Abstract
The unique ligation properties of metal ions are widely exploited by proteins, with approximately one-third of all proteins estimated to be metalloproteins. Although antibodies use various mechanisms for recognition, to our knowledge, none has ever been characterized that uses an interfacial metal. We previously described a family of CD4-reactive antibodies, the archetype being Q425. CD4:Q425 engagement does not interfere with CD4:HIV-1 gp120 envelope glycoprotein binding, but it blocks subsequent steps required for viral entry. Here, we use surface-plasmon resonance to show that Q425 requires calcium for recognition of CD4. Specifically, Q425 binding of calcium resulted in a 55,000-fold enhancement in affinity for CD4. X-ray crystallographic analyses of Q425 in the presence of Ca2+, Ba2+, or EDTA revealed an exposed metal-binding site, partially coordinated by five atoms contributed from four antibody complementarity-determining regions. The results suggest that Q425 recognition of CD4 involves direct ligation of antigen by the Q425-held calcium, with calcium binding each ligating atom of CD4 with ≈1.5 kcal/mol of binding energy. This energetic contribution, which is greater than that from a typical protein atom, demonstrates how interfacial metal ligation can play a unique role in antigen recognition.
Keywords: antibody Q425, CD4, crystal structure, HIV
The humoral immune system employs various mechanisms to generate highly specific antibody (Ab) recognition. Starting from a combinatorial array with a limited number of components with moderate affinity (10-6 M), it rapidly evolves high affinity (10-9 M) (reviewed in refs. 1-3). Recent structural and biochemical studies of Ab-antigen interactions have revealed several tricks that can augment this process including domain swapping (4), Tyr sulfation (5, 6), and nonspecific interaction with membrane (7, 8).
One commonly observed strategy for protein recognition, direct metal ligation, has not been observed with Abs. This absence is perhaps surprising in light of the unique coordination properties of metal ions and the wide exploitation observed throughout nature of the special properties of metals: approximately one-third of structurally characterized proteins contain metals, and it is estimated that about the same proportion of all proteins are metalloproteins (reviewed in refs. 9 and 10).
Abs can distinguish conformational alterations induced by metals in antigens and have been designed or selected to bind metals by direct ligation (11) or indirectly through metal-binding cofactors (12-15). Many of the latter cases of “designed” metal binders have involved “catalytic” Abs (16). Despite the eclectic variety of Abs described to date, to our knowledge, none had been observed in which the Ab contributes directly to the ligand-binding sphere of the metal, with the metal also binding directly to the antigen.
In investigating the CD4-reactive Ab Q425, we were surprised to find that its binding was affected by EDTA. Because CD4 was not known to bind metal ions and the site to which Q425-binding mapped on the third domain of CD4 (residues 249-252) (17) was relatively free of common metal-ligating amino acids (Cys, Asp, Glu, His), this finding suggested that it was the Ab, Q425, that was primarily responsible for binding the metal. Here, we describe surface-plasmon resonance (SPR)-based experiments that characterize the metal binding of Q425 and supplement this information with x-ray crystallography to provide atomic-level detail.
Materials and Methods
Protein Production and Purification. Abs Q425, Q428, and Q4116 were produced from hydribomas as described in ref. 17 and purified over Protein A-Sepharose (Amersham Pharmacia). The four-domain extracellular portion of human CD4 (sCD4) was produced in CHO cells and purified by affinity chromatography on a column composed of Q425 conjugated to Protein A, using EDTA for elution (18). Q425 Fab was prepared by papain digestion of reduced and alklated IgG following methods described in ref. 19.
SPR. SPR experiments were performed on a Biacore biosensor system at 25°C. sCD4 was immobilized on research grade CM5 sensor chips by the recommended standard amine coupling. Binding experiments were carried out in HBSP buffer (10 mM Hepes, pH 7.4/150 mM NaCl/0.005% surfactant P-20) with 1 mM EDTA or at specified concentrations of metal ions. During the association phase, Q425 Fab analytes were passed over the buffer-equilibrated chip surface at a rate of 30 μl/min. After the association phase, bound analytes were allowed to dissociate for 5 min. The chip surface was then regenerated by two 25-μl injections of 10 mM Gly/HCl (pH 3.0) at a flow rate of 50 μl/min. Association and dissociation values were calculated by numerical integration and globally fit to a 1:1 interaction model using biaevaluation 3.0 software (Biacore).
Ab Sequencing and Genomic Analysis. The sequences of Q425, Q428, and Q4116 Abs were determined by using methods described in ref. 20. Briefly, the heavy and light chains of Q425, Q428, and Q4116 were reduced with DTT, separated on SDS/PAGE, and blotted onto poly(vinylidene difluoride) membranes for N-terminal amino acid sequencing. Edman sequencing gave unambiguous sequences for at least the first 10 aa. Primers for N termini of heavy or light chains were designed according to the N-terminal amino acid sequences with reference to mouse genomic sequences. These primers were used in combination with universal C-terminal primers corresponding to conserved constant regions to amplify hybridoma cDNA. Sequences were determined from a minimum of five clones. N-terminal primers for Q425 and Q425-like Abs were as follows: for the heavy chains, 5′-CTGGTGGAGTCTGGGGGAGACTTAGTGAAG-3′; and for the light chains, 5′-GAAACAACTGTGACCCAGTCTCCAGCATCC 3′. C-terminal “Universal” primers used to obtain sequences were as follows: for the heavy chains, 5′-ACAATCCCTGGGCACAATTTTCTTGTCCACC-3′; and for the light chains, 5′-ACACTCATTCCTGTTGAAGCTCTTG-3′.
