Abstract
Recently, we have shown that a cancer causing truncation in adenomatous polyposis coli (APC) (APC1–1450) dominantly interferes with mitotic spindle function, suggesting APC regulates microtubule dynamics during mitosis. Here, we examine the possibility that APC mutants interfere with the function of EB1, a plus-end microtubule-binding protein that interacts with APC and is required for normal microtubule dynamics. We show that siRNA-mediated inhibition of APC, EB1, or APC and EB1 together give rise to similar defects in mitotic spindles and chromosome alignment without arresting cells in mitosis; in contrast inhibition of CLIP170 or LIS1 cause distinct spindle defects and mitotic arrest. We show that APC1–1450 acts as a dominant negative by forming a hetero-oligomer with the full-length APC and preventing it from interacting with EB1, which is consistent with a functional relationship between APC and EB1. Live-imaging of mitotic cells expressing EB1-GFP demonstrates that APC1–1450 compromises the dynamics of EB1-comets, increasing the frequency of EB1-GFP pausing. Together these data provide novel insight into how APC may regulate mitotic spindle function and how errors in chromosome segregation are tolerated in tumor cells.
INTRODUCTION
During mitosis, spindle microtubules probe the three-dimensional space of the cell in a “search and capture” process that is important for the efficient interaction of microtubule plus ends with the cell cortex and kinetochores (reviewed in Kline-Smith and Walczak, 2004). An increase in microtubule dynamics at the onset of mitosis is believed to facilitate the timely orientation and alignment of chromosomes in metaphase. A further change in microtubule dynamics has also been proposed to contribute to the forces that segregate chromosomes and elongate the mitotic spindle in anaphase (reviewed in Scholey et al., 2003). Inhibition of microtubule dynamics by mutations in proteins that associate with microtubule plus ends or by addition of drugs leads to failures in chromosome alignment and segregation (Berlin et al., 1990; Dujardin et al., 1998; Gruss et al., 2002; Maiato et al., 2002; Rogers et al., 2002; Andrews et al., 2004; Cassimeris and Morabito, 2004; Kline-Smith and Walczak, 2004; Vaughan, 2004). Thus, properly regulating the dynamic properties of microtubules is critical for ensuring the accurate segregation of chromosomes in mitosis.
Although the changes in microtubule dynamics during mitosis are well documented, the mechanisms that control microtubule behavior are less clear. A number of microtubule-associated proteins as well as soluble factors emanating from chromosomes have been implicated in regulating microtubule dynamics, suggesting that a complex network of proteins controls microtubules during mitosis. The plus ends of microtubules are an important binding site for proteins that regulate microtubules. The so-called +TIPs family of proteins have been shown to regulate microtubule dynamics in a number of systems and include EB1, CLIP-170, CLASP, dynein, LIS1, dynactin subunit p150glued, and adenomatous polyposis coli (APC; reviewed in Karsenti and Vernos, 2001; Kline-Smith and Walczak, 2004; Vaughan, 2004). In addition to sharing a common localization at the plus ends of microtubules, these proteins typically modulate the transitions between microtubule growth and shrinkage. One of the best characterized +TIPs is EB1, whose function as an “anti-pausing” factor is well conserved. Inhibition of EB1 in a number of systems results in nondynamic microtubules that spend the majority of time in a paused state (Tirnauer et al., 1999, 2002b; Rogers et al., 2002). EB1 immunodepletion experiments in Xenopus extracts results in a dramatic reduction in microtubule length. Similarly, RNAi depletion of EB1 in Drosophila embryos results in reduced microtubule stability and disrupted mitotic spindles (Rogers et al., 2002; Tirnauer et al., 2002b). In contrast, other +TIP proteins, including LIS1, have been reported to suppress microtubule dynamics in vitro by reducing catastrophes; inhibition of LIS1 results in defective kinetochore-microtubule attachments (Faulkner et al., 2000; Coquelle et al., 2002; Tai et al., 2002). Thus, it is likely that a balance of activities at microtubule plus ends optimizes the search and capture process, ensuring that microtubule plus ends efficiently find their attachment sites.
APC can directly interact with microtubules via its basic region or can indirectly interact with microtubules via its association with the kinesin II-associated protein, KAP3a, or with the +TIP, EB1 (Nathke et al., 1996; Mimori-Kiyosue et al., 2000a, 2000b; Mogensen et al., 2002; Etienne-Manneville and Hall, 2003; Wen et al., 2004). By yeast two-hybrid and in vitro binding studies, EB1 has been shown to interact with the carboxy terminus of APC, whereas KAP3a interacts with the amino terminal armadillo domain in APC (Su et al., 1995; Jimbo et al., 2002). The binding of the carboxy terminus of APC to EB1 enhances the ability of EB1 to bind along the length of in vitro-polymerized microtubules, arguing that APC may function to “load” EB1 on microtubule plus ends (Nakamura et al., 2001). The potential physiological connection between APC and EB1 is supported by recent work showing that the interaction between these two proteins is important for the formation of stable microtubules in migrating fibroblasts (Wen et al., 2004). These findings raise the possibility that APC may regulate the activity of +TIPs, like EB1, in response to signals associated with polarized cellular events.
Interestingly, APC has also been implicated in the proper function of the mitotic spindle. APC localizes to the plus ends of kinetochore-microtubules during mitosis, suggesting it may influence microtubule stability or attachment in this context as well. Consistent with this hypothesis, ES cells lacking wild-type APC and cells expressing mutant forms of APC are susceptible to chromosome segregation errors (Fodde et al., 2001; Kaplan et al., 2001; Green and Kaplan, 2003; Dikovskaya et al., 2004; Louie et al., 2004). Recent work demonstrates that chromosome segregation and spindle positioning errors may be due to microtubule plus-end attachment defects in cells expressing mutant forms of APC (Green and Kaplan, 2003; Tighe et al., 2004). It is possible that APC mutants affect the ability of microtubules to locate their binding site, by perturbing microtubule dynamics, or alternatively by affecting the integrity of the binding site. EB1 and APC have also been implicated in spindle positioning in higher eukaryotes (Lu et al., 2001; McCartney et al., 2001; Yamashita et al., 2003). Thus, it is reasonable to hypothesize that EB1 and APC may cooperate at microtubule plus ends to regulate microtubule dynamics during mitosis.
Previously we showed that a truncated form of APC (APC1–1450), similar to the protein expressed in many colorectal cancers, dominantly compromises microtubule plus-end attachments during mitosis, resulting in a disrupted mitotic spindle and errors in chromosome segregation. In this article we investigate the molecular basis underlying this dominant phenotype. We find that inhibition of EB1 or APC causes defects remarkably similar to those observed in cells expressing APC1–1450. Inhibiting both EB1 and APC does not have an additive effect arguing that these proteins function together to regulate mitotic spindles. We find that inhibition of either APC or EB1 causes chromosome alignment defects but no mitotic arrest; in contrast, inhibition of other +TIPS result in both chromosome alignment defects and a robust mitotic arrest. Consistent with a functional relationship between APC and EB1, we find that APC1–1450 forms a hetero-oligomer with endogenous APC and that the formation of this complex precludes EB1 association with APC. Live imaging of EB1-GFP comets reveals a significant increase in the frequency of pausing in cells expressing APC1–1450. Together these results provide compelling evidence that APC regulates the mitotic spindle through EB1; furthermore, tumor-initiating APC mutations can promote chromosome alignment errors while still allowing cell division, thereby potentially contributing to chromosome instability in tumor cells.
MATERIALS AND METHODS
Plasmids and Antibodies
Regions encoding indicated fragments of human APC and a 13myc epitope tag were amplified by PCR from a previously constructed plasmid (gift from I. Nathke and Longtine et al., 1998) and were cloned into the pIND/Hygro ecdysone-inducible mammalian expression vector (Invitrogen, Carlsbad, CA). The coding region of each plasmid was confirmed by DNA sequence analysis (Davis Sequencing Facility, Davis, CA). pIK131 (EB1-GFP) construct was a generous gift from J. Tirnauer (Harvard Medical School). The APC antibody was a gift from I. Nathke. Antibodies against c-Myc (9E10, Santa Cruz Biotechnology, Santa Cruz, CA), tubulin (Tub2.1 or FITC conjugated Tub2.1, Sigma, St. Louis, MO), EB1 (BD Biosciences PharMingen, San Diego CA), LIS1 (BL894, Bethyl Laboratories, Montgomery, TX), CLIP170 (H-300, Santa Cruz Biotechnology), Bub1p (14H5, Chemicon, Temecula, CA), BubR1p (8G1, Chemicon), MAD2 (BD Transduction Laboratories, San Jose, CA) and human centromere antigens (ACA anti-centromere, Antibodies Inc., Davis, CA) were used at manufacturers recommendation.
Cell Culture and Immunofluorescence
Cells were cultured in DMEM high-glucose medium (Cellgro, Herndon, VA) supplemented with 10% fetal calf serum, 2 mg/ml l-glutamine, 1 mM sodium pyruvate, and 50 U/ml penicillin and streptomycin. Cells were maintained at 37°C and 5% CO2. After DNA transfection, using the FuGene 6 reagent (Roche, Indianapolis, IN), stable clones were selected and maintained in DMEM as above, supplemented with 200 μg/ml hygromycin (Sigma). Expression was induced, in stable cell lines, with the addition of ponasterone (5 μM; Invitrogen). Cells were grown as indicated and prepared for fixed immunofluorescence and images were collected using an Applied Precision Delta Vision Restoration, DV3.0 as previously described (Green and Kaplan, 2003). In some cases cells were treated with nocodazole or taxol, as indicated, before imaging or extract preparation.
