Abstract
Mammalian septins constitute a family of at least 12 GTP-binding proteins that can form hetero-oligomers and that are sometimes found in association with actin or microtubule filaments. However, their functions are not understood. Using RNA interference, we found that suppression of septin expression in HeLa cells caused a pronounced increase in microtubule stability. Mass spectroscopic analysis of proteins coprecipitating with Sept6 identified the microtubule-associated protein MAP4 as a septin binding partner. A small, proline-rich region in the C-terminal half of MAP4 bound directly to a Sept 2:6:7 heterotrimer, and to the Sept2 monomer. The trimer blocked the ability of this MAP4 fragment to bind and bundle microtubules in vitro. In intact cells, MAP4 was required for the stabilization of microtubules induced by septin depletion. Moreover, septin depletion increased the number of cells with abnormal nuclei, and this effect was blocked by gene silencing of MAP4. These data identify a novel molecular function for septins in mammalian cells: the modulation of microtubule dynamics through interaction with MAP4.
INTRODUCTION
Septins are a family of GTP-binding proteins that are conserved among all eukaryotes except plants (Hartwell, 1971; Longtine et al., 1996; Nguyen et al., 2000). Septins were originally discovered in the budding yeast Saccharomyces cerevisiae, where they localize to the presumptive bud site and form a ring around the bud neck during cytokinesis (Field and Kellogg, 1999). Septins are postulated to form a scaffold at the bud neck and have a demonstrated role in the localization of other cytokinesis proteins (Chant, 1996; Field and Kellogg, 1999; Castillon et al., 2003; Caviston et al., 2003; Longtine and Bi, 2003). In addition, septins act as a diffusion barrier to segregate proteins and mRNA between mother and daughter cells (Barral et al., 2000; Takizawa et al., 2000). Although originally thought to be unique to yeast, septins have been discovered in Caenorhabditis elegans, Drosophila melanogaster, and vertebrates (Kinoshita et al., 1997; Oegema et al., 1998; Nguyen et al., 2000). To date, at least 12 distinct septins with multiple splice variants have been identified in mammals (reviewed in Macara et al., 2002). Their function is not limited to cytokinesis, because they are expressed at high levels in nondividing cells such as neuronal tissue and platelets (Kinoshita et al., 2000; Dent et al., 2002; Peng et al., 2002). In postmitotic cells, mammalian septins are thought to be involved in the secretory pathway, guiding vesicles to points of exocytosis (Kartmann and Roth, 2001). However, their functions at a molecular level are largely unknown.
During interphase, mammalian septins form filamentous structures throughout the cell. For simplicity, we refer to these as “filaments,” although their exact nature is unknown. In some cell types, these filaments align along actin stress fibers and/or microtubules (MTs); they dissipate during mitosis, and at telophase associate with the midbody (Kinoshita et al., 1997; Joberty et al., 2001; Surka et al., 2002). The filaments are comprised of complexes of multiple different septins, which in fibroblasts and epithelial cells contain Sept2, Sept6, and Sept7 (Macara et al., 2002). These three septins form a heterotrimer when coexpressed in bacteria, and this oligomerization is necessary for the stability of the individual septins (Joberty et al., 2001; Sheffield et al., 2003). Sept2 and Sept9 can align along MTs during interphase in PC12 and HeLa cells (Surka et al., 2002; Nagata et al., 2003; Vega and Hsu, 2003), and Sept2 has been reported to associate with spindle microtubules during mitosis (Spiliotis et al., 2005). Remarkably, even a small decrease in Sept2 level can disrupt spindle attachment to centromeres and cause mitotic defects (Spiliotis et al., 2005). Injection of anti-Sept2 antibodies into mitotic cells also partially blocks cytokinesis (Kinoshita et al., 1997). However, the mechanism underlying these phenotypes is not known.
MTs are highly dynamic, undergoing constant assembly and disassembly. A number of cellular factors regulate this process by binding MTs and affecting their stability (Drewes et al., 1998; Ebneth et al., 1999; Andersen, 2000). One family of regulators consists of the microtubule-associated proteins (MAPs), which bind and stabilize MTs (Mandelkow and Mandelkow, 1995; Andersen, 2000). Three of these proteins, MAP2, MAP4, and tau, are closely related; MAP2 and tau are primarily found in neurons and are important for neuronal differentiation and growth, whereas MAP4 is expressed in a variety of tissues (Ebneth et al., 1999). In addition to effects on MT stability, MAP binding to MTs alters the kinetic properties of several MT motor proteins (Ebneth et al., 1998; Trinczek et al., 1999; Mandelkow et al., 2004). MAP4 has been reported to regulate MT dynamics during cell division (Shiina and Tsukita, 1999a; Chang et al., 2001) and to recruit the cyclin B1/cdc2 kinase complex to MTs during cell division (Ookata et al., 1992, 1993, 1995). These data indicate an important role for MAP4 in the overall regulation of the MT cytoskeleton and its function in the cell.
MAP4 is a multifunctional protein. The N terminus (aa 1–575) contains a large number of acidic amino acids and is postulated to regulate the spacing of individual MTs (Iida et al., 2002). The proline-rich domain (PRD, aa 654–895) contains at least one MT binding site, as well as a region that promotes MT assembly and bundling (Aizawa et al., 1991). This region also binds to cyclin B1 and contains at least two cdk2 phosphorylation sites (Ookata et al., 1995, 1997; Kitazawa et al., 2000). The C-terminal affinity domain (AD, aa 902-1090) contains up to four motifs (depending on the isoform) that are phosphorylated by MARK/Par-1 family kinases. Phosphorylation of MAP4 by cdk2 or MARK/Par-1 kinases inhibits the binding of the MAP to microtubules, causes the MAP4 to dissociate, and leads to decreased MT stability (Drewes et al., 1997). To date, phosphorylation of MAP4 is the only known mechanism of MAP4-MT regulation.
We asked whether septins might regulate MT stability, using small interfering RNAs (siRNA) to deplete endogenous septins. We identify a novel septin binding partner, MAP4, and show that this interaction inhibits the activity of MAP4, by blocking MAP4 binding to MTs. Overall, septins reduce the stability of cellular MTs, and this function seems to be important for normal mitosis and cytokinesis. Together, these data define a novel molecular function for mammalian septins.
MATERIALS AND METHODS
Cell Culture and RNA Interference
Duplex siRNAs were purchased from Dharmacon (Lafayette, CO). Two duplexes targeting different regions of Sept7 (GUCGACAUUAAUCAACUCAdTdT, GGCAGUAUCCUUGGGGUGUdTdT, referred to as Sept7 #1 and #2, respectively), one duplex targeting Sept2 (GGUGAAUAUUGUGCCUGUCdTdT), and one control duplex with no homology to any known gene (GUCGACUGUGGAUUGGCAUAdTdT), were used. MAP4 knockdowns were performed with an anti-MAP4 siRNA pool from Dharmacon. Duplex RNA transfections were performed in HeLa cells, using Oligofectamine (Invitrogen, Carlsbad, CA), and then the cells were incubated for 72 h at 37°C with 5% CO2. Knockdown was confirmed by immunoblotting, using cell lysates normalized for equal protein and separated by SDS-PAGE (Joberty et al., 2000). Immunofluorescence was performed as described previously (Joberty et al., 2001).