Nucleotide sequences of Q425-family Abs were analyzed with the ImMunoGeneTics database (IMGT) (21). Genomic gene usage was determined by homology, and somatic mutations were determined by comparing Ab nucleotide sequences with the genomic allele of the highest homology.
Crystallography and Structural Analysis. Q425 Fab crystals were obtained by vapor-diffusion-hanging drop with 0.5 μl of protein solution combined with 0.5 μl of reservoir solution, which comprised 14% polyethylene glycol 4000, 25 mM Na Acetate (pH 5.5), and 10 mM CaCl2, or 10 mM EDTA or 25 mM BaCl2. All crystallographic data were collected from capillary-mounted crystals, using 1.54-Å x-rays from a rotating copper anode. The calcium data were collected on a Mar area detector, and were processed and reduced with denzo/scalepack (22). The EDTA and barium data were collected on a Xuong-Hamlin area detector and processed with rotavata/agrovata (23).
The structure of Q425 Fab in the presence of EDTA was solved by molecular replacement with the program amore (24). A collection of Fabs with different elbow angles was used for search models. The highest Patterson correlation coefficient (10- to 4-Å data) was found by using the Fab with Protein Data Bank (PDB) ID code 1FVD (25), and it was used as an initial model. By using iterative rounds of model building [xtalview (26)] and refinement [cns (27)], side chains were corrected, and the model was subjected to torsion-angle simulated annealing with slow cooling, iterative manual fitting, water picking, and positional and restrained B-factor refinements. An Rfree test set consisting of 5% of the data was used as a monitor throughout the refinement.
The pH of the crystallization conditions (5.5) was not optimal for divalent ion binding; to increase the occupancy of the metal ion, the 10 mM Ca2+ and 25 mM Ba2+ data sets were collected at pH 7.5. The structures of Q425 Fab with Ba2+ or Ca2+ were solved by molecular replacement using the Q425:EDTA structure as a model and refined as described for the Q425:EDTA structure.
Structure Analysis and Figures. Nonisomorphism prevented standard difference Fourier analysis. To overcome this obstacle, difference Fourier syntheses were calculated after rigid-body refinements. For a difference Fourier comparing Q425 with barium against Q425 with EDTA, rigid-body refinement was carried out of the Q425:EDTA structure against the Q425:Ba2+ data to place the Q425:EDTA structure into the framework of the Q425:Ba2+ crystal lattice. Then the difference Fourier was calculated by using the observed structure factors of Q425:Ba2+ minus the calculated structure factors of the Q425:EDTA structure after rigid-body refinement and using phases provided by the Q425:EDTA after rigid-body refinement. Superpositions were carried out with the program lsqkab in the ccp4 package (23). Figures were made with pymol (DeLano Scientific, San Carlos, CA; 2002), xtalview (26), or raster3d (28).
Results
Metal Dependence of Q425 Binding. SPR was used to characterize the metal dependence of Q425 binding. sCD4 was covalently coupled to the surface of a CM5 chip, and various antigen-binding fragments (Fabs) were passed over the immobilized CD4, either in the presence of a divalent metal ion or in the presence of EDTA (Fig. 1). With Q425, a dramatic change in affinity was observed between calcium- and EDTA-containing solutions, with tight binding only in the presence of calcium.
Fig. 1.
Interaction of Q425 with CD4 requires Ca2+. SPR was used to investigate the calcium dependence of Q425. As controls, the CD4-reactive Ab, L71, and the gp120-reactive Ab, 17b, were used. (A and B) Sensorgrams of Q425 Fab, L71 Fab, and 17b Fab binding to immobilized sCD4 in the presence of 2.5 mM Ca2+ (A) or of 1 mM EDTA (B). (C) Sensorgrams for Q425 Fab binding to immobilized sCD4 in the presence of 2.5 mM Ca2+, 2.5 mM Sr2+, or 2.5 mM Mg2+/1 mM EGTA. The surface density of sCD4 was 2,000 SPR-response units (RU); the buffer was 10 mM Hepes (pH 7.4), 150 mM NaCl, and 0.0005% P-20. Analytes at a concentration of 140 nM were injected and allowed to bind for 120 s (A and B) or 180 s (C) before dissociation. Precise affinities for Q425 are reported in Fig. 2. For L71, tight binding was observed in the presence of either 2.5 mM Ca2+ (KD = 2.7 nM) or 10 mM EDTA (KD = 2.3 nM).
