Abstract
Myosin-Va is an actin-based processive motor that conveys intracellular cargoes. Synaptic vesicles are one of the most important cargoes for myosin-Va, but the role of mammalian myosin-Va in secretion is less clear than for its yeast homologue, Myo2p. In the current studies, we show that myosin-Va on synaptic vesicles interacts with syntaxin-1A, a t-SNARE involved in exocytosis, at or above 0.3 μM Ca2+. Interference with formation of the syntaxin-1A–myosin–Va complex reduces the exocytotic frequency in chromaffin cells. Surprisingly, the syntaxin-1A-binding site was not in the tail of myosin-Va but rather in the neck, a region that contains calmodulin-binding IQ-motifs. Furthermore, we found that syntaxin-1A binding by myosin-Va in the presence of Ca2+ depends on the release of calmodulin from the myosin-Va neck, allowing syntaxin-1A to occupy the vacant IQ-motif. Using an anti-myosin-Va neck antibody, which blocks this binding, we demonstrated that the step most important for the antibody's inhibitory activity is the late sustained phase, which is involved in supplying readily releasable vesicles. Our results demonstrate that the interaction between myosin-Va and syntaxin-1A is involved in exocytosis and suggest that the myosin-Va neck contributes not only to the large step size but also to the regulation of exocytosis by Ca2+.
INTRODUCTION
Myosin-V, a processive molecular motor, conveys vesicles and other organelles along F-actin (Mercer et al., 1991; Espreafico et al., 1992; Cheney et al., 1993; Reck-Peterson et al., 2000; Vale, 2003). This unconventional myosin is a member of the class-V myosins, which are expressed in all eukaryotic species from yeast to mammals (Reck-Peterson et al., 2000; Matsui, 2003; Vale, 2003). Myosin-V is a dimeric protein (Cheney et al., 1993). Each monomer is composed of a head region, a long neck domain containing six tandem IQ-motifs, and a tail region (Reck-Peterson et al., 2000). The head acts as a plus-end ATPase-dependent molecular motor to move myosin-V along F-actin (Reck-Peterson et al., 2000). The long neck region is thought to act as a lever arm to regulate the motor activity of the head and to maintain the large step size of myosin-V via bound light chains. In higher eukaryotes, the light chains consist mainly of calmodulin (CaM) bound to the IQ-motifs in the neck domain (Cheney et al., 1993; Vale, 2003). In addition, the globular tail of myosin-V interacts with membrane-bound vesicles. In these ways, myosin-V plays a central role in intracellular polarized transport (Reck-Peterson et al., 2000; Matsui, 2003; Vale, 2003).
Among the three isoforms of myosin-V in higher vertebrates, myosin-Va is the most abundant, and it is highly enriched in the brain (Espreafico et al., 1992), particularly in the neurons (Tilelli et al., 2003). Several lines of evidence indicate that synaptic vesicles, which undergo the Ca2+-regulated exocytosis, are one of the most important cargoes for myosin-Va (Prekeris and Terrian, 1997; Bridgman, 1999; Tilelli et al., 2003). In addition, Myo2p, a yeast homologue of myosin-Va, directs intracellular transport during secretion and budding through interactions with other proteins (Matsui, 2003). However, the roles of myosin-Va in secretion and Ca2+-regulated exocytosis are not as clear, probably because the myosin-Va-interacting molecules have not been identified in neurons (Reck-Peterson et al., 2000; Matsui, 2003).
In the current studies, we found a novel interaction between myosin-Va, which is present on cortical synaptic vesicles (Prekeris and Terrian, 1997; Bridgman, 1999) and syntaxin-1A, a t-SNARE that participates in exocytosis (Duman and Forte, 2003; Li and Chin, 2003), in presence of micromolar levels of Ca2+. We also found that this unique interaction, linked to Ca2+-dependent release of CaM from the neck region of myosin-Va, is involved in Ca2+-regulated exocytosis.
MATERIALS AND METHODS
Immunoprecipitation Studies
Immunoprecipitation using an antibody against the globular tail of myosin-Va (gift of P. C. Bridgman, Washington University School of Medicine, St. Louis, MO; Evans et al., 1998) was performed as described previously (Ohyama et al., 2002) except that the buffer contained 1 μM CaCl2, 2 mM MgCl2, and 0.5 mM ATP. In some experiments, immunoprecipitation was performed with a pool of myosin-Va and syntaxin-enriched fractions. This material was obtained as fractions 20–26 from a 5–40% sucrose density gradient centrifugation of hypotonically-treated brain P2 fraction, carried out as described by Ohyama et al. (2002) for glycerol density gradient centrifugation. The antibody against myosin-Va tail used for immunoblotting was kindly provided by V. I. Gelfand (University of Illinois, Urbana, IL; Karcher et al., 2001).
Identification of Myosin-Va
The brain homogenate (S2 fraction) was prepared as described by Fujita et al. (1998). Ca2+-dependent syntaxin-1A binding proteins from rat brain were detected using PreScission protease (GE Healthcare, Uppsala, Sweden) as described previously (Ohyama et al., 2002). The 190-kDa syntaxin-1A binding protein was digested with trypsin and analyzed by mass spectrometry. This protein contained the sequence YFATVSGSASEANVEEK, which corresponds to amino acids 179–195 of myosin-Va.
Ternary Complex Formation between Myosin-Va, F-actin, and Syntaxin-1A
Cosedimentation experiments were performed as described by Nascimento et al. (1996). Purified brain myosin-Va (50 nM) was mixed with 500 nM of F-actin in the presence of 10–6 M Ca2+. In some experiments, the mixture was added to glutathione S-transferase (GST)-syntaxin-1A (50 nM). Cosedimentation was confirmed by centrifugation of the protein mixture at 100,000 × g for 1 h (Nascimento et al., 1996), and the pellet and supernatant were analyzed by SDS-PAGE followed by staining with Coomassie Brilliant Blue.
We also examined whether actin can access the complex between myosin-Va and syntaxin-1A to form a ternary complex. Myosin-Va (5 nM) and either actin (50 nM) or syntaxin-1A (5 nM) were first mixed together and incubated for 1 h at 4°C in the presence of 10–6 M Ca2+. The missing third component (syntaxin-1A or actin, respectively) was then added, and the mixture was incubated for another 1 h. Next, immunoprecipitation was carried out as described above using an anti-myosin-Va antibody (1:200) or an anti-syntaxin-1A antibody (1:200). Myosin-Va, syntaxin-1A, and actin were detected by immunoblotting.
Biochemical and Molecular Biological Techniques for Assessing Protein Binding
Native myosin-Va was purified from chick brain (Cheney, 1998). Recombinant myosin-V (DHM5; [1-1193]) was produced in Sf9 cells as described previously (Homma et al., 2000) or by in vitro translation (Promega, Madison, WI) using the mouse dilute cDNA (gift of N. A. Jenkins, University of Sao Paolo, Ribeirao Preto, Brazil; Mercer et al., 1991). DHM5 was detected with anti-myosin-Va head antibody (gift of R. E. Larson, National Cancer Institute, Frederick, MD; Nascimento et al., 1996; Evans et al., 1998). The binding experiments were carried out using GST-syntaxin-1A fusion proteins immobilized on glutathione-Sepharose (Ohyama et al., 2002). In some experiments, His6-DHM5 fusion protein (Homma et al., 2000) was immobilized on a Ni2+-chelating column. Various concentrations of Ca2+ were generated using an EGTA-Ca2+ buffer with the required amounts of CaCl2 and 4 mM EGTA calculated using Max Chelator or WebMaxC (http://www.stanford.edu/~cpatton/maxc.html) software. In the reconstitution study, the purified myosin-Va and GST-syntaxin 1A [1-262] were incubated together for 1 h, followed by an additional 1 h with recombinant SNAP-25, VAMP-2 [1-96], NSF, and α-SNAP (Hohl et al., 1998). The concentration ratio of the proteins (except for DHM5) was determined as described by Hohl et al. (1998). Synaptic vesicles were purified from adult rat brain as described previously (Huttner et al., 1983). Bacterial two-hybrid experiments were carried out using BacterioMatch (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The rabbit anti-myosin-V neck antibody was generated against the neck domain sequence of mouse myosin-Va and was affinity-purified using protein G-Sepharose (Sigma-Aldrich, St. Louis, MO).
Determination of the Stoichiometry for Binding
The binding stoichiometry between syntaxin-1A and myosin-Va was measured using a BIAcore3000 (BIAcore, Uppsala, Sweden) by immobilizing myosin-Va on CH5 carboxymethyl chips and adjusting the resonance units (RU) to ∼10,000 as described in the manufacturer's instructions. Next, syntaxin-1A [1-262] (0.1–25 μM) in HEPES-buffered saline (10 mM HEPES, pH 7.4, 150 mM NaCl) containing 0.005% Tween 20, 0.1 mM dithiothreitol, and pCa = 5.5 was injected into the flow cells of a BIAcore 3000. The sensorgrams were analyzed using BIA evaluation software version 3.1 (BIAcore). The stoichiometry was calculated from changes in RU at the point between association and dissociation on the compensated sensorgram and using 1200 RU as equal to 1.2 ng of mass per flow cell.