Quantification, Measurements, and Statistical Analysis
Evaluation of spindle position and misaligned chromosomes were assessed as previously described (Green and Kaplan, 2003). Analysis of APC fragment expression levels (relative to endogenous APC) was determined by detecting endogenous APC and truncated APC fragments using α-human APC antibodies and directly comparing the Western blot signal (on the same film). Because some of the shorter APC fragments analyzed eliminate the APC epitopes that the antibodies were generated against, the shorter fragments were compared directly to APC1–1450 levels (using α-myc antibodies) to indirectly determine expression level relative to endogenous APC. Kinetochore-to-kinetochore distances were measured in deconvolved z-stacks using tools provided in the SoftWorx image analysis software (API, Issaquah, WA). EB1 comets were counted throughout the entire Z-stack for each spindle analyzed. Mitotic indices were generated by dividing the total number of mitotic cells by the total number of cells observed. In siRNA experiments, only cotransfected (GFP positive) cells were quantified. Statistical analysis of data were performed with GraphPad Prism version 3.0 software (GraphPad Software, San Diego, CA). Nonparametric grouping of data were analyzed by ANOVA and secondary analysis for significance with a Tukey or Newman-Keuls multiple-comparison test. Group comparisons were deemed significant at p < 0.05.
Immunoprecipitation and Immunoblotting
Cells were lysed in 10 mM HEPES, pH 7.2, 1% Triton X-100, 150 mM NaCl, 0.25 mM EDTA, 50 mM NaF, 50 mM β-glycerol-phosphate, 10% glycerol, and a protease inhibitor cocktail (1 mM TPCK, 10 mM phenylmethylsulfonyl fluoride, and 10 μg/ml leupeptin, pepstatin, and chymostatin), proteins were resolved on 3–8% gradient gels using recommended conditions (Invitrogen) and transferred to nitrocellulose overnight as previously described (Nathke et al., 1996). Smaller proteins (EB1) were resolved on 10% SDS-PAGE gels and transferred to nitrocellulose membranes for 6 h. For immunoprecipitation experiments, cells were lysed in 50 mM Tris-HCl at pH 7.5, 150 mM sodium chloride, 5 mM EDTA, 2 mM sodium orthovanadate, 10 mM sodium fluoride, 0.5% Triton-X100, and 10 μg/ml leupeptin, pepstatin, and chymostatin. Lysate (1–2 mg) was incubated with antibodies (0.5 μg) for 2 h at 4°C, followed by addition of GammaBind sepharose (Amersham Biosciences, San Francisco, CA) beads (30 μl) 1 h at 4°C. Beads were harvested by centrifugation and washed three times in immunoprecipitation buffer, and bound proteins were eluted by boiling in Laemmli buffer (4 min). Proteins were resolved as described above. Antibody incubations were carried out as previously described, and bound antibodies were detected using the Amersham ECL detection system (Amersham-Biosciences, Amersham, United Kingdom). Blots were exposed to film for multiple exposure times to ensure the linearity of signal. In some cases, chemiluminescence signals were detected with an Alpha-Innotech imaging system (San Leandro, CA), and light units were quantified using AlphaEaseFC software.
Live Imaging and EB1-GFP Tracking
For live imaging, cells were grown directly on glass-bottom culture dishes in DMEM (MatTek, Ashland, MA), transiently transfected with pIK131 (EB1-GFP; kindly provided by J. Tirnauer), allowed to recover for 48 h, and induced with ponasterone (5 μM) for 24 h before imaging. Media temperature was maintained at 37°C with a microincubator (Medical Systems, Greenvale, NY) fitted to the microscope stage, and pH was buffered by the addition of 10 mM HEPES, pH 7.4. Images were acquired with an Olympus microscope (Melville, NY) equipped with an Ultra-View spinning disk confocal head (Perkin Elmer-Cetus, Fremont, CA) and a 100× 1.35-numerical aperture objective. A sample thickness of 2 μm (in Z-steps of 0.5 μm) was imaged every 2–3 s (for 2–4 min) for prometaphase/metaphase cells. A sample thickness of 1 μm (in Z-steps of 0.5 μm) was imaged every 1–2 s (for 1–2 min) for interphase cells. Z-sections were projected into a single movie file for EB1-GFP tracking. Tracking was performed with software written in MatLab was utilized for EB1-GFP tracking. To measure EB1-GFP pausing we calculated the instantaneous velocity (change in distance/change in time) for each comet during each increment of time imaged. Instantaneous velocities falling below basal movement threshold were determined to be “paused,” whereas values above this threshold were determined to be “growing.” The threshold value (0.03 μm/s) was set, by visual inspection of comet life histories, where clear breaks in comet “growth” were observed (as shown in example, Figure 5B; 95% of control instantaneous velocities lie above this threshold).
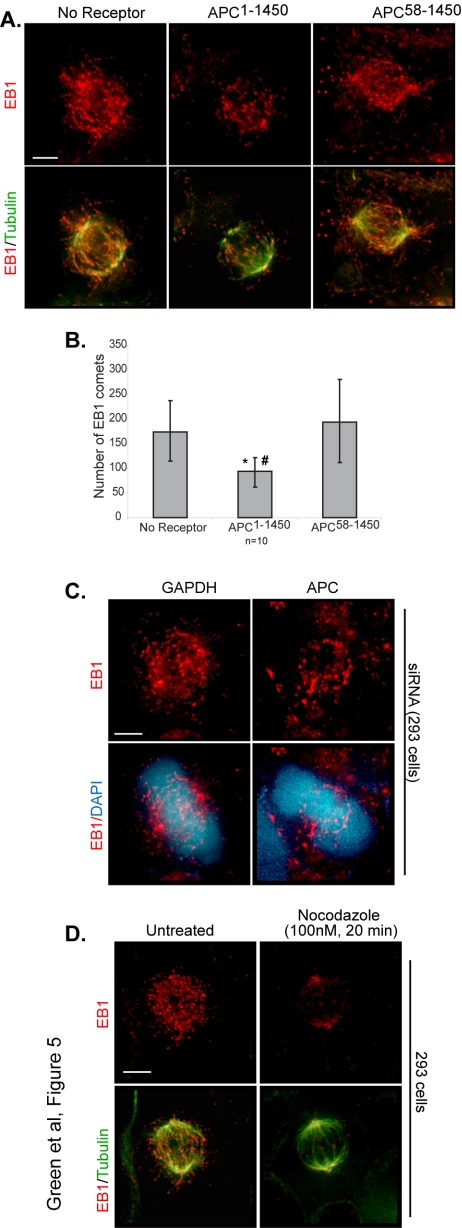
Figure 5.
EB1 does not require APC for loading. Fluorescence data were collected and optical projections are presented (15 Z-sections) of the indicated cell lines stained to visualize endogenous EB1 (red) and tubulin (green) at metaphase (A). (B) The number of EB1 comets were quantified from multiple fixed cells (n = 10). Error bars, SD. Statistical significance between no receptor and APC1–1450: *p < 0.05; statistical significance between APC1–1450 and APC58–1450: # p < 0.01. (C) EB1 (red) continues to localize to comets in APC siRNA-treated cells (48 h). (D) 293 cells were untreated or were treated with low doses of nocodazole (100 nM, 20 min) to disrupt microtubule dynamics, fixed, and imaged for EB1 (red) comet distribution. Scale bars, (D and E) 5 μm.
siRNA
293 cells were transfected using siLentFect (Bio-Rad, Hercules, CA) with APC1 Silencer Validated siRNA (Ambion, Austin, TX) at a final concentration of 100 nM, with EB1 siRNA (synthesized by Dharmacon, Lafayette, CO; sequence previously described by Wen et al., 2004), with 100 nM CLIP-170 siRNA (sequence previously described in Lansbergen et al., 2004), with 100 nM Lis1 siRNA (sequence previously described in Shu et al., 2004), or with 100 nM GAPDH siRNA (Silencer siRNA control, Ambion). For double siRNA experiments, cells were transfected simultaneously with the indicated siRNA at the same concentrations. For immunofluorescence experiments, cells were cotransfected with the plasmid, pFM112, that expresses GFP (kindly provided from Frank McNally) to allow for identification of transfected cells.
Mathematical Modeling
Microtubules were assumed to have three possible states: growing, shrinking, or pausing. Similar to previous work (Verde et al., 1992), transitions between states were assumed to be first order. A set of three differential stochastic equations were written and solved for steady state. For mathematical details see Supplementary Material.
Online Supplementary Material
APC and EB1 siRNA data are shown in titrated extracts and example images in Supplementary Figure S1. Supplementary Figure S2 presents CLIP170 and LIS1 siRNA data. Supplementary Figure S3 presents the localization (α-myc) of APC1–1450, APC58–1450, and APC1–768. Supplementary Figure S4 demonstrates interphase EB1-GFP comet tracking, and the resulting data can be found in Supplementary Table S1. Supplementary Videos 1–3, showing EB1-GFP comet dynamics, are available online. Supplementary Video 1 shows an example of comet behavior in a control cell (no receptor). The Supplementary Video 2 sequence, acquired from a cell stably expressing APC1–1450, shows a growing and paused comet. Supplementary Video 3 shows an example of comet behavior a cell stably expressing APC58–1450. Also available online are the mathematical details for the microtubule dynamics mathematical modeling experiments.
