Abstract
Numerous intestinal diseases are characterized by immune cell activation and compromised epithelial barrier function. We have shown that cytokine treatment of epithelial monolayers increases myosin II regulatory light chain (MLC) phosphorylation and decreases barrier function and that these are both reversed by MLC kinase (MLCK) inhibition. The aim of this study was to determine the mechanisms by which interferon (IFN)-γ and tumor necrosis factor (TNF)-α regulate MLC phosphorylation and disrupt epithelial barrier function. We developed a model in which both cytokines were required for barrier dysfunction. Barrier dysfunction was also induced by TNF-α addition to IFN-γ-primed, but not control, Caco-2 monolayers. TNF-α treatment of IFN-γ-primed monolayers caused increases in both MLCK expression and MLC phosphorylation, suggesting that MLCK is a TNF-α-inducible protein. These effects of TNF-α were not mediated by nuclear factor-κB. However, at doses below those needed for nuclear factor-κB inhibition, sulfasalazine was able to prevent TNF-α-induced barrier dysfunction, MLCK up-regulation, and MLC phosphorylation. Low-dose sulfasalazine also prevented morphologically evident tight junction disruption induced by TNF-α. These data show that IFN-γ can prime intestinal epithelial monolayers to respond to TNF-α by disrupting tight junction morphology and barrier function via MLCK up-regulation and MLC phosphorylation. These TNF-α-induced events can be prevented by the clinically relevant drug sulfasalazine.
A principal function of epithelial surfaces is the maintenance of a barrier to hydrophilic solutes. In intestinal epithelium, this barrier function is compromised in a spectrum of infectious, immune-mediated, and idiopathic diseases.1 In Crohn’s disease these barrier defects, measured as increases in paracellular permeability, may precede clinical evidence of disease,2 can serve as markers of impending disease reactivation,3 and correlate with systemic immune activation.4 Thus, defects in epithelial barrier function are intimately associated with Crohn’s disease pathogenesis.
One feature common to Crohn’s disease and other intestinal diseases with compromised barrier function is TH1-polarized immune activation, with associated elevations of mucosal interferon (IFN)-γ and tumor necrosis factor (TNF)-α. Several lines of evidence suggest that these cytokine elevations are responsible for the observed barrier defects. First, in vitro studies have shown that, at relatively high doses, IFN-γ and TNF-α can induce barrier dysfunction in cultured epithelial monolayers.5–9 At lower doses, these cytokines can synergize to disrupt barrier function in vitro.8,10,11 In vivo, both in human patients and animal models, TNF-α and IFN-γ antagonism can diminish disease severity and restore barrier function.12–15 Thus, TNF-α and IFN-γ are critical to the barrier disruption that occurs both in vitro and in vivo.
Although both in vitro and in vivo data confirm that TNF-α and IFN-γ can cause dysfunction of the epithelial tight junction barrier, the mechanisms of this effect remain unknown. TNF-α can cause epithelial apoptosis, but this does not appear to be the mechanism by which barrier function is compromised.8,11,16,17 Other mechanisms may be involved in cytokine-induced barrier dysfunction, including down-regulation of the tight junction proteins ZO-1 and occludin,18,19 decreased Na+-K+ ATPase activity,20 or nuclear factor (NF)-κB activation.7 Nonetheless, little is known of the biochemical events that mediate TNF-α- and IFN-γ-induced barrier dysfunction.
We and others have shown that myosin II activation, as indicated by phosphorylation of myosin II regulatory light chain (MLC), is involved in physiological and pathophysiological tight junction regulation.21–26 In the course of studies on this topic, we showed that treatment of intestinal epithelial monolayers with IFN-γ and TNF-α induced both barrier dysfunction and increased MLC phosphorylation.11 We have also found that inhibition of MLC kinase (MLCK) both reduces MLC phosphorylation and restores barrier function.11 Thus, our previous report suggests that MLCK-mediated MLC phosphorylation is critical to IFN-γ- and TNF-α-induced barrier dysfunction. However, the mechanism by which MLCK increases MLC phosphorylation was not identified.
In the present studies we sought to define the mechanisms by which IFN-γ and TNF-α cause increases in MLC phosphorylation and concomitant barrier dysfunction in intestinal epithelia. The data show that IFN-γ primes intestinal epithelia to respond to TNF-α by increasing MLCK expression. This is followed by increases in MLC phosphorylation and barrier dysfunction. Sulfasalazine (SSA), an agent effective in the treatment of inflammatory bowel disease, prevents this barrier dysfunction by blocking MLCK up-regulation as well as increased MLC phosphorylation. However, this protective effect of SSA does not require NF-κB-inhibition. Thus, these data identify MLCK as a cytokine-inducible protein and also provide new insight into the mechanisms of epithelial barrier dysfunction in intestinal disease.
Materials and Methods
Monolayer Preparation and TER Measurement
Caco-2 cells were grown as monolayers on collagen-coated polycarbonate membrane Transwell supports (Corning-Costar, Acton, MA) and used 17 to 20 days after confluence, as described previously.26 Transwell supports with 0.33- and 5-cm2 surface areas were used for electrophysiological and biochemical studies, respectively. Cytokines (R&D Systems, Minneapolis, MN), were added to the basal chamber without manipulating the apical media. SSA, 5-aminosalicylic acid, sulfapyridine, and 4-aminosalicylic acid (MP Biochemicals, Aurora, OH) and curcumin, triptolide, capsaicin, BAY 11-7085, SN50, and MG132 (Calbiochem, San Diego, CA) were also added to the basal chamber only. Transepithelial resistance (TER) was measured with an epithelial voltohmmeter (EVOM; World Precision Instruments, Sarasota, FL). In all experiments the TER of control monolayers was ∼240 Ω·cm2 after subtraction of fluid resistance (Figure 1A). To facilitate comparisons between experiments, the TER of all monolayers was typically normalized to that of control monolayers in the same experiment.
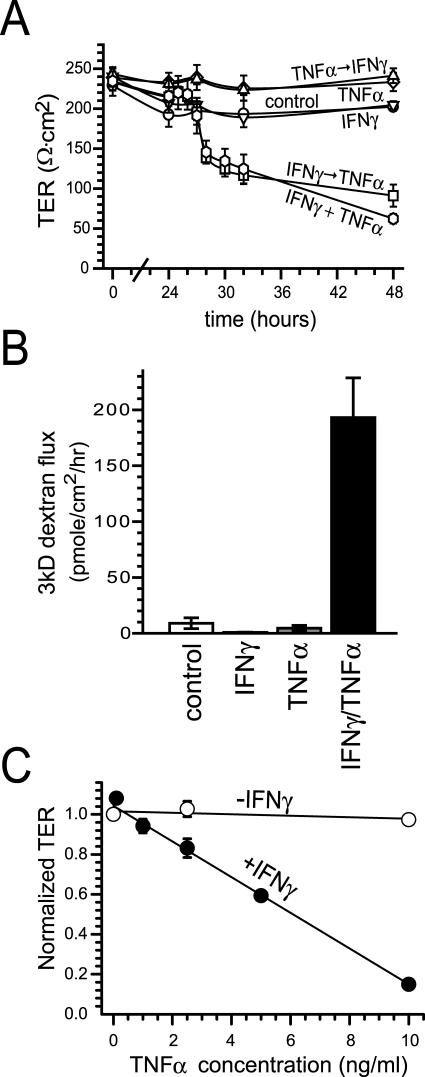
Figure 1.