Nocodazole Stability Assays
The assays were performed essentially as described by Nguyen et al. (1997). HeLa cells were transfected in duplicate as described above. After 72 h, the transfection medium was removed, and the cells were washed once in DMEM with 5% calf serum and 5% fetal calf serum (FCS) (DMEM 5 + 5). The medium in one well of each transfection was replaced with DMEM 5 + 5, and the other well received DMEM 5 + 5 plus 10 μM nocodazole (Sigma-Aldrich). Cells were incubated for 30 min at 37°C, washed in PHEM buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, 2 mM MgCl2, pH 6.9), and stained for α-tubulin as described above. Slides were examined using a Nikon TE200 inverted fluorescence microscope with a 60× (numerical aperture [N.A.] 1.2) water immersion lens. Images from random fields were captured with an ORCA charge-coupled device camera (Hamamatsu, Bridgewater, NJ) controlled by Openlab 3.5 software. The number of cells containing intact microtubules was counted as a percentage of total cells present in each field. Data were analyzed from three independent experiments.
Antibodies
Antibodies against Ran and MAP4 were obtained from BD Transduction Laboratories (Lexington, KY). Anti-α tubulin and anti-acetylated tubulin were purchased from Sigma-Aldrich (St. Louis, MO). Alexa-594-conjugated phalloidin was purchased from Molecular Probes (Eugene, OR).
To generate monoclonal antibody (mAb) 9E7, a tetramer of glutathione S-transferase (GST)-BD3:Sept2:Sept6:Sept7 was expressed in Escherichia coli BL21(DE3) and purified as described previously (Sheffield et al., 2003). Monoclonal antibodies were produced by the Lymphocyte Culture Facility (University of Virginia, Charlottesville, VA) using standard procedures. The resulting antibodies were screened for recognition of BD3, Sept2, Sept6, and Sept7, and discarded if they recognized GST.
GST-tagged Sept2 or -Sept7 was expressed and purified as described previously (Sheffield et al., 2003). Polyclonal antibodies against each protein were generated in rabbits by Cocalico Biologicals (Reamstown, PA).
Where indicated, blots were stripped by incubating in stripping buffer (62.5 mM Tris-HCl, pH 6.9, 2% SDS, 100 mM 2-mercaptoethanol) for 30 min at 50°C. The stripped blots were washed, reblocked in Tris-buffered saline + 0.05% Tween 20 + 5% dry milk, and reprobed as indicated.
Immunoprecipitation and Mass Spectroscopy
Preparative coimmunoprecipitations (coIPs) were performed essentially as described previously (Joberty et al., 2001). All procedures were performed at 4°C. Briefly, HeLa cells from 3 × 150-mm plates were lysed in lysis buffer (25 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 0.5 mM EDTA, 0.5% Triton X-100, 10 mM phenylmethylsulfonyl fluoride, 2 mM dithiothreitol, 25 μg/ml aprotinin, 12.5 μg/ml leupeptin) (Joberty et al., 2000). Cellular debris was removed by centrifugation, and the supernatant was precleared by incubating with protein A-Sepharose beads (GE Healthcare, Piscataway, NJ) for 30 min with agitation. The cleared supernatant was incubated with 20 μg of anti-Sept6 antibody 9E7 or control mouse IgG for 1 h. A 50% protein A-Sepharose slurry (200 μl) preblocked in 5% bovine serum albumin was added to each immunoprecipitation (IP) and agitated for 1 h. The beads were then collected by centrifugation, washed four times in 2 ml of wash buffer (25 mM HEPES, pH 7.4, 450 mM NaCl, 1 mM MgCl2, 0.5 mM EDTA, 0.5% Triton X-100) (Joberty et al., 2000), once in lysis buffer without Triton X-100, and resuspended in 100 μl of Laemmli buffer. Samples were separated by SDS-PAGE, fixed, and stained with Coomassie Brilliant Blue.
Protein bands were excised from the gel and subjected to in-gel digestion as described previously (Shevchenko et al., 1996). Extracted tryptic peptides were purified with Poros R2 (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions (http://protana.com). The peptides were concentrated into a nano-electrospray capillary and placed in the source of a QSTAR Pulsar hybrid mass spectrometer (Applied Biosystems) to derive de novo peptide sequences. Peptide sequences were searched against the protein and DNA nonredundant data bases using the FASTS algorithm (Mackey et al., 2002).
Fragments of MAP4 were amplified by PCR, cloned into pK-myc, and expressed in COS-7 cells using Lipofectamine 2000 (Invitrogen). Cells were incubated for 24 h and then lysed and clarified. Lysates were incubated with 6 μg of anti-Sept6 or control mouse antibody for 1 h. Preblocked protein A-Sepharose slurry (25 μl) was added to each sample and agitated for 1 h. Beads were collected and washed four times in 1 ml of wash buffer and then washed once in lysis buffer without Triton X-100, and resuspended in Laemmli buffer. After transfer to nitrocellulose, the blots were probed with anti-Sept2 to confirm septin complex precipitation, and with horseradish peroxidase (HRP)-conjugated 9E10 (anti-myc) to identify coprecipitating fragments of MAP4.
Bacterial Expression and In Vitro Binding Assays
A MAP4 fragment comprising the PRD and AD (aa 654-1090) was amplified by PCR and cloned into pGEX 4T-1 (GE Healthcare). Competent BL-21(DE3) E. coli were transformed with pGEX 4T-1 MAP4 PRD+AD, pGEX 4T-1, pGEX 2T-BD3, pQE60-Ran (Plafker and Macara, 2002), or a mixture of p15A-Sept2+pT7-His-Sept6::Sept7 plasmids (Sheffield et al., 2003). For small-scale preparations, bacteria were grown to OD600 = 0.6 and then induced with 1 mM isopropyl-β-d-thiogalactopyranoside. They were grown with shaking overnight at 14°C and then harvested by centrifugation and resuspended in MAP4 binding buffer (20 mM PIPES, pH 6.8, 100 mM NaCl, 1 mM EGTA, 1 mM MgCl2, 0.1% NP-40). Resuspended cells were mixed and then lysed by sonication, clarified by centrifugation, and incubated on ice for 30 min. Each tube received 25 μl of a 50% glutathione-Sepharose (GE Healthcare) slurry in MAP4 binding buffer, and the tubes were agitated 1 h at 4°C. The beads were washed four times in MAP4 binding buffer, resuspended in Laemmli buffer, and separated by SDS-PAGE. Samples were transferred to nitrocellulose and probed with anti-GST for GST-MAP4, GST, or GST-BD3 as well as with anti-His6 antibody (QIAGEN, Valencia, CA, and Covance, Princeton, NJ) for septin trimer or Ran. Sept6:7 dimer or Sept2 monomer binding assays were performed the same way, except that His-GFP was substituted for His-Ran in the Sept2 binding assay.
Large-scale purifications for cosedimentation and bundling assays were performed as described above, but with a GSTrap fast-performance liquid chromatography column (GE Healthcare). The column was washed with PEM buffer, and bound protein was eluted in PEM buffer (80 mM PIPES, 1 mM EGTA, 1 mM MgCl2, pH 6.8) with 100 mM glutathione. The proteins were dialyzed overnight against PEM buffer without glutathione and stored at -80°C.
