Abstract
The ADP-ribosylation factors (Arfs) are six proteins within the larger Arf family and Ras superfamily that regulate membrane traffic. Arfs all share numerous biochemical activities and have very similar specific activities. The use of dominant mutants and brefeldin A has been important to the discovery of the cellular functions of Arfs but lack specificity between Arf isoforms. We developed small interference RNA constructs capable of specific depletion of each of the cytoplasmic human Arfs to examine the specificity of Arfs in live cells. No single Arf was required for any step of membrane traffic examined in HeLa cells. However, every combination of the double knockdowns of Arf1, Arf3, Arf4, and Arf5 yielded a distinct pattern of defects in secretory and endocytic traffic, demonstrating clear specificity for Arfs at multiple steps. These results suggest that the cooperation of two Arfs at the same site may be a general feature of Arf signaling and provide candidates at several cellular locations that when paired with data on the localization of the many different Arf guanine nucleotide exchange factors, Arf GTPase activating proteins, and effectors will aid in the description of the mechanisms of specificity in this highly conserved and primordial family of regulatory GTPases.
INTRODUCTION
ADP-ribosylation factors (Arfs) are ∼20-kDa GTP-binding proteins that were originally identified by their ability to support the cholera toxin-catalyzed ADP-ribosylation of the α subunit of the adenylyl cyclase activating G protein Gs (Kahn and Gilman, 1984, 1986). Arf activity also affects morphologies of organelles such as the Golgi apparatus and early endosomes and regulates protein traffic through the secretory and endocytic pathways (for reviews, see Kahn, 2003). Biochemical studies demonstrate that one of the predominant Arf-mediated functions includes the recruitment of coat proteins to membrane surfaces, consequently facilitating vesicle budding (Rothman, 1994). Arfs also activate phospholipase D and phosphatidylinositol 4-kinase and phosphatidylinositol (4) phosphate 5-kinase (Cockcroft et al., 1994; Brown et al., 1995; Godi et al., 1999; Jones et al., 2000). Arfs thus play a number of regulatory roles related to membrane traffic, lipid metabolism, organelle morphology, and cellular signaling. However, these roles have been difficult to dissect, at least in part because of the complexity of the Arf family and the observations that the many biochemical activities ascribed to Arfs to date have not shown clear isoform specificity in in vitro assays.
The importance of the Arfs in cell physiology throughout eukaryotic evolution is highlighted by the finding that the earliest eukaryotes, e.g., Giardia lamblia, express six members of the Arf family, whereas no members of the Ras or heterotrimeric G protein α subunit families were found (Murtagh et al., 1992; Logsdon and Kahn, 2003; Li et al., 2004). Mammals have six Arf isoforms, Arf1–6 (Arf2 has been lost in humans), which have been grouped into three classes based on primary sequence and gene organization. Class I Arfs, Arf1–3, are 96% identical. Class II Arfs, Arf4 and Arf5, are 90% identical to each other and 81% identical to Arf1. Class III is comprised of only Arf6 and is the most divergent of the Arf proteins with 66–70% identity to the other human Arf proteins. Arfs1–5 are soluble proteins that cycle on and off membranes in concert with the binding and hydrolysis of GTP, respectively. In contrast, Arf6 is more stably bound to membranes (Cavenagh et al., 1996) and regulates endocytic traffic and actin at the plasma membrane (D'Souza-Schorey et al., 1995; Peters et al., 1995; Radhakrishna and Donaldson, 1997). Thus, although Arf6 can be distinguished from Arf1–5, the latter are often thought of as interchangeable in location and function.
Arf1 and Arf3 are the most abundantly expressed Arf isoforms in those cells or tissues examined (Cavenagh et al., 1996). Because Arfs have been purified ∼10 times in different laboratories using a variety of assays and it is Arf1 and Arf3 that were obtained, it has been assumed in the past that it is only the class I Arfs that possess the activity used in the purification. However, the lower abundance of class II Arfs in cells and similarities in specific activities of the recombinant Arfs makes this conclusion risky at best. Although the functions of Arf4 and Arf5 remain largely unknown, biochemical assays suggest that class I and class II Arfs play overlapping and redundant roles (Balch et al., 1992; Liang and Kornfeld, 1997). The fungal metabolite brefeldin A (BFA) and dominant mutants of Arf alter Arf activity and provide important tools to analyze cellular functions of Arfs. BFA acts by inhibiting a subset of the Arf guanine nucleotide exchange factors (GEFs), but there is little indication of specificity among the Arfs. Dominant active mutants, [Q71L]Arf1 and homologous mutants, are thought to work in cells by activation of the GTPase and effectors with the potential to sequester and inactivate shared Arf GTPase activating proteins (GAPs) that in turn may produce elevated levels of activation of other Arfs. The dominant negative forms of Arfs, typically the [T31I]Arf1 or homologous mutants in other isoforms is used, purportedly bind to Arf GEFs unproductively, thereby sequestering them from use by that or other Arfs. Thus, the extensive biochemical overlap and inability to distinguish among the different Arf isoforms pharmacologically limits the ability to study the functions of individual Arfs in cells. Therefore, it is unknown whether the individual Arf isoforms are functionally redundant or whether each isoform plays distinct or specific roles in membrane traffic.
Here, we showed that dominant negative and dominant active forms of Arfs produced the same effect on Golgi morphology and recruitment of coat proteins to the Golgi. In contrast, the use of siRNA allowed dissection of the role each Arf isoform plays with respect to Golgi morphology, coat recruitment, and membrane traffic via the secretory and endocytic pathways. Although single Arf isoform knockdowns did not produce observable phenotypes, pairwise double small interference RNA (siRNA) combinations produced a unique combination of altered activities and functions in cells.
MATERIALS AND METHODS
Inducible Cell Lines
Normal rat kidney (NRK) cells were used to generate a number of lines capable of interferon-inducible expression of Arf3, Arf4, Arf5, or point mutants, as described previously for human Arf1 (Zhang et al., 1994).
Small Interference RNA
Four different sequences, each 19 nucleotides (nt) in length, were chosen from the coding regions of Arf1, Arf3, Arf4, or Arf5. Synthetic oligonucleotides were kinased and subcloned into BglII and HindIII sites of the pSUPER-based plasmid (Brummelkamp et al., 2002) that expresses siRNA under control of the histone H1 promoter. Only siRNA plasmids that provided >60% knockdown of the individually targeted Arf were used. The plasmids used for this study and the corresponding 19-nt targeted sequences are as follows: Arf1A, 5′-ACCGTGGAGTACAAGAACA-3′; Arf1B, 5′-TGACAGAGAGCGTGTGAAC-3′; Arf3A, 5′-TGTGGAGACAGTGGAGTAT-3′; Arf3B, 5′-ACAGGATCTGCCTAATGCT-3′; Arf4A, 5′-TCTGGTAGATGAATTGAGA-3′; Arf4B, 5′-AGATAGCAACGATCGTGAA-3′; Arf5A, 5′-TCTGCTGATGAACTCCAGA-3′; Arf5B, 5′-CCATAGGCTTCAATGTAGA-3′. For the single knockdowns, 5 μg of each pSUPER plasmid was transfected and for the double knockdowns, 5 μg of each pSUPER plasmid was cotransfected using Lipofectamine 2000, according to the manufacturer's recommendations. Immunoblots (see below) using previously characterized Arf isoform-specific rabbit polyclonal antibodies (Cavenagh et al., 1996) confirmed that expression levels of Arf1, Arf3, Arf4, and Arf5 were optimally decreased on day 3.
Immunoblotting and Quantitation of Arf Expression
HeLa cells were harvested in phosphate-buffered saline (PBS)/5 mM EDTA; pelleted by centrifugation at 1000 × g; suspended in 50 mM Tris·Cl, pH 8.0, 150 mM NaCl, 1% NP-40, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO); incubated on ice for 30 min with periodic mixing; and clarified by centrifugation at 14,000 × g. The samples were diluted in Laemmli sample buffer, subjected to SDS-PAGE, and transferred to nitrocellulose membranes. Immunoblots for the different Arfs were performed using previously characterized rabbit polyclonal antibodies (Cavenagh et al., 1996). In general, blots were developed using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence. To develop blots for quantitation, primary antibody solutions also contained a β-tubulin mouse antibody (1: 1000; Sigma-Aldrich) to control for loading. Blots were rinsed and incubated in AlexaFluor-680 donkey anti-mouse (1:1000; Molecular Probes, Eugene, OR) and IRDye 800 goat anti-rabbit (1:1000; Rockland, Gilbertsville, PA) secondary antibodies in blocking buffer. Blots were scanned and quantified with an Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE). The Odyssey program calculated the integrated intensity of the bands and subtracted background from the values. Background, or 0% immunoreactivity, is calculated using pixels around the perimeter of the area being quantified. The Arf immunoreactivity was normalized to the loading control within each lane.
Immunofluorescence
Cells grown on poly-d-lysine-coated coverslips were fixed with 2% paraformaldehyde, rinsed, and permeabilized with blocking buffer (0.05% saponin, 5% normal goat serum, and 1% bovine serum albumin diluted in PBS). Cells were incubated in the following primary antibodies diluted in blocking buffer overnight at 4°C: βCOP (1:500; Scheel et al., 1997), calnexin (1:250; StressGen Biotechnologies, Victoria, British Columbia, Canada), ERGIC53 (1:500; gift of Hans-Peter Hauri, Biozentrum University of Basel, Basel, Switzerland), giantin (1:5000; Covance, Berkeley, CA), GM130 (1:100; BD Biosciences, Bedford, MA), and KDEL receptor (1:500; StressGen Biotechnologies). Rinses were performed using 0.05% saponin/phosphate-buffered saline. Goat anti-rabbit or mouse secondary antibodies (Molecular Probes) conjugated to Alexa-488, Alexa-594, or Alexa-633 (1:500) were diluted in blocking buffer. Cells were mounted with Prolong antifade mounting media (Molecular Probes).