IFN-γ and TNF-α synergize to reduce barrier function in Caco-2 cell monolayers. A: Caco-2 monolayers were incubated with the indicated cytokine(s) (IFN-γ = 10 ng/ml, TNF-α = 2.5 ng/ml) added to media in the basal chamber. Conditions were no cytokines (control), continuous IFN-γ (IFN-γ), continuous TNF-α (TNF-α), continuous IFN-γ and TNF-α (IFN-γ + TNF-α), TNF-α for 24 hours followed by IFN-γ (TNF-α → IFN-γ), or IFN-γ for 24 hours followed by TNF-α (IFN-γ → TNF-α). TER fell only in cells exposed to IFN-γ and TNF-α continuously or IFN-γ followed by TNF-α (n = 3 in this representative experiment). B: Caco-2 monolayers were incubated with the indicated cytokine(s) added to the basal chamber, as above. Conditions were no cytokines (control), continuous IFN-γ for 32 hours (IFN-γ), no cytokines for 24 hours followed by TNF-α for 8 hours (TNF-α), or IFN-γ for 24 hours followed by TNF-α for 8 hours (IFN-γ/TNF-α). At that time monolayers were assayed for permeability to 3kD fluorescein isothiocyanate-dextran. Paracellular permeability was increased only in cells exposed to IFN-γ followed by TNF-α (P < 0.001) (n = 6 in this representative experiment). C: Caco-2 monolayers were incubated with (filled circles) or without (open circles) IFN-γ (10 ng/ml) for 24 hours, washed, and transferred to media with TNF-α at indicated concentrations. TER shown is 48 hours after TNF-α addition. TNF-α caused dose-dependent TER decreases in IFN-γ-primed monolayers, but not in monolayers without IFN-γ pretreatment (n = 3 in this representative experiment).
Paracellular Flux Assays
Flux of fluorescein isothiocyanate-labeled dextran (molecular weight, 3kD; Molecular Probes, Eugene, OR) across Caco-2 monolayers was assayed as described previously.10,27 Briefly, monolayers were washed free of media and cytokines and transferred to Hanks’ balanced salt solution. The apical chamber was gently aspirated and replaced with 50 μl of 1 mg/ml of fluorescein isothiocyanate-dextran (1 mg/ml). The monolayers were rotated on an orbital shaker (60 rpm) at 37°C, and samples (50 μl) were removed from the basal chamber after 30 and 60 minutes. Fluorescence of these samples was determined using a fluorescent plate reader (Synergy HT; Bio-Tek Instruments, Winooski, VT). Molar flux was calculated from a standard curve that was prepared daily.
Immunoblotting
Lysates of Caco-2 monolayers grown on 5-cm2 Transwell supports were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad, Hercules, CA) and transferred to polyvinylidene difluoride membranes, as described previously.26 For NF-κB analyses, nuclear fractions were prepared by using the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Pierce Biotechnology, Inc., Rockford, IL). After blocking the membranes were blotted using antibodies to MLCK (clone K36; Sigma, St. Louis, MO), ZO-1, occludin, claudin-1, and NF-κB RelA p65 (Zymed, South San Francisco, CA), caspase-3, caspase-8, and poly ADP-ribose polymerase (Cell Signaling Technology, Beverly, MA), and total or phosphorylated MLC.28 After incubation with peroxidase-conjugated secondary antibodies (Cell Signaling Technology), blots were visualized by enhanced chemiluminescence, as described previously.26 Densitometry of immunoblot data was performed using Metamorph 6.2 (Universal Imaging Corp., Downingtown, PA).
Fluorescence Microscopy
Caco-2 monolayers grown on 0.33-cm2 Transwell supports were fixed with 1% paraformaldehyde and permeabilized in 0.1% Triton X-100 in phosphate-buffered saline. After incubation with mouse anti-ZO-1, rabbit anti-occludin, or rabbit anti-claudin-1 antibodies, monolayers were washed and incubated with Alexa 594-conjugated secondary antibodies and Hoechst 33342 (Molecular Probes), as indicated. Monolayers were mounted in Slowfade (Molecular Probes) and imaged using a Leica DMLB epifluorescence microscope equipped with an 88000 filter set (Chroma Technology, Brattleboro, VT) and a Coolsnap HQ camera (Roper Scientific, Tucson, AZ) controlled by MetaMorph 6.2. Postacquisition deconvolution and serial reconstruction used Autodeblur 9 (AutoQuant Imaging, Inc., Watervliet, NY) for 10 iterations.
NF-κB Activity Assay
pNFκB-TA-Luc (Clontech, Palo Alto, CA) was transiently transfected into freshly trypsinized Caco-2 cells, in suspension, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and the cells were plated on 12-well inserts. This controlled for transfection efficiency because each experiment was performed from a single transfection. Cells were subsequently treated with cytokines or inhibitors, as indicated. Luciferase expression in lysates was detected using luciferin as the substrate (Promega), measured with a Lumat LB 9507 luminometer (Berthold, Oak Ridge, TN), and normalized to total protein (BCA assay, Bio-Rad).
Results
IFN-γ and TNF-α Synergize to Reduce Barrier Function in Caco-2 Monolayers
Previous in vitro work has clearly established that IFN-γ8,20,29,30 and TNF-α7,31–36 are capable of independently reducing barrier function in intestinal epithelial monolayers. However, it is also clear that these cytokines can synergize to induce barrier dysfunction.10,11 Elevations of both cytokines are seen in intestinal disease in vivo.37 Thus, we developed an in vitro model similar to previous reports8–11 in which low, physiologically relevant levels of IFN-γ and TNF-α are both required to decrease barrier function in cultured monolayers of intestinal epithelial Caco-2 cells. In this model TER, a sensitive measure of barrier function, was unaffected by 48 hours of culture with basolateral IFN-γ or TNF-α individually (Figure 1A). However, simultaneous application of both cytokines caused TER to fall, indicating reduced barrier function, within 30 hours (Figure 1A). To determine whether the synergy between IFN-γ and TNF-α required the two cytokines to be present simultaneously, we added one cytokine for 24 hours, washed monolayers free of that cytokine, and then added the second cytokine. Sequential treatment with IFN-γ followed by TNF-α caused TER decreases comparable to those induced by simultaneous treatment with IFN-γ and TNF-α (Figure 1A). In IFN-γ-primed monolayers TER began to decrease within 4 hours after TNF-α addition. In contrast, sequential treatment with TNF-α followed by IFN-γ did not affect TER (Figure 1A). Similarly, paracellular flux of 3-kD dextran was not significantly increased in monolayers treated with either IFN-γ or TNF-α alone, but was increased 21 ± 4-fold in monolayers treated sequentially with IFN-γ followed by TNF-α (Figure 1B). Thus, the synergy between these cytokines is because of the ability of IFN-γ treatment to prime monolayers to respond rapidly to physiologically relevant doses of TNF-α.