Microtubule–MAP4 Cosedimentation
Purified tubulin was obtained from Cytoskeleton (Denver, CO), and in vitro sedimentation assays were performed per the manufacturer's instructions. GST-MAP4 fragments or GST were added, as appropriate, to a final concentration of 160 nM in PEM buffer. Sept2:6:7 trimer (final concentration of 2 μM in septin buffer), or an equal volume of septin buffer (50 mM HEPES, pH 7.4, 400 mM NaCl, 2 mM MgCl2, 10% glycerol), was added as indicated. MTs were pelleted at 100,000 × g in a Beckman Ti-100 ultracentrifuge rotor at 25°C. The resulting soluble and pellet proteins were separated by SDS-PAGE; half of each sample was stained with Coomassie Brilliant Blue to detect tubulin and septin trimer, and half was probed with anti-GST for MAP4 fragments. Quantitation of pellet:soluble ratio was determined by densitometry and ImageJ software.
For cell-based assays, HeLa cells were transfected with control or anti-Sept7 siRNA, incubated 72 h, and lysed by sonication. MT-MAP cosedimentation was performed as described previously (Mary et al., 2002). The resulting fractions were probed for MAP4 and α-tubulin as described above.
Bundling Assay
Rhodamine-labeled tubulin was obtained from Cytoskeleton, and MT assembly assays were performed per the manufacturer's instructions. GST or GST-MAP4 PRD+AD (aa 654-1090) (1 μl, final concentration of 1.25 μM) in 0.5× PEM buffer + 0.5× phosphate-buffered saline was mixed on ice with 0.5 μl of Sept2:6:7 trimer (final concentration 6 μM in septin buffer) or septin buffer. Rhodamine-tubulin (5 μg) was resuspended in 4 μl of PEM buffer, and 1 μl of this solution was used per reaction (final concentration of 0.5 mg/ml). The tubes were incubated for 5 min at 37°C, fixed by adding 22.5 μl of 1% glutaraldehyde in PEM buffer, and incubated for 3 min at room temperature. One hundred microliters of 50% glycerol in PEM buffer was added to each reaction, and then 3 μl of each reaction were spotted onto microscope slides and squashed with glass coverslips. Each field was captured at 60× as described previously, and the number of MT bundles per field was counted.
Fluorescence Recovery after Photobleaching (FRAP) Assay
Chinese hamster ovary (CHO) cells were grown in F12 (HAM) medium containing 10% FCS and penicillin/streptomycin. The cells were transfected with control or anti-Sept2 siRNA as described above, and then transfected 48 h later with GFP-MAP4 using FuGENE6 (Roche Diagnostics, Indianapolis, IN) per manufacturer's instructions. After 24 h, the cells were imaged with a 100× oil lens (N.A. 1.4) and subjected to photobleaching using a Zeiss LSM 510 confocal microscope. Images were collected using a 488-nm laser line at a power of 1%, and the regions of interest (ROIs) were bleached with five iterations at 100% power. Seven cells were selected for each condition. Two prebleach images were collected, and recovery was monitored by capturing images every 4 s for 224 s. Mean fluorescence was determined for each ROI, for the unbleached area of each cell, and background. After background subtraction and correction for photobleaching during image collection, fractional fluorescence recovery of the bleached ROI was determined and plotted against postbleach time.
RESULTS
Septin Depletion Increases MT Stability
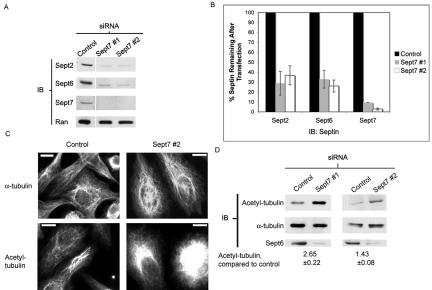
To examine the possible role of septins in microtubule dynamics, RNA interference was used to deplete the endogenous septins. To date, little is known regarding the molecular basis of septin function; it is unclear whether individual septins or their heteromeric complexes are responsible for the activity of these proteins. Moreover, some septins can substitute for others within these complexes, although the functional implications of these transpositions are uncertain. Several groups have reported that Sept7 is present in a variety of septin complexes and that this septin may form part of a “core” around which other septins are added (Peng et al., 2002; Sheffield et al., 2003; Nagata et al., 2004). Two RNA duplexes against different regions of Sept7 were designed, to deplete this septin and limit the possibility of a functional substitution with another septin. These duplexes reduced endogenous expression of Sept7 to 9% and 3% of the level in control-transfected cells, respectively (Figure 1, A and B). As described previously (Kinoshita et al., 2002), knockdown of Sept7 also reduced the expression of Sept2 and Sept6 (Figure 1, A and B). This reduction was not likely to be a result of off-target effects of the siRNAs, because the two regions selected for duplex design were not homologous to each other or to other known proteins. Moreover, knockdown of Sept6 also reduced the expression of Sept7 and Sept2, confirming that these septins exhibit a coordinated regulation of their expression levels (Figure S1).
Figure 1.
Septin depletion increases acetylated tubulin. (A and B) HeLa cells were transfected as described in Materials and Methods and stained for septin expression after 72 h. Ran was used as a loading control. (A) Immunoblot for septin expression after transfection with anti-Sept7 siRNA. (B) Immunoblot bands were scanned, and their intensity was quantified and normalized for Ran expression. Each septin-depleted signal was compared with control-transfected cells from the same experiment. Bars, SEM (n = 3). (C) Immunofluorescence images of control or septin-depleted cells stained with anti-α-tubulin (top) or anti-acetylated tubulin (bottom). Antibody preparations and exposure times were identical between samples. Bar, 10 μm. (D) Control or septin-depleted cells were lysed, separated by SDS-PAGE, and transferred to nitrocellulose. Blots were probed for acetylated tubulin, stripped, and reprobed for α-tubulin. Immunoblot bands were scanned, quantified, and normalized for tubulin expression. The ratios of septin-depleted to control-transfected cells were measured. Each experiment was performed three times, and the mean change ± SEM was determined.
We then asked whether loss of septins might alter MT stability. Control or septin-depleted cells were stained for α-tubulin to assay possible effects of septin knockdown on the microtubule cytoskeleton. Depletion of septins induced a number of changes to the MT architecture. The septin-depleted cells seemed to have more MTs overall, and the perinuclear region was particularly enriched in MTs (Figure 1C, top). To measure this increase in MTs after septin depletion, the total anti-α-tubulin intensity was measured and divided by total cellular area. This per-pixel intensity was elevated in cells transfected with Sept7 siRNA #1 by 33 ± 9% compared with control-transfected cells (p = 3 × 10-6). These changes could be induced by a number of mechanisms, including by an increase in MT stability. To test this possibility, cells were stained for acetylated tubulin, a marker of stabilized MTs (Piperno et al., 1987) (Figure 1C, bottom). Septin-depleted cells seemed to contain more acetylated tubulin, although the effect of the knockdown on acetylated tubulin was difficult to quantify by immunofluorescence. Therefore, control or septin-depleted cells were lysed and blotted for acetylated and total α-tubulin. The two duplexes increased acetylated tubulin by 165 ± 22% (p = 0.03) and 43 ± 8% (p = 0.05), compared with control-transfected cells, after correcting for the total cellular α-tubulin. These results suggest that septin knockdown leads to an increase in MT stability, and that this stabilization increases the total number of MTs in the cell.