The cation specificity of Q425 binding was investigated. For the alkaline earth metal ions (IIa series of divalent cations), enhanced binding was not seen with Mg2+, was highest with Ca2+, weaker with Sr2+, and barely evident with Ba2+ (Fig. 1C). These results indicate that the coordination of the binding sphere is optimal for Ca2+,with smaller or larger ionic radii less well accommodated. Analysis of the transition metal ions demonstrated further selectivity, with only Mn2+ and Cd2+ slightly enhancing Q425 affinity (Table 1).
Table 1. Metal dependence of Q425 interaction with CD4.
| Metal ion* | Concentration, mM | Relative binding† | Ionic radii,‡ Å | Coordination number‡ |
|---|---|---|---|---|
| Ca2+ | 2.5 | 1.00 | 0.99 | 6/7/8 |
| Mg2+ | 2.5 | <0.005§ | 0.72 | 6 |
| Sr2+ | 2.5 | 0.58 | 1.13 | 6/8 |
| Ba2+ | 2.5 | 0.04 | 1.42 | 6 |
| Mn2+ | 2.5 | 0.04 | 0.83 | 6 |
| Co2+ | 2.5 | <0.005 | 0.74 | 6 |
| Ni2+ | 2.5 | <0.005 | 0.69 | 6 |
| Cu2+ | 2.5 | <0.005 | 0.73 | 4 |
| Zn2+ | 2.5 | <0.005 | 0.75 | 4/6 |
| Cd2+ | 2.5 | 0.24 | 0.95 | 4/6 |
| K+ | 2.5 | <0.005 | 1.33 | 4/6/8 |
| K+ | 25 | <0.005 | 1.33 | 4/6/8 |
| K+ | 250 | <0.005 | 1.33 | 4/6/8 |
Mg2+ and K+ were tested in the presence of 1 mM EGTA to chelate potentially contaminating Ca2+ ions
Determined by the equilibrium–SPR response of Q425 to immobilized CD4 (2000 RU)
Data for ionic radii and coordination are from refs. 34 and 35; see also www.webelements.com
Below the detection limit of the SPR response
A series of K+ concentrations was tested, up to 250 mM (Table 1). No binding was observed even at the highest concentration. The inability of the isoelectronic potassium to substitute for calcium revealed that the binding involved a significant electrostatic component. Together, the results characterize the calcium specificity of Q425 binding to CD4, detailing the selectivity for ion size, charge, and coordination geometry.
Q425, CD4, and Ca2+ Binding Affinities. We used SPR to quantify more precisely the binding interactions between Q425, CD4, and Ca2+. CD4 was coupled to a CM5 chip, and its interaction with Q425 Fab was analyzed under a variety of conditions. In the absence of Ca2+, Q425 showed only weak affinity for CD4 (KD = 84.7 ± 2.3 μM) (Fig. 2A). However, in the presence of 25 mM Ca2+, the affinity for Q425 increased 55,000-fold to yield a KD of 1.602 ± 0.008 nM (Fig. 2B). This change in affinity was related to changes in both the association and dissociation rates. The association rate changed from 1,730 M-1·s-1 in the absence of calcium to 487,000 M-1·s-1 in 25 mM Ca2+. The dissociation rate changed from 1.42 s-1 in the absence of calcium to 0.000734 s-1 in 25 mM Ca2+.
Fig. 2.
Free energy of interaction for Q425, Ca2+, and CD4. (A) Sensorgram overlays of Q425 Fab binding to immobilized sCD4 in 10 mM EDTA. Q425 Fab at 2-fold serial dilutions from 7,720 to 193 nM was injected for 120 s before dissociation. The surface density of CD4 was 2,000 RU, and the maximal response (calibrated with Q425 in the presence of calcium) was determined to be 472 RU. Fitting (smooth thin line) gave an overall χ2 of 0.452, and an affinity of Q425 for CD4 in the absence of calcium of 84.7 ± 2.3 μM. (B) Sensorgram overlays of Q425 Fab binding to immobilized sCD4 in 25 mM Ca2+. Q425 Fab at 2-fold dilutions starting from 24 to 6 nM was injected for 120 s before dissociation. The surface density of CD4 was 200 RU. Fitting (dotted line) gave an overall χ2 of 0.327 and an affinity of Q425 for CD4 in the presence of calcium of 1.602 ± 0.008 nM. (C) Sensorgram overlays of Q425 Fab binding to immobilized sCD4 at various concentrations of calcium. The concentration of Q425 was fixed at 22 nM and the concentration of calcium tested at 25 mM and at 2-fold serial dilutions from 2.5 to 0.039 mM. As can be seen, the Koff remained essentially constant as long as the concentration of calcium was >0.2 mM, indicating that at these higher concentrations of calcium, the dissociation of the ternary complex could be attributed almost entirely to the release of the Q425:Ca2+ complex. To reduce the possible influence of any alterations in the Koff as a function of the concentration of calcium, only the first 20 s of the association were used in measuring the observed on rate. (D) Fit of the concentration of Q425:Ca2+ as a function of the concentration of Ca2+. We rearranged KD = [Q425][Ca2+]/[Q425:Ca2+] as a function of [Q425:Ca2+] and fit the resultant quadratic equation with data from the initial association observed in C. The fit gave an R2 residual of 0.937, with a Q425 affinity for calcium of KD = 187 ± 16 μM. (E) The complete free energy (ΔG) cycle of Q425:CD4:Ca2+ interaction. Kinetic constants from A-C were used to derive free energy values (ΔG). The calcium affinity for the Q425:CD4 complex was derived these values.