Priming of Caco-2 monolayers with IFN-γ requires at least 18 hours of IFN-γ treatment (data not shown). Increasing IFN-γ treatment duration (up to 36 hours) or dose (up to 100 ng/ml) did not augment subsequent TNF-α-induced TER decreases (data not shown). In contrast, TER decreases induced by TNF-α addition to IFN-γ-primed monolayers were strongly correlated with TNF-α dose (r = 0.996, Figure 1C). Therefore, IFN-γ primes intestinal epithelial monolayers to respond to TNF-α in a dose-dependent manner.
IFN-γ- and TNF-α-Induced Barrier Dysfunction Is Associated with Morphological and Biochemical Tight Junction Disruption
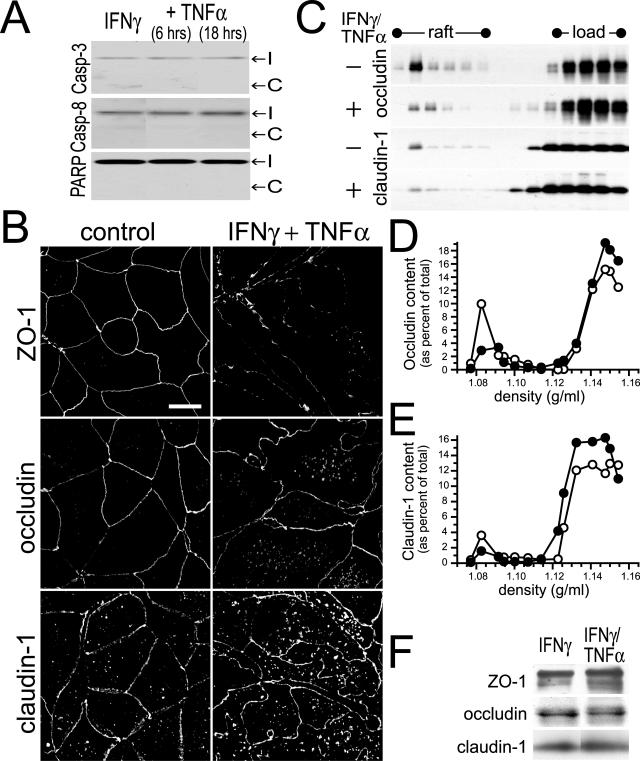
Some studies have suggested that TNF-α reduces epithelial barrier function by inducing apoptosis.38,39 However, others have shown barrier function to be maintained in the face of apoptosis,16,17 and we11 and others8 have shown that this is not the mechanism by which IFN-γ and TNF-α induce barrier dysfunction. Consistent with this, caspase-3, caspase-8, and PARP cleavage were not increased by IFN-γ and TNF-α treatment of Caco-2 monolayers (Figure 2A). Thus, we considered the possibility that the barrier dysfunction observed represents disruption of the tight junction, the primary determinant of barrier function in intact monolayers. We first assessed the morphological distribution of tight junction proteins before and after cytokine treatment. In control monolayers the tight junction plaque protein ZO-1 was restricted to the tight junction, whereas the transmembrane proteins occludin and claudin-1 were detected at the tight junction, along lateral membranes, and within intracellular vesicular pools (Figure 2B). These distributions were not changed by incubation with IFN-γ (10 ng/ml) for 24 hours (data not shown). Treatment of IFN-γ-primed monolayers with TNF-α caused striking reorganization of ZO-1, occludin, and claudin-1 such that the en face xy profiles became irregular (Figure 2B). The intensity of staining for ZO-1 and occludin at the tight junction was also reduced, and expanded intracellular pools of occludin and claudin-1 were apparent after treatment of IFN-γ-primed monolayers with TNF-α (Figure 2B). This morphologically evident removal of occludin and claudin-1 from the tight junction was also associated with removal of occludin and claudin-1 from tight junction-enriched lipid raft fractions40 (Figure 2C). The occludin and claudin-1 content within the raft fractions (operationally defined as those with densities between 1.077 and 1.094 g/ml) decreased by 50% and 51%, respectively (Figure 2, D and E). This loss of tight junction proteins from the raft fractions was not because of degradation because total cellular ZO-1, occludin, and claudin-1 were unchanged (Figure 2F). Thus, treatment of IFN-γ-primed Caco-2 monolayers with TNF-α causes both morphological and biochemical redistribution of tight junction proteins that correlates with loss of barrier function.
Figure 2.
IFN-γ- and TNF-α-induced barrier dysfunction is associated with apoptosis-independent tight junction disruption. A: Caco-2 monolayers were treated with IFN-γ (10 ng/ml) for 24 hours followed by TNF-α (2.5 ng/ml) for times indicated. Cell lysates were analyzed for cleavage of caspase-3, caspase-8, and PARP as markers of apoptosis. No increases in cleaved caspase-3, caspase-8, or PARP were detected in Caco-2 cells treated with the indicated cytokine(s). The positions of intact (I) and cleaved (C) proteins are shown. Data are representative of four similar experiments, each performed in triplicate. B: Caco-2 monolayers were treated with IFN-γ (10 ng/ml) for 24 hours followed by TNF-α (2.5 ng/ml) for 8 hours. Tight junction proteins (ZO-1, occludin, and claudin-1) were detected by immunofluorescence microscopy. Treatment of IFN-γ-primed monolayers with TNF-α caused obvious disruptions of the distribution of tight junction proteins. The tight junction staining was decreased in intensity and became irregular. Increased intracellular pools of occludin and claudin-1 were also apparent. Data are representative of six similar experiments, each performed in duplicate. C: Caco-2 monolayers were treated with IFN-γ (10 ng/ml) for 24 hours followed by TNF-α (2.5 ng/ml) for 8 hours, and harvested at 4°C in TBS with 1% Triton X-100. Cell lysates (adjusted to 40% sucrose) were loaded under 0 to 40% continuous sucrose gradients and centrifuged at 180,000 × g for 18 hours.40 SDS-PAGE immunoblots for occludin and claudin-1 were performed. Fractions from 1.06 to 1.16 g/ml are shown, with lipid raft and load regions designated. Treatment with IFN-γ and TNF-α caused both occludin and claudin-1 to be removed from the raft fraction. Data are representative of three similar experiments, each performed in duplicate. D: Immunoblots detecting occludin (Figure 2C) were analyzed quantitatively. The amount of occludin present in each fraction is shown as a percentage of the sum of occludin detected in all fractions. The peak occludin content within raft fractions was detected at a density of 1.082 g/ml and was 9.9% of total in control monolayers (open symbols) but only 2.9% of total in monolayers treated with IFN-γ and TNF-α (filled symbols). Data are representative of three similar experiments, each performed in duplicate. E: Immunoblots detecting claudin-1 (Figure 2C) were analyzed quantitatively. The amount of claudin-1 present in each fraction is shown as a percentage of the sum of claudin-1 detected in all fractions. The peak claudin-1 content within raft fractions was detected at a density of 1.082 g/ml and was 3.6% of total in control monolayers (open symbols) but only 1.6% of total in monolayers treated with IFN-γ and TNF-α (filled symbols). Data are representative of three similar experiments, each performed in duplicate. F: Caco-2 monolayers were treated with IFN-γ (10 ng/ml) for 24 hours or IFN-γ for 24 hours followed by TNF-α (2.5 ng/ml) for 18 hours. Cell lysates were analyzed for the expression of tight junction proteins ZO-1, occludin, and claudin-1 by SDS-PAGE immunoblot. Expression of these tight junction proteins was not significantly changed by cytokine treatment. Data are representative of four similar experiments, each performed in triplicate.