For cell surface labeling, fixed cells were incubated for 15 min with concanavalin A-Alexa-594 (ConA) (5 μg/ml; Molecular Probes) diluted in PBS followed by four rinses with PBS.
Confocal microscopy (Figures 3, 5, and 7) was performed on a Zeiss LSM 510 as described previously (Volpicelli et al., 2001).
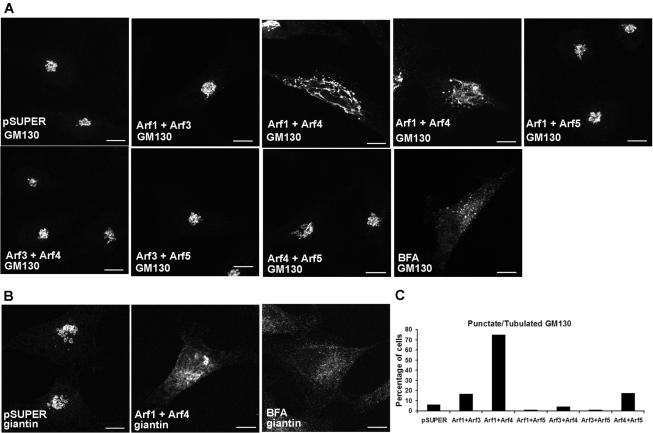
Figure 3.
Decreased expression of both Arf1 and Arf4 caused tubulation and vesiculation of the Golgi. (A) HeLa cells were transfected with pSUPER control or siRNA constructs targeting dual combinations of Arfs and 3 d later were fixed, labeled with antibodies to GM130 to visualize the Golgi apparatus, and confocal images were collected. With the exception of the Arf1+Arf4 double knockdown, all combinations of Arf siRNAs yielded Golgi morphologies that were indistinguishable from controls. In cells cotransfected with Arf1+Arf4, GM130 staining seemed more punctate with elongated tubules extending from the Golgi. For comparison, GM130 staining in HeLa cells treated with BFA (5 μg/ml) for 30 min showed a punctate, dispersed pattern. Bar, 10 μm. (B) HeLa cells were labeled with antibodies to giantin, which showed a perinuclear ribbon-like appearance in pSUPER-transfected control cells. Decreased expression of Arf1+Arf4 caused giantin staining to seem dispersed throughout the cell, similar to the effects of BFA treatment on giantin localization. Bar, 10 μm. (C) The percentage of cells showing punctate and/or tubulated GM130 localization without the condensed perinuclear morphology visualized in control cells were counted (pSUPER, n = 150; Arf1+Arf3, n = 150; Arf1+Arf4, n = 150; Arf1+Arf5, n = 100; Arf3+Arf4, n = 100; Arf3+Arf5, n = 100; and Arf4+Arf5, n = 150).
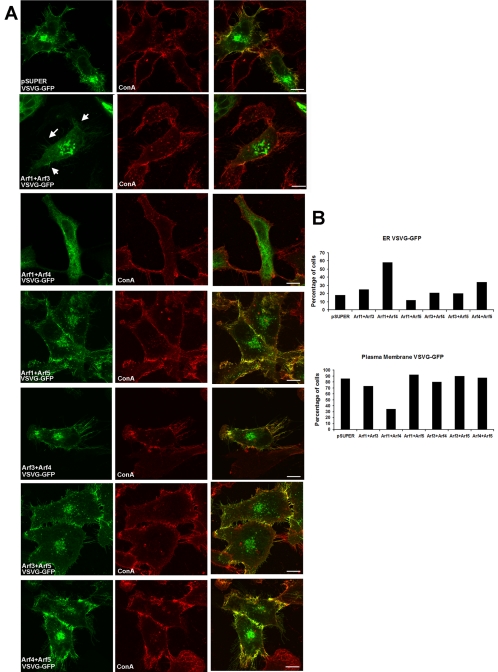
Figure 5.
Decreased expression of Arf1+Arf4 and Arf1+Arf3 altered traffic of ts045-VSVG-GFP. HeLa cells were cotransfected with pSUPER-based plasmids on day 0. After 2 d, cells were transfected with the plasmid directing expression of ts045-VSVG-GFP. Cells were then incubated at the restrictive temperature (40°C) for 16 h before switching to the permissive temperate (32°C) for 90 min before fixing, before collection of confocal images. (A) Equal amounts of pSUPER control or the two plasmids directing knockdown of pairs of Arfs, as indicated in each panel, were used for transfections. Nonpermeabilized cells were incubated with ConA-Alexa594 to label the cell surface. Colocalization of ts045-VSVG-GFP (green) and ConA (red) was visualized as yellow in the merged images. In Arf1+Arf4 knockdown cells, ts045-VSVG-GFP displayed an ER-like appearance, did not localize to the cell surface and did not colocalize with ConA. In Arf1+Arf3 knockdown cells, ts045-VSVG-GFP localized to enlarged puncta. Although ts045-VSVG-GFP traveled to the cell surface in these cells (arrows), the amount of staining at the cell surface seemed diminished. Bar, 10 μm. (B) Percentage of cells in which ts045-VSVG-GFP showed a reticular/ER distribution throughout the entire cell (i.e., seemed similar to the distribution of the ER marker calnexin) was quantified (pSUPER, n = 100; Arf1+Arf3, n = 100; Arf1+Arf4, n = 150; Arf1+Arf5, n = 100; Arf3+Arf4, n = 100; Arf3+Arf5, n = 100; Arf4+Arf5, n = 150). The percentage of cells displaying fluorescence at the plasma membrane also was quantified (pSUPER, n = 150; Arf1+Arf3, n = 100; Arf1+Arf4, n = 150; Arf1+Arf5, n = 100; Arf3+Arf4, n = 100; Arf3+Arf5, n = 100; and Arf4+Arf5, n = 100). Cells were scored a 0 if they showed no cell surface localization and a 1 if they showed any localization to the plasma membrane regardless of the intensity. Therefore, these cell counts are a conservative estimate of whether ts045-VSVG-GFP travels to the plasma membrane under these conditions but do not reflect the amount of ts045-VSVG-GFP at the cell surface.
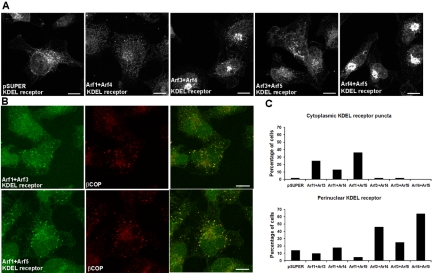
Figure 7.
Double knockdowns alter the localization of the retrograde transport KDEL receptor. Cells were transfected with pSUPER-based and ts045-VSVG-GFP plasmids and incubated as described in the figure legend to Figure 5. (A) Cells were fixed, incubated with antibodies to the KDEL receptor, and confocal images were collected, as described under Materials and Methods. In control cells and those depleted of Arf1+Arf4 or Arf3+Arf5, the KDEL receptor localized to small puncta distributed throughout the cytoplasm with a slight concentration near the nucleus. In Arf4+Arf5 knockdown cells, the KDEL receptor was substantially more concentrated in the perinuclear region. This effect was also seen in Arf3+Arf4 knockdown cells. All images were captured at the same gain. Bar, 10 μm. (B) In Arf1+Arf3 or Arf1+Arf5 knockdown cells, the KDEL receptor (green) in a proportion of cells localized to larger cytoplasmic puncta that colocalized with βCOP (red). (C) The percentage of cells showing cytoplasmic KDEL receptor puncta and perinuclear KDEL receptor were quantified (pSUPER, n = 100; Arf1+Arf3, n = 100; Arf1+Arf4, n = 100; Arf1+Arf5, n = 100; Arf3+Arf4, n = 100; Arf3+Arf5, n = 100; Arf4+Arf5, n = 100).
Transferrin (Tfn) Recycling Assays
Cells were incubated with Alexa-488-conjugated Tfn (25 μg/ml; Molecular Probes) diluted in medium for 60 min. For recycling experiments, cells were incubated with Tfn Alexa-488 for 60 min, placed on ice, rinsed four times with ice-cold DMEM, and incubated at 37°C in DMEM alone for 60 min. The cells were then placed on ice, rinsed one time with cold PBS, incubated with cold stripping buffer (0.5M NaCl, 0.5% acetic acid) for 1 min on ice, rinsed one time with ice-cold PBS, fixed, and mounted onto slides as described above. Confocal images were captured using the same resolution, zoom, pinhole size, and amplitude offset. All cells were captured at the same gain. Using the MetaMorph Imaging System (Universal Imaging, Downingtown, PA), the average grayscale pixel intensity + 1 SD was measured in a small region in which no staining was present. This value was defined as background and the threshold of the green channel was set to this value. Single cells were manually traced. The average pixel intensity + 1 SD was determined, and the threshold was then set at this new value. The resulting average gray scale intensity was recorded. The extent of Tfn recycling for each double Arf knockdown was calculated by measuring the average pixel intensity for the 60-min time point and subtracting the average pixel intensity from the recycling experiment from this value. Data are expressed as a percentage of the pSUPER control.
ts045-VSVG-GFP Transport Assays
Cells were transfected with pSUPER-based plasmids and plated onto coverslips. Two days later, they were transfected with the plasmid directing expression of ts045-VSVG-GFP. The cells were rinsed with fresh media and then incubated at 40°C for 16 h before switching to 32°C for 90 min and then fixed.