The affinity of Ca2+ was difficult to measure directly by SPR because the mass of calcium is <1/1,000 the mass of Q425. We used the large difference in affinity between free and calcium-bound Q425 and relatively high concentrations of calcium to select appropriate concentration of Q425 and calcium such that the formation of the Q425:Ca2+:CD4 ternary complex could be attributed almost entirely to the concentration of the Q425:Ca2+ complex (Fig. 2C). Quantification of the concentration of Q425:Ca2+ as a function of the concentration of calcium established a Q425 affinity for calcium corresponding to KD = 0.187 ± 0.016 mM.
These experiments defined the free energies of ternary complex formation for three legs of a thermodynamic cycle (Fig. 2E). The missing fourth leg, the affinity of calcium for the Q425:CD4 complex, was difficult to measure directly, because the Q425 affinity for CD4 in the absence of calcium was weak. Definition of three legs of the cycle, however, permitted calculation of the missing fourth leg, the affinity of calcium for the Q425:CD4 complex. The tightness of this affinity, KD = 3.5 ± 0.3 nM, suggested that the calcium was fully coordinated in the Q425:Ca2+:CD4 ternary complex.
Crystal Structures of Q425. To gain an atomic-level picture of the interaction of calcium and Q425, Fab Q425 was screened for crystallization. Tetragonal crystals of Fab Q425 could be grown from PEG 4000, in space group P43212, in the presence of 10 mM Ca2+ or 10 mM EDTA. To definitively mark the position of calcium, we also grew crystals of Fab Q425 in the presence of 25 mM Ba2+. Crystals from Ca2+,Ba2+, or EDTA diffracted similarly to minimum Bragg spacings of 2.5-2.9 Å. Data were collected, the structures were determined by molecular replacement, and atomic-level models were built with the Q425 sequence (Fig. 3). Refinement yielded reasonable R values with good geometry for each of the three differently liganded crystals (Table 2).
Fig. 3.
Q425 sequence. DNA sequences for both heavy and light chains of Q425 (black) are aligned with V (green), D (purple, heavy chain), and J (orange) genes. Gene mutations, deletions, and insertions in the Q425 DNA sequences compared with the VDJ genes are colored in red. The corresponding Q425 amino acid sequences are displayed according to the Chothia numbering scheme (33). CDRs (Chothia definition) are shown in blue. Red triangles highlight residues involved in Ca2+ as described in Fig. 4.
Table 2. X-ray crystallographic data.
| Q425 Crystal | EDTA | Calcium | Barium |
|---|---|---|---|
| Data collection | |||
| Space group | P43212 | P43212 | P43212 |
| Unit cell dimensions, Å | a = b = 97.7 c = 112.0 | a = b = 97.2 c = 111.0 | a = b = 97.2 c = 110.3 |
| No. of molecules per ASU | 1 | 1 | 1 |
| Wavelength, Å | 1.5418 | 1.5418 | 1.5418 |
| Resolution, Å | 20–2.5 | 20–2.9 | 20–2.8 |
| Completeness,* % | 94.4 (78.8) | 92.0 (82.8) | 97.4 (78.0) |
| No. of total reflections | 70,089 | 61,311 | 50,884 |
| No. of unique reflections | 18,319 | 10,984 | 12,855 |
| I/σ* | 7.3 (2.9) | 17.1 (2.0) | 5.5 (2.2) |
| Rsym*† | 0.098 (0.26) | 0.21 (0.83) | 0.13 (0.35) |
| Refinement statistics (|F| > 0σ) | |||
| Resolution, Å | 20–2.5 | 20–2.9 | 20–2.8 |
| Rcryst,‡ % | 20.8 | 21.4 | 21.1 |
| Rfree,ठ% | 24.9 | 29.6 | 26.9 |
| Rmsd bond length, Å | 0.0066 | 0.0077 | 0.0071 |
| Rmsd bond angles, ° | 1.37 | 1.55 | 1.43 |
| Average B factor, Å2 | 31.0 | 71.1 | 41.6 |
| Ramachandran favored,¶ % | 95.22 | 88.07 | 90.21 |
| Ramachandran allowed,¶ % | 99.52 | 97.14 | 99.05 |
Values in parentheses are for the highest resolution shell
Rsym = Σ|I–〈I〉|/Σ〈I〉, where I is the observed intensity, and 〈I〉 is the average intensity of multiple observations of symmetry related reflections
R = Σhkl||Fobs|–|Fcalc||/Σhkl|Fobs|
Rfree is calculated from 5% of the reflections excluded from refinement