RESULTS
APC and EB1 Function Together in Mitosis to Regulate Spindle Integrity and Detection of Misaligned Chromosomes
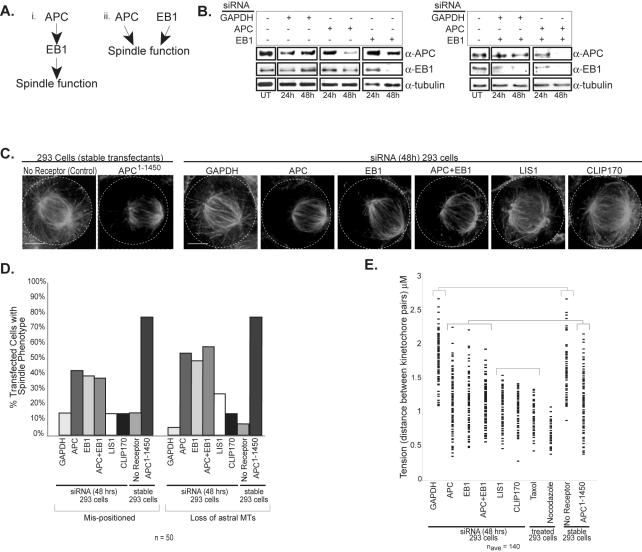
Recent work has shown that truncating mutations in APC (e.g., APC1–1450) act dominantly to promote mitotic spindle defects, possibly by compromising microtubule dynamics (Green and Kaplan, 2003; Tighe et al., 2004). The previously reported interaction between APC and EB1 led us to examine the possibility that dominant APC mutants might affect EB1 function (Su et al., 1995; Berrueta et al., 1998; Morrison et al., 1998). One prediction of this hypothesis is that directly inhibiting either APC or EB1 will act to phenocopy, or mimic, the mitotic defects observed in cells expressing APC1–1450 (see schematic in Figure 1A). To test this prediction, we transfected 293 cells with siRNA directed against EB1, APC, or GAPDH as a negative control. Immunoblot analysis of cell lysates 48 h after transfection showed a reduction in the levels of the targeted proteins (APC 82% and EB1 84% of control treatment; see Materials and Methods), suggesting that their function should also be largely inhibited; in contrast, no change in APC or EB1 protein levels were observed in cells transfected with siRNA directed against GAPDH (Figure 1B, Supplementary Figure S1). Our previous characterization of dominant APC1–1450 showed that a reduction of astral microtubules and the mispositioning of mitotic spindles was the most sensitive read-out for the dominant activity (Green and Kaplan, 2003). Therefore, we used immunofluorescence microscopy to characterize astral microtubules and spindle positioning in the siRNA-transfected mitotic cells. Both EB1 and APC siRNA-treated cells exhibit a fourfold increase in mispositioned spindles (see Materials and Methods) and a ninefold increase in loss of astral microtubules compared with control cells (Figure 1D). These phenotypes are remarkably similar to those observed in cells expressing APC1–1450 (Figure 1C and Green and Kaplan, 2003) but are notably less severe (see Figure 1, C and D), highlighting the potency of APC1–1450 allele.
Figure 1.
Inhibition of EB1, APC, or both results in spindle defects similar to defects caused by APC1–1450 expression. (A) The two proposed pathways depict (i) APC and EB1 working in the same pathway or (ii) in separate pathways to regulate mitotic spindle function. (B) Human 293 cells were transfected with siRNA directed against APC, EB1, or GAPDH (panel 1), or double siRNA combinations (panel 2); extracts were prepared 24 and 48 h posttransfection, and immunoblots were performed with antibodies against APC, EB1, or tubulin. (C) Control cells or cells stably expressing APC1–1450 were fixed and stained for tubulin. Cells were transfected with siRNA directed against the indicated target, fixed at 48 h posttransfection, and stained to visualize tubulin. Projections of Z-sections containing the spindle are presented. The outlines of the cells have been indicated by the dashed line. The percentage of metaphase cells with mispositioned spindles and/or lacking astral microtubules (D) was determined for cells transfected with siRNA directed against GAPDH, APC, EB1, APC+EB1, LIS1, and CLIP170 (n = 50). For reference no receptor control and APC1–1450 stable cells are also included. (E) Quantification of changes in tension (kinetochore to kinetochore distance) in response to siRNA treatment, microtubule poisons or dominant APC1–1450 expression as indicated (naverage = 140 kinetochore pairs sampled from several cells). Each mark represents a separate distance measured for a pair of kinetochores.
Multiple comparison analysis was performed and statistically similar groups were plotted together (brackets). Scale bar, (C) 5 μm.
We have previously shown that cells expressing APC1–1450 have compromised midzone microtubules and defective kinetochore attachments (Green and Kaplan, 2003). However, we only observe a modest reduction in the levels of midzone microtubules. We reasoned that this partial reduction is due to the failure to completely deplete APC or EB1 after siRNA treatment (Figure 1C, Supplementary Figure S1, and unpublished data). To detect more subtle defects in kinetochore-microtubule attachments, we measured the microtubule-mediated separation of sister kinetochores (tension) in metaphase cells. In control cells, the mean distance between sister kinetochores in metaphase is 1.8 ± 0.32 μm. Treatment of cells with taxol or nocodazole to eliminate tension reduced the mean distance between sister kinetochores to 0.92 ± 0.19 or 0.70 ± 0.13 μm, respectively. Cells either expressing APC1–1450 or treated with siRNA directed against EB1 and APC showed a similar intermediate loss of tension between sister kinetochores in metaphase, with a mean distance of 1.2 ± 0.40, 1.1 ± 0.33, and 1.1 ± 0.38 μm, respectively (Figure 1E). These results suggest cells inhibited for APC and EB1 exhibit a similar level of kinetochore microtubule attachment defects compared with cells expressing APC1–1450. Together, these results argue that APC1–1450 is a potent dominant negative mutant and are consistent with APC and EB1 functioning together to regulate both astral and kinetochore spindle microtubules.
The similarity of APC and EB1 siRNA phenotypes led us to speculate that APC and EB1 work together to regulate mitotic spindles. This led us to predict that inhibiting both APC and EB1 will not have an additive effect on mitotic spindle defects. Alternatively, if APC and EB1 work separately to regulate mitotic spindles, then inhibiting both together should exacerbate the spindle defects we observe (Figure 1A). To distinguish between these two possibilities, we performed double siRNA treatment against both APC and EB1 and examined mitotic spindles (Figure 1B). We observed no increase in the severity of the microtubule or tension phenotypes (Figure 1, C–E), arguing that APC and EB1 work in the same pathway to regulate the mitotic spindle (Figure 1Ai).
Next, we examined whether APC and EB1 have a distinct role in regulating mitotic spindles or whether all +TIPs function in this manner. To address this question, we inhibited CLIP170 and LIS1, two +TIPs believed to interact and function in a common pathway (Tai et al., 2002). When we used siRNA to reduce the levels of LIS1 or CLIP170, we observed changes in the mitotic spindle that were distinct from changes associated with the loss of APC or EB1. Spindle microtubules appear bundled and disorganized but do not exhibit a drastic decrease in astral or midzone microtubules (Figure 1C and Supplementary Figure S2). In fact, we often observed extra long microtubules that wrap around the cell cortex or bypass the kinetochores, suggesting that LIS1 and CLIP170 normally reduce the stability of microtubules. Interestingly, inhibition of LIS1 and CLIP170 results in a more uniform loss of tension at sister kinetochores (mean distance between sister kinetochores 0.99 ± 0.28 and 0.99 ±0.19 μm, respectively) than inhibition of APC or EB1, suggesting that stabilizing microtubules has a dramatic effect on kinetochore-microtubule attachments (Figure 1E). These distinct effects on spindle microtubules argue that LIS1 and CLIP170 function independently of APC and EB1.
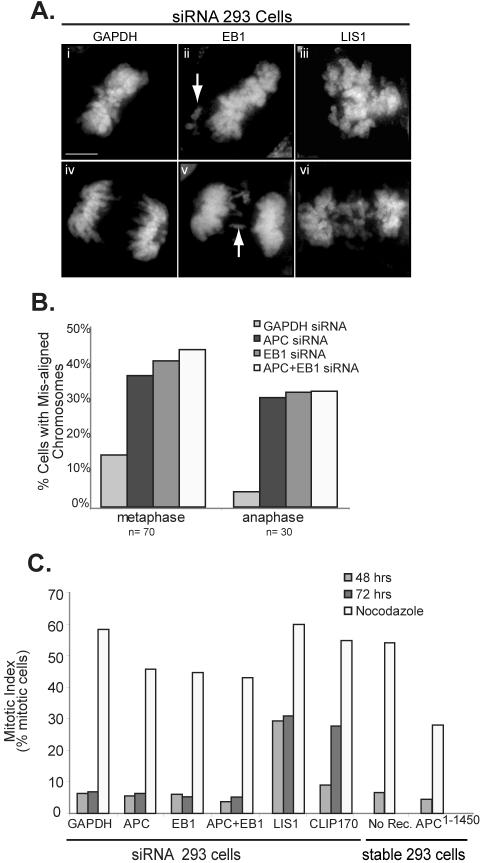
We have previously shown that mitotic cells expressing APC1–1450 have frequent misalignment (75% at metaphase and 85% at anaphase) of a small number of chromosomes; Green and Kaplan, 2003). Similarly, in APC and EB1 siRNA-treated cells, we frequently observed a few misaligned chromosomes that fail to associate with the metaphase spindle or that are left behind in the middle of the cell during anaphase (see arrows; Figure 2A). We observed misaligned chromosomes in 38% (APC), 41% (EB1), and 45% (EB1 and APC) of metaphase cells and 30% (APC), 31% (EB1), and 32% (APC and EB1) anaphase cells compared with 13 and 4% in controls, respectively (Figure 2B). In contrast, inhibition of LIS1 and CLIP170 causes a severe defect in chromosome congression, thus preventing cells from reaching a characteristic metaphase alignment of chromosomes (see Figure 2A and Supplementary Figure S2). The massive failure in chromosome congression is consistent with the inability of LIS1 or CLIP170 inhibited cells to establish tension at the kinetochore and is clearly distinct from the more subtle alignment defects observed after inhibiting APC and EB1.
Figure 2.
siRNA-mediated inactivation of EB1, APC, or both results in mitotic abnormalities similar to those observed upon APC1–1450 expression. (A) Chromosomes stained with DAPI highlight the extent of chromosomal alignment and segregation phenotypes associated with control (panels i and iv), inhibition of APC or EB1 (examples shown for EB1, panels ii and v) and inhibition of LIS1 or CLIP170 (example shown for LIS1, panels iii and vi; see Supplemental Figure 2 for more examples). (B) After the indicated siRNA treatment, the percentage of metaphase or anaphase cells with misaligned chromosomes were determined (n = 70 and 30, respectively). (C) The mitotic index was calculated for siRNA-treated cells and for APC1–1450-expressing cells in the presence or absence of nocodazole (n = 500). Nocodazole (100 nM, 20 h) was administered 24 h posttransfection. Scale bar, (A) 5 μm.