Quantitation of Changes in Organelle Morphology and Statistical Analyses
For the analysis of organelle morphologies, cells were given a value of 0 or 1 depending on whether they showed a particular phenotype (for example, 0, normal Golgi; 1, dispersed Golgi). If a cell showed an obvious phenotype, it was scored a 1. If a phenotype was at all questionable, it was scored a 0. Specific criteria for each phenotype are included in the figure legends. In addition, transfection frequency of HeLa cells under the conditions used was determined to be 70–90% and no adjustments were made in the scoring of phenotypes. Therefore, the data presented are a conservative measurement of the phenotypes described.
Statistical Analyses and Reproducibility
For the Tfn experiments, data were analyzed with SPSS software using analysis of variance with Dunnett's post hoc test. The cell count data represent dichotomous variables, i.e., cells were scored a 1 if they showed a particular phenotype or a 0 if they did not show a phenotype. Every experiment reported was performed at least twice with similar results. In addition, for most phenotypes described for a combination of paired Arf siRNA plasmids was confirmed with at least one different combination of siRNA plasmids directing knockdown of the same two Arf isoforms, as a control against off-target effects.
RESULTS
Effects of Dominant Mutants of Arfs on Golgi Morphology and Coat Protein Recruitment
BFA and dominant mutant forms of Arf1 have been used to demonstrate important roles for Arf1 in the morphology of the Golgi apparatus and recruitment of coat proteins to the Golgi. BFA causes the rapid (<2 min at 5 μg/ml) loss of coat proteins, such as COPI (viewed with an antibody to the β-subunit, βCOP), from Golgi membranes to the cytosol and causes the slower (>10-min) dissolution of the Golgi in intact cells. Similarly, dominant negative forms of Arf1 exhibit a BFA-like phenotype in which the Golgi collapses and coat proteins bound to the Golgi become soluble. Conversely, the dominant active [Q71L]Arf1 causes the vesiculation and expansion of the Golgi and increased stability of COPI association with Golgi membranes (Zhang et al., 1994). To determine whether other Arfs perform similar roles in Golgi morphology and coat protein recruitment, we created NRK cells stably transfected with wild type, dominant active, and dominant negative forms of human Arf3 and Arf4 under control of the interferon-inducible Mx1 promoter. We were able to generate stable cell lines capable of inducible expression of Arf5, but not either of the Arf5 mutants. The reason for our failure to obtain mutant Arf5-expressing clones is unknown but may result from leakiness (although no increases in any Arf proteins have been observed by immunoblotting in the absence of induction) of the Mx1 promoter and greater sensitivity of NRK cells to Arf5 mutants than the mutants of other Arf isoforms.
Interferon induction increased expression of wild type and mutant forms of human Arf3 and Arf4 over endogenous protein (Figure 1A) with very similar kinetics to each other and to Arf1 constructs (Zhang et al., 1994). The induced proteins reached maximal levels of induction within 8–12 h and were stable for at least an additional 24 h (Figure 1A). Although changes in the levels of the dominant active mutants were subtler, the appearance of altered Golgi morphologies correlated temporally with protein induction.
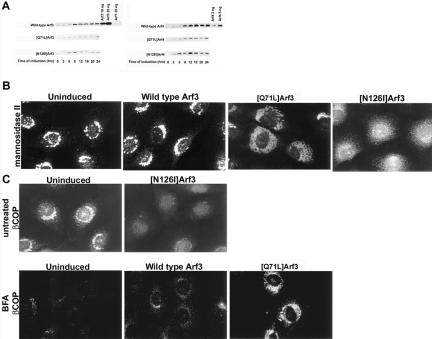
Figure 1.
[Q71L]Arf3 expression caused an expansion of the Golgi and delayed the BFA-induced release of βCOP, whereas [N126I]Arf3 caused a dispersion of the Golgi and dissociation of βCOP. (A) NRK cells stably transfected with wild-type and mutant forms of Arf3 and Arf4 were incubated for varying times with interferon and the levels of Arf3 and Arf4 proteins were determined by immunoblotting. (B) Uninduced or induced cells were stained for mannosidase II to analyze the effect of Arf3, [Q71L]Arf3, and [N126I]Arf3 on Golgi morphology. (C) Uninduced or induced cells were stained for βCOP. Expression of [N126I]Arf3 caused a release of βCOP from the Golgi in untreated cells, whereas expression of [Q71L]Arf3 slowed the release of βCOP in cells treated with 10 μM BFA for 10 min.
Immunofluorescent staining of the lumenal Golgi protein mannosidase II showed that induction of dominant active [Q71L]Arf3 caused an expansion of the Golgi apparatus (Figure 1B). Induction of wild-type Arf3 did not produce any detectable changes in Golgi morphology. Expression of [Q71L]Arf3 also caused a delayed release of βCOP from the Golgi in response to BFA (Figure 1C). In contrast, expression of dominant negative [N126I]Arf3 caused the Golgi to show a dispersed localization throughout the cell (Figure 1B) and caused βCOP to redistribute from the Golgi to the cytosol (Figure 1C). Induction of dominant active and dominant negative Arf4 produced changes in Golgi and βCOP distribution similar to mutant forms of Arf3 (data not shown). However, at any time point, only a smaller percentage of cells was affected. The changes in Golgi morphology and sensitivity to BFA produced by [Q71L]Arf3 and [N126I]Arf3 were similar to those described previously for dominant mutant forms of Arf1 (Zhang et al., 1994). Therefore, BFA, dominant active and dominant negative forms of Arf1, Arf3, and Arf4 produced similar effects on Golgi morphology and βCOP localization. These data suggest that there were no isoform-dependent effects of dominant mutants of Arfs1–4 on Golgi morphology or coat protein recruitment.
Decreasing Expression of Individual Arfs Produced Isoform-specific Effects on Golgi Morphology
Because at least some Arf GAPs and Arf GEFs can act on multiple Arfs (for reviews, see Donaldson and Jackson, 2000; Jackson and Casanova, 2000), the inactivation of these enzymes by one Arf isoform can lead to indirect changes in the level of activation of other Arf subtypes. Thus, neither BFA nor the dominant mutants of Arfs can be assured of selectively altering activity of a single Arf isoform.
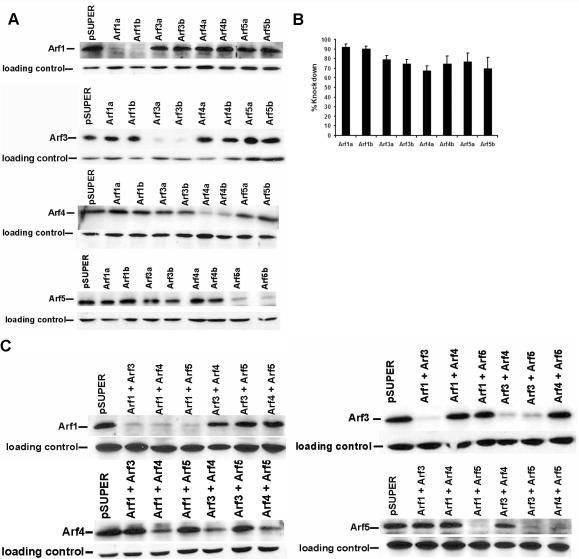
We used siRNAs expressed by pSUPER-based plasmids to specifically decrease expression of Arf1, Arf3, Arf4, or Arf5 individually or in paired combinations. For each Arf isoform four different sequences, each 19 nt in length, were chosen from the coding regions. Only plasmids that provided >60% knockdowns of the individually targeted Arf were used. This resulted in two different plasmids that expressed siRNAs targeted to different regions of the Arf sequence (Arf1a, Arf1b, Arf3a, Arf3b, Arf4a, Arf4b, Arf5a, Arf5b). Having two independent plasmids for each Arf isoform thus allowed us to confirm that the phenotypes observed were produced by decreased expression of the respective Arf and not a result of off-target effects produced by expression of an individual siRNA. Expression of siRNAs maximally decreased expression of each Arf by 3 d after transfection and expression remained reduced 4 d after transfection.
Each siRNA plasmid was transfected into HeLa cells, and total cell lysates were analyzed by immunoblotting to determine the effectiveness of each siRNA plasmid. Expression levels were decreased as follows: Arf1a, 92%; Arf1b, 90%; Arf3a, 79%; Arf3b, 75%; Arf4a, 68%; Arf4b, 75%; Arf5a, 77%; and Arf5b, 70% (Figure 2, A and B). Knockdown of each Arf was specific in that only the targeted Arf was decreased in expression (Figure 2A) as determined by quantitative immunoblotting. Therefore, siRNAs allowed specific knockdown of endogenous Arf expression, providing a powerful tool for analyzing the functions of individual Arfs.
Figure 2.