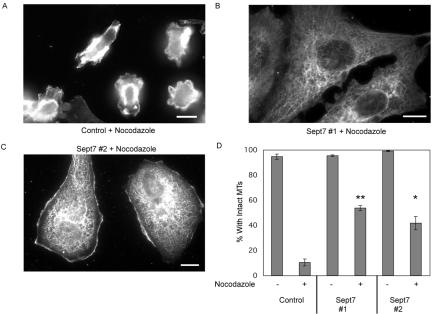
To confirm this stabilizing effect, control or septin-depleted cells were treated with nocodazole and scored for the number of cells with intact microtubules. As seen in the previous experiment, both control and septin-depleted cells had well-defined MT arrays in the absence of nocodazole. However, after treatment with the MT-depolymerizing drug, only 10% of control-transfected cells maintained an MT array, compared with 54 and 42% of siRNA #1- or siRNA #2-transfected cells, respectively (Figure 2, A and B). In addition, septin-depleted cells maintained their cell size and shape after nocodazole treatment, whereas most control-transfected cells began to round up after loss of their MTs (compare Figure 2A with B and C). These results suggest that the increase in acetylated tubulin seen in Figure 1 correlated with an overall stabilization of MTs after septin knockdown. Moreover, these data suggest that septin expression destabilizes MTs in vivo.
Figure 2.
Septin depletion increases MT stability to nocodazole. (A–C) Cells were fixed and stained for α-tubulin after treatment for 30 min with 10 μM nocodazole. Images were captured using a 60× water immersion lens (N.A. 1.2). Bar, 10 μm. (D) At least 250 cells per experiment were counted and scored for the presence or absence of intact MTs as described previously (Nguyen et al., 1997). Graph indicates three independent assays for each condition. Bars, SEM. *p = 0.02, **p = 0.01 (two-tailed Student's t test), compared with control-transfected cells treated with nocodazole.
Mammalian Septins Interact with MAP4
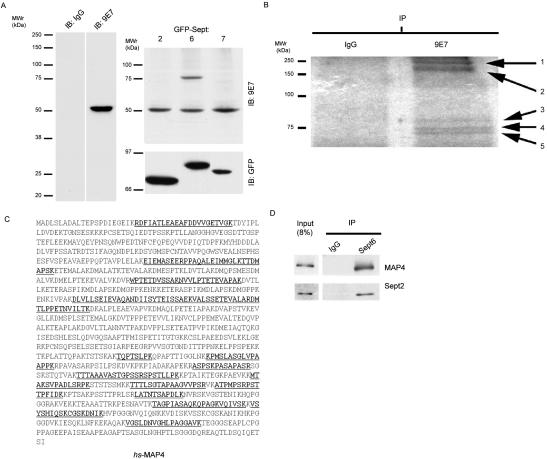
mAb clone 9E7 was generated against recombinant Sept6. In an immunoblot of a HeLa cell lysate, 9E7 recognized a single band of ∼50 kDa, which is the expected size of Sept6 (Figure 3A). Moreover, 9E7 recognized a 75-kDa protein in cells expressing green fluorescent protein (GFP)-Sept6, but it did not react with GFP-tagged Sept2 or Sept7. These results confirm that, of the septins examined in this study, the 9E7 antibody is specific for Sept6 (Figure 3A). Because septins form hetero-oligomers, the immunoprecipitation of any single septin results in copurification of other septins (Kinoshita et al., 2002). Therefore, to test the ability of 9E7 to isolate septin complexes, we performed an immunoprecipitation on HeLa cell lysates with the 9E7 MAb and blotted for Sept2. Sept2 was coprecipitated with 9E7 but not with a nonspecific mouse IgG (Figure 3D).
Figure 3.
Sept6 forms a complex with MAP4. (A) mAb 9E7 recognizes Sept6 but not Sept2 or Sept7. Left, Madin-Darby canine kidney (MDCK) cell lysate (10 μg) was probed with control or 9E7 antibody. Right, MDCK cells expressing Sept2-, Sept6-, or Sept7-GFP were lysed and 10 μg of each lysate was probed with antibody 9E7. The 50-kDa band corresponds to the endogenous 50-kDa band (left). The larger band in the Sept6-GFP lane corresponds to the size of the fusion protein. (B) Sept6 and interacting proteins were precipitated with antibody 9E7 or control antibody and separated by SDS-PAGE. Bands were excised and sequenced by tandem mass spectroscopy. Band 2 corresponds to MAP4. (C) Peptide sequence of human MAP4. Bold/underlined amino acids were identified in the analysis of band 2. (D) Endogenous Sept6 coimmunoprecipitates with endogenous MAP4. Sept6 from HeLa cell lysate was precipitated with 9E7 or control antibody, and the precipitates were probed for MAP4 or Sept2.
To identify Sept6 interaction with nonseptin binding partners, antibody 9E7 or control mouse IgG were used to immunoprecipitate endogenous proteins from HeLa cell lysate. Five protein bands larger than 50 kDa were cut from the gel and analyzed by mass spectroscopy (Figure 3B). Band 1 yielded small fragments of low homology to any known protein. Band 2, with an apparent molecular mass of 220 kDa, was MAP4. Twenty-four peptides, representing 26% of the total protein, were identified (Figure 3C). Bands 3, 4, and 5 were heat-shock protein (Hsp)70, and Hsp70-like proteins; these protein chaperones were not studied further.
Based on the mass spectroscopy data, we focused on MAP4 as a potential binding partner for septins. MAP4 is a well-characterized MT binding protein responsible for stabilization and assembly of MTs. To confirm the sequencing results, endogenous Sept6 was immunoprecipitated from HeLa cell lysate, and the resulting protein complex was probed for MAP4. As shown in Figure 3D, endogenous MAP4 coprecipitated with the endogenous septin complex.
Septins Interact Directly with MAP4 through Its Proline-rich Domain
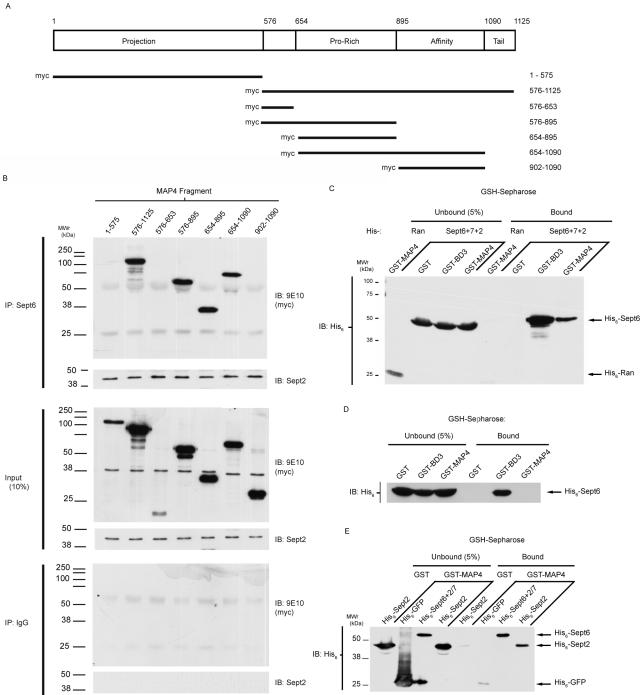
MAP4 is a large protein, with multiple structural, functional, and regulatory domains. To narrow down the region of septin interaction, we generated a variety of MAP4 fragments that were transiently expressed as myc-tagged proteins in COS-7 cells (Figure 4A). Sept6 was immunoprecipitated from lysates of these cells, and the precipitates were probed for the myc epitope. All of the myc-MAP4 fragments were expressed (Figure 4B, middle), but only those that contain the PRD were coprecipitated with endogenous Sept6 (Figure 4B, top).