Amperometry
Amperometric measurement of exocytotic catecholamine release was performed as described previously (Ohyama et al., 2002; Quetglas et al., 2002) except that the chromaffin cells were stimulated with 60 mM KCl. Microinjection was performed using a 6-d-old culture of chromaffin cells on collagen-coated coverslips. In each experiment, the cytosol of 50–150 cells was microinjected using an Eppendorf injection system. The cytosolic concentration of the injected fragments was estimated to be 60–120 μg/ml. For cells injected with the syntaxin-1A fragment [191-240] or its L222E mutant, the microinjected cells were stimulated by 60 mM KCl for 4 s. Cells injected with the anti-myosin-Va neck and normal antibodies were stimulated with KCl for 5 min, and the exocytotic frequency during the initial (0- to 1-min) and sustained (1- to 5-min) phases was compared with determine the step of exocytosis regulated by the interaction between myosin-Va and syntaxin-1A.
Effects of Syntaxin-1A Binding on Myosin-Va Properties
Myosin-Va ATPase activity was determined in a reaction mixture containing 50 μg/ml myosin-Va, 420 μg/ml F-actin, 20 mM imidazole-HCl, pH 7.2, 75 mM KCl, 2.5 mM MgCl2, 2 mM ATP, 4 mM EGTA, enough CaCl2 to generate the desired pCa (between 8 and 5) at 25°C, and the presence or absence of 30 μg/ml syntaxin-1A [1-262]. The time course was measured by removing an aliquot every 3 min. The ATPase activity was calculated from the concentration of the released Pi (Chifflet et al., 1988) per mole of myosin-Va per second. The assay of myosin-Va motility was carried out using rhodamine-phalloidin-labeled F-actin as described previously (Rock et al., 2000). After blocking the flow cells with bovine serum albumin, myosin-Va (20–30 μg/ml) was added and adsorbed to the cells for 2 min at room temperature. The flow buffer contained Ca2+ (pCa = 6) in the presence or absence of 1 μM syntaxin-1A. Similar results were obtained with 1 μM and higher concentrations (e.g., 10 μM) of syntaxin-1A (our unpublished data).
The 80-kDa SNARE complex (i.e., the SDS-resistant complex) was isolated as described previously (Igarashi et al., 1997). Briefly, the immobilized 1 μM GST-syntaxin-1A was incubated for 1 h with an equal amount of recombinant SNAP-25 and VAMP-2 and eluted by cleavage with PreScission protease. The eluted proteins were then incubated with or without 1 μM syntaxin-1A [191-240] for 0.5 h and then treated with SDS-sample buffer at 60°C for 5 min (which does not break up the SNARE complex). The 80-kDa protein complex was analyzed by immunoblotting with antibodies specific to syntaxin-1A, SNAP-25, and VAMP-2, which are components of the neuronal SNARE complexes.
Morphological Studies Using Atomic Force Microscopy (AFM)
AFM was carried out as described previously (Mizuta et al., 2003). Myosin-Va was diluted to 5–10 μg/ml in 10 mM HEPES, pH 7.4, containing 2 mM MgCl2. Next, 5 μl of the sample was dripped onto freshly cleaved mica and dried with compressed air. Two minutes later, Milli-Q water (10 μl) was dripped onto the mica surface to remove salts, and the surface was immediately air-dried. The cantilevers (SI-DF40-AL; Seiko Instruments, Neu Isenburg, Germany) used were rectangular, the force constant was 40 Nm–1, and the resonance frequency was 250–390 kHz.
RESULTS
Myosin-Va Associates with Syntaxin in a Ca2+-dependent Manner
In the current studies, we isolated a synaptosomal fraction from cortex and used it as a source for synaptic vesicle protein complexes (Huttner et al., 1983). After solubilization with a nonionic detergent, we performed immunoprecipitation with a myosin-Va antibody. We found that myosin-Va was associated with a 35-kDa protein in the presence of 10–6 M Ca2+ and Mg2+/ATP. This 35-kDa protein was recognized by antibody against syntaxin-1A, a membrane protein that is also known as t-SNARE and is involved in regulated exocytosis (Li and Chin, 2003). Association of syntaxin-1A with myosin-Va required Ca2+ and Mg2+/ATP (Figure 1A). After treatment with Ca2+, myosin-Va remained associated with synaptic vesicles purified from the cortex (Figure 1B).
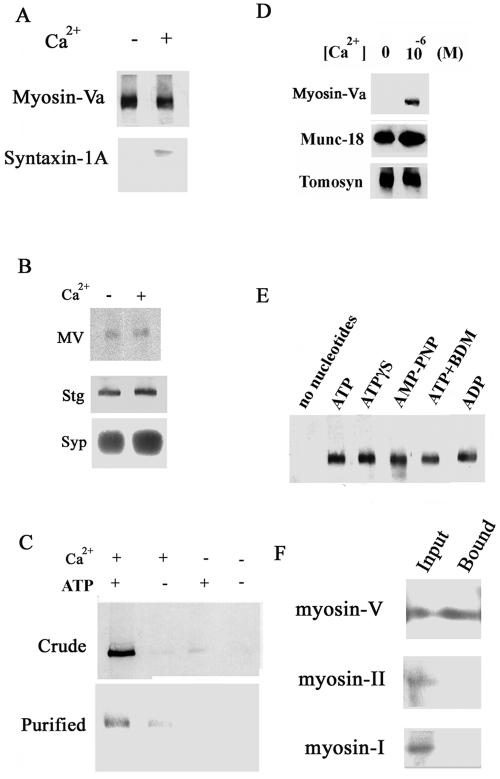
Figure 1.
Ca2+-dependent binding of myosin-Va to syntaxin-1A. (A) Immunoprecipitation of the myosin-Va–syntaxin complex from brain homogenate using an anti-myosin-V antibody. The immunoprecipitation was carried out in the presence or absence of 10–6 MCa2+/2 mM Mg2+/0.5 mM ATP. (B) Myosin-Va (MV) is localized and remains on synaptic vesicles purified from cortex even after addition of 1 μM Ca2+. Myosin-Va was immunoprecipitated from brain homogenate in the presence and absence of 1 μM Ca2+, and myosin-Va, synaptotagmin I, and synaptophysin were detected by immunoblotting. Synaptotagmin I (Stg) and synaptophysin (Syp) are shown as synaptic vesicle markers. (C) Confirmation of the 10–6 M Ca2+/2 mM Mg2+/0.5 mM ATP-dependent binding of syntaxin-1A to myosin-Va in brain homogenate (Crude) or to purified myosin-Va (Purified). Binding of myosin-Va to GST-Syntaxin-1A was assessed in the presence and absence of Ca2+ and Mg-ATP using a GST pull-down assay, followed by anti-myosin-Va immunoblotting. (D) Ca2+-dependence of myosin-Va binding to syntaxin-1A. Brain homogenate was incubated with immobilized GST-syntaxin-1A in the presence or absence of 10–6 M Ca2+/2 mM Mg2+/0.5 mM ATP. Bound myosin-Va was detected by immunoblotting. Myosin-Va binding to syntaxin-1A required 10–6 M Ca2+. In contrast, binding of tomosyn and Munc-18 to syntaxin-1A does not require Ca2+. (E) Binding of myosin-Va by syntaxin-1A requires ATP analogues in presence of 10–6 M Ca2+. As shown by immunoblotting, myosin-Va bound to syntaxin-1A only in the presence of 0.5 mM ATP, ADP, or the nonhydrolyzable ATP analogues adenosine 5′-O-[3-thiotriphosphate] (ATPγS), 5′-adenylylimidodiphosphate (AMP-PNP), or 0.5 mM ATP with 5 mM 2,3-butanedione monoxime (BDM; a myosin-ATPase inhibitor). (F) Myosin-I (myr1A) and myosin-IIB do not bind to syntaxin-1A, even though they are present in the brain homogenate. Rat brain homogenate was mixed with mixed with GST-syntaxin-1A. Equal amounts (15 μl) of the rat brain homogenate (1 μg/μl; Input) and of the fraction bound to GST-syntaxin-1A (Bound; see C) were analyzed by immunoblotting with antibodies against myosin-Va, myosin-IIB (gift of T. Shirao, Gunma University Graduate School of Medicine, Maebashi, Gunma, Japan), or myr 1A (gift of M. Bähler, Westfalische Wilhelms University, Münster, Germany).
A GST pull-down study using syntaxin-1A mixed with rat brain homogenate in (Ohyama et al., 2002) revealed a 190-kDa protein that bound specifically to syntaxin-1A in the presence of Ca2+ and ATP. Using mass spectroscopy, we confirmed that this protein was myosin-Va. Furthermore, binding of brain myosin-Va to syntaxin-1A required the presence of both Ca2+ and ATP (Figure 1C). This association of syntaxin-1A and myosin-Va required at least 10–6 M Ca2+, corresponding to a physiological elevation of Ca2+, whereas two other syntaxin-1A-binding proteins, Munc-18, and tomosyn (Ohyama et al., 2002), bound to syntaxin-1A in the absence of Ca2+ (Figure 1D). Although the interaction between myosin-Va and syntaxin-1A required ATP (Figure 1C), nonhydrolyzable analogues of ATP and ADP also enhanced this binding (Figure 1E). Kinetic analysis of this binding using plasmon resonance revealed that the stoichiometry of binding was 0.77 ± 0.12 (mean ± SD; n = 7), implying a 1:1 interaction between syntaxin-1A and myosin-Va dimer. Rat brain contained other myosins, such as myosin-I and -IIB, but these did not bind to syntaxin-1A (Figure 1F).