Failures of spindle microtubules to properly align and segregate chromosomes typically trigger the spindle checkpoint, delaying metaphase until these errors have been corrected (reviewed in Lew and Burke, 2003). Interestingly, inhibition of APC or EB1 does not result in an accumulation of mitotic cells (Figure 2C). This result is identical for cells expressing APC1–1450; a high percentage of APC1–1450 expressing mitotic cells exhibit defective spindles and misaligned chromosomes, yet cells do not arrest in mitosis. In contrast, we observed an increase in the mitotic index of cells inhibited for LIS1 48 h after transfection and a similar increase in the mitotic index of cells inhibited for CLIP170 72 h after transfection (28 and 27%, respectively, compared with 6% in control cells; Figure 2C). The delay in mitotic arrest in CLIP170 inhibited cells may be due to a slowing of the cell cycle and is consistent with the slow accumulation of mitotic cells after inhibition of CLIP170 in HEp2 cells (see Wieland et al., 2004). To examine if inhibition of APC or EB1 abolishes the spindle checkpoint, we treated cells with nocodazole overnight. Although we consistently observed a modest decrease in the mitotic index in cells inhibited for EB1 or APC when compared with controls, the accumulation of mitotic cells suggests that the spindle checkpoint is functional (Figure 2C). These findings are consistent with previous work that showed that APC mutations modulate but do not eliminate spindle checkpoint function (Kaplan et al., 2001; Tighe et al., 2004). Whether the loss of APC and EB1 activity compromises the spindle checkpoint is not clear (see Discussion) but these effects are distinct from the response to misaligned chromosomes after inhibition of CLIP170 and LIS1. Together these findings add further support to the argument that APC and EB1 work together to regulate mitotic spindle function.
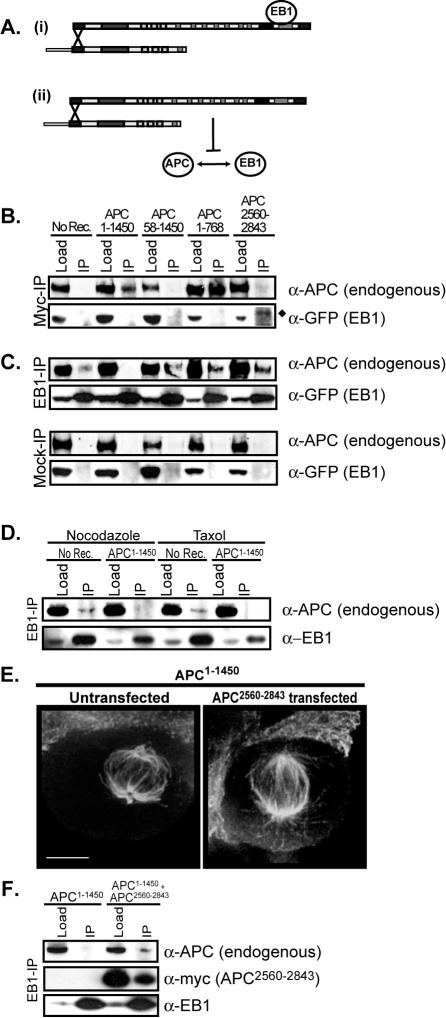
The APC1–1450 Mutant Acts Dominantly to Block EB1-APC Interaction
Our genetic data predicts that dominant negative mutations in APC that compromise mitotic spindles interfere with EB1 function, possibly by disrupting APC-EB1 complexes. To better understand the dominant negative mechanism of APC1–1450, we created stable cell lines using constructs that allow the ecdysone-regulated expression of a series of deletions in APC1–1450, all of which are fused to 13-myc epitope tags (see schematic, Figure 3A). In all cases, multiple stable clones were isolated and those expressing near endogenous APC levels were selected for further analysis (Figure 3B). For reference, APC1–1450 is expressed approximately threefold less than endogenous APC (see Materials and Methods and Green and Kaplan, 2003). Stable cell lines containing the expression plasmid for APC1–1450 but not the ecdysone receptor plasmid, which is required for APC1–1450 expression, were used as a negative control (labeled “no receptor”). As shown previously, control metaphase cells have a robust mitotic spindle with the majority of cells exhibiting extended astral microtubules (>95% of mitotic cells; Figure 3, C and D) and centrally positioned spindles (>80% of mitotic cells; Figure 3E), whereas more than 70% of mitotic cells expressing APC1–1450 exhibit a dramatic loss of visible astral microtubules and mispositioned mitotic spindles (Figure 3, Ci, D, and E; Green and Kaplan, 2003).
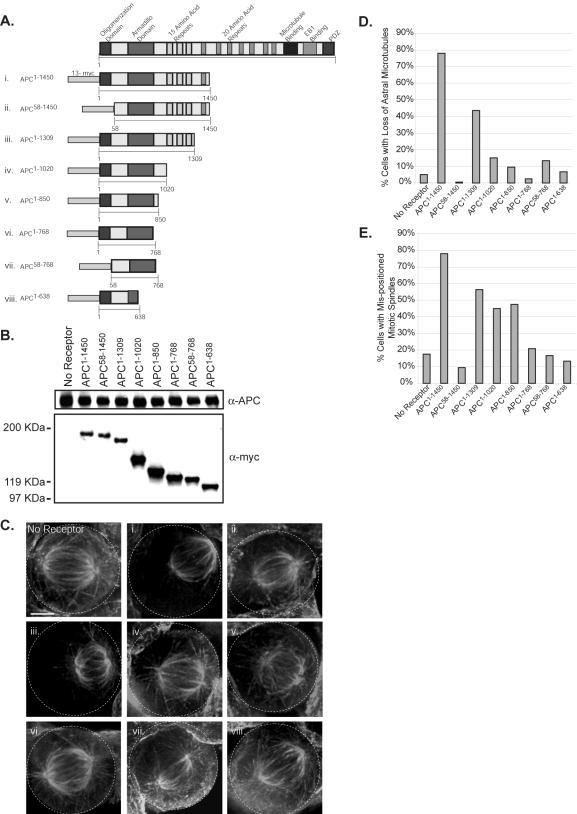
Figure 3.
The oligomerization domain and amino acids 768-1309 are required for the dominant function of APC1–1450. (A) The diagram shows a schematic of the 13myc-tagged APC fragments (i–viii; referred to subsequently by APCamino acids) that were expressed stably in human 293 cells. (B) Stable cell lines carrying the APC1–1450 plasmid without the ecdysone receptor (no receptor) or carrying the indicated APC fragments with the ecdysone receptor were grown in the presence of ponasterone for 72 h and lysed, and immunoblots were performed with antibodies against the myc epitope (α-myc) or against endogenous APC (α-APC). Positions of the nearest molecular weight standards are shown. (C) The stable cell lines (roman numerals (i–viii) corresponding to the APC fragments in A) were grown in the presence of ponasterone for 72 h, fixed, and stained to visualize microtubules. Projections of Z-sections containing the spindle are presented. The percentage of metaphase cells lacking astral microtubules (D) or with mispositioned spindles (E) was determined for the indicated stable cell lines (n = 40). Scale bars, (C) 5 μm.
Previous data has demonstrated that APC oligomerizes through its first 58 amino acids (Joslyn et al., 1993; Su et al., 1993). Therefore, we hypothesized that the oligomerization domain could facilitate the dominant negative activity of APC1–1450. To test this possibility, we deleted the first 58 amino acids (APC58–1450). Mitotic spindles in cells expressing APC58–1450 have normal astral microtubules, no defect in positioning spindles and are comparable to the no receptor control in fixed preparations (Figure 3C, “no receptor” vs. Figure 3, Cii, D, and E). These data strongly suggest that the formation of oligomers is important for the dominant activity of APC1–1450.
Deletions of the carboxy terminal regions of APC1–1450 revealed a gradual reduction in the dominant activity of APC1–1450. Although cells expressing APC1–1309 have severely compromised mitotic spindles, quantification of cells with reduced astral microtubules and mispositioned spindles suggest that the dominant activity of APC1–1309 is slightly diminished compared with cells expressing APC1–1450 (Figure 3, Ciii, D, and E). Although astral microtubules appear normal in cells expressing APC1–1020 or APC1–850, a significant percentage of mitotic cells have mispositioned spindles compared with control cells, suggesting that a subtle astral microtubule defect may exist in cells expressing shorter APC truncations (45 and 47% compared with 18%; Figure 3, D and E). However, cells expressing APC1–768, a truncation that retains the armadillo domain, exhibit wild-type spindles, normal astral microtubules, and control levels of mispositioned mitotic spindles (Figure 3, Cvi, D, and E). From these analyses, we conclude that the region between amino acids 768 and 1309 is important for the dominant activity of APC1–1450. Therefore there are two domains important for the dominant negative activity of APC1–1450, the oligomerization domain and the region between amino acids 768 and 1309.
As the deletions did not completely eliminate the ability of APC mutants to localize to the mitotic spindle (see Supplementary Figure S3), we assessed the roles of the oligomerization domain (amino acids 1–58) and the 768-1309 domain in the dominant activity of APC1–1450 on the formation of APC-complexes. We hypothesized that oligomerization of APC1–1450 with endogenous full-length APC might result in the formation of a dominant complex. As diagrammed in Figure 4A, we entertained two possibilities for how the dominant complex might act: 1) it might trap APC associated proteins (e.g., EB1) in a nonfunctional complex or 2) it might sequester endogenous APC so that it is unable to interact with its binding partner(s) (e.g., EB1). In both models, we predict the interaction between APC1–1450 and full-length APC. To test this possibility, we immunopurified myc-tagged APC mutants from extracts made from stable cell lines and monitored the ability of these mutants to interact with the full-length endogenous APC protein. As anticipated, mutants containing the oligomerization domain (APC1–1450 and APC1–768) associate with full-length APC, while APC58–1450 and APC2560–2843, which lack the oligomerization domain, do not associate with endogenous APC (Figure 4B, top panel). The apparent increase in endogenous APC-APC1–768 interaction is likely a consequence of higher expression levels of this mutant (compare APC1–1450 and APC1–768 levels in Figure 3B). We conclude that APC1–1450 forms a hetero-oligomer with the endogenous, full-length APC.
Figure 4.