Knockdown of each Arf isoform by siRNA is effective and specific. (A) HeLa cells were transfected with pSUPER-based plasmids directing expression of siRNAs targeted to human Arf1, Arf3, Arf4, or Arf5 or the empty pSUPERvector. Two different plasmids expressing siRNAs targeting different sequences within the open reading frame of each Arf isoform were used. The level of expression of each Arf was assessed 3 d after transfection, using antisera specific to each Arf isoform. Loading controls for each blot are presented beneath the blot for the respective Arf isoform, as described under Materials and Methods. (B) Immunoblots were scanned and quantified using the Odyssey infrared imaging system. The pixel intensity of each band was normalized to its respective loading control and quantified as described under Materials and Methods. The data represent the average knockdown (±SEM) from the following number of independent experiments: Arf1, n = 5; Arf3, n = 4; Arf4, n = 5; and Arf5, n = 4. (C) HeLa cells were cotransfected with the following combinations of pSUPER-based plasmids: pSUPER control, Arf1a+Arf3b, Arf1a+Arf4b, Arf1a+Arf5b, Arf3b+Arf4b, and Arf3b+Arf5b, Arf4b+Arf5b. Immunoblots show that the double transfectants result in knockdowns of each targeted Arf isoform that are comparable in magnitude to those seen with single knockdowns.
Decreasing Arf activity with BFA or dominant negative Arf mutants causes substantial changes to the morphology of the various organelles and association of coat proteins with membranes. We found, however, that the loss of any one Arf did not produce a similar result. Decreased expression of Arf1, Arf3, Arf4, or Arf5 did not produce any obvious effects on the morphology of the Golgi apparatus or early endosomes as visualized by immunofluorescent staining for giantin or the transferrin receptor, respectively. In addition, individual Arf knockdowns did not noticeably affect localization of the coat proteins COPI, visualized by βCOP staining, or AP-1, visualized by γ-adaptin staining (our unpublished data). These data were interpreted to suggest either that there exist “spare” Arfs and that up to 92% depletion of a single Arf was still not sufficient to make the cell functionally null for that protein or that there is a level of functional redundancy within the four soluble human Arfs. Note also that the extent of knockdown achieved is limited by the transfection frequency of HeLa cells. Thus, with 92% depletion in the entire cell population and <100% transfection efficiency, we expect many cells to be essentially 100% depleted for ARF1 and thus the lack of change in COPI or AP-1 staining can be taken as strong evidence for redundancy in the roles of ARF1 in recruitment of these coats.
To test the hypothesis that functional redundancy exists among the four soluble Arfs in intact cells, we asked whether knocking down two Arf isoforms results in phenotypes that might be reminiscent of BFA or negative dominant mutant expression. Coexpression of each pair of Arfs substantially decreased expression of the respective isoforms (Figure 2C).
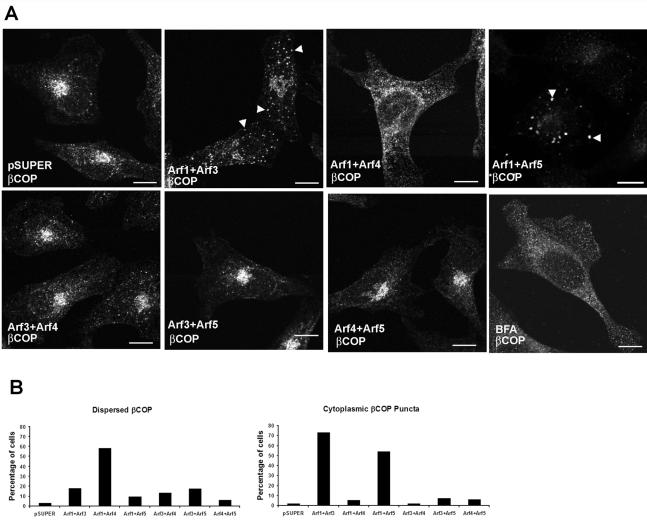
Effects on the cis/medial-Golgi
Decreasing cellular Arf activity with BFA or dominant negative Arf mutants causes dramatic changes in Golgi morphology and functions (Fujiwara et al., 1988; Lippincott-Schwartz et al., 1989). Therefore, we asked whether the double knockdowns produced a similar result. In untreated, control (empty pSUPER vector)-transfected cells, the Golgi exhibited a perinuclear localization, as visualized by immunofluorescent staining of the cis/medial marker GM130 (Figure 3A). Decreased expression of Arf1+Arf4 caused a dramatic change in Golgi morphology; it seemed more punctate with elongated tubules extending from the perinuclear region (Figure 3A, top third and fourth from the left). Quantitation revealed that ∼75% of Arf1+Arf4 knockdown cells showed a punctate/tubulated Golgi (Figure 3C), whereas only ∼5% of cells in the vector-transfected control cells and <20% of every other pairwise double siRNA combinations showed such a staining pattern for the Golgi. Note that because the transfection efficiency of our HeLa cells is <100%, and we do not correct for this in scoring phenotypes, the actual percentages of doubly transfected cells showing any of the phenotypes we quantify is actually greater than the numbers indicated. None of the other paired combinations of Arf knockdowns caused a perceptible change in GM130 staining. BFA treatment caused GM130 to localize to puncta distributed throughout the cytoplasm (Figure 3A, bottom, fourth from left) (Nakamura et al., 1995), whereas giantin seemed completely dispersed throughout the cytoplasm (Figure 3B, third from left) (Linstedt and Hauri, 1993). Similar to the effects of BFA, decreased expression of Arf1+Arf4 caused giantin to seem dispersed (Figure 3B, second from left). The specificity of these changes to the combination of Arf1 and Arf4 were confirmed with independent sets of plasmids; i.e., Arf1a+Arf4b and Arf1b+Arf4a produced the same effects. These data suggest that the effects of BFA on Golgi morphology result from inhibition of Arf1 and Arf4, although changes resulting from depletion of Arf1+Arf4 are not identical to those from BFA treatment.
Effects of Decreased Arf Expression on COPI Localization
In addition to effects on Golgi morphology, Arfs recruit COPI to the Golgi. βCOP is a component of the COPI coatomer complex that mediates traffic between the Golgi cisternae, from the intermediate compartment to the cis-Golgi, and from the cis-Golgi back toward the ER. Dominant negative Arf1 (Zhang et al., 1994), dominant negative Arf3 (Figure 1), or BFA (Figure 6B) caused a redistribution of βCOP from the Golgi to the cytosol. Impaired COPI recruitment may contribute to the changes in Golgi morphology because coats have been proposed to induce membrane curvature and mediate budding of vesicles. We thus tested the effects of decreased expression of Arf isoforms on recruitment of COPI to the Golgi.
Figure 6.
Reduced levels of Arf1+Arf3 and Arf1+Arf4 trap ts045-VSVG-GFP early in the secretory pathway. HeLa cells were cotransfected with pSUPER-based and ts045-VSVG-GFP plasmids and incubated as described in the legend to Figure 5. (A) Cells were then incubated with antibodies to calnexin or ERGIC53 to label the ER or VTCs, respectively. Control cells show no colocalization of ts045-VSVG-GFP (green) with calnexin (red) and a minimal amount of colocalization with ERGIC53 (red). In cells depleted of Arf1+Arf4 ts045-VSVG-GFP colocalized extensively with calnexin (visualized as yellow in the merged images). In some Arf1+Arf4 knockdown cells ts045-VSVG-GFP was also seen in large puncta that colocalized with ERGIC53 (arrows). (B) Cells were prepared as described in A before staining with antibodies to ERGIC53 (red) or βCOP (blue). Ts045-VSVG-GFP, ERGIC53 and βCOP colocalized in the perinuclear region of control cells (visualized as white in the merged images). In Arf1+Arf3 depleted cells, ts045-VSVG-GFP localized to large puncta that costained with ERGIC53 and βCOP (white). Arrows point out examples of colocalization. In Arf1+Arf5 knockdown cells, ERGIC53 localization seemed similar to control cells and ts045-VSVG-GFP and ERGIC53 did not colocalize. Bar, 10 μm.
In pSUPER-transfected control cells and the Arf3+Arf4, Arf3+Arf5 and Arf4+Arf5 double knockdowns, βCOP showed perinuclear localization, consistent with its unchanged localization to the Golgi and to small puncta throughout the cytoplasm (Figure 4A). In contrast, decreased expression of Arf1+Arf4 caused βCOP to seem dispersed throughout the cytoplasm (Figure 4A, top, third from left), similar to the effects of BFA treatment (Figure 4A, bottom, fourth from left). These data were confirmed with independent sets of plasmids, i.e., Arf1a+Arf4b and Arf1b+Arf4a produced the same effects. Cell counts revealed that ∼58% of Arf1+Arf4 knockdown cells showed dispersed βCOP localization (Figure 4B), whereas <5% of cells in the vector-transfected control cells or <20% of any of the other combinations of double siRNA combinations showed such a staining pattern for the βCOP.
Figure 4.