Syntaxin-1A Binding Alters the ATPase Activity but Not the Motility of Myosin-Va
We next examined whether the properties of myosin-Va are altered by F-actin. Syntaxin-1A cosedimented with both actin and myosin-Va (Figure 2A). Syntaxin-1A could bind to the myosin-Va–actin complex, and actin could associate with the myosin-Va–syntaxin-1A complex (Figure 2B), indicating that these three proteins can form a complex. Myosin-Va ATPase is activated by Ca2+ and actin (Cheney et al., 1993), and, interestingly, this enhancement of ATPase was completely inhibited by syntaxin-1A binding at pCa = 6 (Figure 2C). In contrast, the F-actin myosin Va-dependent sliding motility was unchanged under these conditions (Figure 2D). Thus, at pCa = 6, the binding of myosin-Va to syntaxin-1A occurs without a large loss of ATP due to hydrolysis and without an effect on motility.
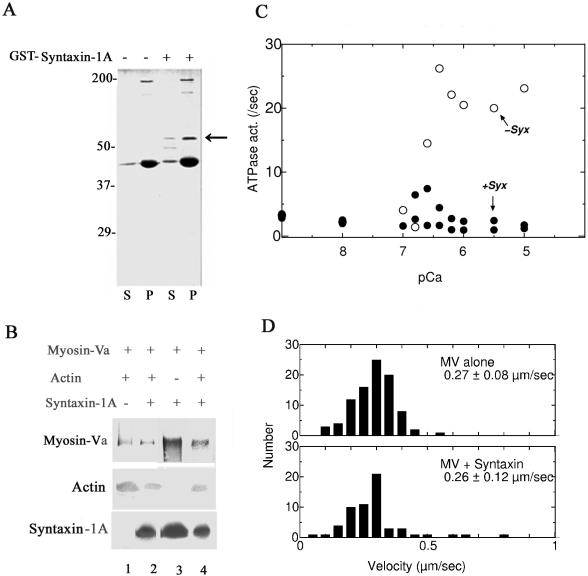
Figure 2.
Syntaxin-1A interacts with myosin-Va independently of F-actin and regulates myosin-Va ATPase activity without affecting its motility. (A) In the presence of 10–6 M Ca2+, GST-syntaxin-1A [1-262] (52-kDa; indicated by an arrow) cosedimented with myosin-Va (190 kDa) in the presence of F-actin. Brain homogenate was incubated with or without GST-syntaxin-1A [1-262] and in the presence of 10–6 M Ca2+. The bound proteins were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. The molecular masses are shown on the left. S, supernatant; P, pellet. The thick 43-kDa band consists of monomeric actin (Nascimento et al., 1996). (B) The myosin-Va–actin complex recruits syntaxin-1A, and the syntaxin-1A–myosin-Va complex recruits actin. Purified brain myosin-Va was first incubated with actin or recombinant syntaxin-1A, after which the binary protein complexes were immunoprecipitated with an anti-syntaxin-1A antibody (lanes 3 and 4) and an anti-myosin-Va antibody (lanes 1 and 2). In some reactions, recombinant syntaxin-1A was added to the myosin-Va–actin binary complexes, and in others, actin was added to the myosin-Va-syntaxin-1A binary complexes. Finally, the ternary protein complex, composed of myosin-Va, syntaxin-1A, and actin, was isolated by immunoprecipitation, and myosin-Va, actin, and syntaxin-1A were detected by immunoblotting. Lanes 1 and 3, the immunoprecipitated binary complex composed of myosin-Va and either actin (lane 1) or syntaxin-1A (lane 3); lanes 2 and 4, the immunoprecipitated ternary complex formed by the addition of syntaxin-1A to the myosin-Va-actin binary complex (lane 2) or by the addition of actin to the myosin-Va-syntaxin-1A binary complex (lane 4). (C) Ca2+/actin-dependent enhancement of myosin-Va ATPase activity is completely blocked by syntaxin-1A binding. In the presence or absence of 30 μg/ml syntaxin-1A [1-262], the ATPase activity was determined in a reaction mixture containing myosin-Va (50 μg/ml) and F-actin (420 μg/ml), by calculation of the released Pi concentration (see Materials and Methods), In the presence of syntaxin-1A (closed circles), actin-activated ATPase activity was suppressed even at high [Ca2+] (pCa = 6), whereas in the absence of syntaxin-1A, the actin-activated ATPase activity remained Ca2+ dependent (open circles). (D) The motility of myosin-Va was not altered by binding of syntaxin-1A at pCa = 6. The assay of myosin-Va motility was carried out using rhodamine-phalloidin-labeled F-actin as described previously (Rock et al., 2000). Purified myosin-Va (20–30 μg/ml) was added and adsorbed to the cells for 2 min at room temperature. The flow buffer contained Ca2+ (pCa = 6) in the presence or absence of 1 μM syntaxin-1A. The average sliding velocities were measured. Each value represents the mean of 70 determinations.
Interference with Syntaxin-1A–Myosin-Va Complex Formation Inhibits Exocytosis from Chromaffin Cells
We found that the binding site of myosin-Va lies between amino acids 191 and 240 of syntaxin-1A, which comprises the first two-thirds of its H3 domain (Figure 3; A; Li and Chin, 2003). We also screened the mutated fragments derived from syntaxin-1A [191-240] without the myosin-Va-binding activity and found that the L222E mutant (syntaxin-1A [191-240 L222E]) lacks myosin-Va binding activity (Figure 3, A and B). Specifically, silver staining showed that, in the presence of Ca2+, the only protein that was unique to syntaxin-1A [191-240] was of 190 kDa (Figure 3A). This 190-kDa protein was identified as myosin-Va using mass spectrometry. A separate experiment confirmed that myosin-Va bound specifically to syntaxin-1A [191-240] but not to syntaxin-1A [191-240 L222E] (Figure 3, B and C). Furthermore, complex formation between myosin-Va and syntaxin-1A was inhibited by syntaxin-1A [191-240] but not by syntaxin-1A [191-240 L222E] (Figure 3D). We confirmed that syntaxin-1A [191-240] did not affect the SNARE complex formation (i.e., the SDS-resistant 80-kDa complex; Hayashi et al., 1995; Figure 3, E and F). Thus, the myosin-Va-binding fragment [191-240] inhibits the association of myosin-Va and syntaxin-1A.
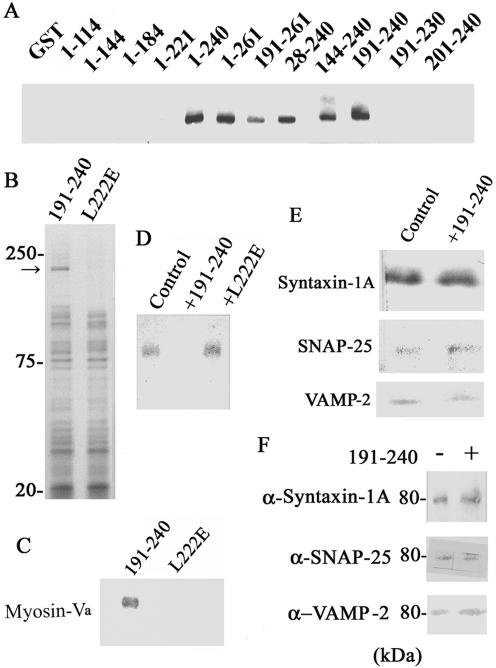
Figure 3.
Inhibition of myosin-Va–syntaxin-1A complex formation by the myosin-V-binding fragment (syntaxin-1A [191-240]) reduces the frequency of exocytosis from chromaffin cells. (A) The myosin-Va binding site on syntaxin-1A is localized in the first two-thirds (amino acids 191–240) of its H3 domain. Various GST-syntaxin-1A constructs were incubated with purified brain myosin-Va, and myosin-Va binding was detected by immunoblotting. The numbers indicate the residue numbers encoded by the GST–syntaxin-1A constructs. (B) Ca2+-dependent binding of brain proteins to syntaxin-1A [191-240] and syntaxin-1A [191-240 L222E]. The immobilized GST-syntaxin-1A [191-240] or GST-syntaxin-1A [191-240 L222E] was incubated with the brain homogenate, and bound proteins were eluted by PreScission protease (GE Healthcare), and detected by SDS-PAGE and silver staining. The molecular masses are shown in kilodaltons on the left. Only binding of the 190-kDa protein, identified as myosin-Va, was different between the two forms of syntaxin-1A. (C) Syntaxin-1A [191-240] but not syntaxin-1A [191-240 L222E] binds myosin-Va. Syntaxin-1A [191-240] or syntaxin-1A [191-240 L222E] were incubated with brain homogenate, after which syntaxin-1A was immunoprecipitated, and bound myosin-Va was detected by immunoblotting. (D) Competitive inhibition of myosin-Va binding to syntaxin-1A by syntaxin-1A [191-240]. Immobilized GST-syntaxin-1A [1-262] was incubated with chromaffin cell lysate, 0.1 mM Ca2+, and no addition (Control), 10 μM syntaxin-1A [191-240] (+191-240), or 10 μM syntaxin-1A [191-240 L222E] (+L222E). Bound myosin-Va was detected by immunoblotting. (E) Syntaxin-1A [191-240] does not inhibit formation of the SNARE complex. GST-syntaxin-1A [1-262] (0.2 μM) was incubated for 2 h at 4°C with SNAP-25 (0.2 μM) or VAMP-2 (0.2 μM) in the absence (Control) or presence of 10 μM syntaxin-1A [191-240] (+191-240). The formed SNARE complex was immobilized on glutathione-Sepharose and analyzed by SDS-PAGE, followed by silver staining. (F) Syntaxin-1A [191-240] does not disrupt preformed SNARE complexes. Isolated SNARE complex was incubated with and without syntaxin-1A [191-240] and then analyzed by immunoblotting for syntaxin-1A (α-Syntaxin), SNAP-25 (α-SNAP-25), or VAMP-2 (α-VAMP-2).