Figure 4.
Septins interact directly with the PRD of MAP4. (A) Map of MAP4 isoform used in this study, showing relevant domains. Lines beneath the map indicate myc-tagged constructs used throughout this study. (B) Endogenous Sept6 coprecipitations with MAP4 fragments containing the PRD. Myc-tagged MAP4 fragments were expressed in COS-7 cells. Sept6 was purified by immunoprecipitation with antibody 9E7, and interacting proteins were identified with anti-Sept2 or anti-Myc. Top, anti-Sept6 IP; middle, unpurified sample, representing 10% of the protein added to each IP to demonstrate expression; and bottom, control using nonspecific IgG, demonstrating specificity of each reaction. (C) Recombinant septin trimer copurifies with recombinant MAP4(PRD+AD). Proteins were expressed in E. coli as described in Materials and Methods, and GST-tagged proteins were isolated onto glutathione-Sepharose beads. The proteins were separated by SDS-PAGE, transferred, and probed with anti-His6 antibody to determine interaction between His6-Ran or His6-Sept6:Sept2:Sept7 trimer with GST, GST-BD3, or GST-MAP4(PRD+AD). (D) Recombinant Sept6:Sept7 dimer does not copurify with recombinant MAP4(PRD+AD). Proteins were expressed and purified as described above, transferred, and probed with anti-His6 antibody to detect interaction between His6-Sept6: Sept7 dimer with GST, GST-BD3, or GST-MAP4(PRD+AD). (E) Recombinant Sept2 monomer interacts with MAP4(PRD+AD). Proteins were expressed recombinantly, purified, separated, and transferred. The proteins were probed with anti-His6 to determine interaction between His6-GFP, His6-Sept6:Sept2:Sept7 trimer, or His6-Sept2 monomer with GST or GST-MAP4(PRD+AD).
We next assayed whether the septin-MAP association was direct. Using bacterially expressed proteins, GST-MAP4 aa 654-1090 (PRD + AD) was incubated with His6-Sept6:Sept2: Sept7 trimer (tagged Sept6) or with His6-Ran as a negative control and then captured on glutathione-Sepharose beads. As a positive control for septin binding, we used GST-BD3. BD3 is the septin-binding domain of the Borg proteins, which interacts with the Sept6:Sept7 heterodimer and with the Sept2:Sept6:Sept7 heterotrimer (Joberty et al., 2001; Sheffield et al., 2003). Bound proteins were probed by immunoblot with an anti-His6 antibody, to detect the His-tagged Sept6 within the trimer (Figure 4C). The septin trimer was not bound by GST alone, and MAP4 did not bind to His-tagged Ran protein. However, the Sept2:Sept6:Sept7 trimer was precipitated by both GST-BD3 and GST-MAP4(PRD+AD). Therefore, the binding of the septin trimer to MAP4 is specific and direct.
Next, we attempted to identify which septin bound to MAP4. Because Sept6 and Sept7 monomers rapidly precipitate out of solution, we expressed these two septins as a heterodimer. Bacterially expressed His6-Sept6:Sept7 dimer was incubated with recombinant GST, GST-BD3, or GST-MAP4(PRD+AD) and then captured on glutathione-Sepharose beads and probed for His6-Sept6, as described above. The Sept6:Sept7 dimer did not bind to GST-MAP4, or to GST, but it was coprecipitated with GST-BD3 (Figure 4D). To examine whether Sept2 mediates the binding of the septin trimer to MAP4, we expressed His6-GFP, His6-Sept6: Sept2:Sept7 trimer, and His6-Sept2 in bacteria and assayed for any interaction of these proteins with recombinant GST or GST-MAP4(PRD+AD) (Figure 4E). In this assay, Sept2 monomer bound specifically to MAP4(PRD+AD). We also detected an interaction between the Sept2:Sept6:Sept7 trimer and MAP4 and between Sept2 monomer and MAP4 but not between Sept6:Sept7 dimer and MAP4. These results show that MAP4 does not interact with Sept6 or Sept7, but can bind directly to Sept2, either as an isolated monomer or in a Sept2:6:7 trimer.
Septins Inhibit MT–MAP4 Interaction
One possible explanation for the increase in MT stability caused by knockdown of Sept7 is that there is a coordinate regulation of MAP4 and septin expression levels, such that loss of septins causes an increase in MAP4. However, MAP4 expression remained unchanged after septin depletion (Figure 5A). We therefore asked whether the interaction of MAP4 with septins alters the ability of MAP4 to bind microtubules.
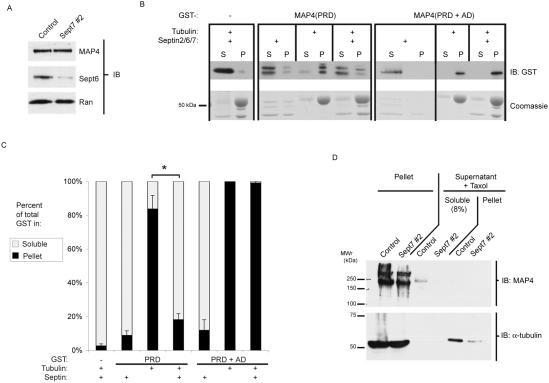
Figure 5.
Septins interrupt MAP4–MT interaction. (A) Control or septin-depleted cells were lysed, separated by SDS-PAGE (10 μg/lane), transferred to nitrocellulose, and probed with anti-MAP4, anti-Sept6, or anti-Ran antibodies. (B and C) In vitro sedimentation assays. (B) GST or GST-MAP4 fragments (160 nM final concentration) were coincubated with and without septin trimer (2 μM final concentration) and in vitro polymerized MTs (200 ng of tubulin per reaction). MTs were sedimented by centrifugation, the soluble fraction and pellet were each divided in half, and the duplicated samples were separated by duplicate SDS-PAGE. One sample was transferred to nitrocellulose and probed for GST to identify the distribution of GST and GST-MAP4 (top row). The other sample was fixed and Coomassie stained to visualize the distribution of septin trimer (triplet found approximately equally in soluble and pellet) and tubulin (larger, darker band predominantly in pellet). S, soluble fraction; P, pellet fraction. The - indicates GST alone, without a fusion partner. (C) Quantitation of above results. Anti-GST immunoblots were scanned to quantify the distribution of GST or GST-MAP4 fragments. The amount of protein in each fraction (S or P) is presented as a percentage of the total protein in each incubation. Bars, SEM (n = 3). *p <0.03, as determined by a two-tailed Student's t test. (D) In vivo MT spindown assay. Control or septin-depleted cells were lysed by sonication, their intact cytoskeleton sedimented, and the soluble tubulin was repolymerized with 10 μM taxol + 0.5 mM GTP. The repolymerized MTs and any binding partners were then sedimented by ultracentrifugation. The pellets were solubilized; the pellets and 8% of the soluble fraction were separated by SDS-PAGE, transferred to nitrocellulose, and probed with anti-MAP4 (top) and anti-α-tubulin (bottom).