An APC-APC1–1450 hetero-oligomer prevents EB1-APC association. (A) Two possible dominant mechanisms for APC1–1450: (i) the APC-APC1–1450 hetero-oligomer binds to EB1 in a nonproductive state; (ii) APC-APC1–1450 hetero-oligomer prevents EB1 association with endogenous APC. Immunoprecipitations from the indicated stable cell lines expressing EB1-GFP were performed using myc antibodies (B), EB1 antibodies or no antibodies (mock-IP; C), and immunoblots were used to identify APC (α-APC) and EB1-GFP (α-GFP). (♦) The slower migration of EB1-GFP compared with the load is due to the position of the immunoglobulin heavy chain. (D) Lysates were prepared from no receptor or APC1–1450 after 2 h treatment with either nocodazole (1 μM) or Taxol (0.5 μM). Immunoprecipitations were performed with EB1 antibodies, and immunoblots were done to identify APC (α-APC) and EB1 (α-EB1). (E) APC1–1450 cells were transiently transfected with 13myc-APC2560–2843, cells were fixed and stained with antibodies against tubulin (E), or lysates were prepared, followed by EB1-immunoprecipitation and immunoblotting with antibodies against APC, EB1, and myc (F). Scale bars, (E) 5 μm.
The genetic relationship between APC and EB1 led us to examine the possibility that the APC hetero-oligomer compromises APC-EB1 interactions. Previous studies have indicated that the endogenous APC-EB1 interaction is extremely difficult to detect biochemically and typically requires the overexpression of one or both proteins (Su et al., 1995; Morrison et al., 1998; Barth et al., 2002). Thus, GFP-EB1 was transiently overexpressed in stable cell lines expressing the APC mutants to allow us to assess APC-EB1 interactions. Because the endogenous, full-length APC contains the carboxy-terminal EB1 binding domain, we asked whether a hetero-oligomer containing APC1–1450 and full-length APC could also interact with EB1. Although EB1-GFP copurified with full-length APC or the region of APC that contains the EB1-interaction domain (APC2560–2843), we failed to observe copurification of EB1-GFP with APC1–1450 (Figure 4, B, bottom panel, and C, and unpublished data). This result suggests that in the context of the hetero-oligomer, full-length APC is unable to interact with EB1 and argues against the first model (Figure 4Ai).
To test if APC1–1450 prevents endogenous APC from interacting with EB1, we immunoisolated EB1-GFP from cell extracts and immunoblotted for endogenous APC (Figure 4C). As expected, a small but significant amount of full-length APC was observed to copurify with EB1-GFP from control cells (compare “no receptor” lanes in “mock-IP” an “EB1-IP”; Figure 4C). However, we observed that the expression of APC1–1450 prevents the interaction between EB1-GFP and full-length APC. Importantly, APC1–768 and APC58–1450, which do not compromise spindle function (Figure 3C, vi and ii), do not interfere with the full-length APC-EB1 interaction. These results suggest that amino acids 1–58 and 768-1450 are important for disrupting full-length APC-EB1 interaction (Figure 4C). The effect of APC1–1450 on full-length APC interactions is specific to EB1 as KAP3a interaction with full-length APC is not affected by expression of APC1–1450 (unpublished data; Jimbo et al., 2002). The failure of APC to interact with EB1 in cells expressing APC1–1450 could be caused indirectly by changes in microtubule stability. To test this possibility, we treated control cells with nocodazole or taxol before isolating EB1 by immunoprecipitation. These treatments had no effect the ability of APC to copurify with EB1, arguing that APC1–1450 directly interferes with the ability of APC and EB1 to interact (Figure 4D). On the basis of these data, we propose that APC1–1450 prevents the full-length APC from interacting with EB1 by forming a heterooligomer with the full-length APC, and the loss of APC-EB1 interaction leads to compromised mitotic spindles (see diagram in Figure 4Aii and Discussion).
Previous studies from a number of labs have demonstrated that the carboxy terminus of APC (amino acids 2560–2843) is sufficient to bind to and affect the activity of EB1 (Askham et al., 2000; Nakamura et al., 2001; Louie et al., 2004; Wen et al., 2004). If the interaction between APC and EB1 is critical for mitotic spindle function, we predict that restoring this interaction in cells expressing APC1–1450 will rescue the mitotic spindle defects. Consistent with this prediction, we observed that expression of the C-terminus of APC, containing the EB1-binding domain (APC2560–2843) but not the microtubule-binding domain, in APC1–1450 cells partially rescues spindle microtubules (Figure 4E). As expected, we also observed that APC2560–2843 interacts with EB1 under these conditions, an interaction that may be sufficient to properly regulate mitotic spindle formation (Figure 4F). However, somewhat surprisingly we observed that the expression of APC2560–2843 also partially restored the ability of full-length APC to interact with EB1 (Figure 4F), raising the possibility that the full-length APC-EB1 interaction is the relevant one with respect to rescuing mitotic spindle formation. In either case, the ability of APC2560–2843 to partially rescue the dominant negative effects of APC1–1450 strongly argues that APC-EB1 interactions are required for proper spindle formation.
APC1–1450 Acts as a Dominant Negative by Inhibiting EB1 Function
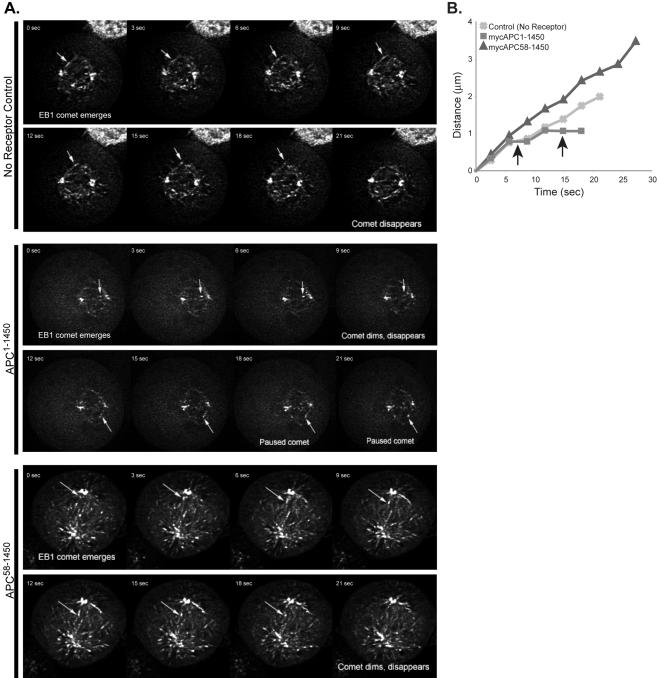
Previous work has demonstrated that the interaction between APC and EB1 enhances the ability of EB1 to associate with and “stabilize” microtubules in vitro (Nakamura et al., 2001). Thus, changes in APC-EB1 interaction, due to expression of APC1–1450, could alter the ability of EB1 to associate with spindle microtubules. To address this possibility, we examined the ability of EB1 to localize in its characteristic cometlike pattern in cells expressing APC1–1450, APC58–1450 or in the no receptor control. Cells expressing APC1–1450 retain EB1 comets (Figure 5A), arguing that APC is not required to load EB1 on microtubule plus ends. However, we did notice a modest but reproducible reduction in the number of EB1 comets in cells expressing APC1–1450 (Figure 5B); a similar phenotype was observed in APC siRNA-treated cells as well as in live imaging experiments of EB1-GFP in cells expressing APC1–1450 (see Figures 5C and 6). Although a reduction in the number of comets might reflect a decrease in the total number of spindle microtubules, microtubule regrowth experiments indicate that microtubule nucleation, and therefore the number, is normal in these cells (Green and Kaplan, 2003). Because EB1 is reported to strongly associate with polymerizing microtubule plus ends, we entertained the possibility that the observed reduction in the number of EB1 comets might reflect fewer growing microtubules. A reduction in the number of growing microtubules could result from APC1–1450 inhibiting the antipausing activity of EB1 which, in turn, alters microtubule growth, or by directly affecting the microtubules themselves. To address the latter possibility, we examined the effect of inhibiting microtubule dynamics on EB1-comets. After treating cells with low concentrations of nocodazole (100 nM) for 20 min to inhibit dynamics, we observed a partially disrupted mitotic spindle that is very similar to those observed in cells expressing APC1–1450. However, low doses of nocodazole completely eliminated EB1 from comets, an obviously different outcome compared with cells expressing APC1–1450 (Figure 5D). We suggest from this comparison that the subtle change in EB1 comets in cells expressing APC1–1450 is not simply due to a global decrease in microtubule dynamics but may instead reflect the failure to properly modulate the antipause activity of EB1 on microtubules.
Figure 6.
Expression of APC1–1450 promotes frequent pausing of EB1-GFP comets. Stable 293 cell lines expressing APC1–1450, APC58–1450, or the no receptor control were transfected with EB1-GFP, and mitotic cells expressing minimal amounts of EB1-GFP were imaged for 2–5 min. (A) Representative time-lapse sequences of plus-end GFP-EB1 tracking at 3-s intervals (see Supplementary Material for corresponding movies). Arrow denotes the growing (no receptor, APC1–1450 and APC58–1450) or paused (APC1–1450) EB1-GFP comet tracked in the inset (as noted). (B) A representative example of EB1-GFP comet life histories for each cell line analyzed is presented. Arrows indicate periods of EB1-GFP pausing. Scale bar, (A) 5 μm.