Chromaffin cells are a typical model system for analyzing exocytosis, and they are more easily studied than other systems such as central neurons (Burgoyne and Morgan, 2003). Moreover, it is the most suitable system for examining whether a biochemical interaction plays a physiological role in exocytosis (Fisher et al., 2001; Ohyama et al., 2002; Quetglas et al., 2002). For these reasons, we used chromaffin cell exocytosis to investigate the function of the Ca2+-dependent interaction between syntaxin-1A and myosin-Va. Amperometric measurements were used to examine the physiological role of myosin-Va–syntaxin-1A binding because it is a powerful method not only for quantitative measurement of exocytosis but also for characterizing the mechanism of exocytosis (Segre et al., 2000; Fisher et al., 2001). We therefore performed an amperometric assay of catecholamine release for dense-core vesicles in chromaffin cells (Ohyama et al., 2002; Quetglas et al., 2002), which are known to possess myosin-Va (Rosé et al., 2003). We found that the syntaxin-1A [191-240] specifically reduced the exocytotic frequency, whereas syntaxin-1A [191-240 L222E] fragment had no effect (Figure 4, A and B).
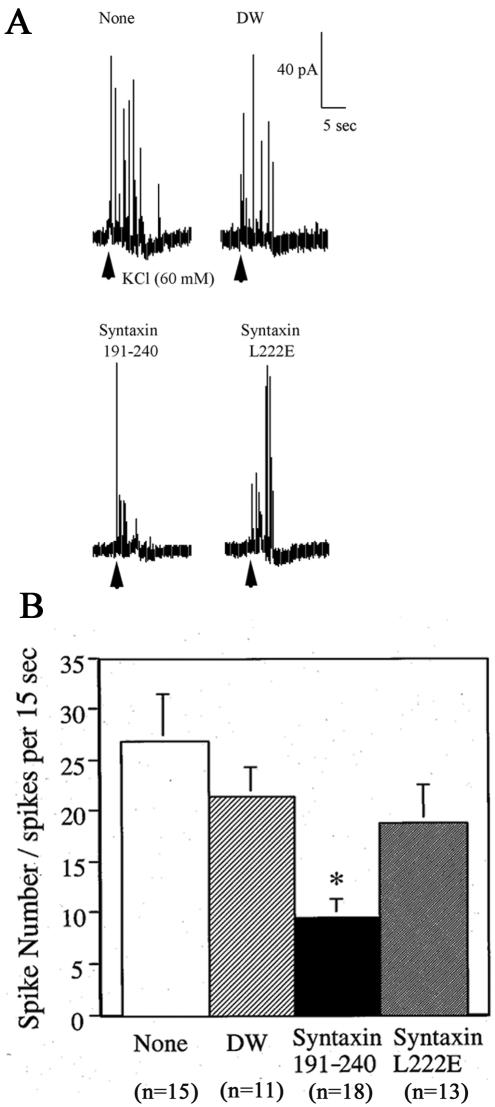
Figure 4.
Effects of the myosin-Va binding fragment of syntaxin-1A on catecholamine release from chromaffin cells as determined by amperometry. Chromaffin cells were microinjected with syntaxin-1A fragments with potent (syntaxin-1A [191-240]) or lacking potent (syntaxin-1A [191-240 L222E]) myosin-Va-binding activity and then stimulated for 4 s with 60 mM KCl. As controls, cells also were microinjected with distilled water (DW) or not injected (none). (A) Amperometric wave patterns. (B) Number of spikes of catecholamine release. The values represent the means ± SEM, and the numbers of determinations (n) were as follows: None, 15; DW, 11; syntaxin-1A [191-240], 18; and L222E syntaxin-1A, 13. The asterisk (*) represents a significant difference between the results using the two syntaxin-1A fragments (p < 0.05).
Syntaxin-1A Binds to the Neck Domain of myosin-Va after Ca2+-dependent CaM Release
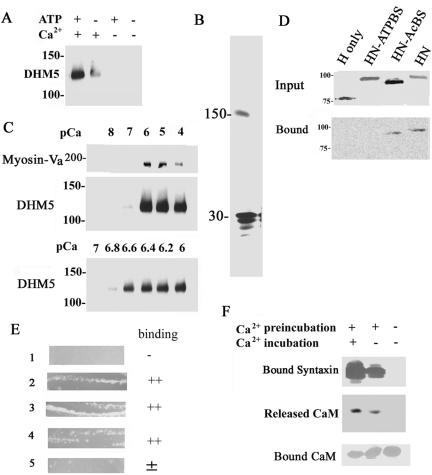
All of the known membrane-associated myosin-Va-binding proteins bind to its globular tail (Reck-Peterson et al., 2000). Therefore, to investigate where on myosin-Va syntaxin-1A binds, we first examined the binding of syntaxin-1A to DHM5, a recombinant myosin-Va protein the lacks the globular tail (Homma et al., 2000). DHM5 did not bind VAMP-2 (our unpublished data) because it lacks a VAMP-2-binding site (Ohyama et al., 2001). Surprisingly, DHM5 bound syntaxin-1A in the presence of Ca2+ (Figure 5A). The Ca2+ dependence of DHM5 binding by syntaxin-1A was similar to that of native brain myosin-Va (Figures 1C and 5B), and the minimal requirement of Ca2+ for binding was pCa = 6.6 (Figure 5C). In addition, using truncated myosin-Va produced by in vitro translation, we confirmed that the whole head-and-neck portion could bind to syntaxin-1A in the presence of Ca2+ and ATP. Removal of the neck region resulted in a substantial loss of syntaxin-1A binding. In addition, a head-and-neck fragment lacking the actin binding site still bound syntaxin-1A, but binding was absent when the ATP binding site was deleted (Figure 5D). Further studies in a bacterial two-hybrid assay, which is a modification of the yeast two-hybrid method, indicated that the six IQ-motifs of the neck bind to syntaxin-1A as well as CaM, although the first IQ alone does not mediate binding (Figure 5E). Collectively, these results demonstrate that the binding site for syntaxin-1A is in the neck of myosin-Va.
Figure 5.
The syntaxin-1A-binding site is located in the neck domain of myosin-Va. (A) Recombinant myosin-Va without the globular tail (DHM5) can bind syntaxin-1A in a Ca2+-dependent manner in the presence of and MgATP. DHM5 was incubated with GST-syntaxin-1A in the presence and absence of 10–6 M Ca2+ and with or without MgATP. DHM5 binding was detected by immunoblotting with an antibody against anti-myosin-Va head. The binding specificity of the recombinant tailless myosin-Va (DHM5) is similar to the full-length brain myosin-Va (see Figure 1D). (B) Syntaxin-1A binds to immobilized tailless myosin-Va (DHM5). Syntaxin-1A [1-262] (30-kDa) was incubated with Ni2+-NTA-immobilized His6-DHM5 in the presence of 10–6 M Ca2+ (see Figure 1C) and eluted with SDS-sample buffer. The eluted sample was separated by SDS-PAGE and silver stained. The 150-kDa band is DHM5. (C) The binding of truncated myosin-Va (DHM5) by syntaxin-1A requires a pCa of 6.6. Syntaxin-1A [1-262] was incubated with DHM5 and the purified myosin-Va from brain in the presence of various concentrations of Ca2+. Binding of myosin-Va and DHM5 was detected by immunoblotting with an antibody against myosin-Va. Top and middle, dose response of Ca2+ for binding of syntaxin-1A by DHM5 between pCa 4 and 8. Brain myosin-Va (Myosin-V) and DHM5 show a similar dependence on Ca2+ for the binding of syntaxin-1A. Bottom, dose response of Ca2+ for binding of syntaxin-1A by DHM5 between pCa 6 and 7. (D) The neck domain of myosin-Va is necessary for syntaxin-1A binding. Truncated forms of myosin-Va were produced by biotin-labeled in vitro translation of mouse myosin-Va cDNA (dilute): H, head domain only (amino acids 1–755); HN-ATPBS, head and neck domains (amino acids 1–911) lacking the ATP-binding site (amino acids 164–171); HN-AcBS, head and neck domain lacking the actin-binding site (amino acids 643–666); and HN, head and neck domains (Espreafico et al., 1992). Top, in vitro-translated proteins. Bottom, binding of these constructs to syntaxin-1A in the presence of 10–6 M Ca2+ and 0.5 mM ATP. Unlike the head domain alone, the head-and-neck portion can bind to syntaxin-1A. Note that the ATP-binding site but not the actin-binding site is also necessary for the binding. The biotin-labeled in vitro-translated proteins were visualized by the streptavidin-conjugated alkaline phosphatase. (E) A bacterial two-hybrid assay (BacterioMatch) reveals that the neck domain of myosin-Va contains the syntaxin-1A-binding site. Reporter strains of Escherichia coli (Stratagene) were cotransfected with myosin-Va and syntaxin-1A constructs and incubated at 30°C for 24 h. Lane 1, pBT+pTRG (manufacturer's negative control); lane 2, pBT-LGF2 + pTRG-GAL11p (manufacturer's positive control); lane 3, pBT-6IQ (amino acids 764–908 of mouse myosin-Va) + pTRG-CaM; lane 4, pBT-6IQ + pTRG-syntaxin-1A [1-262]; lane 5, pBT-1IQ (amino acids 764–787 of mouse myosin Va) + pTRG-syntaxin-1A [1-262]. Lanes 2–4 were judged to be the binding-positive plates. (F) Ca2+ requirement for binding of syntaxin-1A to myosin-V is due to Ca2+-dependent release of CaM from the neck domain of myosin-Va. Immobilized His6-DHM5, which copurified with CaM from Sf9 cells (Homma et al., 2000), was first preincubated for 1 h with phosphate-buffered saline (PBS) containing 10–6 M Ca2+ (Ca2+-phosphate-buffered saline; +) or with Ca2+-free PBS (–). The His6-DHM5-CaM complex was then incubated for another 1 h with syntaxin-1A in Ca2+-phosphate-buffered saline (+) or Ca2+-free PBS (–). Top, detection of syntaxin-1A binding by immunoblotting. After the preincubation with Ca2+, which released CaM from DHM5, Ca2+ was no longer necessary for the binding of syntaxin-1A by DHM5. Immunoblotting for CaM confirmed that, after the preincubation with Ca2+, CaM was released into the supernatant (middle) and no longer bound to DHM5 (bottom).