MAP4 regulates MT stability by binding to MTs and inhibiting their depolymerization (Mandelkow and Mandelkow, 1995; Andersen, 2000). We therefore examined the role of septins on the in vitro association of MAP4 with MTs, using a MT spindown assay. Purified, taxol-stabilized MTs were incubated with MAP4 fragments together with or without septin trimer. The MTs were then pelleted by centrifugation, and the distribution of the MAP4 was examined by immunoblot. GST-MAP4(PRD) is soluble in the absence of taxol-stabilized MTs (Figure 5, B and C). When incubated with MTs, this fusion protein partitions into the pellet. However, when the GST-MAP4(PRD) was incubated with septin trimer before addition of the MTs, the MAP4 remained in the soluble fraction. These results indicate that septin binding to the proline-rich domain of MAP4 prevents the PRD from associating with MTs.
Unexpectedly, the inhibition by septins was observed only for the isolated PRD. The larger MAP4(PRD+AD) fragment contains at least two distinct MT binding sites, because each domain can bind independently to MTs (Aizawa et al., 1991), and the incubation of this GST-fusion fragment with septin trimer did not alter its cosedimentation with MTs (Figure 5, B and C). These data demonstrate that septins can interact directly with MAP4 through its PRD and block the binding of this domain to MTs.
To analyze the effect of septins on MAP4 binding to MTs in a more biological context, we used a spin-down method described previously (Mary et al., 2002) to copurify endogenous MTs and MAPs from cell lysates. Our model suggests that septins can bind to MAP4 and reduce the affinity of at least one of its domains for MTs. Therefore, silencing of septin expression by RNA interference (RNAi) should increase the fraction of MAP4 that is complexed with MTs. As shown in Figure 5D, this was the observed effect. The majority of tubulin was purified in the first pellet, presumably as intact MTs (Figure 5D, bottom, left two lanes). In both control and septin-depleted cells, MAP4 was detected in this first pellet (Figure 5D, top, left two lanes). However, control-transfected cells also contained a significant portion of their endogenous MAP4 in a form that did not precipitate in the initial pellet, whereas no soluble MAP4 was observed in the septin-depleted cells (Figure 5D, top, “soluble” fraction). Note that only 8% of the soluble fraction was separated on the gel, whereas the entire pellet fraction was loaded. Moreover, the soluble MAP4 did not cosediment with repolymerized MTs (Figure 5D, top, rightmost two lanes), suggesting that some fraction of MAP4 in control-transfected cells had lost the ability to bind and copurify with MTs. This is consistent with the hypothesis that the septins in the control cells bind and sequester MAP4 away from MTs. Furthermore, septin depletion seemed to increase MT stability in this assay, because septin-depleted cells contained less free tubulin after lysis, as observed by the decrease in tubulin staining in the repolymerized MT fraction (Figure 5D, bottom, rightmost two lanes).
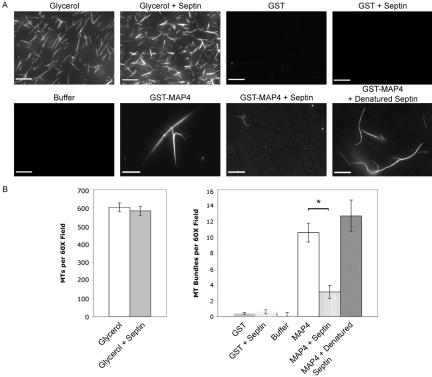
We next examined the effects of septins on other MAP4 functions. We had demonstrated that septins bind to MAP4 via its PRD (Figure 4A) and that this interaction is sufficient to disrupt MAP4-MT binding in vitro and in vivo (Figure 5) and alter MT stability in vivo (Figure 1D). First, we asked whether septins directly alter the formation of MTs in vitro. Glycerol reduces the critical concentration of tubulin required for the spontaneous assembly of microtubules, as can be seen in Figure 6A (top left; compare with addition of GST, top right). The addition of Sept2:6:7 trimer did not have any significant effect on this nonspecific induction of MTs (Figure 6A, top middle, and B). Because the PRD of MAP4 induces assembly and bundling of MTs (Aizawa et al., 1991), we next tested whether septins alter this activity of MAP4. As expected, GST-MAP4 PRD+AD induced the formation of large, thick aggregates of rhodamine-tubulin when incubated in the absence of septins. These MTs were qualitatively different from MTs induced by glycerol, because of the bundling activity of the MAP4 fragment (Figure 6A, bottom). When Sept2:6:7 trimer and MAP4 were added to the rhodamine-tubulin, the bundling effect disappeared, and the resulting mixture looked similar to incubation of rhodamine-tubulin with GST or buffer alone (Figure 6). This effect seemed to be specific to native septin trimer. When the septin proteins were boiled and cooled before coincubation with GST-MAP4 PRD+AD and rhodamine tubulin, MT assembly and bundling was restored to similar levels observed with GST-MAP4 PRD+AD alone. Sample 60× microscope fields are shown in Figure 6A, and quantitation of this effect is shown in Figure 6B.
Figure 6.
Septins reduce MAP4-dependent MT assembly and bundling. Rhodamine-labeled tubulin (0.5 mg/ml final concentration) was incubated with 10% glycerol, GST, or GST-MAP4 (PRD+AD) (final concentration of GST proteins 1.25 μM) in the presence or absence of recombinant Sept2:6:7 trimer (6 μM final concentration). The resulting MTs were fixed, squashed, and sealed under glass coverslips. Images were captured using a 60× water immersion lens (N.A. 1.2). (A) Representative images of polymerized rhodamine-tubulin. Bar, 10 μm. (B) Quantitation of bundling seen in A. Ten 60× fields were randomly imaged, and the number of MTs was counted in each. Bars, SEM. *p = 0.001, as determined by a two-tailed Student's t test.
Septin-mediated MT Stabilization Requires MAP4
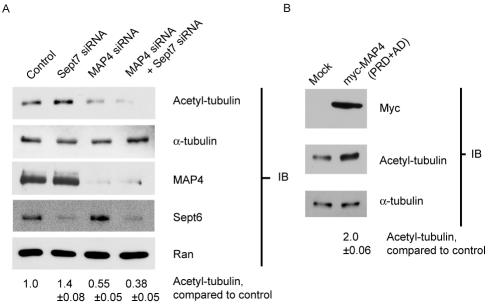
To further examine whether MT stabilization is regulated through septin interaction with MAP4, MAP4 expression was silenced by siRNAs. Cells were transfected with control siRNA or with siRNAs directed against Sept7, MAP4, or both Sept7 plus MAP4. Cell lysates were probed with antibodies against MAP4, Sept6, and Ran to confirm knockdown, and with anti-acetylated tubulin and anti-α-tubulin antibodies to assay for stabilized MTs. In addition, cells were fixed, stained for MAP4, and directly visualized to confirm knockdown (Figure S2).