Decreased expression of Arf1+Arf4 caused βCOP to disperse throughout the cytoplasm, whereas Arf1+Arf3 and Arf1+Arf5 double knockdowns caused βCOP to localize to large cytoplasmic puncta. (A) After 3 d, control pSUPER- and siRNA-transfected HeLa cells were labeled with antibodies to βCOP to visualize the localization of the COPI coat complex. In cells depleted of Arf1+Arf4, βCOP was dispersed throughout the cell, similar to what was observed in cells treated for 3 min with BFA (row 2, panel 4). In cells depleted of Arf1+Arf3 or Arf1+Arf5, the cytoplasmic βCOP puncta seemed larger. Bar, 10 μm. (B) Cells were counted as having a “dispersed” βCOP localization if staining was cytoplasmic with no perinuclear localization, and the percentage of such cells are shown in the bar graph to the left (pSUPER, n = 250; Arf1+Arf3, n = 250; Arf1+Arf4, n = 250; Arf1+Arf5, n = 150; Arf3+Arf4, n = 150; Arf3+Arf5, n = 150; Arf4+Arf5, n = 150). The percentage of cells showing enlarged cytoplasmic βCOP-positive puncta was counted in cells expressing ts045-VSVG-GFP after an overnight incubation at 40°C, and 1.5 h at 32°C, and are shown in the bar graph shown at the right (pSUPER, n = 100; Arf1+Arf3, n = 100; Arf1+Arf4, n = 100; Arf1+Arf5, n = 100; Arf3+Arf4, n = 100; Arf3+Arf5, n = 100; Arf4+Arf5, n = 100).
In Arf1+Arf3 and Arf1+Arf5 knockdown cells, the peripheral cytoplasmic puncta to which βCOP localized seemed larger compared with pSUPER-transfected control cells. We identified these puncta as belonging to the ER-Golgi intermediate compartment (ERGIC, aka vesicular tubular clusters, VTCs; see below and Figures 6 and 7). Quantitation demonstrated that ∼73% of Arf1+Arf3 and ∼54% of Arf1+Arf5 knockdown cells showed βCOP localization to enlarged, cytoplasmic puncta (Figure 4B), whereas <10% of cells in the vector-transfected control cells or the other double siRNA combinations showed such a staining pattern for βCOP. It is notable that accumulation of βCOP in cytoplasmic puncta in the Arf1+Arf3 and Arf1+Arf5 knockdowns was more apparent in the ts045-VSVG-GFP experiments (see below), indicating that a bolus of protein transiting the secretory pathway exacerbates the effects of Arf depletions on βCOP localization in these cells. Therefore, decreased expression of both Arf1 and Arf4 caused βCOP to disperse throughout the cytosol, similar to the effects of BFA, whereas Arf1+Arf3 and Arf1+Arf5 knockdowns cause accumulation of βCOP in enlarged VTCs. Together, these data indicate that Arf1 plays a central role in traffic through the Golgi but that each of the other cytoplasmic Arfs (Arf3–5) makes specific contributions to the cellular functions of Arfs at different steps in the early secretory pathway.
Effects of Decreasing Arf Expression on Anterograde Traffic through the Secretory Pathway
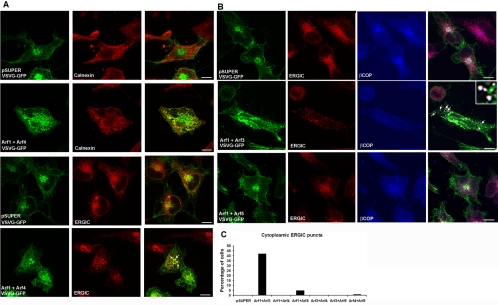
Dominant mutants of Arf1 or BFA treatment inhibit traffic of secreted proteins, such as the vesicular stomatitis G protein (VSVG), through the secretory pathway (Doms et al., 1989; Dascher and Balch, 1994; Zhang et al., 1994; Aridor et al., 1995; Rowe et al., 1996). However, other treatments that alter Golgi morphology, such as dispersion of the Golgi by microtubule depolymerization, do not impair secretory traffic (Cole et al., 1996). We therefore used the temperature-sensitive mutant of ts045-VSVG, tagged with green fluorescent protein (ts045-VSVG-GFP), to determine altered Golgi structure and COPI localization produced by the Arf double knockdowns correlated with altered protein traffic through the early secretory pathway. At 40°C, ts045-VSVG-GFP is misfolded and retained in the ER (Scales et al., 1997). After switching to the permissive temperature for ts045-VSVG-GFP folding (1.5 h at 32°C), ts045-VSVG-GFP in control cells was no longer in the ER but rather was seen at the plasma membrane, where it colocalized with the cell surface marker ConA, and a perinuclear location, consistent with Golgi (Figure 5A, top, Hirschberg et al., 2000). No differences from controls were found in the Arf1+Arf5, Arf3+Arf4, Arf3+Arf5, and Arf4+Arf5 cells. However, in Arf1+Arf4 knockdown cells, ts045-VSVG-GFP did not travel to the plasma membrane and did not colocalize with ConA (Figure 5A, third row from top). Instead, after shifting to the permissive temperature, ts045-VSVG-GFP in the Arf1+Arf4 knockdowns remained in the ER, where it colocalized with the ER marker calnexin (Figure 6A), similar to effects of BFA treatment and expression of dominant negative Arf1 on ts045-VSVG-GFP traffic. These data were confirmed with independent sets of plasmids, i.e., Arf1a+Arf4b and Arf1b+Arf4a produced the same effects. Quantitation revealed that ∼58% of Arf1+Arf4 knockdown cells showed ER localization of ts045-VSVG-GFP after 90 min at the permissive temperature (Figure 5B).
The exit of proteins from the ER is mediated by Sar1 (a distant member of the Arf family) and COPII at ER exit sites (Barlowe et al., 1994; Kuge et al., 1994) and ER-derived vesicles exchange COPII for COPI in an Arf-dependent manner and subsequently differentiate into VTCs that then travel to the Golgi (Aridor et al., 1995; Rowe et al., 1996; Scales et al., 1997). We therefore performed double-labeling immunofluorescence experiments to determine at which step in the secretory pathway ts045-VSVG-GFP traffic is inhibited by decreased expression of Arf1+Arf4. Confocal images demonstrate that 90 min after shifting from 40°C to the permissive temperature of 32°C, the ts045-VSVG-GFP colocalized with the ER marker calnexin in cells in which Arf1 and Arf4 expression were reduced (Figure 6A, second row from top). In some cells, ts045-VSVG-GFP also colocalized with ER-GIC53, a marker for VTCs, which mediates traffic from the ER to the Golgi (Figure 6A, fourth row from top). Therefore, Arf1+Arf4 knockdowns inhibited transport of ts045-VSVG-GFP at a very early step in transport from the ER.
In Arf1+Arf3 double knockdown cells, ts045-VSVG-GFP localized to the perinuclear compartment. However, unlike control cells, the perinuclear ts045-VSVG-GFP looked like dramatically enlarged puncta (Figure 5B). Although ts045-VSVG-GFP trafficked to the plasma membrane in Arf1+Arf3 knockdown cells (Figure 5, second row, panel 1, see arrows), the amount of ts045-VSVG-GFP at the cell surface seemed diminished. These data were confirmed with independent sets of plasmids, i.e., Arf1a+Arf3b and Arf1b+Arf3a produced the same effects. Therefore, decreased expression of Arf1+Arf4 inhibited transport of ts045-VSVG-GFP at an early step in transport from the ER, whereas decreased levels of Arf1+Arf3 caused accumulation of ts045-VSVG-GFP in large perinuclear puncta.
In cells in which Arf1+Arf3 were depleted, the localization of ERGIC53 and thus the morphology of the ER-Golgi intermediate compartment/VTCs was dramatically altered. In control cells, ERGIC53 localized to small puncta localized throughout the cell (Figure 6B, top row). In Arf1+Arf3 knockdowns, ERGIC53 localized to large puncta (Figure 6B, second row), similar to those formed after BFA treatment (Lippincott-Schwartz et al., 1990). These large ERGIC53-positive puncta colocalized with ts045-VSVG-GFP and βCOP. Although decreased expression of Arf1+Arf5 also resulted in the formation of enlarged βCOP-positive puncta, relative to control cells (Figure 6B), the pattern of ERGIC53 staining seemed normal and ts045-VSVG-GFP did not colocalize in these cells. Quantitation of costaining in confocal images revealed that 42% of Arf1+Arf3 knockdown cells showed ERGIC53 localization to large cytoplasmic puncta (Figure 6C), whereas <5% of cells in the vector-transfected control cells or the other double siRNA combinations showed such a staining pattern for ERGIC53. Therefore, decreased expression of Arf1+Arf3 selectively impaired traffic of βCOP-positive VTC's to the cis-Golgi.
Effects of Decreased Arf Expression on Retrograde Traffic from Golgi to ER
Arfs also play a role in COPI-mediated retrograde traffic from the cis-Golgi back to the ER. We therefore determined the effects of the double knockdowns on the localization of the KDEL receptor, which retrieves proteins containing the KDEL sequence from the cis-Golgi and returns them to the ER. In pSUPER-transfected control cells, the KDEL receptor localized to small puncta that are more concentrated near the nucleus (Figure 7A, far left). Localization of the KDEL receptor seemed similar to control cells in Arf1+Arf4 and Arf3+Arf5 double knockdowns. In Arf3+Arf4 and even more so in Arf4+Arf5 cells, there is a dramatically increased concentration of the KDEL receptor at the cis-Golgi (Figure 7A, third and fifth from the left). Approximately 46 and 64% of Arf3+Arf4 and Arf4+Arf5 double knockdown cells, respectively, show enhanced Golgi localization of the KDEL receptor (Figure 7C, bottom). Because the KDEL receptor normally cycles between the ER and cis-Golgi this change in steady-state distribution likely reflects a decrease in the rate of exit from the cis-Golgi back to the ER.