Calculated from ref. 36; see also http://kinemage.biochem.duke.edu
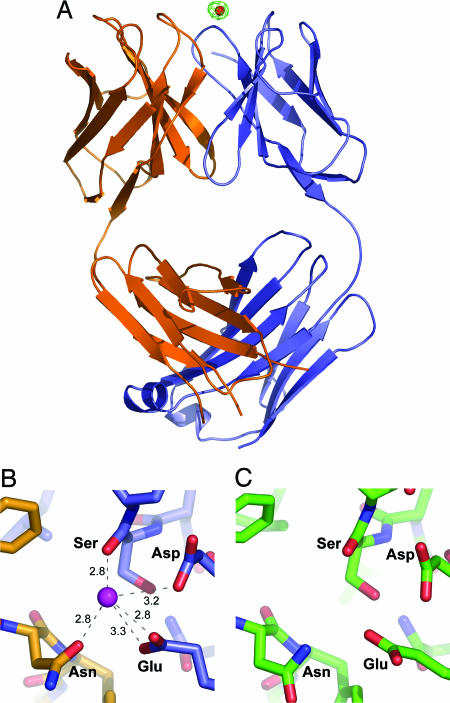
The structures of Q425 in the presence of Ca2+,Ba2+, or EDTA were extremely similar. Despite this similarity in structure, crystals were sufficiently nonisomorphous to prevent direct difference Fourier analysis. Rigid-body refinement of the Q425:EDTA structure against the Q425:Ba2+ data permitted difference Fourier calculations, resulting in a single unique peak of 9σ near four complementarity-determining regions (CDR) (Fig. 4A). Examination of the Q425:Ca2+ structure showed that this site was occupied by a single calcium ion, coordinated by three side chains, Asn-100a of the CDR H3 loop, Asp-32 of the CDR L1, and Glu-50 of the CDR L2. These side chains contributed four coordinating atoms. A fifth coordinating oxygen was contributed by the backbone carbonyl of Ser-91 of the CDR L3 (Fig. 4B).
Fig. 4.
Structure of Fab Q425. (A) Overall structure. The Q425:Ba2+ complex is shown in ribbon representation, with Ba2+ ion in red, heavy chain in orange, and light chain in blue. Contoured in green at 6σ is the rigid-body difference Fourier (Ba2+ vs. EDTA; see details in Materials and Methods), marking the position of the Ba2+ ion. (B) Q425:Ca2+ structure, showing details of the Ca2+ binding site. A close-up of the Q425-calcium binding site is shown in an orientation related to A by a 90° rotation around a horizontal axis. The Ca2+ ion is colored purple, oxygen atoms are colored red, nitrogen atoms are in dark blue, carbon atoms of the heavy chain are in yellow, and carbon atoms of the light chain are in blue. Coordinating ligands are side-chain oxygens from Asn (residue 100a from the CDR H3), Asp (residue 32 from the CDR L1), and Glu (residue 50 from the CDR L2), as well as the backbone carbonyl of residue 92 (in the CDR L3). Ligand distances are shown in Å. (C) Q425:EDTA structure, showing details of the same site shown in B but in the presence of 10 mM EDTA.
Analysis of the Q425-calcium binding site in the presence of EDTA showed minimal alteration (Fig. 4C). The primary change was the absence of ordered electron density in the calcium-binding pocket, with the closest nonprotein electron density, a water molecule, 5.5 Å distal from the location of calcium in the bound structure. Within the binding pocket, analysis of the hydrogen-bonding donors/acceptors suggested that the primary protein structural change involved Asn-100a switching side-chain amide and carbonyl positions. The results indicate that the binding of calcium minimally perturbed the Q425 structure and suggest that the Ca2+ effect on Q425 affinity results from direct coordination of CD4 by the Q425-held Ca2+.
Genomic Analysis of Q425 and Other Q425-Like Abs. In addition to Q425 several other Abs with similar virus neutralization phenotypes have been identified (17), including Q428 and Q4116. We found that Q425, Q428, and Q4116 all had similar sequences, with the same V-D-J and V-J combination for heavy and light chains respectively (see Fig. 7, which is published as supporting information on the PNAS web site).
In Q428, one of the amino acids identified in Q425 as coordinating calcium (Asn-100a of the heavy chain) was changed to a Ser. Replacement of the coordinating Asn observed in Q425 with a Ser would place the Ser hydroxyl 3.9 Å from the calcium, too far for direct ligation. The binding of Q428 and Q4116 to CD4, however, remained calcium dependent (Fig. 5). The results suggest either that Ser-100a of Q428 is able to coordinate indirectly through an intermediate water molecule or that the Q428 site does not require five coordinating ligands to bind calcium. In either case, the results show that much of the calcium coordination is defined by the four coordinating ligands of the light chain, which are specified directly by its Vκ17 germ-line sequence (Fig. 3).
Fig. 5.