To test the possibility that APC modulates the activity of EB1, we transiently transfected no receptor control, APC1–1450, and APC58–1450 cells with EB1-GFP and recorded the movement of EB1-GFP comets on microtubules using a spinning disk confocal microscope (see Material and Methods). Importantly, we show that overexpression of EB1-GFP did not change the spindle phenotype in cells expressing APC1–1450 (Figure 6 and unpublished data), thus allowing us to make conclusions about the effect of APC1–1450 on comet movement. As an additional precaution, we analyzed cells that had the minimal amount of EB1-GFP expression necessary to allow the visualization of comets. We collected Z-information from a 2-μm-thick region in the middle of the spindle, and comets were tracked until they disappeared; we chose comets that extended from the pole on a vector that minimized the chances it would leave the 2-μm focal plane (Figure 6A, arrow; Supplementary Movies and Supplementary Figure S4A). Even with this precaution, we cannot rule out that some of the EB1-GFP comets enter or leave the focal plane during a time sequence. However, as we make comparative measurements, errors from out-of-focus plus ends will be constant between cell lines and therefore not affect our conclusions about the relative behavior of EB1-GFP comets. Analysis of time-lapse movies revealed that no receptor control, APC1–1450, and APC58–1450 cells have similar comet velocities (Table 1 and Figure 6B). EB1-GFP comets have a measured velocity of ∼0.16 μm/s (9.6 μm/min), which is comparable to previously reported microtubule polymerization velocities in LLCPK and PTK1 cells (Rusan et al., 2001; Tirnauer et al., 2002a). Comets in cells expressing APC1–1450 were observed to track for shorter distances than in no receptor and APC58–1450 controls (1.35 ± 0.48 μm compared with 2.13 ± 0.72 and 2.73 ± 0.83 μm, respectively). Although brightly staining comets were most often associated with growing microtubules, we observed many instances of dimmer EB1 comets that were neither growing nor shrinking. We classified these EB1 comets as “paused,” and we observed that they persisted for relatively short periods of time (2–6 s) before they decayed, presumably from dissociation or movement of the plus end out of the plane of focus. This behavior is not unprecedented and may reflect the ability of EB1 to associate with plus-end “walls” of microtubules as previously observed in vitro (Tirnauer et al., 2002b). Analysis of comet dynamics revealed that in APC1–1450 -expressing cells, comets spend about fourfold more time paused than no receptor control and APC58–1450 cells (see Table 1). These findings are consistent with the findings of Rogers et al. (2002), where inhibition of EB1 function, in Drosophila S2 cells, resulted in static or paused interphase microtubules. Interestingly, comets in cells expressing APC58–1450 extend for longer periods and to greater lengths and have a reduced pause frequency compared with the no receptor control, suggesting that this fragment of APC may have a stabilizing effect on EB1 comets (Figure 6, Table 1; Supplementary Data, Supplementary Table S1, Supplementary Figure S4). In interphase cells, similar trends are observed; however, the differences in EB1-comet pause frequency is more subtle, perhaps because of reduced dynamicity during interphase (see Supplementary Data, Figure S4 and Supplementary Table S1; Rusan et al., 2001). As we observe all EB1-comets associated with microtubule plus ends in the cell lines examined by fixed immunofluorescence (see Figure 5A), we conclude that APC1–1450 alters EB1-comet behavior at the plus ends of microtubules.
Table 1.
Dynamic properties of EB1-GFP-positive microtubule plus ends in mitosis
| No receptor (n = 45) | APC1-1450 (n = 43) | APC58-1450 (n = 40) | |
|---|---|---|---|
| Mitosisa | |||
| Average velocity of growth (μm/s) | 0.177 ± 0.065 | 0.157 ± 0.050 | 0.164 ± 0.038 |
| Average distance (μm) | 2.13 ± 0.72 | 1.35 ± 0.48 | 2.73 ± 0.83 |
| Average duration (s) | 14.32 ± 5.24 | 12.31 ± 5.09 | 17.95 ± 5.57 |
| Average time in growth (s) | 13.79 ± 4.80 | 9.72 ± 3.88 | 17.36 ± 5.35 |
| Average time in pause (s) | 0.92 ± 1.53 | 2.95 ± 2.98 | 0.93 ± 1.47 |
| Pause frequency (% time in pause) | 6.00 | 22.20 | 5.06 |
Values are mean ± SD.
Although a number of reports suggest that the measurement of EB1 behavior and microtubule behavior is equivalent in other cells lines (Tirnauer et al., 2002b, 2004; Piehl and Cassimeris, 2003), our attempts to track GFP-labeled microtubules or other +TIPs (GFP-CLIP170) in these mitotic cells were unsuccessful (unpublished data); thus, we were unable to directly correlate EB1-comet behavior and microtubule behavior under our live imaging conditions. However, given close association of EB1-comets and microtubule plus ends, it is probable that the increased number of paused EB1-comets in cells expressing APC1–1450 reflects changes in the underlying microtubule dynamics (see Discussion).
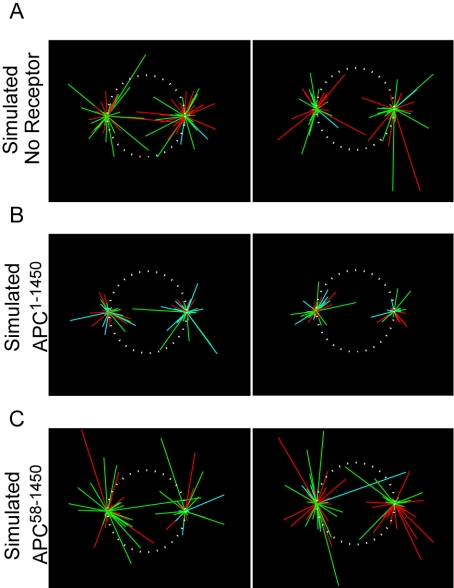
Can an increase in microtubule pausing account for the defective spindle phenotype that we observe upon APC1–1450 expression? To address this question, we developed a mathematical model of microtubule dynamics to assess the effect of microtubule pausing on overall microtubule length distribution. Previous mathematical analyses of dynamic instability approximated microtubule behavior considering two states of dynamics: growing or shrinking (Verde et al., 1992). However, our in vivo measurements predict a subpopulation of paused microtubules; therefore we expanded on existing models that include all three states (Grego et al., 2001): growing, shrinking, and pausing (for details, see Supplementary Material). For these experiments, we assumed that EB1-comet dynamics accurately reflected microtubule plus-end dynamics. Our analysis indicates that the measured dynamic instability parameters (Table 1) would result in a 33% decrease in average microtubule length for cells expressing APC1–1450 and a 25% increase in average microtubule length for cells expressing APC58–1450, under the assumption of a negligible number of rescue events in mitosis (Rusan et al., 2001). Higher rescue frequencies exacerbated the differences in microtubule length, suggesting that our approach may understate the situation in cells (unpublished data). When these parameters were run through a mathematical simulation, we found the APC1–1450-simulated spindles are phenotypically similar to the actual spindles in cells expressing APC1–1450, with reduced astral and kinetochore microtubules (Figure 7B). Conversely, the no receptor control and APC58–1450 simulated spindles are more robust (Figure 7, A and C). This result suggests that even the modest increase (fourfold) in plus-end pause events that we observe in cells expressing APC1–1450 can affect microtubule growth and therefore give rise to the dramatic phenotypic changes we observe in the mitotic spindle.
Figure 7.
Mathematical simulation of microtubule dynamics. Two representative outcomes from mathematical simulations of microtubule dynamics using measured EB1-GFP parameters for simulated control cells (A), cells expressing APC1–1450 (B), or cells expressing APC58–1450 (C). The dynamic states of microtubules are indicated by colors: pausing (blue), growing (green), or shrinking (red). Also see Supplementary Material.
DISCUSSION
Proper regulation of the mitotic spindle is essential for accurate spindle orientation and segregation of chromosomes. Although correlative studies have linked APC to EB1 and to the regulation of microtubules, there has been little direct evidence that this link is physiologically relevant, especially in mitosis (Munemitsu et al., 1994; Mimori-Kiyosue et al., 2000b; Nakamura et al., 2001; Zumbrunn et al., 2001; Mogensen et al., 2002; Wen et al., 2004). In this study we use genetic approaches to demonstrate that APC and EB1 work together to regulate the mitotic spindle. We show that APC is not required for the loading of EB1 onto microtubule plus ends but may instead act to modulate the antipausing activity of EB1. Furthermore, we demonstrate EB1-APC interactions are specifically disrupted by the expression of APC1–1450, a dominant APC truncation fragment that mimics mutations commonly found in human colorectal cancer. Interestingly, our data also suggest that defects in APC and EB1 compromise the ability of cells to arrest in mitosis in the presence of misaligned chromosomes. Together, these results imply that APC mutations in colorectal cancers may act dominantly to inhibit normal APC-EB1 interactions, promote spindle defects and allow cells to divide in the presence of failed kinetochore-microtubule attachments.
APC Regulation of EB1 Function
The genetic and biochemical data presented here strongly suggest that APC regulates the mitotic function of EB1. Depletion of APC, EB1, or APC and EB1 together, but not LIS1 or CLIP170, produce spindle phenotypic defects comparable to cells expressing the dominant APC1–1450 fragment, arguing that APC and EB1 work together to regulate the mitotic spindle (Figures 1 and 2). Of course, these results do not rule out the ability of APC and EB1 to also work independently from one another to affect a separate cellular process (e.g., regulation of β-catenin). We further demonstrate that EB1-APC association is important for spindle function, because expression of APC1–1450 perturbs this interaction and gives rise to mitotic spindle defects (Figures 3, C–E, and 4C). However, inhibition of APC by siRNA or by expression of APC1–1450 results in only a subtle change in the distribution of EB1 comets, arguing against APC serving a primary role in loading EB1 on microtubule plus ends during mitosis. Rather, the fourfold increase in EB1-GFP pausing in cells expressing the dominant negative APC1–1450 allele argues that APC normally stimulates the antipause activity of EB1. Given that EB1-comets have only ever been observed on the plus ends of growing microtubules, it is reasonable to extrapolate that the microtubules labeled with EB1 are similarly compromised in their dynamics. Our inability to measure dynamics of non-EB1-labeled microtubules may mean that we are underestimating the effects of APC1–1450 on microtubule dynamics. Nonetheless, our modeling of microtubule dynamics suggests that a small increase in microtubule pausing can have dramatic effects on the robustness of the mitotic spindle.
