M, Lin E, Unutzer J, Simon G, Walker E, Ludman E, Bush T. Population-based care of depression: effective disease management strategies to decrease prevalence. Gen Hosp Psychiatry. 1997;19:169–178. doi: 10.1016/s0163-8343(97)00016-9. [DOI] [PubMed] [Google Scholar]
- 39.Craske MG, Barlow DH. Mastery of Your Anxiety and Panic. 3rd ed. Boulder, Colo: Graywind Publication; 2000.
- 40.Barlow DH, Craske MG. Agoraphobia Supplement. 3rd ed. Boulder, Colo: Graywind Publication; 2000.
- 41.Roy-Byrne P, Stein M, Bystrisky A, Katon W. Pharmacotherapy of panic disorder: proposed guidelines for the family physician. J Am Board Fam Pract. 1998;11:282–290. doi: 10.3122/jabfm.11.4.282. [DOI] [PubMed] [Google Scholar]
- 42.Wells KB. The design of partners in care: evaluating the cost-effectiveness of improving care for depression in primary care. Soc Psychiatry Psychiatr Epidemiol. 1999;34:20–29. doi: 10.1007/s001270050107. [DOI] [PubMed] [Google Scholar]
- 43.Clark DM, Salkovskis PM, Hackmann A, Middleton H, Anastasiades P, Gelder M. A comparison of cognitive therapy, applied relaxation and imipramine in the treatment of panic disorder. Br J Psychiatry. 1994;164:759–769. doi: 10.1192/bjp.164.6.759. [DOI] [PubMed] [Google Scholar]
- 44.DiNardo P, Barlow D. Anxiety Disorders Interview Schedule–Revised (ADIS-R) San Antonio, Tex: Graywind Publications Inc, The Psychological Corp; 1988.
- 45.Marks IM, Mathews AM. Brief standard self-rating for phobic patients. Behav Res Ther. 1979;17:263–267. doi: 10.1016/0005-7967(79)90041-x. [DOI] [PubMed] [Google Scholar]
- 46.Epping-Jordan J, Ustun B. The WHODAS!! leveling the playing field for all disorders. WHO Bull Ment Health. 2000;6:5–6. [Google Scholar]
- 47.Ware JEJ, Kosinski M, Keller SD. How To Score the SF-12 Physical and Mental Health Summary Scales Boston, Mass: The Health Institute, New England Medical Center; 1995.
- 48.Otto MW, Reilly-Harrington N. The impact of treatment on anxiety sensitivity. In: Taylor S, ed. Anxiety Sensitivity: Theory, Research, and Treatment of the Fear of Anxiety Mahwah, NJ: Lawrence, Erlbaum; 1999:321–336.
- 49.Schmidt NB, Lerew DR, Joiner TE., Jr Prospective evaluation of the etiology of anxiety sensitivity: test of a scar model. Behav Res Ther. 2000;38:1083–1095. doi: 10.1016/s0005-7967(99)00138-2. [DOI] [PubMed] [Google Scholar]
- 50.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychological Meas. 1977;1:385–401. [Google Scholar]
- 51.Steketee G, Perry C, Goisman RM, Warshaw MG, Massion AO, Peterson LG, Langford L, Weinshenker N, Farreras IG, Keller MB. The psychosocial treatments interview for anxiety disorders: a method for assessing psychotherapeutic procedures in anxiety disorders. J Psychother Pract Res. 1997;6:194–210. [PMC free article] [PubMed] [Google Scholar]
- 52.Shi M, Weiss RE, Taylor JMG. An analysis of pediatric AIDS CD4 counts using flexible random curves. Appl Stat. 1996;45:151–163. [Google Scholar]
- 53.Gelman A, Carlin J, Stern H, Rubin D. Bayesian Data Analysis London, England: Chapman and Hall; 1995.
- 54.Balk EM, Bonis PA, Moskowitz H, Schmid CH, Ioannidis JP, Wang C, Lau J. Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. JAMA. 2002;287:2973–2982. doi: 10.1001/jama.287.22.2973. [DOI] [PubMed] [Google Scholar]
- 55.Carlin B, Louis T. Bayes and Empirical Bayes Methods for Data Analysis. 2nd ed. London, England: Chapman and Hall; 2000.
- 56.Gilks W, Richardson S, Spiegelhalter D. Markhov Chain Monte Carlo Methods in Practice London, England: Chapman and Hall; 1996.
- 57.WinBUGS User Manual Version 1.4 London, England: MRC Biostatistics Unit; 2003.
- 58.Schmidt NB, Lerew DR, Jackson RJ. Prospective evaluation of anxiety sensitivity in the pathogenesis of panic: replication and extension. J Abnorm Psychol. 1999;108:532–537. doi: 10.1037//0021-843x.108.3.532. [DOI] [PubMed] [Google Scholar]
- 59.Roy-Byrne P, Russo J, Dugdale DC, Lessler D, Cowley D, Katon W. Undertreatment of panic disorder in primary care: role of patient and physician characteristics. J Am Board Fam Pract. 2002;15:443–450. [PubMed] [Google Scholar]
- 60.Simon GE, Ludman EJ, Tutty S, Operkalski B, Von Korff M. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment. JAMA. 2004;292:935–942. doi: 10.1001/jama.292.8.935. [DOI] [PubMed] [Google Scholar]
- 61.Gilbody S, Whitty P, Grimshaw J, Thomas R. Educational and organizational interventions to improve the management of depression in primary care: a systematic review. JAMA. 2003;289:3145–3151. doi: 10.1001/jama.289.23.3145. [DOI] [PubMed] [Google Scholar]
- 62.Bower P, Rowland N, Hardy R. The clinical effectiveness of counseling in primary care: a systematic review and meta-analysis. Psychol Med. 2003;33:203–215. doi: 10.1017/s0033291702006979. [DOI] [PubMed] [Google Scholar]
- 63.Otto MW, Deckersbach T. Cognitive-behavioral therapy for panic disorder: theory, strategies, and outcome. In: Rosenbaum JF, Pollack MH, eds. Panic Disorder and Its Treatment New York, NY: Marcel Dekker; 1998:181–203.