Calcium dependence of other Q425-like Abs binding to CD4. CD4 was immobilized on a CM5 chip, and the binding of Q425, Q428, and Q4116 IgG was tested. Sensorgrams are shown for Q425, Q428, and Q4116 in the presence of 2.5 mM Ca2+ and also in the presence of 1 mM EDTA. IgG (144 nM) was passed over the chip starting at 80 s and allowed to associate for 170 s, with the dissociation followed for 5 min.
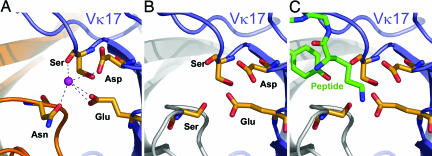
We searched the PDB for examples of Ab coordination similar to that observed with Q425. Because four of the five coordinating ligands derive from the light chain, we expected other Vκ17 genes to have similarly oriented amino acids. We found one structure with a Vκ17 light chain, with PDB ID code 1i8i (29), the complex of a variable domain (Fv) with an epidermal-growth-factor peptide.
Superposition of the variable light-chain regions from the Fv-peptide complex and from Q425 revealed a site on the Fv quite similar to that observed with Q425. Interestingly, as with Q428, the heavy chain of the Fv had a Ser, but in this case, the orientation of the heavy chain differed from that observed in Q425, such that the Ser hydroxyl would be positioned within hydrogen-bonding distance of a potential calcium. Even with this favorable five-coordinated potential site of ligation, the Fv-peptide structure did not contain a metal ligand; instead, the site identified in Q425 as binding calcium bound to a Lys of the epidermal-growth-factor peptide (Fig. 6). It may be that small changes in the positions of the Vk17 ligating atoms in the Fv reduced its affinity for calcium or that the direct binding of antigen displaced the more weakly bound calcium. In either case, the Fv-peptide complex serves to demonstrate some of the difficulties of interfacial metal coordination, from maintaining high occupancy of the partially coordinated metal to providing a selective advantage over direct binding.
Fig. 6.
Calcium binding motif of Vκ17 in Q425 and in an Fv-peptide complex. (A) Q425. The calcium-binding site of Q425 is shown with Ca2+ in purple and ligating amino acids colored according to the chemistry of their atoms: yellow (carbon), red (oxygen), and blue (nitrogen). The backbone ribbons for the light and heavy chains are in blue and orange, respectively. (B) Fv. The same site in 1i8i (29), an Fv-peptide complex, is shown. Orientation and coloring are the same as in A, except that the backbone ribbon for the heavy chain is gray. Note that the identity of all of the ligating atoms is preserved, except that Asn-100a of the Q425 heavy chain in the lower left of the image has been changed to a Ser. It is not known whether the unliganded Fv binds calcium. (C) Fv-peptide. The site depicted in B is shown binding peptide (green). A Lys at the N terminus of the peptide binds to the pocket, which in Q425 holds calcium.
Discussion
In the case of the coordination of the calcium-held Q425 atom, we would expect two or three CD4 atoms to complete the full seven- or eight-atom coordination shell. Because the affinity of calcium for the Q425:CD4 complex was -11.5 kcal/mol, this result would indicate that the energy contributed by each coordinating atom is 1.4-1.6 kcal/mol. The strength of such interaction would seem to provide an advantage in selecting for binding of high affinity, and the constraints of coordination geometry would seem to offer advantages of specificity.
In light of these advantages, why has interfacial metal binding not been observed previously with Abs? One possibility is that interfacial metals must satisfy two criteria: they must bind to the Ab with sufficient affinity to comprise part of the recognition surface, but at the same time they must do so with only a partially filled coordination shell to permit additional ligation by antigen. In Q425, the five-coordinated half-site would seem to be almost optimal. Of the coordinating ligands, two are negatively charged (Asp and Glu), fulfilling both hydrogen bonding and electrostatic interactions. Even with such optimal partial coordination, the KD of Q425 for calcium was 0.19 mM, only 8-fold greater than the physiological concentration of calcium. In terms of other metal ions, only Mg2+ is present at such high concentration, with extracellular concentrations of other metal ions typically found in proteins (e.g., Co2+, Cu2+, Fe3+, Mn2+, Ni2+, Zn2+) present at much lower concentrations. Thus, partial coordination of a metal ion at an affinity sufficient to allow substantial occupancy during antigen selection is a hurdle that is easily satisfied only by calcium and magnesium.
An analysis of Ab structures finds many with partially coordinated divalent metal ions: ≈20% of Ab structures in the PDB contain metal ions. Many of these ions are crystallization artifacts. Still, their high frequency suggests that partially coordinated metals can easily bind to the surface of Abs. The commonality of such surface coordination and the lack of previously observed interfacial metals suggest that metal coordination offers little advantage in most types of recognition. The 1i8i Fv-peptide structure shows that even with a light-chain gene that has high intrinsic potential for calcium coordination, direct antigen binding may be preferred over interfacial metal coordination.