Although APC has been found localized at microtubule plus ends in a number of systems, several of our observations are more consistent with its regulation of EB1 in the cytosol. In mitotic cells, only low levels of wild-type APC are found along microtubules and at their plus ends. In cells expressing APC1–1450, we more often observe clusters of APC at plus ends, suggesting a change in the affinity of APC for its binding partner on microtubules. In contrast, EB1 is found very clearly in comets on the majority of spindle microtubules. Although it is possible that APC regulates only a subset of EB1 comets at any given time, the ability of APC to interact with EB1 even in nocodazole-treated cells leads us to propose that the relevant APC-EB1 interactions is independent of microtubules (see Figure 4D). This idea is consistent with current models that postulate the preassembly and copolymerization of +TIPs with microtubules (reviewed in Carvalho et al., 2003). Finally, the ability of APC2560–2843 to partially suppress the dominant phenotype of APC1–1450 also argues for a microtubule-independent interaction, because APC2560–2843 does not contain the microtubule-binding domain and fails to localize to microtubules in mitosis (Green and Kaplan, 2003). Therefore, we propose that APC regulates EB1 activity before it associates with microtubule plus ends, although we cannot rule out that it has secondary effects on EB1 in comets.
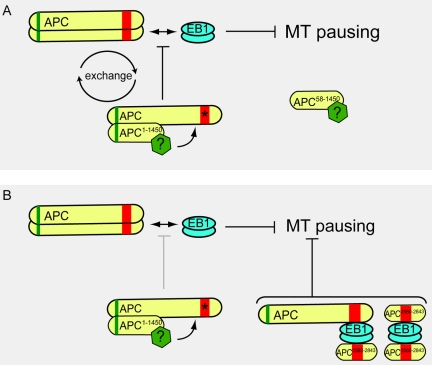
The behavior of APC mutants in mitosis highlights the transient nature of APC-EB1 interactions. APC1–1450 associates with only a fraction (∼5–10%) of the total endogenous full-length APC in cells, yet completely abrogates APC-EB1 interaction. One interpretation of this observation is that the dominant APC1–1450 hetero-oligomer “poisons” the full-length APC. To explain this observation, we propose that the exchange of APC subunits from the hetero-oligomer complex globally inhibits the APC-EB1 interaction (Figure 8A). Although other scenarios are possible, we favor the idea that a negative regulator associates with the APC hetero-oligomer and modifies full-length APC to prevent its interaction with EB1. The loss of the dominant phenotype in APC1–768 raises the possibility that the negative regulator interacts with a domain encompassing amino acids 768-1309. In this model, eliminating the oligomerization domain of APC prevents the “poisoning” of full-length APC as we observe but also predicts that APC58–1450 remains associated with the putative negative regulator (Figure 8A). Consistent with this prediction, we observe a decrease in microtubule pausing in cells expressing APC58–1450 (see Figure 6B and Table 1). Determining the nature of APC “poisoning” in this context will provide important insights into its normal regulation with respect to microtubule dynamics.
Figure 8.
Model for APC regulation of EB1 anti-pausing activity. (A) APC positively regulates the antipausing activity of EB1. The dominant negative APC1–1450 allele forms a hetero-oligomer with full-length APC and a putative negative regulator (green polygon) modifies full-length APC; exchange of modified APC (arrows) “poisons” the normal APC pool and prevents it from interacting with EB1. APC58–1450 can no longer dimerize with full-length APC but remains associated with the putative negative regulator (green polygon). (B) APC2560–2843 interacts with either EB1 dimers alone or with an EB1 dimer and the full-length APC. Either type of complex or both may be sufficient to restore the antipausing activity of EB1, allowing for the partial rescue of spindle microtubules we observe.
The partial rescue of the dominant APC1–1450 spindle phenotype by expression of APC2560–2843 further argues for the importance of the APC-EB1 interaction. Biochemical analysis shows that APC2560–2843 interacts with EB1 and rescues the interaction between full-length APC and EB1. As illustrated in the model (Figure 8B), it is possible that APC2560–2843 interacts with EB1, with EB1 associated with the full-length APC or in both of types of complexes. Recent structural studies of EB1 suggest that it may form a dimer when associated with APC (Honnappa et al., 2005; Slep et al., 2005); we speculate that such arrangements may sequester full-length APC from the hetero-oligomer complex, preventing it from being negatively modified and thereby partially restoring the antipausing activity of EB1. The behavior of these mutant APC fragments with respect to EB1 begs the question how the APC-EB1 interaction is normally regulated. The link between APC and the Rho family of small G-proteins and their ability to regulate interactions between formins, APC and EB1, raise the possibility that microtubule dynamics are similarly regulated during mitosis (Gundersen et al., 2004; Watanabe et al., 2004; Wen et al., 2004).
Tumor Cell Biology
APC mutations that result in a truncated gene product are frequent in sporadic and inherited forms of colorectal cancers. We initially showed that even in the heterozygous state, these mutations (e.g., APC1–1450) perturb normal microtubule function (Green and Kaplan, 2003). More recently, Tighe et al. (2004) made a similar observation in the human colorectal tumor cell line, HCT116. Tighe et al. reported that APC1–750 compromised mitotic spindles based on changes in pole-pole distance and a reduction in tension between sister kinetochores; this trend was exacerbated with constructs containing longer regions of APC (i.e., APC1–1309 and APC1–1807) and is therefore consistent with our analyses of APC truncations. For example, our measurements of mispositioned spindles shows that APC1–850 has a modest but significant defect and this defect increases by more than twofold in cells expressing APC1–1309 (see Figure 3). Interestingly, the truncations that give the most severe defects in mitotic spindles correlate with mutational hot spot regions found in patients with either familial or sporadic cases of colorectal cancer (for review see Fearnhead et al., 2001). Relatively few alleles are found in human cancers that are shorter than APC1–768 and many of these may represent secondary lesions in APC, or alternatively, may give rise to tumors through pathways independent of spindle function, such as failure in β-catenin regulation.
Although the importance of chromosome instability in cancer progression is controversial, it is intriguing to consider the consequences for cells that divide with compromised mitoses. This includes inhibited microtubule dynamics, misaligned chromosomes that fail to be detected by the spindle checkpoint, and mispositioned spindles. Such defects in mitotic fidelity may combine to perturb the normal division pattern in the intestine, perhaps shaping selective pressures that promote the “evolution” of tumors. Particularly intriguing is the failure of both APC- and EB1-inhibited cells to arrest in response to misaligned mitotic chromosomes. Although this failure may be due to merotelic attachments, it is difficult to clearly observe these defective attachments in 293 cells. Nonetheless, at times we fail to observe microtubules in the region of these chromosomes, suggesting that at least in some cases these chromosomes are not merotelically attached to the spindle. Furthermore, the misaligned chromosomes we observe in APC or EB1 inhibited cells exhibit very low tension between sister kinetochores, suggesting that they have failed to form functional kinetochore-microtubule attachments (unpublished data). Our work and the work of Tighe et al. (2004) has shown that checkpoint proteins are still effectively recruited to misaligned chromosomes and that nocodazole or taxol treatment can activate the checkpoint, arguing against a wholesale failure of the spindle checkpoint in cells inhibited for APC or EB1 (Green and Kaplan, 2003; Tighe et al., 2004). Our observation that inhibition of EB1 also prevents misaligned chromosomes from inducing mitotic arrest in cells further argues that APC and EB1 work together and raises the intriguing possibility that +TIPs interface with the spindle checkpoint in ways not previously appreciated. Thus, simultaneous failure to properly align chromosomes and the inability of the spindle checkpoint to sense these failures represents a potent combination that could contribute to chromosome instability in tumor cells.
Supplementary Material
Acknowledgments
We thank F. McNally, J. Scholey, and L. Rose for helpful discussions and comments on the manuscript. We also acknowledge the support of the Sidney Kimmel Cancer Foundation, an Institutional Research Grant (IRG) grant from the American Cancer Society (IRG-95-125-04), Grant RSG-02-035-01-CCG from the American Cancer Society and the National Institutes of Health, Molecular, and Cellular Biology Training program (T32-GM-07377) for support of R.A.G.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–03–0259) on July 19, 2005.