CaM is known to be released from the neck of myosin-Va in the presence of micromolar Ca2+ (Cameron et al., 1998; Homma et al., 2000). We confirmed that preincubation of myosin-Va with Ca2+ released the bound CaM (Figure 5F). In addition, after this treatment, syntaxin-1A could bind myosin-Va in the absence of Ca2+ (Figure 5F). Thus, we suspected that the Ca2+-dependent syntaxin-1A-myosin-Va binding is due to the Ca2+-dependent release of CaM from the myosin-Va neck; in other words, the binding of syntaxin-1A occurs at the same site as CaM.
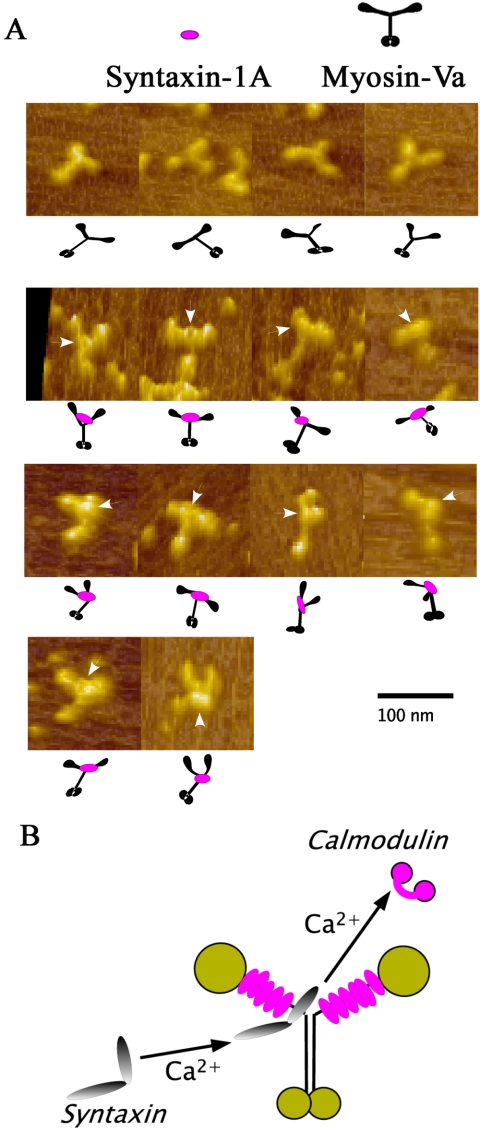
Next, we directly visualized syntaxin-1A-myosin-Va binding by AFM, a new technology for imaging biological molecules at nanometer resolution (Horber and Miles, 2003). The AFM studies show that syntaxin-1A binding occurs between two heads of the myosin dimer and not in the head or tail (Figure 6, A and B; Cheney et al., 1993). Similar to these AFM findings, rotary shadowing views of this complex reveal that the binding site was between the two heads and distinct from the head or the tail (Katayama, Watanabe, and Igarashi, unpublished observations). This is also the first report that the IQ-motif binds proteins other than the myosin light chains or CaM family proteins (Cheney et al., 1993; Vale, 2003). Homma et al. (2000) suggested that Ca2+-dependent CaM release most likely occurs at the sixth IQ motif. Our AFM results provide further support for this possibility because they showed that syntaxin-1A binds close to bifurcation of the neck region of myosin-Va (Figure 6, A and B).
Figure 6.
(A) AFM views of native myosin-Va alone (four views in the top row of images) and syntaxin-1A-bound myosin-Va (14 views in the next four rows). The 14 views of the complex show that the bound syntaxin-1A is located around the bifurcation of the two necks, forming a knot-like structure (arrowheads) that can be clearly distinguished from the heads and the globular tails. Illustrations depicting the arrangement of syntaxin-1A and the heads and tails of myosin-Va are shown under each micrograph. Bars, 50 nm. (B) Putative model of the complex between myosin-Va and syntaxin-1A. The neck region of myosin-Va senses the Ca2+ elevation its neck and exchanges one CaM molecule for one syntaxin-1A molecule.
Myosin-Va Can Bind to the SNARE Complex via Syntaxin-1A
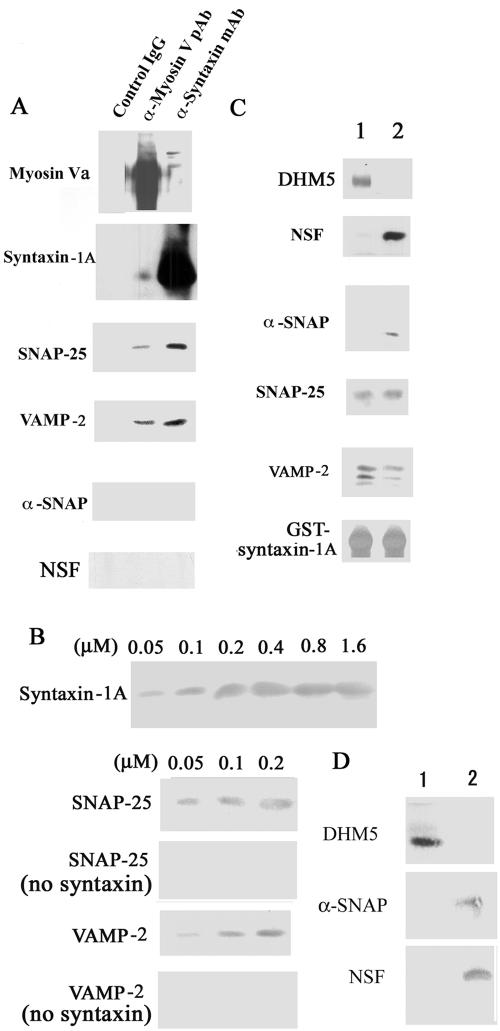
Immunoprecipitation from brain homogenate further showed that the myosin-Va–syntaxin-1A complex bound SNAP-25 and VAMP-2, two neuronal SNAREs involved in exocytosis (Figure 7A). Because VAMP-2 binds to the tail of myosin-V (Prekeris and Terrian, 1997; Ohyama et al., 2001), we examined whether the SNARE complex can be bound by a complex between syntaxin-1A and DHM5, the truncated form of myosin-Va lacking a tail (Figure 5, B and C). We first confirmed that the binding of syntaxin-1A to DHM5 saturated at a 1:1 ratio. At concentrations below saturation, VAMP-2 and SNAP-25 bound to the DHM5–syntaxin-1A complex quantitatively (Figure 7B). Immunoprecipitation further showed that the myosin-Va–syntaxin-1A complex did not associate with NSF or α-SNAP, proteins that dissociate the SNARE complex (Duman and Forte, 2003; Figure 5A), and reconstitution studies revealed that, in the presence of VAMP and SNAP-25, syntaxin-1A associates with either α-SNAP/NSF or DHM5 (Figure 7C). Similarly, we found that the SNARE complex interacts with either α-SNAP/NSF or DHM5 (Figure 7D). These results demonstrate that myosin-Va can bind the SNARE complex including VAMP-2 and SNAP-25 and that NSF/α-SNAP can release myosin-Va from the SNARE complex.
Figure 7.
Myosin-Va interacts with the SNARE complex via syntaxin-1A. (A) The myosin-Va–syntaxin-1A complex contains other SNAREs. The myosin-Va–syntaxin-1A complex was immunoprecipitated with an anti-syntaxin-1A antibody (α-syntaxin monoclonal antibody), an anti-myosin-Va antibody (α-Myosin-V pAb), or a control IgG from a pool of myosin-Va and syntaxin-1A-enriched fractions, which were obtained by 5–40% sucrose density gradient fractionation of hypotonically treated and Triton X-100-solubilized brain P2 fraction. The immunoprecipitated complexes were analyzed by immunoblotting. (B) Reconstitution study using recombinant SNAREs. Protein binding was assessed by immunoblotting. Top, His6-DHM5 (0.2 μM of dimer) immobilized on Ni2+-NTA resin was incubated with 0.05–1.6 μM of recombinant syntaxin-1A in the presence of 10–6 M Ca2+, and syntaxin-1A binding was assessed by immunoblotting DHM5 binding saturated at 0.2 μM syntaxin-1A. Bottom four, His6-DHM5-syntaxin-1A complex was formed with 0.05, 0.1, or 0.2 μM of syntaxin-1A and then incubated with the equal concentrations of SNAP-25 or VAMP-2. Controls contained no syntaxin-1A. Bound SNAP-25 and VAMP-2 were eluted with SDS-sample buffer and detected by immunoblotting. Note that SNAP-25 and VAMP-2 did not bind to myosin-Va in the absence of syntaxin-1A. (C) Reconstitution study using recombinant SNAREs, NSF, and α-SNAP. Immobilized GST-syntaxin-1A (0.2 μM) was mixed with (lane 1) or without (lane 2) DHM5 (0.2 μM dimer), and in the presence of 10–6 M Ca2+. NSF was incubated with α-SNAP for 2 h at 4°C to form a complex. This NSF–α-SNAP complex was mixed with VAMP-2 and SNAP-25 and then incubated for 2 h with GST-syntaxin-1A-DHM5 in the presence of 10–6 M Ca2+. Proteins bound to syntaxin-1A were visualized by immunoblotting. DHM5 bound to syntaxin-1A in the presence of SNAP-25 and VAMP-2 but not in the presence of NSF/α-SNAP. (D) The SNARE complex interacts with either α-SNAP/NSF or DHM5. The SNARE complex (0.2 μM) was formed as in B and C and then incubated for 1 h with α-SNAP/NSF (0.2 μM). This complex was then incubated for 1 h with DHM5 (1 μM) in the presence of 10–6 M Ca2+ (lane 1). Alternatively, DHM5 (0.2 μM) was first incubated with the SNARE complex followed by α-SNAP/NSF (1 μM) (lane 2). Bound proteins were detected by immunoblotting.