One case where interfacial metal ligation may be useful is where high-affinity binding is required but only limited surface accessibility is available. Such a situation may exist with highly glycosylated viral envelopes like that of HIV, where the exposed glycan-free surface is kept to a minimum (30). Another case where interfacial metals might offer advantages would be with catalytic Abs, where the specific properties of metal coordination cannot easily be replicated by standard protein chemistry. The failure to observe interfacial metals despite designed metal-binding sites (31, 32), however, suggests that interfacial metal ligation is not trivial to obtain.
Our analysis of the Q425 family of calcium-dependent Abs lends insight to these issues. First, the Ab must bind metal with sufficient affinity to induce high occupancy using only a partially coordinated site, and, second, the interfacial coordination of the metal must have a selective advantage over direct binding. In this latter case, the random mutagenesis of affinity maturation may select more easily for single amino acid alterations, each of which improves affinity. The multiple interdependent requirements of metal ligation would be at a selective disadvantage in the same manner that random mutagenesis to stabilize protein structure rarely evolves disulfide bonds. In the case of variable genes with genomically encoded metal-binding sites (like the calcium-binding motif of Vκ17 that we describe here in Q425), the precise coordination geometry required to complete the metal ligation reduces the flexibility of antigen recognition. Thus, although interfacial metal ligation may represent an optimum from the perspective of specificity and localized affinity, the multicoordinated nature of the ligation site and the decreased flexibility of antigen recognition place it at a selective disadvantage during affinity maturation. It will be interesting to see whether in circumstances where localized affinities are a premium, such as with HIV-envelope glycoprotein recognition or with enzymatic catalysis, the advantages of interfacial metal coordination will be sufficient to allow its selection.
Supplementary Material
Acknowledgments
We thank R. Axel for use of cell-culture facilities; H. Faison for assistance with manuscript preparation; M. Gawinowicz for N-terminal protein sequencing; C. Huang for assistance with kinetic analysis; C. Klee, D. Myszka, L. Shapiro, and D. Van Ryk for critical readings of the manuscript; and M. Venturi for assistance with Ab sequencing. This work was supported in part by the Intramural Research Program of the National Institutes of Health Vaccine Research Center (T.Z. and P.D.K.) and National Cancer Institute (D.H.H.). W.A.H. was supported in part by National Institutes of Health Grant AI40895, and Q.J.S. was supported by grants from the Medical Research Council and the Department for International Development (United Kingdom).
Abbreviations: CDR, complementarity-determining region; Fab, antigen-binding Ab fragment; Fv, variable domain; PDB, Protein Data Bank; RU, response units; SPR, surface-plasmon resonance.
Data deposition: The atomic coordinates of Q425 in the presence of calcium, barium, and EDTA have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 2ADJ, 2ADI, and 2ADG, respectively). The nucleotide sequences for Q425, Q428, and Q4116 have been deposited in the GenBank database (accession nos. DQ132636-DQ132641, respectively).
References
- 1.Schultz, P. G., Yin, J. & Lerner, R. A. (2002) Angew. Chem. Int. Ed. 41, 4427-4437. [DOI] [PubMed] [Google Scholar]
- 2.Tonegawa, S. (1983) Nature 302, 575-581. [DOI] [PubMed] [Google Scholar]
- 3.Wabl, M. & Steinberg, C. (1996) Curr. Opin. Immunol. 8, 89-92. [DOI] [PubMed] [Google Scholar]
- 4.Calarese, D. A., Scanlan, C. N., Zwick, M. B., Deechongkit, S., Mimura, Y., Kunert, R., Zhu, P., Wormald, M. R., Stanfield, R. L., Roux, K. H., et al. (2003) Science 300, 2065-2071. [DOI] [PubMed] [Google Scholar]
- 5.Choe, H., Li, W., Wright, P. L., Vasilieva, N., Venturi, M., Huang, C., Grundner, C., Zwick, M. B., Wang, L., Rosenberg, E. S., Kwong, P. D., et al. (2003) Cell 114, 161-170. [DOI] [PubMed] [Google Scholar]