In 25% of Arf1+Arf3 knockdowns and 36% of Arf1+Arf5 knockdowns, the KDEL receptor seemed less concentrated at the perinuclear region and localized to enlarged puncta in the cell periphery. This finding is similar to the effects of BFA on KDEL receptor localization, which has been shown to arrest the KDEL receptor and other rapidly recycling proteins in the ERGIC (Hauri et al., 2000). These KDEL receptor-positive puncta colocalized with βCOP-positive puncta (Figure 7B), suggesting a blockage in ERGIC/VTC to cis-Golgi traffic. But because COPI and the KDEL receptor are each involved in traffic in both directions between the ER and Golgi, we cannot confidently assign directionality to the defects in traffic observed. Overall, these data are interpreted as showing that decreased expression of Arf3+Arf4 or Arf4+Arf5 retards or arrests the KDEL receptor in the cis-Golgi, whereas in Arf1+Arf3 or Arf1+Arf5 double knockdowns, the KDEL receptor is trapped in the ERGIC/VTCs.
Effects of Decreased Arf Expression on Endosome-to-Plasma Membrane Traffic
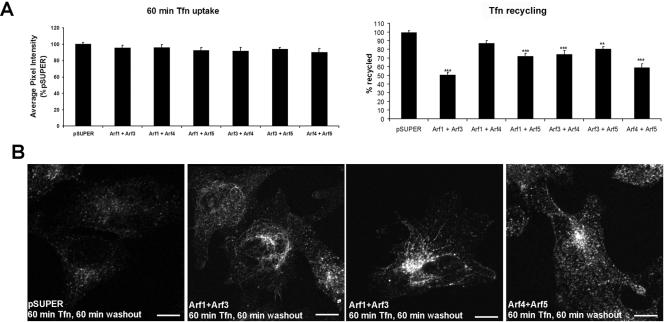
Arf6 plays a role at the plasma membrane in endocytic traffic (D'Souza-Schorey et al., 1995; Peters et al., 1995; Radhakrishna and Donaldson, 1997). The roles of Arfs1–5 in endocytic traffic have received scant attention despite previous studies implicating these Arfs in endosomal pathways (Lenhard et al., 1992; Zhang et al., 1994; Faundez et al., 1998; Gaynor et al., 1998). We thus asked whether Arfs1–5 play a role in the endocytic pathway by analyzing the receptor-mediated uptake of transferrin and its recycling back to the cell surface.
To analyze Tfn uptake, cells were continuously treated with Tfn Alexa 488 for 60 min, before cells were washed, fixed, and analyzed. To measure Tfn recycling cells were incubated with Tfn Alexa 488 for 60 min, and then rinsed and incubated in media alone for an additional 60 min. The amount of intracellular Tfn was measured using confocal images and quantitation of the average pixel intensity within the cells (see Materials and Methods). Recycling was calculated as the average pixel intensity after washout, subtracted from the average pixel intensity after 60 min uptake, normalized to the average amount of recycling in the pSUPER-transfected control cells. The double Arf knockdowns did not affect the extent of Tfn endocytosis (Figure 8A, left bar graph). In contrast, every combination except Arf1+Arf4 (Arf1+Arf3, Arf1+Arf5, Arf3+Arf4, Arf3+Arf5, and Arf4+Arf5) significantly impaired Tfn recycling (Figure 8A, right bar graph). The effect was most pronounced with Arf1+Arf3 (∼50%) and Arf4+Arf5 (∼45%). In the Arf1+Arf3 knockdowns, the endosomes labeled by Tfn Alexa-488 seemed extensively tubulated and after 60 min of Tfn washout, the increased retention of Tfn was evident within these tubules (Figure 8B, middle). In contrast to Arf1+Arf3 double knockdowns, decreased expression of Arf4+Arf5 also increased the retention of Tfn, but in this case, it was trapped in the perinuclear recycling endosomes. The differences in the sites at which Tfn is retained may indicate evidence for distinct roles and sites of action for Arfs in receptor recycling.
Figure 8.
Double Arf knockdowns alter the recycling of Tfn. (A) Cells were incubated with Tfn-Alexa488 for 60 min and either fixed or rinsed several times and incubated in media for another 60 min to allow internalized Tfn to return to the cell surface and be released into the medium. Cells were scanned using confocal microscopy and the average pixel intensity (±SEM) corresponding to the amount of Alexa-488 Tfn in the cells was determined. All images were captured at the same gain. Tfn recycling was calculated as the average pixel intensity in the recycling experiment subtracted from the average pixel intensity of the 60-min time point. The data are expressed as a percentage of control cells. The double knockdowns produced no significant changes in the extent of Tfn uptake under these conditions. **p < 0.01, ***p < 0.001. (B) Confocal images (captured at the same gain) show the localization of Tfn-Alexa 488 after 60-min uptake and 60-min washout, as described under Materials and Methods. In control cells, Tfn-Alexa 488 localized to small puncta in the cytoplasm. Two examples of the Arf1+Arf3 knockdowns show that Tfn-positive endosomes are extensively tubulated. In Arf4+Arf5 knockdown cells, Tfn Alexa 488 is concentrated in the perinuclear compartment. (C) The percentage of cells showing tubulated endosomes after 60-min Tfn uptake was determined. The endosomes were counted as tubulated if the TfnR staining showed long tubules or finger-like projections extending from the perinuclear region (pSUPER, n = 150; Arf1+Arf3, n = 150; Arf1+Arf4, n = 150; Arf1+Arf5, n = 100; Arf3+Arf4, n = 100; Arf3+Arf5, n = 100; and Arf4+Arf5, n = 100).
DISCUSSION
This study demonstrates a new level of complexity in the regulation of membrane traffic by the highly conserved Arf family of regulatory GTPases that was discovered from the use of siRNA to reduce endogenous expression of Arfs in paired combinations. Although no effects were observed for any single Arf knockdown, the paired knockdowns caused distinct and dramatic effects on the morphology of the Golgi and on protein traffic through the secretory and endocytic pathways. These findings help begin to define distinct roles for the different Arf isoforms in membrane traffic and cell signaling. Arf1 and Arf4 were required for the integrity of the Golgi structure, association of COPI with the Golgi, and exit of secreted proteins from the ER. Arf1 and Arf3 in combination were required for traffic from VTCs/ERGIC to the cis-Golgi. The combined depletions of Arf1+Arf3 or Arf1+Arf5 also impaired retrograde traffic from VTCs back to the ER, whereas decreased expression of Arf4+Arf5 or, to a lesser extent, Arf3+Arf4 impaired retrograde traffic at the level of the cis-Golgi. In addition, each paired combination of Arf knockdowns, with the exception of Arf1+Arf4, impaired recycling of the TfnR from early endosomes back to the plasma membrane. The extent of inhibition was the greatest for the Arf1+Arf3 and Arf4+Arf5 knockdowns, which caused the Tfn-labeled endosomes to become tubulated or trapped in the perinuclear compartment, respectively. These differences may indicate different mechanisms by which each combination of Arfs regulates receptor recycling. Figure 9 provides a model summarizing the Arfs involved in various pathways of membrane traffic in the cell (also summarized in Table 1). We conclude that specific combinations of Arfs display a unique profile of activities in cells to control the morphology and functions of organelles of the secretory and endocytic pathway. Specificity in Arf GEF and Arf GAP actions in cells or additional Arf effectors now need to be identified to explain these actions and specificity among Arf isoforms.
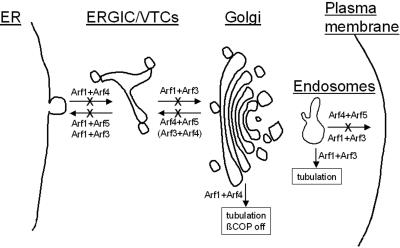
Figure 9.
Model showing the sites of action described for different combinations of Arfs in traffic between the ER and Golgi and in endosome recycling to the plasma membrane. Note that only paired combinations are shown because no single Arf knockdown yielded a discernible effect. An “X” through a line indicates a defect in that step was observed with depletion of that combination of Arfs. Pairs shown within parentheses yielded effects but less than the other pair shown.
Table 1.
Phenotypes described for paired knockdown in expression of Arfs 1, 3, 4, and 5 in HeLa cells
| Knockdown | GM130 | Giantin | βCOP | VSVG-GFP; 3 h at 32°C | KDELR | TfnR |
|---|---|---|---|---|---|---|
| Control | Perinuclear / Golgi | Perinuclear / Golgi membranes | Perinuclear / Golgi + small peripheral puncta | Plasma membrane and perinuclear / Golgi | Small puncta concentrated near nucleus | Perinuclear / endosomal localization |
| Arf1+Arf3 | Same as control | Same as control | Larger peripheral, cytoplasmic puncta/VTCs | Less at plasma membrane, large intracellular puncta that colocalize with ERGIC53 and βCOP | Cytoplasmic puncta that colocalize with βCOP | Impaired recycling (50%), localizes to tubules |
| Arf1+Arf4 | Cytoplasmic puncta and tubules | Dispersed small puncta | Dispersed, small cytoplasmic puncta | Less at plasma membrane, retention in ER/VTCs | Same as control | No effect on recycling |
| Arf1+Arf5 | Same as control | Same as control | Larger peripheral cytoplasmic puncta/no change in ERGIC | Same as control | Cytoplasmic puncta that colocalize with βCOP | Impaired recycling (28%) |
| Arf3+Arf4 | Same as control | Same as control | Same as control | Same as control | Increased perinuclear localization | Impaired recycling (26%) |
| Arf3+Arf5 | Same as control | Same as control | Same as control | Same as control | Same as control | Impaired recycling (20%) |
| Arf4+Arf5 | Same as control | Same as control | Same as control | Same as control | Increased perinuclear localization | Impaired recycling (40%), increased perinuclear localization |
HeLa cells were transfected with either empty vector (control) or paired plasmids directing knockdown in Arf messages, as described under Materials and Methods. Six different assays for organelle morphology, membrane association of coat proteins, early secretory membrane traffic, and endosomal traffic were used to assess consequences of knockdowns are summarized.