IFN-γ- and TNF-α-Dependent TER Decreases Are Accompanied by Increases in MLC Phosphorylation and MLCK Expression
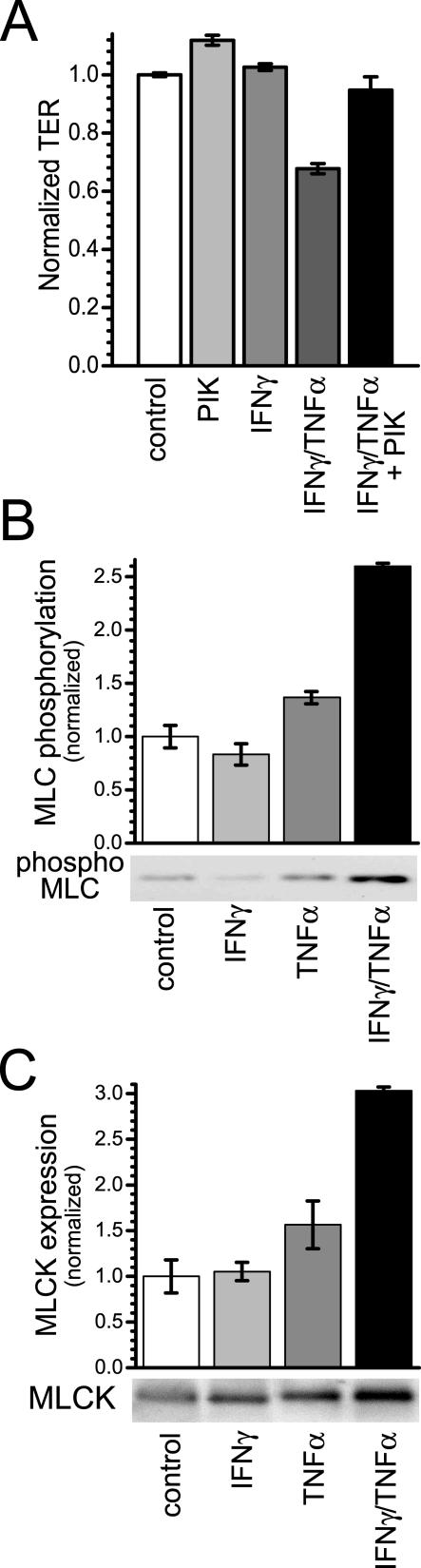
We have previously shown that barrier dysfunction induced by simultaneous IFN-γ and TNF-α treatment is accompanied by increased myosin II regulatory light chain (MLC) phosphorylation11 and that inhibition of MLCK reverses both increased MLC phosphorylation and decreased TER.11 The TER loss induced by treatment of IFN-γ-primed monolayers with TNF-α was also reversed by MLCK inhibition using PIK, a highly specific membrane-permeant inhibitor of MLC kinase (Figure 3A). As shown in Figure 3B, treatment of IFN-γ-primed monolayers with TNF-α increased MLC phosphorylation by 159 ± 4% (P < 0.05). In contrast, when IFN-γ pretreatment was omitted TNF-α induced only a 36 ± 9% statistically insignificant increase in MLC phosphorylation (P > 0.05).
Figure 3.
IFN-γ- and TNF-α-dependent TER decreases are accompanied by increases in MLC phosphorylation and MLCK expression. A: Caco-2 monolayers were incubated in media with or without IFN-γ (10 ng/ml) for 24 hours and then transferred to media with or without TNF-α (2.5 ng/ml), as indicated. In the designated conditions, 200 μmol/L PIK, a highly specific membrane-permeant inhibitor of MLC kinase, was added to the apical media 2 hours after TNF-α addition. As we have shown previously,11 PIK alone induced a small increase in TER. However, in monolayers treated sequentially with IFN-γ and TNF-α, PIK was able to nearly completely prevent TER loss (P < 0.02) (n = 3 in this representative experiment). B: Caco-2 monolayers were incubated in media with or without IFN-γ (10 ng/ml) for 24 hours and then transferred to media with or without TNF-α (2.5 ng/ml), as indicated. Monolayers were harvested 8 hours after TNF-α addition. MLC phosphorylation was assessed by SDS-PAGE immunoblot. TNF-α (2.5 ng/ml) caused a small increase in MLC phosphorylation in monolayers without IFN-γ (10 ng/ml) pretreatment (P > 0.05), but a significant increase in MLC phosphorylation in cells with IFN-γ pretreatment (P < 0.05) (n = 2 in this representative experiment). C: Caco-2 monolayers were incubated in media with or without IFN-γ (10 ng/ml) for 24 hours and then transferred to media with or without TNF-α (2.5 ng/ml), as indicated. Monolayers were harvested 8 hours after TNF-α addition. MLCK protein expression was analyzed by SDS-PAGE immunoblot. TNF-α (2.5 ng/ml) caused a slight increase MLCK expression in monolayers without IFN-γ (10 ng/ml) pretreatment (P > 0.05), but a significant increase in MLCK expression occurred after TNF-α treatment of monolayers primed with IFN-γ (P < 0.05) (n = 2 in this representative experiment).
MLCK is primarily responsible for MLC phosphorylation in intestinal epithelia and inhibition of MLCK with PIK prevented TER loss after IFN-γ and TNF-α treatment (Figure 3A). Thus, we asked whether increases in MLCK expression accompanied increased MLC phosphorylation after TNF-α-treatment. In IFN-γ-primed monolayers, TNF-α increased MLCK expression by 203 ± 5% (Figure 3C, P < 0.05). When IFN-γ was omitted, TNF-α did not significantly increase MLCK protein expression (Figure 3C, P > 0.05). Thus, TER decreases correlated with up-regulation of MLCK, suggesting that increased MLCK expression may be a central mechanism of IFN-γ- and TNF-α-induced barrier dysfunction.
Barrier Defects Induced by TNF-α and IFN-γ Are Not Abrogated by NF-κB Inhibition
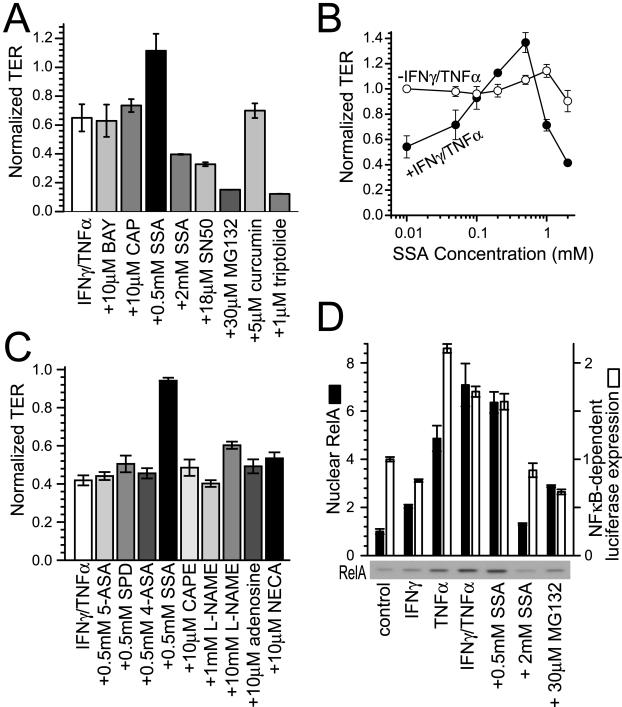
The data above suggest that transcriptional up-regulation of MLCK may be a mechanism of TNF-α-induced barrier dysfunction. Thus, we considered the possibility that MLCK up-regulation might be mediated by NF-κB. This would be consistent with a recent report suggesting that NF-κB activation is required for TNF-α-induced epithelial barrier dysfunction,7 but would conflict with a separate study suggesting that NF-κB is necessary for recovery of barrier function after TNF-α treatment.36 To determine the role of NF-κB in IFN-γ- and TNF-α-induced barrier dysregulation, we first examined the ability of known NF-κB inhibitors to prevent barrier dysfunction. A variety of inhibitors that prevent NF-κB activation failed to prevent IFN-γ- and TNF-α-dependent TER decreases (Figure 4A). In some cases, these inhibitors even exacerbated TER decreases (eg, SN50, MG132, triptolide).