Anti-Myosin-V Neck Antibody, Which Blocks the Interaction between Myosin-V and Syntaxin-1A, Affects the Late Step of Exocytosis
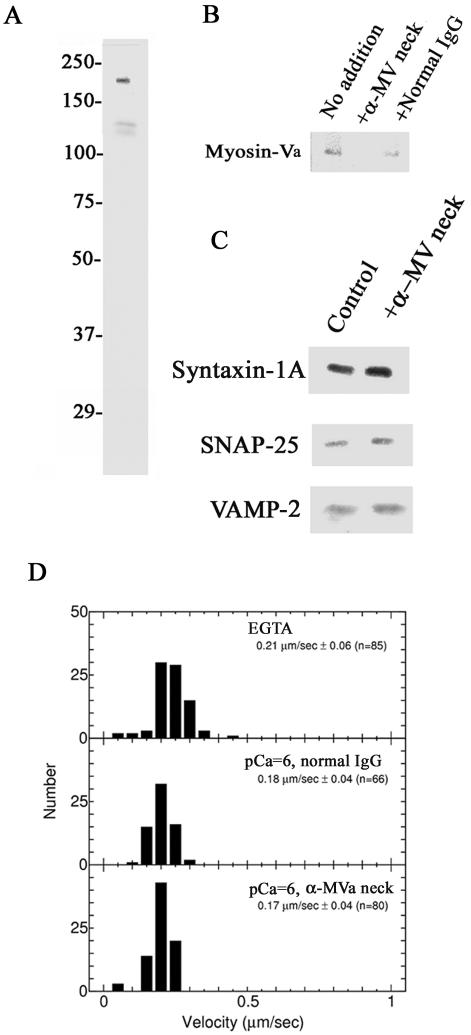
We generated an antibody specific to the neck domain of myosin-V (Figure 8A). This antibody inhibits the myosin-Va–syntaxin-1A interaction as effectively as syntaxin-1A [191-240] (Figure 8B). The antibody did not affect formation of the SNARE complex (Figure 8C) nor did it significantly reduce the sliding velocity of myosin-Va (0.24 ± 0.15 μm/s; n = 70; p > 0.1 based on Student's t test; Figure 8D; see also Figure 2D).
Figure 8.
Inhibition of myosin-Va–syntaxin-1A complex formation by anti-myosin-Va neck antiserum reduces the frequency of exocytosis from chromaffin cells. (A) Characterization of the myosin-V-neck antiserum by immunoblotting. The antiserum recognized the 190-kDa myosin-V and weakly detected (<10% of the 190-kDa protein), its 130-kDa proteolytic fragment. (B) Anti-myosin-V neck antiserum inhibits the binding of myosin-Va by syntaxin-1A. Immobilized GST-syntaxin-1A [1-262] was incubated with chromaffin cell lysate and 1:200 anti-myosin-Va neck antiserum (+α-MV neck antibody), normal rabbit serum (+Normal serum), or no serum (No addition) in the presence of 1 μM Ca2+. Bound myosin-Va was detected by immunoblotting using an anti-myosin-Va globular tail antibody. (C) SNARE complex formation is not affected by the anti-myosin-Va neck antibody. Experiments were carried out in the absence (Control) or presence of 1:200 anti-myosin-Va neck antibody (+α-MV neck antibody) as described in B. (D) The anti-myosin-Va neck antibody does not alter myosin-Va-based sliding velocity. Velocity measurements were carried out as described in Figure 2D in the presence of 1:200 anti-myosin-Va neck antibody (bottom; n = 80) or normal IgG (middle; n = 66) at pCa = 6. These results were similar to those obtained in the presence of the Ca2+ chelator EGTA and no added antibody (top; n = 85).
Like syntaxin-1A [191-240], the neck-specific antibody reduced the exocytotic frequency as measured by amperometry (Figure 9, A–C). In this particular experiment, we stimulated the cells for 5 min and analyzed the exocytotic frequency in the initial (0–1 min) and sustained phases (1–5 min) to detect in which step the syntaxin-1A–myosin-Va interaction is involved. The anti-myosin-Va neck antibody reduced the total number events during the full 5 min of stimulation (Figure 9B). Interestingly, the reduction of the event frequency was predominant not in the initial phase but in the sustained phase (Figure 9C).
Figure 9.
Effect of the anti-myosin-Va neck antibody on amperometric measurements. Amperometric analysis of the exocytotic response of chromaffin cells to a 5-min stimulation with 60 mM KCl in the absence (Control) and presence of anti-myosin-Va neck antibody (α-MV neck) or normal mouse IgG (Normal IgG). (A) Amperometric patterns and the (B) total frequency of exocytotic events in response to the 5-min stimulation with 60 mM KCl. (C) Amperometric analysis of the event frequency in the initial and sustained phase of the response reveals that anti-myosin-Va neck antibody predominantly affects the sustained phase. The values represent the means ± SEM (n = 11 [Control]; n = 13 [α-MV neck]; and n = 16 [Normal IgG]). Open bars, control; closed bars, anti-myosin-Va neck antibody; hatched bars, no addition. A significant difference was obtained only for the sustained phase (1–5 min). Asterisks (*) in B and C indicate significant differences between the results with the control and anti-myosin-Va neck antibodies (p < 0.05).
To further define at which step this interaction is involved, chromaffin cells were injected with the anti-myosin-Va neck antibody and stimulated with high K+ for 5 min. This stimulation was for a much longer time than we used for studies of inhibition by syntaxin-1A [191-240] (4 s; Figure 4). The wave patterns reveal that the anti-neck antibody inhibited exocytosis (Figure 9A). Also, the sum of events over the entire 5-min period shows that anti-myosin-Va neck antibody significantly reduced exocytosis compared with the normal IgG (Figure 9B). Interestingly, exocytotic release during the first minute (0–1 min; initial phase) was not affected by the anti-myosin-Va neck antibody, but this antibody severely attenuated exocytosis during the next 4 min (1–5 min; sustained phase) (Figure 9C).
DISCUSSION
The current model of exocytosis, based on the SNARE mechanism (Duman and Forte, 2003), does not completely account for the fact that Ca2+ is required at several steps of vesicular recycling (Burgoyne and Morgan, 2003). In this study, we found a submicromolar Ca2+-dependent interaction between myosin-Va, a putative molecular motor for synaptic vesicles, and syntaxin-1A, a neuronal membrane t-SNARE. We also presented evidence that this interaction contributes to the regulation of exocytosis in chromaffin cells. Our current study is the first clear and detailed demonstration that the Ca2+-dependent binding site for syntaxin-1A is neck rather than its tail of myosin-Va.
Is the Interaction between Myosin-Va and Syntaxin-1A Physiologically Important for Exocytotic Regulation?
We applied two probes to inhibit this interaction specifically: the myosin-Va-binding fragment (syntaxin-1A [191-240]), and an anti-myosin-Va neck antibody. We found that the exocytotic frequency was reduced by both probes, indicating that they inhibited the association of myosin-Va and syntaxin-1A. Furthermore, these results confirmed this interaction participates in the regulation of exocytosis.
We next asked in which step of exocytosis this interaction functions. Exocytotic vesicles are classified into readily releasable and reserve pools. The readily released pool is released first, and the reserve pool is released after the former is depleted (Rettig and Neher, 2002). In amperometric analysis, the frequency of the exocytotic response in the initial phase corresponds to the number of docked or readily releasable vesicles, and the frequency in the sustained phase represents the release of the newly recruited vesicles (Kumakura et al., 2004). The pronounced inhibition of the frequency in the sustained phase by the anti-myosin-Va neck antibody indicates that the interaction between myosin-Va and syntaxin-1A affects the recruitment of vesicles to the readily releasable pool. Therefore, these results, together with the fact that myosin-Va is a cargo-conveying motor molecule (Reck-Peterson et al., 2000), suggest that the interaction between myosin-Va and syntaxin-1A affects the process of vesicle mobilization from the reserve pool (i.e., replenishment of the docked vesicle pool).