No single Arf isoform was essential for any step in membrane traffic assayed in this study. However, because incomplete (68–92%) depletion of Arfs was achieved by siRNA, it is possible that cells maintain sufficient Arfs to maintain functionality. The findings that double knockdowns, obtained using the same plasmids, resulted in dramatic phenotypes make this possibility unlikely. Arf1 and Arf3 are typically expressed to ∼5–10 times the levels of the other Arfs so the largest decrease in total cellular Arfs is found with the Arf1+Arf3 knockdown. Although the Arf1+Arf3 double knockdowns produced unique phenotypes compared with other Arf siRNA combinations, many phenotypes were observed with other combinations. It is also notable that there was no consistent pattern in effects of paired knockdowns yielding phenotypes that might suggest the general need for a class I Arf and a class II Arf. We interpret these data as evidence for specificity in the actions of different Arf isoforms at different points in early secretory and endocytic membrane traffic and the need for at least two different Arfs at each of the steps investigated here.
Arf1 and Arf4 are required for exit of ts045-VSVG-GFP from the ER and VTCs, consistent with previous data demonstrating a block in exit from the ER upon expression of a dominant negative mutant of Arf1 (Dascher and Balch, 1994) or the addition of peptides derived from the N termini of Arf1 or Arf4 that inhibited in vitro assays of ER-Golgi traffic (Balch et al., 1992). The exit of proteins from the ER is mediated by Sar1 (a distant member of the Arf family) and COPII at ER exit sites (Barlowe et al., 1994; Kuge et al., 1994), thus it is unlikely that Arf1 and Arf4 play a role in the initial exit of VSVG from the ER. The ER-derived vesicles exchange COPII for COPI in an Arf-dependent manner and subsequently differentiate into VTCs that then travel to the cis-Golgi (Aridor et al., 1995; Rowe et al., 1996; Scales et al., 1997). In Arf1+Arf4 double knockdowns, COPI shows a dispersed localization throughout the cell, indicating impaired recruitment to membranes. Impaired exchange of COPI in the Arf1+Arf4 knockdowns may prevent the stabilization of ER-derived vesicles, causing them to collapse back into the ER, as has been shown previously (Garcia-Mata et al., 2003).
Thus, a combination of Arf1 and Arf4 are required at the earliest site of Arf dependence in the secretory pathway. These data also provide likely candidates for testing of specificity of Arf GEFs at different cellular locations, e.g., the Arf GEF GBF1 is implicated in VTC to cis-Golgi traffic (Garcia-Mata et al., 2003), and our data thus implicate Arf1 and Arf4 as likely substrates.
Different combinations of Arf isoforms seem to play a role in retrograde traffic from the cis-Golgi, as visualized by tracking the KDEL receptor. The increased staining of the KDEL receptor at the Golgi in Arf3+Arf4 and in Arf4+Arf5-depleted cells indicates a defect in retrograde traffic from the cis-Golgi. Similarly, because the KDEL receptor is found in enlarged cytoplasmic puncta that costain with βCOP but not ts045-VSVG-GFP in Arf1+Arf3 or Arf1+Arf5 knockdown cells, we interpret this as blockage in retrograde traffic between the VTCs and ER. Note that this blockage need not be complete because we are visualizing proteins that cycle between different cellular compartments so that a change in kinetics of the rate-limiting step is all that would be required to observe changes such as those described here. We conclude that there exist differences in specificities for Arf isoforms between traffic in each direction of ER-Golgi traffic (Figure 9).
Our data also suggest that each of the Arfs is required for the recycling of proteins from endosomes back to the plasma membrane. Because almost each of the paired knockdowns in Arfs resulted in a statistically significant defect in Tfn receptor (TfnR) recycling (Figure 8A, right bar graph), specificity was less obvious than at the ER-Golgi. However, two different staining patterns were observed in cells that displayed the greatest retention of Tfn after washout: tubulation in the Arf1+Arf3-depleted cells (also observed after BFA treatment; Lippincott-Schwartz et al., 1991) and increased staining at the perinuclear region in Arf4+Arf5-depleted cells. Therefore, different Arfs may regulate receptor retrieval at two different steps in the recycling pathway. Such a possibility is strengthened by the observations that two different Arf-dependent coat complexes have been shown to be involved in sorting and recycling of proteins in endosomes; COPI (Daro et al., 1997; Gu et al., 1997; Gu and Gruenberg, 2000) and AP-1 (Pagano et al., 2004). Previous data also support the requirement for Arf1, Arf3, and the Arf GEF BIG2 in preventing endosome tubulation (Shin et al., 2004). In contrast, the defect in exit from recycling endosomes seen in Arf4+Arf5 knockdown cells may be mediated by the class II-specific Arf arfophilins, which also bind to Rab11a found predominantly at perinuclear recycling endosomes (Shin et al., 1999; Hickson et al., 2003).
Not only is more than one Arf required at each step but also several Arfs are required at more than one site of membrane traffic analyzed here. Because membrane traffic is bidirectional at all sites and several of the protein components are used at more than one site it is possible that one or more of the phenotypes observed represent secondary effects, e.g., depletion of a required component at one site leading to a change at a different site. Is the tubulation of Golgi membranes in Arf1+Arf4 knockdown cells the result of βCOP dissociation at the Golgi or from the decrease in ER-Golgi traffic? Is the receptor recycling back to the plasma membrane seen in Arf1+Arf3 cells a direct effect or secondary to the decrease in secretory traffic? These are very difficult questions to resolve definitively but must be considered when interpreting such data. What clearly emerges in any case is the evidence for specificity among the Arfs at each step. Our results provide a number of testable hypotheses as the field moves forward in the description of specificity in components of vesicle generation and fusion.
Although dominant mutants of Arfs and BFA have provided important clues regarding cellular functions of Arfs, the specific depletion of endogenous proteins is of unparalleled utility in tests designed to assess specificity among closely related proteins. The overlapping specificity of Arf GEFs and Arf GAPs for Arfs, at least in vitro, makes it likely that BFA and dominant mutants of Arf alter the activity of more than one isoform. We interpret the observation that BFA and negative dominant mutants of Arf1, Arf3, or Arf4 yield indistinguishable effects on Golgi morphology (Zhang et al., 1994; Figure 1) as demonstrating the lack of specificity in the use of such mutants and the potential for secondary effects of these reagents. Although the use of siRNA to study the Arf family is not without its own complications in interpretation, it clearly provides insights not possible with these other techniques and the basis for a number of hypotheses that will require further testing in both in vitro and cell-based assays.
Acknowledgments
We thank the many colleagues who donated their antibodies, without which the work could not have been completed, including Gareth Griffiths (βCOP), Marilyn Gist Farquhar and Kelly Moremen (mannosidase II), and Hans-Peter Hauri (ERGIC53). We also thank Jennifer Lippincott-Schwartz for the generous gifts of the ts045-VSVG-GFP plasmid. We also appreciate and value the contribution of helpful discussions with Victor Faundez and members of the Kahn laboratory during this work and preparation of the manuscript. This work was supported by National Institutes of Health Grants GM-067226 (to R.A.K.) and F32 AG023990 (to L.V.D.) and the Alzheimer's Association 03-5206.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–12–1042) on July 19, 2005.
Abbreviations used: Arf, ADP-ribosylation factor; BFA; brefeldin A; ConA, concanavalin A; ER, endoplasmic reticulum; GAP, GTPase activating protein; GEF, guanine nucleotide exchange factor; Tfn, transferrin; TfnR, transferrin receptor; VSVG, vesicular stomatitis G protein; VTC, tubulo-vesicular cluster.