Figure 4.
Barrier defects induced by TNF-α and IFN-γ are not abrogated by NF-κB inhibition. A: Caco-2 monolayers were cultured with IFN-γ (10 ng/ml) for 24 hours and transferred to media with 10 μmol/L BAY 11-7085 (BAY), 10 μmol/L capsaicin (CAP), 0.5 mmol/L SSA, 2 mmol/L SSA, 18 μmol/L SN50, 30 μmol/L MG132, 5 μmol/L curcumin, or 1 μmol/L triptolide with or without TNF-α (2.5 ng/ml). None of the NF-κB inhibitors significantly altered TER without TNF-α treatment. TER of monolayers treated with TNF-α is normalized to identical monolayers, treated with the same NF-κB inhibitor, but without TNF-α. SSA (0.5 mmol/L) prevented TER decreases, but 2 mmol/L SSA, 18 μmol/L SN50, 30 μmol/L MG132, and 1 μmol/L triptolide exacerbated TER decreases. BAY 11-7085 (10 μmol/L), 10 μmol/L capsaicin, and 5 μmol/L curcumin had no significant effects (n = 3 in this representative experiment). B: Caco-2 monolayers were cultured with (closed circles) or without (open circles) IFN-γ (10 ng/ml) for 24 hours and transferred to TNF-α (2.5 ng/ml). SSA was also included at indicated doses (0.01 to 2 mmol/L). Maximal barrier protection occurred at 0.5 mmol/L. Higher SSA doses were not protective and, as shown in A, 2 mmol/L SSA exacerbated TER loss (n = 3 in this representative experiment). C: Caco-2 monolayers were cultured with IFN-γ (10 ng/ml) for 24 hours and transferred to TNF-α (2.5 ng/ml) with 0.5 mmol/L SSA, 0.5 mmol/L 5-aminosalicylic acid (5-ASA), 0.5 mmol/L sulfapyridine (SPD), 0.5 mmol/L 4-aminosalicylic acid (4-ASA), 10 μmol/L caffeic acid phenethyl ester, 1 mmol/L and 10 mmol/L NG-nitro-l-arginine-methyl-ester (L-NAME), 10 μmol/L adenosine, and 10 μmol/L 5′-N-ethylcarboxamidoadenosine (NECA). TER of monolayers treated with TNF-α is normalized to identical monolayers, treated with the same drug, but without TNF-α. Only SSA prevented barrier dysfunction induced by IFN-γ and TNF-α (n = 3 in this representative experiment). D: NF-κB RelA p65 (closed bars) was detected by SDS-PAGE immunoblot of nuclear fractions isolated 10 minutes after TNF-α (2.5 ng/ml) and drug addition to monolayers with or without IFN-γ (10 ng/ml) pretreatment for 24 hours, as indicated. A representative immunoblot of nuclear fractions is shown below the graph. NF-κB (RelA p65) nuclear translocation was inhibited by 2 mmol/L SSA and 30 μmol/L MG132, but not by 0.5 mmol/L SSA [n = 2 in this experiment which is representative of nine similar experiments, each with duplicate samples (total n = 18)]. NF-κB-dependent luciferase expression (open bars) was assessed in Caco-2 cells transiently transfected with pNFκB-TA-Luc reporter construct. Monolayers were then treated identically to the translocation assay, but harvested 8 hours after TNF-α addition to allow for luciferase synthesis. NF-κB-dependent luciferase expression was inhibited by 2 mmol/L SSA and 30 μmol/L MG132, but not by 0.5 mmol/L SSA [n = 2 in this experiment which is representative of four similar experiments, each with duplicate samples (total n = 8)].
One inhibitor, the clinically relevant agent SSA stood out in that it was able to prevent IFN-γ- and TNF-α-induced TER decreases, but only at low doses; higher doses enhanced TER decreases (Figure 4, A and B). SSA is generally considered to be a prodrug that is metabolized by colonic bacteria into 5-ASA and sulfapyridine. Thus, we tested the abilities of 5-ASA, sulfapyridine, and the related compound 4-ASA to prevent IFN-γ- and TNF-α-induced TER decreases. Unlike SSA, the other three related compounds did not prevent TER decreases (Figure 4C). Thus, because the inhibitory effects of SSA on lipoxygenase and cyclooxygenase are shared by 5-ASA, sulfapyridine, and 4-ASA,41 this is not the mechanism by which SSA prevents TER decreases. SSA, 5-ASA, sulfapyridine, and 4-ASA, have also been reported to have antioxidant properties.42 Although 5-ASA, sulfapyridine, and 4-ASA did not prevent TER decreases, we tested the ability of caffeic acid phenethyl ester, which has antioxidant properties and also inhibits ornithine decarboxylase, protein tyrosine kinase, lipoxygenase, and NF-κB activities to prevent TER decreases. At doses from 10 μmol/L (Figure 4C) to 50 μmol/L caffeic acid phenethyl ester did not prevent IFN-γ- and TNF-α-induced TER decreases. SSA, 5-ASA, and sulfapyridine may also inhibit nitric oxide synthase expression.43 To determine whether this could be a mechanism of SSA action, we tested the ability of NG-nitro-l-arginine-methyl-ester, which inhibits nitric oxide synthesis, to prevent TER loss. At doses from 1 mmol/L to 10 mmol/L NG-nitro-l-arginine-methyl-ester was unable to prevent IFN-γ- and TNF-α-induced TER decreases (Figure 4C). Finally, SSA has been reported to induce the release of micromolar concentrations of adenosine.44 Thus, we directly tested the ability of 10 μmol/L to 100 μmol/L adenosine and similar concentrations of the adenosine receptor agonist 5′-N-ethylcarboxamidoadenosine to prevent IFN-γ- and TNF-α-induced TER decreases. Neither compound was able to prevent these TER decreases (Figure 4C). Thus, these reported mechanisms of SSA action cannot explain the ability of this drug to prevent IFN-γ- and TNF-α-induced barrier dysfunction.