- 6.Huang, C., Venturi, M., Majeed, S., Moore, M. J., Phogat, S., Zhang, M. Y., Dimitrov, D. S., Hendrickson, W. A., Robinson, J., Sodroski, J., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 2706-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ofek, G., Tang, M., Sambor, A., Katinger, H., Mascola, J., Wyatt, R. & Kwong, P. D. (2004) J. Virol. 78, 10724-10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso, R. M., Zwick, M. B., Stanfield, R. L., Kunert, R., Binley, J. M., Katinger, H., Burton, D. R. & Wilson, I. A. (2005) Immunity 22, 163-173. [DOI] [PubMed] [Google Scholar]
- 9.Holm, R. H., Kennepohl, P. & Solomon, E. I. (1996) Chem. Rev. 96, 2239-2314. [DOI] [PubMed] [Google Scholar]
- 10.Barondeau, D. P. & Getzoff, E. D. (2004) Curr. Opin. Struct. Biol. 14, 765-774. [DOI] [PubMed] [Google Scholar]
- 11.Iverson, B. I., Iverson, S. A., Roberts, V. A., Getzoff, E. D., Tainer, J. A., Benkovic, S. J. & Lerner, R. A. (1990) Science 249, 659-662. [DOI] [PubMed] [Google Scholar]
- 12.Brummer, O., Hoffman, T. Z. & Janda, K. D. (2001) Bioorg. Med. Chem. 9, 2253-2258. [DOI] [PubMed] [Google Scholar]
- 13.Iverson, B. I. & Lerner, R. A. (1989) Science 243, 1184-1188. [DOI] [PubMed] [Google Scholar]
- 14.Nicolas, K. M., Wentworth, P. J., Harwig, C. W., Wentworth, A. D., Shafton, A. & Janda, K. D. (2002) Proc. Natl. Acad. Sci. USA 99, 2648-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nimri, S. & Keiman, E. (1999) J. Am. Chem. Soc. 121, 8978-8982. [Google Scholar]
- 16.Lerner, R. A., Benkovic, S. J. & Schultz, P. G. (1991) Science 252, 659-667. [DOI] [PubMed] [Google Scholar]
- 17.Healy, D., Dianda, L., Moore, J. P., McDougal, J. S., Moore, M. J., Estess, P., Buck, D., Kwong, P. D., Beverly, P. C. L. & Sattentau, Q. J. (1990) J. Exp. Med. 172, 1233-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu, H., Kwong, P. D. & Hendrickson, W. A. (1997) Nature 387, 527-530. [DOI] [PubMed] [Google Scholar]
- 19.Kwong, P. D., Wyatt, R., Desjardins, E., Robinson, J., Culp, J. S., Hellmig, B. D., Sweet, R. W., Sodroski, J. & Hendrickson, W. A. (1999) J. Biol. Chem. 274, 4115-4123. [DOI] [PubMed] [Google Scholar]
- 20.Kwong, P. D., Doyle, M. L., Casper, D. J., Cicala, C., Leavitt, S. A., Majeed, S., Steenbeke, T. D., Venturi, M., Chaikin, I., Fung, M., et al. (2002) Nature 420, 678-682. [DOI] [PubMed] [Google Scholar]
- 21.LeFranc, M. P. (2003) Nucleic Acids Res. 31, 307-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otwinowski, Z. & Minor, W. (1997) Methods Enzymol. 276, 307-326. [DOI] [PubMed] [Google Scholar]
- 23.Collaborative Computational Project, No. 4 (1994) Acta Crystallogr. 50, 760-763. [Google Scholar]
- 24.Navaza, J. (1994) Acta Crystallogr. A 50, 157-163. [Google Scholar]
- 25.Eigenbrot, C., Presta, L., Randal, M. & Kossiakoff, A. A. (1993) J. Mol. Biol. 229, 969-995. [DOI] [PubMed] [Google Scholar]
- 26.McRee, D. E. (1999) J. Struct. Biol. 125, 156-165. [DOI] [PubMed] [Google Scholar]
- 27.Brunger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S., et al. (1998) Acta Crystallogr. D 54, 905-921. [DOI] [PubMed] [Google Scholar]
- 28.Merritt, E. A. & Bacon, D. J. (1997) Methods Enzymol. 277, 505-524. [DOI] [PubMed] [Google Scholar]
- 29.Landry, R. C., Klimovicz, A. C., Lavictoire, S. J., Borisova, S., Kottachchi, D. T., Lorimer, I. A. & Evans, S. V. (2001) J. Mol. Biol. 308, 883-893. [DOI] [PubMed] [Google Scholar]
- 30.Wei, X., Decker, J. M., Wang, S., Hui, H., Kappes, J. C., Wu, X., Salazar-Gonzalez, J. F., Salazar, G. M., Kilby, M. J., Saag, M. S., et al. (2003) Nature 422, 307-312. [DOI] [PubMed] [Google Scholar]
- 31.Barbas, C. F., III, Rosenblum, J. S. & Lerner, R. A. (1993) Proc. Natl. Acad. Sci. USA 90, 6385-6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts, V. A., Iverson, B. I., Iverson, S. A., Benkovic, S. J., Lerner, R. A., Getzoff, E. D. & Tainer, J. A. (1990) Proc. Natl. Acad. Sci. USA 87, 6654-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chothia, C. & Lesk, A. M. (1987) J. Mol. Biol. 196, 901-917. [DOI] [PubMed] [Google Scholar]
- 34.Glucker, J. P., Katz, A. K. & Bock, C. W. (1999) Rigaku J. 16, 8-16. [Google Scholar]
- 35.Rulisek, L. & Vondrasek, J. (1998) J. Inorg. Biochem. 71, 115-127. [DOI] [PubMed] [Google Scholar]
- 36.Lovell, S. C., Davis, I. W., Arendall, W. B., de Bakker, P. I. W., Word, J. M., Prisant, M. G., Richardson, J. S. & Richardson, D. C. (2003) Proteins Struct. Funct. Genet. 50, 437-450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.