References
- Aridor, M., Bannykh, S. I., Rowe, T., and Balch, W. E. (1995). Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J. Cell Biol. 131, 875–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch, W. E., Kahn, R. A., and Schwaninger, R. (1992). ADP-ribosylation factor is required for vesicular trafficking between the endoplasmic reticulum and the cis-Golgi compartment. J. Biol. Chem. 267, 13053–13061. [PubMed] [Google Scholar]
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M. F., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77, 895–907. [DOI] [PubMed] [Google Scholar]
- Brown, H. A., Gutowski, S., Kahn, R. A., and Sternweis, P. C. (1995). Partial purification and characterization of Arf-sensitive phospholipase D from porcine brain. J. Biol. Chem. 270, 14935–14943. [DOI] [PubMed] [Google Scholar]
- Brummelkamp, T. R., Bernards, R., and Agami, R. (2002). A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553. [DOI] [PubMed] [Google Scholar]
- Cavenagh, M. M., Whitney, J. A., Carroll, K., Zhang, C., Boman, A. L., Rosenwald, A. G., Mellman, I., and Kahn, R. A. (1996). Intracellular distribution of Arf proteins in mammalian cells. Arf6 is uniquely localized to the plasma membrane. J. Biol. Chem. 271, 21767–21774. [DOI] [PubMed] [Google Scholar]
- Cockcroft, S., Thomas, G. M., Fensome, A., Geny, B., Cunningham, E., Gout, I., Hiles, I., Totty, N. F., Truong, O., and Hsuan, J. J. (1994). Phospholipase D: a downstream effector of ARF in granulocytes. Science 263, 523–526. [DOI] [PubMed] [Google Scholar]
- Cole, N. B., Sciaky, N., Marotta, A., Song, J., and Lippincott-Schwartz, J. (1996). Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol. Biol. Cell 7, 631–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daro, E., Sheff, D., Gomez, M., Kreis, T., and Mellman, I. (1997). Inhibition of endosome function in chinese hamster ovary cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J. Cell Biol. 139, 1747–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher, C., and Balch, W. E. (1994). Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J. Biol. Chem. 269, 1437–1448. [PubMed] [Google Scholar]
- Doms, R. W., Russ, G., and Yewdell, J. W. (1989). Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J. Cell Biol. 109, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson, J. G., and Jackson, C. L. (2000). Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol. 12, 475–482. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey, C., Li, G., Colombo, M. I., and Stahl, P. D. (1995). A regulatory role for ARF6 in receptor-mediated endocytosis. Science 267, 1175–1178. [DOI] [PubMed] [Google Scholar]
- Faundez, V., Horng, J. T., and Kelly, R. B. (1998). A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell 93, 423–432. [DOI] [PubMed] [Google Scholar]
- Fujiwara, T., Oda, K., Yokota, S., Takatsuki, A., and Ikehara, Y. (1988). Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 263, 18545–18552. [PubMed] [Google Scholar]
- Garcia-Mata, R., Szul, T., Alvarez, C., and Sztul, E. (2003). ADP-ribosylation factor/COPI-dependent events at the endoplasmic reticulum-Golgi interface are regulated by the guanine nucleotide exchange factor GBF1. Mol. Biol. Cell 14, 2250–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor, E. C., Chen, C. Y., Emr, S. D., and Graham, T. R. (1998). ARF is required for maintenance of yeast Golgi and endosome structure and function. Mol. Biol. Cell 9, 653–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi, A., Pertile, P., Meyers, R., Marra, P., Di Tullio, G., Iurisci, C., Luini, A., Corda, D., and De Matteis, M. A. (1999). ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat. Cell Biol. 1, 280–287. [DOI] [PubMed] [Google Scholar]
- Gu, F., Aniento, F., Parton, R. G., and Gruenberg, J. (1997). Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J. Cell Biol. 139, 1183–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, F., and Gruenberg, J. (2000). ARF1 regulates pH-dependent COP functions in the early endocytic pathway. J. Biol. Chem. 275, 8154–8160. [DOI] [PubMed] [Google Scholar]
- Hauri, H. P., Kappeler, F., Andersson, H., and Appenzeller, C. (2000). ER-GIC-53 and traffic in the secretory pathway. J. Cell Sci. 113, 587–596. [DOI] [PubMed] [Google Scholar]
- Hickson, G. R., Matheson, J., Riggs, B., Maier, V. H., Fielding, A. B., Prekeris, R., Sullivan, W., Barr, F. A., and Gould, G. W. (2003). Arfophilins are dual Arf/Rab 11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol. Biol. Cell 14, 2908–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg, K., Phair, R. D., and Lippincott-Schwartz, J. (2000). Kinetic analysis of intracellular trafficking in single living cells with vesicular stomatitis virus protein G-green fluorescent protein hybrids. Methods Enzymol. 327, 69–89. [DOI] [PubMed] [Google Scholar]
- Jackson, C. L., and Casanova, J. E. (2000). Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 10, 60–67. [DOI] [PubMed] [Google Scholar]
- Jones, D. H., Morris, J. B., Morgan, C. P., Kondo, H., Irvine, R. F., and Cockcroft, S. (2000). Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1 and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the Golgi compartment. J. Biol. Chem. 275, 13962–13966. [DOI] [PubMed] [Google Scholar]
- Kahn, R. A. (2003). ARF Family GTPases, Norwell, MA: Kluwer Academic Publishers.
- Kahn, R. A., and Gilman, A. G. (1984). Purification of a protein cofactor required for ADP-ribosylation of the stimulatory regulatory component of adenylate cyclase by cholera toxin. J. Biol. Chem. 259, 6228–6234. [PubMed] [Google Scholar]
- Kahn, R. A., and Gilman, A. G. (1986). The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J. Biol. Chem. 261, 7906–7911. [PubMed] [Google Scholar]
- Kuge, O., Dascher, C., Orci, L., Rowe, T., Amherdt, M., Plutner, H., Ravazzola, M., Tanigawa, G., Rothman, J. E., and Balch, W. E. (1994). Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J. Cell Biol. 125, 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard, J. M., Kahn, R. A., and Stahl, P. D. (1992). Evidence for ADP-ribosylation factor (ARF) as a regulator of in vitro endosome-endosome fusion. J. Biol. Chem. 267, 13047–13052. [PubMed] [Google Scholar]
- Li, Y., Kelly, W. G., Logsdon, J. M., Jr., Schurko, A. M., Harfe, B. D., Hill-Harfe, K. L., and Kahn, R. A. (2004). Functional genomic analysis of the ADP-ribosylation factor family of GTPases: phylogeny among diverse eukaryotes and function in C. elegans. FASEB J 18, 1834–1850. [DOI] [PubMed] [Google Scholar]
- Liang, J. O., and Kornfeld, S. (1997). Comparative activity of ADP-ribosylation factor family members in the early steps of coated vesicle formation on rat liver Golgi membranes. J. Biol. Chem. 272, 4141–4148. [DOI] [PubMed] [Google Scholar]
- Linstedt, A. D., and Hauri, H. P. (1993). Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell 4, 679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Donaldson, J. G., Schweizer, A., Berger, E. G., Hauri, H. P., Yuan, L. C., and Klausner, R. D. (1990). Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell 60, 821–836. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Yuan, L., Tipper, C., Amherdt, M., Orci, L., and Klausner, R D. (1991). Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67, 601–616. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Yuan, L. C., Bonifacino, J. S., and Klausner, R. D. (1989). Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56, 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon, J. M., and Kahn, R. A. (2003). The Arf family tree. In: Arf Family GTPases, vol. 1, ed. R. A. Kahn, Dordrecht, The Netherlands: Kluwer Academic Publishers, 1–22. [Google Scholar]
- Murtagh, J. J., Jr., Mowatt, M. R., Lee, C. M., Lee, F. J., Mishima, K., Nash, T. E., Moss, J., and Vaughan, M. (1992). Guanine nucleotide-binding proteins in the intestinal parasite Giardia lamblia. Isolation of a gene encoding an approximately 20-kDa ADP-ribosylation factor. J. Biol. Chem. 267, 9654–9662. [PubMed] [Google Scholar]
- Nakamura, N., Rabouille, C., Watson, R., Nilsson, T., Hui, N., Slusarewicz, P., Kreis, T. E., and Warren, G. (1995). Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131, 1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano, A., Crottet, P., Prescianotto-Baschong, C., and Spiess, M. (2004). In vitro formation of recycling vesicles from endosomes requires adaptor protein-1/clathrin and is regulated by rab4 and the connector rabaptin-5. Mol. Biol. Cell 15, 4990–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, P. J., Hsu, V. W., Ooi, C. E., Finazzi, D., Teal, S. B., Oorschot, V., Donaldson, J. G., and Klausner, R. D. (1995). Overexpression of wild-type and mutant ARF1 and ARF 6, distinct perturbations of non-overlapping membrane compartments. J. Cell Biol. 128, 1003–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna, H., and Donaldson, J. G. (1997). ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 139, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman, J. E. (1994). Mechanisms of intracellular protein transport. Nature 372, 55–63. [DOI] [PubMed] [Google Scholar]
- Rowe, T., Aridor, M., McCaffery, J. M., Plutner, H., Nuoffer, C., and Balch, W. E. (1996). COPII vesicles derived from mammalian endoplasmic reticulum microsomes recruit COPI. J. Cell Biol. 135, 895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales, S. J., Pepperkok, R., and Kreis, T. E. (1997). Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell 90, 1137–1148. [DOI] [PubMed] [Google Scholar]
- Scheel, J., Pepperkok, R., Lowe, M., Griffiths, G., and Kreis, T. E. (1997). Dissociation of coatomer from membranes is required for brefeldin A-induced transfer of Golgi enzymes to the endoplasmic reticulum. J. Cell Biol. 137, 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, H. W., Morinaga, N., Noda, M., and Nakayama, K. (2004). BIG2, a guanine nucleotide exchange factor for ADP-ribosylation factors: its localization to recycling endosomes and implication in the endosome integrity. Mol. Biol. Cell 15, 5283–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, O. H., Ross, A. H., Mihai, I., and Exton, J. H. (1999). Identification of arfophilin, a target protein for GTP-bound class II ADP-ribosylation factors. J. Biol. Chem. 274, 36609–36615. [DOI] [PubMed] [Google Scholar]
- Volpicelli, L. A., Lah, J. J., and Levey, A. I. (2001). Rab5-dependent trafficking of the m4 muscarinic acetylcholine receptor to the plasma membrane, early endosomes, and multivesicular bodies. J. Biol. Chem. 276, 47590–47598. [DOI] [PubMed] [Google Scholar]
- Zhang, C. J., Rosenwald, A. G., Willingham, M. C., Skuntz, S., Clark, J., and Kahn, R. A. (1994). Expression of a dominant allele of human ARF1 inhibits membrane traffic in vivo. J. Cell Biol. 124, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]