To determine whether the effects of low-dose (0.5 mmol/L) SSA are because of NF-κB inhibition, we examined nuclear translocation of RelA p65 and as well as NF-κB-dependent transcription from a consensus κB promoter. Both RelA nuclear translocation and NF-κB-dependent transcription were increased by TNF-α treatment, either with or without IFN-γ pretreatment (Figure 4D). SSA (0.5 mmol/L) did not prevent RelA translocation or NF-κB-dependent luciferase transcription. In contrast, 2 mmol/L SSA and 30 μmol/L MG132 were able to inhibit both RelA translocation and NF-κB-dependent luciferase transcription (Figure 4D). Thus, the one agent that protected IFN-γ-primed monolayers from TNF-α-induced barrier dysfunction, 0.5 mmol/L SSA, did not prevent NF-κB activation, whereas two agents that did inhibit NF-κB activation (2 mmol/L SSA and 30 μmol/L MG132) exacerbated TNF-α-induced barrier dysfunction. These data suggest that NF-κB activation is not an intermediate in TNF-α-induced barrier dysfunction.
Low-Dose SSA Prevents Morphological Tight Junction Disruption and Increases in MLC Phosphorylation and MLCK Expression
Given that 0.5 mmol/L SSA prevented TNF-α-induced barrier dysfunction, we asked if 0.5 mmol/L SSA also prevented morphological tight junction disruption. As shown in Figure 5A, 0.5 mmol/L SSA completely prevented ZO-1, occludin, and claudin-1 redistribution in TNF-α-treated IFN-γ-primed monolayers. Thus, SSA prevented TNF-α-induced tight junction disruption, both functionally and morphologically. Based on this remarkable protective effect of 0.5 mmol/L SSA, we asked whether SSA prevented IFN-γ- and TNF-α-induced barrier dysfunction and tight junction disruption by blocking increases in MLC phosphorylation. Consistent with the protective effect on barrier function, 0.5 mmol/L SSA prevented increases in MLC phosphorylation in IFN-γ-primed TNF-α-treated monolayers (Figure 5B, P < 0.01). In contrast, 2 mmol/L SSA and 30 μmol/L MG132 only minimally reduced MLC phosphorylation (P > 0.05 for 2 mmol/L SSA, P = 0.04 for MG132). We then considered the possibility that 0.5 mmol/L SSA prevents MLC phosphorylation by inhibiting up-regulation of MLCK expression. SSA (0.5 mmol/L) completely prevented TNF-α-dependent increases in MLCK expression (Figure 5C, P < 0.02). Consistent with their slight effect on MLC phosphorylation, 2 mmol/L SSA and 30 μmol/L MG132 caused small statistically insignificant reductions in MLCK expression (P > 0.05). Thus, the effects of 0.5 mmol/L SSA, 2 mmol/L SSA, and 30 μmol/L MG132 on MLCK expression and MLC phosphorylation correlate directly with their effects on TER loss after TNF-α treatment of IFN-γ-primed monolayers. In contrast, the ability of these inhibitors to block NF-κB activation correlates inversely with their efficacy in preventing TER loss. These data, therefore, show that 0.5 mmol/L SSA prevents barrier dysfunction by inhibiting MLCK up-regulation via mechanisms that do not depend on NF-κB signaling.
Figure 5.
Low dose SSA prevents morphological tight junction disruption, increased MLC phosphorylation, and increased MLCK expression. A: Caco-2 monolayers were treated with IFN-γ (10 ng/ml) for 24 hours followed by TNF-α (2.5 ng/ml) and 0.5 mmol/L SSA for 8 hours. Tight junction proteins (ZO-1, occludin, and claudin-1) were detected by immunofluorescence microscopy. 0.5 mmol/L SSA completely prevented ZO-1, occludin, and claudin-1 redistribution in IFN-γ-primed TNF-α-treated monolayers. Data are representative of three similar experiments, each performed in triplicate. B: Caco-2 monolayers were incubated with IFN-γ (10 ng/ml) for 24 hours and then transferred to media with TNF-α (2.5 ng/ml) and 0.5 mmol/L SSA, 2 mmol/L SSA, or 30 μmol/L MG132, as indicated. Monolayers were harvested 8 hours after TNF-α addition. Lysates were assayed for phosphorylated MLC by SDS-PAGE immunoblot. SSA (0.5 mmol/L) prevented increases in MLC phosphorylation induced by IFN-γ and TNF-α (P < 0.01). In contrast, 2 mmol/L SSA and 30 μmol/L MG132 failed to prevent increases in MLC phosphorylation (P > 0.05 for 2 mmol/L SSA, P = 0.04 for 30 μmol/L MG132) (n = 2 in this representative experiment). C: Caco-2 monolayers were incubated with IFN-γ (10 ng/ml) for 24 hours and then transferred to media with TNF-α (2.5 ng/ml) and 0.5 mmol/L SSA, 2 mmol/L SSA, or 30 μmol/L MG132, as indicated. Monolayers were harvested 8 hours after TNF-α addition. Lysates were assayed for MLCK by SDS-PAGE immunoblot. SSA (0.5 mmol/L) prevented increases in MLCK expression induced by IFN-γ and TNF-α (P < 0.02). In contrast, 2 mmol/L SSA and 30 μmol/L MG132 failed to prevent increases in MLCK expression (P > 0.05 for 2 mmol/L SSA or 30 μmol/L MG132) (n = 2 in this representative experiment).
Discussion
Intestinal barrier function is compromised in inflammatory, infectious, ischemic, and immune-mediated intestinal disease.1 In many cases, this barrier dysfunction is closely associated with elevations of mucosal TNF-α and IFN-γ. Although antagonism of TNF-α signaling can normalize intestinal barrier function in human and animal disease and correlates with improvement in disease symptoms,12–15 the mechanisms by which TNF-α disrupts barrier function are poorly characterized. One clue may be found in our recent report that TNF-α and IFN-γ cause increased phosphorylation of MLC and that inhibition of MLCK is sufficient to both reverse increased MLC phosphorylation and restore barrier function11. This suggests that MLC phosphorylation is central to cytokine-induced barrier dysfunction.
We therefore considered the possibility that MLCK protein expression is increased by TNF-α treatment of IFN-γ-primed monolayers. The data show that MLCK expression is up-regulated in association with barrier dysfunction in cytokine-treated monolayers. The implications of this observation are not limited to intestinal epithelial barrier dysfunction because MLCK isoforms are expressed ubiquitously and regulate diverse cellular functions. Thus, MLCK up-regulation may in part explain other events, such as enhanced epithelial cell migration,45 triggered by proinflammatory cytokines.