Abbreviations used: ACA, anti-centromere antibody; APC, adenomatous polyposis coli; CIN, chromosome instability; CLIP170, cytoplasmic linker protein 170; EB1, end binding 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IP, immunoprecipitation; KAP3a, kinesin-associated protein 3a; LIS1, lissencephaly 1; siRNA, small interfering RNA; +TIPs, plus-end tracking protein.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Andrews, P. D., Ovechkina, Y., Morrice, N., Wagenbach, M., Duncan, K., Wordeman, L., and Swedlow, J. R. (2004). Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253–268. [DOI] [PubMed] [Google Scholar]
- Askham, J. M., Moncur, P., Markham, A. F., and Morrison, E. E. (2000). Regulation and function of the interaction between the APC tumour suppressor protein and EB1. Oncogene 19, 1950–1958. [DOI] [PubMed] [Google Scholar]
- Barth, A. I., Siemers, K. A., and Nelson, W. J. (2002). Dissecting interactions between EB1, microtubules and APC in cortical clusters at the plasma membrane. J. Cell Sci. 115, 1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin, V., Styles, C. A., and Fink, G. R. (1990). BIK1, a protein required for microtubule function during mating and mitosis in Saccharomyces cerevisiae, colocalizes with tubulin. J. Cell Biol. 111, 2573–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta, L., Kraeft, S. K., Tirnauer, J. S., Schuyler, S. C., Chen, L. B., Hill, D. E., Pellman, D., and Bierer, B. E. (1998). The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules. Proc. Natl. Acad. Sci. USA 95, 10596–10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, P., Tirnauer, J. S., and Pellman, D. (2003). Surfing on microtubule ends. Trends Cell Biol. 13, 229–237. [DOI] [PubMed] [Google Scholar]
- Cassimeris, L., and Morabito, J. (2004). TOGp, the human homolog of XMAP215/Dis1, is required for centrosome integrity, spindle pole organization, and bipolar spindle assembly. Mol. Biol. Cell 15, 1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquelle, F. M. et al. (2002). LIS1, CLIP-170's key to the dynein/dynactin pathway. Mol. Cell. Biol. 22, 3089–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikovskaya, D., Newton, I. P., and Nathke, I. S. (2004). The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts. Mol. Biol. Cell 15, 2978–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin, D., Wacker, U. I., Moreau, A., Schroer, T. A., Rickard, J. E., and De Mey, J. R. (1998). Evidence for a role of CLIP-170 in the establishment of metaphase chromosome alignment. J. Cell Biol. 141, 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2003). Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature 421, 753–756. [DOI] [PubMed] [Google Scholar]
- Faulkner, N. E., Dujardin, D. L., Tai, C. Y., Vaughan, K. T., O'Connell, C. B., Wang, Y., and Vallee, R. B. (2000). A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat. Cell Biol. 2, 784–791. [DOI] [PubMed] [Google Scholar]
- Fearnhead, N. S., Britton, M. P., and Bodmer, W. F. (2001). The ABC of APC. Hum Mol. Genet. 10, 721–733. [DOI] [PubMed] [Google Scholar]
- Fodde, R. et al. (2001). Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat. Cell Biol. 3, 433–438. [DOI] [PubMed] [Google Scholar]
- Green, R. A., and Kaplan, K. B. (2003). Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J. Cell Biol. 163, 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grego, S., Cantillana, V., and Salmon, E. D. (2001). Microtubule treadmilling in vitro investigated by fluorescence speckle and confocal microscopy. Biophys. J. 81, 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss, O. J., Wittmann, M., Yokoyama, H., Pepperkok, R., Kufer, T., Sillje, H., Karsenti, E., Mattaj, I. W., and Vernos, I. (2002). Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol. 4, 871–879. [DOI] [PubMed] [Google Scholar]
- Gundersen, G. G., Gomes, E. R., and Wen, Y. (2004). Cortical control of microtubule stability and polarization. Curr. Opin. Cell Biol. 16, 106–112. [DOI] [PubMed] [Google Scholar]
- Honnappa, S., John, C. M., Kostrewa, D., Winkler, F. K., and Steinmetz, M. O. (2005). Structural insights into the EB1-APC interaction. EMBO J. 24, 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimbo, T., Kawasaki, Y., Koyama, R., Sato, R., Takada, S., Haraguchi, K., and Akiyama, T. (2002). Identification of a link between the tumour suppressor APC and the kinesin superfamily. Nat. Cell Biol. 4, 323–327. [DOI] [PubMed] [Google Scholar]
- Joslyn, G., Richardson, D. S., White, R., and Alber, T. (1993). Dimer formation by an N-terminal coiled coil in the APC protein. Proc. Natl. Acad. Sci. USA 90, 11109–11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, K. B., Burds, A. A., Swedlow, J. R., Bekir, S. S., Sorger, P. K., and Nathke, I. S. (2001). A role for the adenomatous polyposis coli protein in chromosome segregation. Nat. Cell Biol. 3, 429–432. [DOI] [PubMed] [Google Scholar]
- Karsenti, E., and Vernos, I. (2001). The mitotic spindle: a self-made machine. Science 294, 543–547. [DOI] [PubMed] [Google Scholar]
- Kline-Smith, S. L., and Walczak, C. E. (2004). Mitotic spindle assembly and chromosome segregation: refocusing on microtubule dynamics. Mol. Cell 15, 317–327. [DOI] [PubMed] [Google Scholar]
- Lansbergen, G. et al. (2004). Conformational changes in CLIP-170 regulate its binding to microtubules and dynactin localization. J. Cell Biol. 166, 1003–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, D. J., and Burke, D. J. (2003). The spindle assembly and spindle position checkpoints. Annu. Rev. Genet. 37, 251–282. [DOI] [PubMed] [Google Scholar]
- Longtine, M. S., McKenzie, A., 3rd, Demarini, D. J., Shah, N. G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J. R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961. [DOI] [PubMed] [Google Scholar]
- Louie, R. K., Bahmanyar, S., Siemers, K. A., Votin, V., Chang, P., Stearns, T., Nelson, W. J., and Barth, A. I. (2004). Adenomatous polyposis coli and EB1 localize in close proximity of the mother centriole and EB1 is a functional component of centrosomes. J. Cell Sci. 117, 1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, B., Roegiers, F., Jan, L. Y., and Jan, Y. N. (2001). Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature 409, 522–525. [DOI] [PubMed] [Google Scholar]
- Maiato, H., Sampaio, P., Lemos, C. L., Findlay, J., Carmena, M., Earnshaw, W. C., and Sunkel, C. E. (2002). MAST/Orbit has a role in microtubule-kinetochore attachment and is essential for chromosome alignment and maintenance of spindle bipolarity. J. Cell Biol. 157, 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney, B. M., McEwen, D. G., Grevengoed, E., Maddox, P., Bejsovec, A., and Peifer, M. (2001). Drosophila APC2 and Armadillo participate in tethering mitotic spindles to cortical actin. Nat. Cell Biol. 3, 933–938. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue, Y., Shiina, N., and Tsukita, S. (2000a). Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J. Cell Biol. 148, 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue, Y., Shiina, N., and Tsukita, S. (2000b). The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr. Biol. 10, 865–868. [DOI] [PubMed] [Google Scholar]
- Mogensen, M. M., Tucker, J. B., Mackie, J. B., Prescott, A. R., and Nathke, I. S. (2002). The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells. J. Cell Biol. 157, 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, E. E., Wardleworth, B. N., Askham, J. M., Markham, A. F., and Meredith, D. M. (1998). EB1, a protein which interacts with the APC tumour suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene 17, 3471–3477. [DOI] [PubMed] [Google Scholar]
- Munemitsu, S., Souza, B., Muller, O., Albert, I., Rubinfeld, B., and Polakis, P. (1994). The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 54, 3676–3681. [PubMed] [Google Scholar]
- Nakamura, M., Zhou, X. Z., and Lu, K. P. (2001). Critical role for the EB1 and APC interaction in the regulation of microtubule polymerization. Curr. Biol. 11, 1062–1067. [DOI] [PubMed] [Google Scholar]
- Nathke, I. S., Adams, C. L., Polakis, P., Sellin, J. H., and Nelson, W. J. (1996). The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol. 134, 165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl, M., and Cassimeris, L. (2003). Organization and dynamics of growing microtubule plus ends during early mitosis. Mol. Biol. Cell 14, 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, S. L., Rogers, G. C., Sharp, D. J., and Vale, R. D. (2002). Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J. Cell Biol. 158, 873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusan, N. M., Fagerstrom, C. J., Yvon, A. M., and Wadsworth, P. (2001). Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-alpha tubulin. Mol. Biol. Cell 12, 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey, J. M., Brust-Mascher, I., and Mogilner, A. (2003). Cell division. Nature 422, 746–752. [DOI] [PubMed] [Google Scholar]
- Shu, T., Ayala, R., Nguyen, M. D., Xie, Z., Gleeson, J. G., and Tsai, L. H. (2004). Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron. 44, 263–277. [DOI] [PubMed] [Google Scholar]
- Slep, K. C., Rogers, S. L., Elliott, S. L., Ohkura, H., Kolodziej, P. A., and Vale, R. D. (2005). Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the microtubule plus end. J. Cell Biol. 168, 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, L. K., Burrell, M., Hill, D. E., Gyuris, J., Brent, R., Wiltshire, R., Trent, J., Vogelstein, B., and Kinzler, K. W. (1995). APC binds to the novel protein EB1. Cancer Res. 55, 2972–2977. [PubMed] [Google Scholar]
- Su, L. K., Johnson, K. A., Smith, K. J., Hill, D. E., Vogelstein, B., and Kinzler, K. W. (1993). Association between wild type and mutant APC gene products. Cancer Res. 53, 2728–2731. [PubMed] [Google Scholar]
- Tai, C. Y., Dujardin, D. L., Faulkner, N. E., and Vallee, R. B. (2002). Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J. Cell Biol. 156, 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe, A., Johnson, V. L., and Taylor, S. S. (2004). Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. J. Cell Sci. 117, 6339–6353. [DOI] [PubMed] [Google Scholar]
- Tirnauer, J. S., Canman, J. C., Salmon, E. D., and Mitchison, T. J. (2002a). EB1 targets to kinetochores with attached, polymerizing microtubules. Mol. Biol. Cell 13, 4308–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer, J. S., Grego, S., Salmon, E. D., and Mitchison, T. J. (2002b). EB1-microtubule interactions in Xenopus egg extracts: role of EB1 in microtubule stabilization and mechanisms of targeting to microtubules. Mol. Biol. Cell 13, 3614–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer, J. S., O'Toole, E., Berrueta, L., Bierer, B. E., and Pellman, D. (1999). Yeast Bim1p promotes the G1-specific dynamics of microtubules. J. Cell Biol. 145, 993–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer, J. S., Salmon, E. D., and Mitchison, T. J. (2004). Microtubule plus-end dynamics in Xenopus egg extract spindles. Mol. Biol. Cell 15, 1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, K. T. (2004). Surfing, regulating and capturing: are all microtubule-tip-tracking proteins created equal? Trends Cell Biol. 14, 491–496. [DOI] [PubMed] [Google Scholar]
- Verde, F., Dogterom, M., Stelzer, E., Karsenti, E., and Leibler, S. (1992). Control of microtubule dynamics and length by cyclin A- and cyclin B-dependent kinases in Xenopus egg extracts. J. Cell Biol. 118, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, T., Wang, S., Noritake, J., Sato, K., Fukata, M., Takefuji, M., Nakagawa, M., Izumi, N., Akiyama, T., and Kaibuchi, K. (2004). Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev. Cell 7, 871–883. [DOI] [PubMed] [Google Scholar]
- Wen, Y., Eng, C. H., Schmoranzer, J., Cabrera-Poch, N., Morris, E. J., Chen, M., Wallar, B. J., Alberts, A. S., and Gundersen, G. G. (2004). EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 6, 820–830. [DOI] [PubMed] [Google Scholar]
- Wieland, G., Orthaus, S., Ohndorf, S., Diekmann, S., and Hemmerich, P. (2004). Functional complementation of human centromere protein A (CENP-A) by Cse4p from Saccharomyces cerevisiae. Mol. Cell. Biol. 24, 6620–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, Y. M., Jones, D. L., and Fuller, M. T. (2003). Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301, 1547–1550. [DOI] [PubMed] [Google Scholar]
- Zumbrunn, J., Kinoshita, K., Hyman, A. A., and Nathke, I. S. (2001). Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr. Biol. 11, 44–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.