Depletion of MAP4 reduced the cellular pool of acetylated tubulin (Figure 7A). The reverse was also found, because expression of the MAP4(PRD+AD) fragment caused an increase in overall acetylated tubulin (Figure 7B), suggesting that this approach was valid for examining overall MT stability. More importantly, targeting both Sept7 and MAP4 for knockdown eliminated the increase in MT stability seen after septin depletion (Figures 1D and 7A). When both septins and MAP4 were depleted, overall acetylated tubulin decreased to a level similar to that seen with MAP4 knockdown alone. MAP4 depletion reduced acetylated tubulin by 45 ± 5% (p = 0.02); MAP4 and Sept7 codepletion reduced acetylated tubulin by 62 ± 5% (p = 0.01). This suggests that MAP4 acts in a pathway downstream of, or parallel to and necessary for, septin regulation of MT stability and formation.
Figure 7.
MAP4 alters MT stability. (A) MAP4 depletion blocks septin knockdown-mediated increase in acetylated tubulin. HeLa cells were transfected with control, Sept7, MAP4, or Sept7 + MAP4 siRNA as indicated in Materials and Methods and incubated for 72 h. The cells were lysed, and 4 μg of each lysate was separated by SDS-PAGE and transferred to nitrocellulose. The blots were probed with anti-acetylated tubulin, stripped, and reprobed with anti-α-tubulin. Identical blots were probed with anti-MAP4, anti-Sept6, and anti-Ran to confirm knockdown. Acetyl-tubulin bands from three experiments were scanned, quantitated, and normalized for α-tubulin expression. The ratio of siRNA- to control-transfected cells was determined for each condition. The numbers below each lane indicate the mean ratio from three experiments ± SEM. (B) MAP4 overexpression increases cellular levels of acetylated tubulin. HeLa cells were transfected with pK-myc or pK-myc-MAP4 (PRD+AD). The cells were lysed, separated, transferred, and probed for acetylated tubulin. Identical blots were probed with HRP-conjugated 9E10 antibody to confirm MAP4 expression. The increase in acetylated tubulin was determined from three experiments ± SEM.
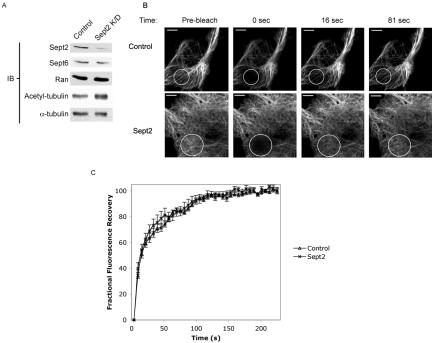
Septin Depletion Does Not Alter GFP-MAP4 FRAP
We next examined the effect of septin depletion on MAP4-MT dynamics in living cells. CHO cells were transfected with control- or Sept2-specific siRNA, incubated for 48 h, and then transfected with a GFP-full length MAP4 construct (Olson and Olmsted, 1999). After an additional 24 h to allow expression of GFP-MAP4, the cells were subjected to FRAP analysis. Results of septin depletion in CHO cells mirrored the results seen in other cell lines (Figure 1); expression of both Sept2 and Sept6 decreased (by 89 and 46%, respectively), whereas acetylation of tubulin increased (Figure 8A). At low expression levels, GFP-MAP4 was observed decorating the MTs of transfected cells (Figure 8B). Because the level of septin knockdown could not be assayed in individual cells under the experimental conditions used, Sept2 siRNA-transfected cells were chosen for FRAP analysis based on morphological characteristics seen after septin depletion: large, rounded, flat cells with multiple nuclei. GFP-MAP4 was bleached with five cycles at 100% laser power and imaged at 4-s intervals for 224 s. As seen in Figure 8B, the bleached area recovered as a whole, rather than in a “treadmilling” manner. These results confirm that MAP4 binding to MTs is dynamic and that MT-bound MAP4 is constantly exchanged with a cytoplasmic MAP4 pool, as has been suggested previously (Olmsted et al., 1989). GFP-MAP4 recovery onto MTs was rapid, with a t1/2 of ∼16 s (Figure 8). Despite the changes in acetylated tubulin, there seemed to be little difference in fluorescence recovery between control-transfected and septin-depleted cells. These results suggest that septin binding to MAP4 does not alter the rate of dissociation from MTs.
Figure 8.
Septin depletion does not alter MAP4-MT FRAP. (A) Sept2 depletion in CHO cells increases acetylated tubulin. CHO cells were transfected with control or Sept2 siRNA as indicated in Materials and Methods and incubated for 72 h. The cells were lysed, and 5 μg of each lysate was separated by SDS-PAGE and transferred to nitrocellulose. The blots were probed with anti-acetylated tubulin, stripped, and reprobed with anti-α-tubulin. Identical blots were probed with anti-Sept2, anti-Sept6, and anti-Ran to confirm knockdown. (B and C) GFP-MAP4 photobleaching in CHO cells. CHO cells were transfected with control- or anti-Sept2 siRNA, incubated 48 h, and then transfected with GFP-MAP4 as described in Materials and Methods. The marked region was photobleached, and the recovery of GFP-MAP4 into the bleached region was imaged every 4s for 224s. Bar, 5 μm. (C) For each condition, the fractional fluorescence recovery was collected and plotted against postbleach time.
Mitotic Defects Caused by Septin Depletion Are Suppressed by MAP4 Depletion
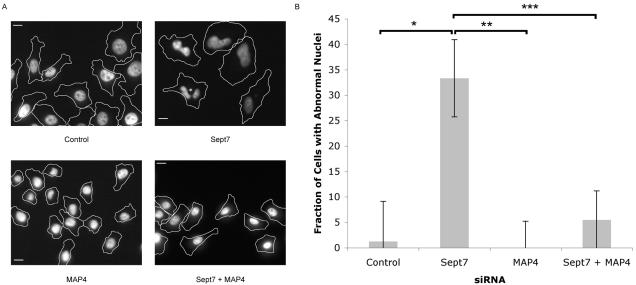
Multiple groups have shown that septin depletion yields fragmented or multinucleated cells consistent with defects in mitosis and failed cytokinesis; however, the mechanism by which septins regulate cell division is unknown (Kinoshita et al., 1997; Nagata et al., 2003; Spiliotis et al., 2005). To examine whether the septin–MAP4 interaction is important for this process, HeLa cells were transfected with siRNAs targeted against Sept7 or MAP4 or against both Sept7 and MAP4. Half of the cells were lysed to confirm knockdown, and the remaining cells were fixed and stained with phalloidin (to determine the periphery of each cell) and Hoechst dye (to observe the nuclei). Random fields were imaged, the Hoechst and phalloidin channels were merged, and the images were scored blindly for cells with multiple or fragmented nuclei. Typical results are shown in Figure 9. Aberrant nuclei were very rare in control-transfected cells. In contrast, septin-depleted cells contained distorted nuclei, nuclear fragments or double nuclei, consistent with previous observations (Kinoshita et al., 1997; Nagata et al., 2003; Spiliotis et al., 2005). In contrast, the nuclei of cells transfected with anti-MAP4 siRNA alone seemed to be normal. This result did not seem to be due to a failure of cell division, as the average number of cells per field was similar between control-transfected cells and MAP4-depleted cells (12.4 ± 5.1 versus 15.2 ± 5.3, respectively, for the data in Figure 9B). More importantly, MAP4 depletion suppressed the mitotic defects induced by silencing of septin expression; the number of cells with multiple nuclei was not significantly different between control-transfected and septin + MAP4-depleted cells. These results suggest that septins act through MAP4 to regulate cell division.