Given that TNF-α activates NF-κB signaling and a recent study suggested that TNF-α-induced barrier dysfunction could be prevented by NF-κB inhibitors, we considered the hypothesis that TNF-α triggers NF-κB-dependent transcriptional up-regulation of MLCK. To our surprise, none of the seven NF-κB inhibitors tested were able to prevent TNF-α-induced barrier dysfunction. In fact, several actually exacerbated TNF-α-induced barrier dysfunction, including the clinically useful drug SSA (2 mmol/L), that has been reported to inhibit IκB kinases.46 However, we also found that, at low doses insufficient to inhibit NF-κB activation (0.5 mmol/L), SSA prevented barrier dysfunction. Low-dose SSA also prevented MLCK up-regulation, increased MLC phosphorylation, and morphological tight junction disruption. Thus, SSA prevents barrier dysfunction by a mechanism that does not depend on NF-κB inhibition. We considered other potential mechanisms by which SSA might prevent barrier dysfunction, including antioxidant effects,42,47 inhibition of cyclooxygenase and lipoxygenase,41 and extracellular adenosine release.44 None of these explained the ability of SSA to prevent barrier dysfunction. SSA is broken down by colonic bacteria into 5-ASA and sulfapyridine, and the latter are generally considered to be the active metabolites of SSA. We found, however, that neither 5-ASA nor sulfapyridine were able to prevent barrier dysfunction in IFN-γ/TNF-α-treated monolayers, suggesting that the intact molecule has unique properties not shared by the metabolites. This observation has also been made in other contexts, in which SSA, but not 5-ASA or sulfapyridine, inhibits IκB kinases and cystine transporters.48,49 The data presented here show that SSA, but not 5-ASA or sulfapyridine, prevents barrier dysfunction in IFN-γ/TNF-α-treated monolayers and can, therefore, be added to the growing list of properties unique to SSA. Although the precise mechanism of SSA action in this case remains to be defined, it is clear that SSA blocks IFN-γ/TNF-α-induced barrier dysfunction at least in part by preventing increased MLCK expression and subsequent MLC phosphorylation.
The conclusion that TNF-α-induced barrier dysfunction does not require NF-κB contrasts with a recent publication suggesting that NF-κB mediates TNF-α-induced barrier dysfunction.7 However, other reports of in vitro models have suggested the alternative, that NF-κB activation is required for maintenance of barrier function after TNF-α treatment.36 In the latter study neither pharmacological inhibition of NF-κB activation nor transfection of a dominant-negative IκBα mutant prevented TNF-α-induced barrier dysfunction.36 Consistent with our observations, that study also found that TNF-α treatment of NF-κB-inhibited monolayers increased ZO-1 redistribution and barrier dysfunction.36 We also observed that some NF-κB inhibitors exacerbated TNF-α-induced barrier dysfunction in IFN-γ-primed monolayers. Thus, our data and those of the previous study support the hypothesis that NF-κB may actually serve a protective role to limit TNF-α-induced epithelial dysfunction. This protective role for NF-κB may actually explain the unusual dose-dependence of the SSA protective effect. We propose that although 0.5 mmol/L SSA inhibits barrier dysfunction, when the SSA dose is increased to 2 mmol/L NF-κB is inhibited, thereby exacerbating epithelial injury and overriding the protective effect of 0.5 mmol/L SSA. This concept is not without precedent. For example, NF-κB has been suggested to serve a protective role in vivo in intestinal ischemia-reperfusion injury.50 Studies of ischemia-reperfusion injury, in which TNF-α levels increase and intestinal barrier dysfunction occurs,10,51,52 show that mice with an intestinal epithelial-specific knockout of IκB kinase-β suffer increased intestinal damage relative to wild-type mice.50 Although future studies will be necessary to specifically define the role(s) of NF-κB in TNF-α-induced barrier function, a protective role must be considered.
In summary, our data show that IFN-γ primes intestinal epithelia to respond to TNF-α by disrupting tight junction structure and barrier function. This response is associated with increased MLCK expression and MLC phosphorylation. Thus, MLCK expression is regulated by inflammatory cytokines in a manner that does not require NF-κB activation. Finally, the clinically effective drug SSA is a potent protective agent that prevents disruption of tight junction structure and barrier function by blocking MLCK up-regulation.
Footnotes
Address reprint requests to Jerrold R. Turner, Department of Pathology, The University of Chicago, 5841 South Maryland Ave., MC 1089, Chicago, IL 60637. E-mail: jturner@bsd.uchicago.edu.
Supported by the National Institutes of Health (grant DK61931, grant DK42086 to The University of Chicago Digestive Disease Center, and grant CA14599 to The University of Chicago Cancer Center) and the Crohn’s & Colitis Foundation of America.
References
- Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn’s disease in a subject with familial risk. Gastroenterology. 2000;119:1740–1744. doi: 10.1053/gast.2000.20231. [DOI] [PubMed] [Google Scholar]
- Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- Yacyshyn BR, Meddings JB. CD45RO expression on circulating CD19+ B cells in Crohn’s disease correlates with intestinal permeability. Gastroenterology. 1995;108:132–137. doi: 10.1016/0016-5085(95)90017-9. [DOI] [PubMed] [Google Scholar]
- Madara JL. Loosening tight junctions. J Clin Invest. 1989;83:1089–1094. doi: 10.1172/JCI113987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin JM, Snock KV. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res. 1990;50:2172–2176. [PubMed] [Google Scholar]
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112:137–146. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor alpha. Gastroenterology. 1998;114:657–668. doi: 10.1016/s0016-5085(98)70579-7. [DOI] [PubMed] [Google Scholar]
- Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- Musch MW, Clarke LL, Mamah D, Gawenis LR, Zhang Z, Ellsworth W, Shalowitz D, Mittal N, Efthimiou P, Alnadjim Z, Hurst SD, Chang EB, Barrett TA. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J Clin Invest. 2002;110:1739–1747. doi: 10.1172/JCI15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GR, Lindberg G, Meddings J, Silva M, Beutler B, Thiele D. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology. 1999;116:593–601. doi: 10.1016/s0016-5085(99)70181-2. [DOI] [PubMed] [Google Scholar]
- Ferrier L, Mazelin L, Cenac N, Desreumaux P, Janin A, Emilie D, Colombel JF, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–804. doi: 10.1016/s0016-5085(03)01057-6. [DOI] [PubMed] [Google Scholar]
- Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am J Gastroenterol. 2002;97:2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J Membr Biol. 1990;116:177–184. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. 2000;113:2085–2090. doi: 10.1242/jcs.113.11.2085. [DOI] [PubMed] [Google Scholar]
- Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol. 1999;276:G1279–G1288. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]