As in trafficking via the Golgi apparatus, we anticipate that the exocytotic vesicle tethering process is mediated by a long coiled-coil protein that regulates the vesicle-target membrane distance at a point before fusion (Li and Chin, 2003; Gillingham and Munro, 2003). Myosin-V, which has a long coiled-coil shaft, is likely involved in this process (Cheney et al., 1993). Myosin-Va on the vesicles binds to syntaxin-1A at the plasma membrane, and, along with other putative tethering molecules (i.e., Rab proteins and/or the exocyst complex), induces vesicular tethering and exocytosis. It also is thought that myosin-VI, a minus-end motor, plays a role in endocytosis (Hasson, 2003). Thus, as depicted in Figure 10, it is plausible that myosin-Va, a plus-directed motor (Cheney et al., 1993), is involved in exocytotic events. This possibility is strongly supported by a very recent report that movements of insulin-containing dense-core secretory vesicles along the cortical actin network depend on myosin-Va and are essential for regulated exocytosis (Varadi et al., 2005). In addition, syntaxin-1 is localized close to the site of exocytosis (Stanley et al., 2003; Ohara-Imaizumi et al., 2004), where it could participate in the process of exocytosis by interacting with myosin-Va.
Figure 10.
Putative model summarizing the results. Myosin-Va, which mediates F-actin-dependent conveyance to the plasma membrane (cf. Giner et al., 2005), is on the surface of the vesicle and binds to syntaxin-1A when the intracellular [Ca2+] increases to 0.3–0.4 μM. After the complex between myosin-Va and syntaxin-1A tethers the vesicle to the membrane, it recruits other SNARE proteins to form the SNARE complex. A second larger elevation in [Ca2+] induces vesicular fusion (Rettig and Neher, 2002) and stimulates the exchange of myosin-Va for NSF/α-SNAP (Our unpublished data; see Figure 5) in the SNARE complex. Myosin-VI, a reverse motor, mediates endocytosis, whereas myosin-Va, an orthotropic motor, probably participates in exocytosis.
A previous report indicated that hippocampal slices of dilute lethal mice, which lack myosin-Va, do not show a significant defect in glutamate release (Schnell and Nicoll, 2001), which seems to contradict our current results. This may be due to differences in the myosin-Va dependence of glutamate and catecholamine release from dense-core vesicles. Despite this contradictory data, our findings are consistent with recent reports that retinal neurotransmitter release is abnormal in dilute lethal mice (Libby et al., 2004) and that dilute lethal mutant mice have a defect in basal neurotransmitter release and presynaptic plasticity (Trinchese et al., 2003). This latter report used cultured neurons derived from 1-d-old mice, whereas Schnell and Nicoll (2001) used tissue slices obtained from 12- to 19-d-old mice, suggesting that the differences may be due to the age of the mice used in the studies. Furthermore, according to a recent detailed immunohistochemical study the brain (Tilelli et al., 2003), the hippocampus does not express much myosin-Va, and most of the myosin-Va immunoreactivity is associated with the neuronal cell bodies rather than the neuropila, which are enriched with synaptic terminals. Thus, differences in the levels of myosin-Va in hippocampal synapses mutant between wild-type and the dilute lethal may be subtle. Finally, other proteins, such as additional myosin-V isoforms, may compensate for the loss of myosin-Va function in the dilute lethal hippocampal slices (Schnell and Nicoll, 2001; Vale, 2003), but not in cell culture.
CaM-dependent Regulation of Exocytosis through Ca2+-dependent Interaction between Myosin-Va and Syntaxin-1A
CaM binds to the myosin-Va neck as a light chain via its IQ-motifs (Cheney et al., 1993) but is released when the intracellular Ca2+ rises to micromolar concentrations (Cameron et al., 1998; Homma et al., 2000). We found that, after Ca2+-dependent release of CaM, syntaxin-1A can bind myosin-Va even in the absence of Ca2+. Thus, we suspected that the apparent Ca2+ dependence of myosin Va-syntaxin-1A binding is due to Ca2+-dependent release of CaM from the myosin-Va neck, and exposure of an otherwise concealed syntaxin-1A binding site. Our results further indicate that CaM can sense submicromolar Ca2+ through the release of CaM from myosin-Va (Cameron et al., 1998; Homma et al., 2000); the binding between myosin-Va and syntaxin-1A requires at least 0.3 μM intracellular Ca2+ (Figure 4B), which corresponds to the level of Ca2+ when secretory granules enter the readily releasable pool (Burgoyne and Morgan, 2003).
CaM, the most abundant Ca2+-sensitive protein, may be widely responsible for micromolar Ca2+ sensitivity (Burgoyne and Clague, 2003). We demonstrated previously that exocytosis is regulated by Ca2+/CaM-dependent protein kinase II (CaMKII), which binds to syntaxin-1A in a Ca2+-dependent manner when it is autophosphorylated (Ohyama et al., 2002). Although the CaM-binding sites of myosin-Va and CaMKII are distinct (Bähler and Rhoads, 2002), their Ca2+-dependencies for syntaxin-1A binding are very similar. Our current studies also could explain the involvement of CaM in exocytosis (Sakaba and Neher, 2001) and other CaM-dependent interactions (Junge et al., 2004).
CONCLUSIONS
Our results demonstrate that submicromolar Ca2+ concentrations induce the 1:1 binding of syntaxin-1A to the myosin-Va neck. This further suggests that syntaxin-1A might mimic CaM for binding at the vacant IQ-motifs. Our results suggest that this complex modulates SNARE-dependent interactions and regulates at least the exocytosis of dense-core vesicles by forming a link between vesicles and their targets; in other words, the complex regulates the recruitment of the vesicles to the readily releasable pool during sustained secretion. Recently, the Ca2+/CaM-dependent interaction between the cargo-conveying tail and the head of myosin-Va was shown to be an important factor regulating its conformation (Krementsov et al., 2004; Li et al., 2004, 2005; Wang et al., 2004). Ca2+/CaM is an important regulator of both exocytosis and the cargo-conveying activity of myosin-Va, and further studies of this novel interaction should help elucidate the roles of myosin-Va in Ca2+-regulated exocytosis.
Acknowledgments
We thank all of the donors for the cDNAs and the antibodies; E. Akaishi-Onodera and M. Sato-Igarashi for technical assistance; and T. Abe, T. Ando, O. Arancio, P. C. Bridgman, E. Katayama, K. Hoshino, M. Norita, and M. Takahashi for helpful discussions. This work was supported by Grants-in-Aid from the Ministry of Education, Sciences, Culture, Sports, and Technology of Japan (#15029218, #16015240, #16044216, and #17023019 to M. I.); the Japan Society for the Promotion of Sciences (#15300123 to M. I.); the Life Science Foundation (to M. I.); the Brain Science Foundation (to M. I.); the Yujin Memorial Foundation (to M. I.); the project-promoting grant from Niigata University (to M. I.); and the Tsukada Milk Foundation for Medical Research (to M. W.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–03–0252) on July 19, 2005.
Abbreviations used: AFM, atomic force microscopy; CaM, calmodulin; CaMKII, Ca2+/CaM-dependent protein kinase II; GST, glutathione S-transferase; RU, resonance units.