- Sugi K, Musch MW, Field M, Chang EB. Inhibition of Na+,K+-ATPase by interferon gamma down-regulates intestinal epithelial transport and barrier function. Gastroenterology. 2001;120:1393–1403. doi: 10.1053/gast.2001.24045. [DOI] [PubMed] [Google Scholar]
- Hecht G, Pestic L, Nikcevic G, Koutsouris A, Tripuraneni J, Lorimer DD, Nowak G, Guerriero V, Jr, Elson EL, Lanerolle PD. Expression of the catalytic domain of myosin light chain kinase increases paracellular permeability. Am J Physiol. 1996;271:C1678–C1684. doi: 10.1152/ajpcell.1996.271.5.C1678. [DOI] [PubMed] [Google Scholar]
- Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- Turner JR, Angle JM, Black ED, Joyal JL, Sacks DB, Madara JL. Protein kinase C-dependent regulation of transepithelial resistance: the roles of myosin light chain and myosin light chain kinase. Am J Physiol. 1999;277:C554–C562. doi: 10.1152/ajpcell.1999.277.3.C554. [DOI] [PubMed] [Google Scholar]
- Turner JR, Black ED, Ward J, Tse CM, Uchwat FA, Alli HA, Donowitz M, Madara JL, Angle JM. Transepithelial resistance can be regulated by the intestinal brush border Na+-H+ exchanger NHE3. Am J Physiol. 2000;279:C1918–C1924. doi: 10.1152/ajpcell.2000.279.6.C1918. [DOI] [PubMed] [Google Scholar]
- Turner JR, Cohen DE, Mrsny RJ, Madara JL. Noninvasive in vivo analysis of human small intestinal paracellular absorption: regulation by Na+-glucose cotransport. Dig Dis Sci. 2000;45:2122–2126. doi: 10.1023/a:1026682900586. [DOI] [PubMed] [Google Scholar]
- Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- Sanders SE, Madara JL, McGuirk DK, Gelman DS, Colgan SP. Assessment of inflammatory events in epithelial permeability: a rapid screening method using fluorescein dextrans. Epithelial Cell Biol. 1995;4:25–34. [PubMed] [Google Scholar]
- Berglund JJ, Riegler M, Zolotarevsky Y, Wenzl E, Turner JR. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na+-glucose cotransport. Am J Physiol. 2001;281:G1487–G1493. doi: 10.1152/ajpgi.2001.281.6.G1487. [DOI] [PubMed] [Google Scholar]
- Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RB, Planchon SM, Roche JK. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356–2363. [PubMed] [Google Scholar]
- Hiribarren A, Heyman M, L’Helgouac’h A, Desjeux JF. Effect of cytokines on the epithelial function of the human colon carcinoma cell line HT29 cl 19A. Gut. 1993;34:616–620. doi: 10.1136/gut.34.5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano CW, Laughlin KV, Russo LM, Soler A Peralta, Mullin JM. Long-term effects of tumor necrosis factor on LLC-PK1 transepithelial resistance. J Cell Physiol. 1993;157:519–527. doi: 10.1002/jcp.1041570311. [DOI] [PubMed] [Google Scholar]
- Yoo J, Nichols A, Song JC, Mammen J, Calvo I, Worrell RT, Cuppoletti J, Matlin K, Matthews JB. Bryostatin-1 attenuates TNF-induced epithelial barrier dysfunction: role of novel PKC isozymes. Am J Physiol. 2003;284:G703–G712. doi: 10.1152/ajpgi.00214.2002. [DOI] [PubMed] [Google Scholar]
- Mullin JM, Laughlin KV, Marano CW, Russo LM, Soler AP. Modulation of tumor necrosis factor-induced increase in renal (LLC-PK1) transepithelial permeability. Am J Physiol. 1992;263:F915–F924. doi: 10.1152/ajprenal.1992.263.5.F915. [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Heyman M, Candalh C, Blaton MA, Bouchaud C. Tumour necrosis factor-alpha induces morphological and functional alterations of intestinal HT29 cl. 19A cell monolayers. Cytokine. 1995;7:441–448. doi: 10.1006/cyto.1995.0060. [DOI] [PubMed] [Google Scholar]
- Soler AP, Marano CW, Bryans M, Miller RD, Garulacan LA, Mauldin SK, Stamato TD, Mullin JM. Activation of NF-kappaB is necessary for the restoration of the barrier function of an epithelium undergoing TNF-alpha-induced apoptosis. Eur J Cell Biol. 1999;78:56–66. doi: 10.1016/s0171-9335(99)80007-7. [DOI] [PubMed] [Google Scholar]
- Agnholt J, Kaltoft K. Infliximab downregulates interferon-gamma production in activated gut T-lymphocytes from patients with Crohn’s disease. Cytokine. 2001;15:212–222. doi: 10.1006/cyto.2001.0919. [DOI] [PubMed] [Google Scholar]
- Abreu MT, Palladino AA, Arnold ET, Kwon RS, McRoberts JA. Modulation of barrier function during Fas-mediated apoptosis in human intestinal epithelial cells. Gastroenterology. 2000;119:1524–1536. doi: 10.1053/gast.2000.20232. [DOI] [PubMed] [Google Scholar]
- Bojarski C, Gitter AH, Bendfeldt K, Mankertz J, Schmitz H, Wagner S, Fromm M, Schulzke JD. Permeability of human HT-29/B6 colonic epithelium as a function of apoptosis. J Physiol. 2001;535:541–552. doi: 10.1111/j.1469-7793.2001.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. J Cell Sci. 2000;113:1771–1781. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- Tornhamre S, Edenius C, Smedegard G, Sjoquist B, Lindgren JA. Effects of sulfasalazine and a sulfasalazine analogue on the formation of lipoxygenase and cyclooxygenase products. Eur J Pharmacol. 1989;169:225–234. doi: 10.1016/0014-2999(89)90019-8. [DOI] [PubMed] [Google Scholar]
- Miles AM, Grisham MB. Antioxidant properties of aminosalicylates. Methods Enzymol. 1994;234:555–572. doi: 10.1016/0076-6879(94)34128-1. [DOI] [PubMed] [Google Scholar]
- Hasko G, Szabo C, Nemeth ZH, Deitch EA. Sulphasalazine inhibits macrophage activation: inhibitory effects on inducible nitric oxide synthase expression, interleukin-12 production and major histocompatibility complex II expression. Immunology. 2001;103:473–478. doi: 10.1046/j.1365-2567.2001.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Montesinos MC, Weissmann G. Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine-dependent mechanism that is independent of inhibition of prostaglandin synthesis and p105 of NFkappaB. Proc Natl Acad Sci USA. 1999;96:6377–6381. doi: 10.1073/pnas.96.11.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corredor J, Yan F, Shen CC, Tong W, John SK, Wilson G, Whitehead R, Polk DB. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am J Physiol. 2003;284:C953–C961. doi: 10.1152/ajpcell.00309.2002. [DOI] [PubMed] [Google Scholar]
- Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn BO, Ko KH, Oh TY, Cho H, Kim WB, Lee KJ, Cho SW, Hahm KB. Efficacy of use of colonoscopy in dextran sulfate sodium induced ulcerative colitis in rats: the evaluation of the effects of antioxidant by colonoscopy. Int J Colorectal Dis. 2001;16:174–181. doi: 10.1007/s003840000282. [DOI] [PubMed] [Google Scholar]
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)-cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633–1640. doi: 10.1038/sj.leu.2402238. [DOI] [PubMed] [Google Scholar]
- Weber CK, Liptay S, Wirth T, Adler G, Schmid RM. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology. 2000;119:1209–1218. doi: 10.1053/gast.2000.19458. [DOI] [PubMed] [Google Scholar]
- Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- Takeyoshi I, Iwanami K, Ohwada S, Kawashima Y, Kawata K, Aiba M, Kobayashi J, Koyama T, Matsumoto K, Satoh S, Morishita Y. Effect of FR167653 on small bowel ischemia-reperfusion injury in dogs. Dig Dis Sci. 1999;44:2334–2343. doi: 10.1023/a:1026633510390. [DOI] [PubMed] [Google Scholar]
- Tamion F, Richard V, Lyoumi S, Daveau M, Bonmarchand G, Leroy J, Thuillez C, Lebreton JP. Gut ischemia and mesenteric synthesis of inflammatory cytokines after hemorrhagic or endotoxic shock. Am J Physiol. 1997;273:G314–G321. doi: 10.1152/ajpgi.1997.273.2.G314. [DOI] [PubMed] [Google Scholar]