References
- Bähler, M., and Rhoads, A. (2002). Calmodulin signaling via the IQ motif. FEBS Lett. 513, 107–113. [DOI] [PubMed] [Google Scholar]
- Bridgman, P. C. (1999). Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex. J. Cell Biol. 146, 1045–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne, R. D., and Clague, M. J. (2003). Calcium and calmodulin in membrane fusion. Biochim. Biophys. Acta 1641, 137–143. [DOI] [PubMed] [Google Scholar]
- Burgoyne, R. D., and Morgan, A. (2003). Secretory granule exocytosis. Physiol. Rev. 83, 581–632. [DOI] [PubMed] [Google Scholar]
- Cameron, L. C., Carvalho, R. N., Araujo, J. R., Santos, A. C., Tauhata, S. B., Larson, R. E., and Sorenson, M. M. (1998). Calcium-induced quenching of intrinsic fluorescence in brain myosin V is linked to dissociation of calmodulin light chains. Arch. Biochem. Biophys. 355, 35–42. [DOI] [PubMed] [Google Scholar]
- Cheney, R. E. (1998). Purification and assay of myosin V. Methods Enzymol. 298, 3–18. [DOI] [PubMed] [Google Scholar]
- Cheney, R. E., O'Shea, M. K., Heuser, J. E., Coelho, M. V., Wolenski, J. S., Espreafico, E. M., Forscher, P., Larson, R. E., and Mooseker, M. S. (1993). Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell 75, 13–23. [DOI] [PubMed] [Google Scholar]
- Chifflet, S., Torriglia, A., Chiesa, R., and Tolosa, S. (1988). A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: application to lens ATPases. Anal. Biochem. 168, 1–4. [DOI] [PubMed] [Google Scholar]
- Duman, J. G., and Forte, J. G. (2003). What is the role of SNARE proteins in membrane fusion? Am. J. Physiol. 285, C237–C249. [DOI] [PubMed] [Google Scholar]
- Espreafico, E. M., Cheney, R. E., Matteoli, M., Nascimento, A. A., De Camilli, P. V., Larson, R. E., and Mooseker, M. S. (1992). Primary structure and cellular localization of chicken brain myosin-V (p190), an unconventional myosin with calmodulin light chains. J. Cell Biol. 119, 1541–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, L. L. Lee, A. J., Bridgman, P. C., and Mooseker, M. S. (1998). Vesicle-associated brain myosin-V can be activated to catalyze actin-based transport. J. Cell Sci. 111, 2055–2066. [DOI] [PubMed] [Google Scholar]
- Fisher, R. J., Pevsner, J., and Burgoyne, R. D. (2001). Control of fusion pore dynamics during exocytosis by Munc18. Science 291, 875–878. [DOI] [PubMed] [Google Scholar]
- Fujita, Y., Shirataki, H., Sakisaka, T., Asakura, T., Ohya, T., Kotani, H., Yokoyama, M., Mizoguchi, A., Scheller, R. H., and Takai, Y. (1998). Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron 20, 905–915. [DOI] [PubMed] [Google Scholar]
- Gillingham, A. K., and Munro, S. (2003). Long coiled-coil proteins and membrane traffic. Biochim. Biophys. Acta 1641, 71–85. [DOI] [PubMed] [Google Scholar]
- Giner, D., Ñeco, P., del Mar Francés, M., López, I., Viniegra, S., and Giltiérrez, L. M. (2005). Real-time dynamics of the F-actin cytoskeleton during secretion from chromaffin cells. J. Cell Sci. 118, 2871–2880. [DOI] [PubMed] [Google Scholar]
- Hasson, T. (2003). Myosin VI: two distinct roles in endocytosis. J. Cell Sci. 116, 3453–3461. [DOI] [PubMed] [Google Scholar]
- Hayashi, T., Yamasaki, S., Nauenburg, S., Binz, T., and Niemann, H. (1995). Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J. 14, 2317–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl, T. M., Parlati, F., Wimmer, C., Rothman, J. E., Söllner, T. H., and Engelhardt, H. (1998). Arrangement of subunits in 20 S particles consisting of NSF, SNAPs, and SNARE complex. Mol. Cell 2, 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma, K., Saito, J., Ikebe, R., and Ikebe, M. (2000). Ca2+-dependent regulation of the motor activity of myosin V. J. Biol. Chem. 275, 34766–34771. [DOI] [PubMed] [Google Scholar]
- Horber, J. K., and Miles, M. J. (2003). Scanning probe evolution in biology. Science 302, 1002–1005. [DOI] [PubMed] [Google Scholar]
- Huttner, W. B., Schiebler, W., Greengard, P., and De Camilli, P. (1983). Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J. Cell Biol. 96, 1374–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi, M., Tagaya, M., and Komiya, Y. (1997). The SNARE complex in growth cones: molecular aspects of the axon terminal development. J. Neurosci. 17, 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge, H. J., Rhee, J. S., Jahn, O., Varoqueaux, F., Spiess, J., Waxham, M. N., Rosenmund, C., and Brose, N. (2004). Calmodulin and Munc13 form a Ca2+ sensor/effector complex that controls short-term synaptic plasticity. Cell 118, 389–401. [DOI] [PubMed] [Google Scholar]
- Karcher, R. L., Rol, J. T., Zappacosta, F., Huddleston, M. J., Annan, R. S., Carr, S. A., and Gelfand, V. I. (2001). Cell cycle regulation of myosin-V by calcium/calmodulin-dependent protein kinase II. Science 293, 1317–1320. [DOI] [PubMed] [Google Scholar]
- Kumakura, K., Sasakawa, N., Murayama, N., and Ohara-Imaizumi, M. (2004). Spatio-temporal regulation of neurotransmitter release by protein kinase C: studies in adrenal chromaffin cells. Crit. Rev. Neurobiol. 16, 173–179. [DOI] [PubMed] [Google Scholar]
- Krementsov, D. N., Krementsova, E. B., and Trybus, K. M. (2004). Myosin V: regulation by calcium, calmodulin, and the tail domain. J. Cell Biol. 164, 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L., and Chin, L. S. (2003). The molecular machinery of synaptic vesicle exocytosis. Cell. Mol. Life Sci. 60, 942–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Mabuchi, K., Ikebe, R., and Ikebe, M. (2004). Ca2+-induced activation of ATPase activity of myosin Va is accompanied with a large conformational change. Biochem. Biophys. Res. Commun. 315, 538–545. [DOI] [PubMed] [Google Scholar]
- Li, X., Ikebe, R., and Ikebe, M. (2005). Activation of myosin Va function by melanophilin, a specific docking partner of myosin Va. J. Biol. Chem. 280, 17815–17822. [DOI] [PubMed] [Google Scholar]
- Libby, R. T., Lillo, C., Kitamoto, J., Williams, D. S., and Steel, K. P. (2004). Myosin Va is required for normal photoreceptor synaptic activity. J. Cell Sci. 117, 4509–4515. [DOI] [PubMed] [Google Scholar]
- Matsui, Y. (2003). Polarized distribution of intracellular components by class V myosins in Saccharomyces cerevisiae. Int. Rev. Cytol. 229, 1–42. [DOI] [PubMed] [Google Scholar]
- Mercer, J. A., Seperack, P. K., Strobel, M. C., Copel, N. G., and Jenkins, N. A. (1991). Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature 349, 709–713. [DOI] [PubMed] [Google Scholar]
- Mizuta, R., Iwai, K., Shigeno, M., Mizuta, M., Uemura, T., Ushiki, T., and Kitamura, D. (2003). Molecular visualization of immunoglobulin switch region RNA/DNA complex by atomic force microscope. J. Biol. Chem. 278, 4431–4434. [DOI] [PubMed] [Google Scholar]
- Nascimento, A. A., Cheney, R. E., Tauhata, S. B., Larson, R. E., and Mooseker, M. S. (1996). Enzymatic characterization and functional domain mapping of brain myosin-V. J. Biol. Chem. 271, 17561–17569. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi, M., Nishiwaki, C., Kikuta, T., Kumakura, K., Nakamichi, Y., and Nagamatsu, S. (2004). Site of docking and fusion of insulin secretory granules in live MIN6 β cells analyzed by TAT-conjugated anti-syntaxin 1 antibody and total internal reflection fluorescence microscopy. J. Biol. Chem. 279, 8403–8408. [DOI] [PubMed] [Google Scholar]
- Ohyama, A., Komiya, Y., and Igarashi, M. (2001). Globular tail of myosin-V is bound to VAMP/synaptobrevin. Biochem. Biophys. Res. Commun. 280, 889–891. [DOI] [PubMed] [Google Scholar]
- Ohyama, A., et al. (2002). Regulation of exocytosis through Ca2+/ATP-dependent binding of autophosphorylated Ca2+/calmodulin-activated protein kinase II to syntaxin 1A. J. Neurosci. 22, 3342–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris, R., and Terrian, D. M. (1997). Brain myosin V is a synaptic vesicle-associated motor protein: evidence for a Ca2+-dependent interaction with the synaptobrevin-synaptophysin complex. J. Cell. Biol. 137, 1589–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quetglas, S., Iborra, C., Sasakawa, N., De Haro, L., Kumakura, K., Sato, K., Lévèque, C., and Seagar, M. (2002). Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis. EMBO J. 21, 3970–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson, S. L., Provance, D. W., Jr., Mooseker, M. S., and Mercer, J. A. (2000). Class V myosins. Biochim. Biophys. Acta 1496, 36–51. [DOI] [PubMed] [Google Scholar]
- Rettig, J., and Neher, E. (2002). Emerging roles of presynaptic protein in Ca2+-triggered exocytosis. Science 298, 781–785. [DOI] [PubMed] [Google Scholar]
- Rock, R. S., Rief, M., Mehta, A. D., and Spudich, J. A. (2000). In vitro assays of processive myosin motors. Methods 22, 373–381. [DOI] [PubMed] [Google Scholar]
- Rosé, S. D., Lejen, T., Casaletti, L., Larson, R. E., Pene, T. D., and Trifaró, J. M. (2003). Myosins II and V in chromaffin cells: myosin V is a chromaffin vesicle molecular motor involved in secretion. J. Neurochem. 85, 287–298. [DOI] [PubMed] [Google Scholar]
- Sakaba, T., and Neher, E. (2001). Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron 32, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Schnell, E., and Nicoll, R. A. (2001). Hippocampal synaptic transmission and plasticity are preserved in myosin Va mutant mice. J. Neurophysiol. 85, 1498–1501. [DOI] [PubMed] [Google Scholar]
- Segre, F., Brioso, M. A., Gómez, J. F., Machado, J. D., and Borges, R. (2000). Automatic analysis for amperometrical recording of exocytosis. J. Neurosci. Methods 103, 151–156. [DOI] [PubMed] [Google Scholar]
- Stanley, E., Reese, T. S., and Wang, G. Z. (2003). Molecular scaffold reorganization at the neurotransmitter release site with vesicle exocytosis or botulinum toxin C1. Eur. J. Neurosci. 18, 2403–2407. [DOI] [PubMed] [Google Scholar]
- Tilelli, C. Q., Martins, A. R., Larson, R. E., and Garcia-Cairasco, N. (2003). Immunohistochemical localization of myosin Va in the adult rat brain. Neuroscience 121, 573–586. [DOI] [PubMed] [Google Scholar]
- Trinchese, F., Rao, M., Peterhoff, C., Kumar, A., Liu, S., Nixon, R., and Arancio, O. (2003). Myosin Va is required for neurotransmitter release during basal synaptic transmission and synaptic plasticity. Mol. Biol. Cell 14, 179a. [Google Scholar]
- Vale, R. D. (2003). The molecular motor toolbox for intracellular transport. Cell 112, 467–480. [DOI] [PubMed] [Google Scholar]
- Varadi, A. Tsuboi, T., and Ruttner, G. A. (2005). Myosin Va transports dense core secretory vesicles in pancreatic MIN6 β-cells. Mol. Biol. Cell 16, 2670–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F., Thirumurugan, K., Stafford, W. F., Hammer, J. A., 3rd, Knight, P. J., and Sellers, J. R. (2004). Regulated conformation of myosin V. J. Biol. Chem. 279, 2333–2336. [DOI] [PubMed] [Google Scholar]