Figure 9.
MAP4 is necessary for mitotic defects resulting from septin depletion. (A and B) Sept7 depletion induces an abnormal nuclear phenotype in HeLa cells, which is suppressed by concomitant MAP4 depletion. HeLa cells were transfected with control or Sept7 siRNA and/or MAP4 siRNA, as indicated in Materials and Methods, and incubated for 72 h. The cells were fixed and stained with Alexa 594-conjugated phalloidin and Hoechst dye. Random fields were imaged, and cells were scored for the presence of multiple nuclei or nuclear fragments in a blinded manner. Sample fields are shown in A. The Hoechst channel is shown, with the outline of each cell as indicated by phalloidin staining indicated by the solid lines. Bar, 10 μm. (B) Quantitation of above-mentioned results. One representative experiment of three is shown. Bar, SEM. *p = 1.8 × 10-5, **p = 3 × 10-7, ***p = 1 × 10-7 (two-tailed Student's t test).
DISCUSSION
In interphase cells, mammalian septins often colocalize with the actin or microtubule cytoskeletons, with which they show some level of interdependence for structural integrity. However, the roles of these associations have yet to be determined. To identify key functions for septins in cytoskeletal regulation, we suppressed cellular septin expression by RNAi. As described previously (Kinoshita et al., 2002), knockdown of a single septin results in the coordinate reduction in levels of other tested septins. Septins both in vivo and in vitro assemble into multioligomeric filaments (Joberty et al., 2001; Kinoshita et al., 2002; Surka et al., 2002; Sheffield et al., 2003), and stable expression in vitro requires the presence of at least a Sept6:Sept7 dimer (Joberty et al., 2001; Sheffield et al., 2003), so it is possible that in the absence of Sept7 the remaining septins cannot assemble correctly into oligomers and are destroyed.
The organization of the MT network was not visibly perturbed in septin-depleted cells but, unexpectedly, the level of acetylated tubulin was significantly increased, which is indicative of MT stabilization. Consistent with this change, the disassembly of MTs by nocodazole was blocked in cells lacking septins. Therefore, it seems that one function of septins is to destabilize MTs.
The cellular MT array is in a constant state of flux, and the magnitude of this flux varies both temporally, through the cell cycle (Rusan et al., 2001; Rubin and Atweh, 2004), and spatially, as in cell migration and axonal extension (Riederer et al., 1997; Gupton et al., 2002; Suter et al., 2004). Cells contain many proteins that regulate MTs, often by interacting directly with MTs/tubulin to alter the rates of assembly, disassembly, or both (Chang et al., 2001; Rubin and Atweh, 2004). To seek the mechanism by which septins alter MT stability, we identified Sept6-associated proteins from immunoprecipitates by mass spectrometry and found that septins interact with MAP4, a ubiquitously expressed, type II, microtubule-binding protein homologous to the neuronal protein tau (Katsuki et al., 1999). Although septins have been shown previously to colocalize with MTs (Surka et al., 2002; Nagata et al., 2003; Vega and Hsu, 2003), the conditions used to prepare the cell lysate would cause disassembly of MTs. Therefore, it is unlikely that the coprecipitation of MAP4 was mediated indirectly by MTs. In addition, in an in vitro MT sedimentation assay, the fraction of septins found in the pellet remained unchanged in the presence or absence of polymerized MTs (Figure 5, B and C). Moreover, bacterially expressed septin trimer and Sept2 monomer bind recombinant MAP4 (Figure 4). Together, these data demonstrate that septin-MAP4 interaction is direct and is not mediated by MTs or other MT-binding proteins.
MAP4 is a large protein that contains several functionally distinct domains. We localized the septin-binding region to the PRD, which binds to MTs and can promote both nucleation and bundling (Aizawa et al., 1991; Nguyen et al., 1999). Septins directly inhibit the binding of the PRD to MTs and inhibit PRD-dependent MT formation and bundling in vitro. However, MAP4 contains a second MT-binding domain, called AD, that does not bundle MTs (Aizawa et al., 1991), and septins do not interfere with this domain. Thus, coincubation with septin trimer does not block MT binding by the MAP4(PRD+AD) fragment, but it does still inhibit bundling. Nonetheless, almost all of the MAP4 in septin-depleted cells is bound to MTs, whereas a fraction of MAP4 remains unbound in control cells. This observation indicates that septins are capable of displacing MAP4 from MTs in the context of the intact cell, even though in vitro the AD is sufficient to retain the association in the presence of septins. One explanation for this observation is that other factors within the cell coordinate with septins to regulate MAP4-MT binding.
Previous studies of MAP4 regulation have focused on the roles of protein kinases in regulating the MAP4-MT interaction, and a number of kinases with differing specificities have been identified. Cdc2 kinase phosphorylates at least two serines in the PRD (Ookata et al., 1997; Kitazawa et al., 2000), whereas kinases of the MARK/Par-1 family phosphorylate multiple serines in the AD (Drewes et al., 1997, 1998; Ebneth et al., 1999). These phosphorylation events inhibit the MAP4–MT interaction and lead to destabilization of MTs (Drewes et al., 1997; Drewes et al., 1998; Ebneth et al., 1999). We speculate that septins and MARK/Par-1 kinases might work coordinately to displace MAP4 from MTs, because each regulates a distinct MT binding domain.
Our data suggested two possible mechanisms for septin–MAP4 interaction that are not mutually exclusive. First, septins might bind to soluble MAP4 and prevent its binding to MTs. Alternately, septins might bind to MT-associated MAP4 and induce its dissociation. To differentiate between these two models, we performed FRAP analysis. Septin depletion did not alter the rate of GFP-MAP4 fluorescence recovery into the bleached area, consistent with the idea that septins bind to cytoplasmic MAP4 and prevent its association with MTs, rather than induce dissociation of MAP4 from the MTs.
Functions for mammalian septins have been described for two distinct steps in cell division: first during chromosome segregation, and, later, during cytokinesis (Kinoshita et al., 1997; Spiliotis et al., 2005). Depletion of septins by RNAi induces defects in both of these processes, although with rather low penetrance, and in neither case has the mechanism been determined. MAP4 regulates microtubule dynamics during cell division, and the overexpression of certain MAP4 mutants can inhibit progression through the cell cycle (Shiina and Tsukita, 1999a,b; Chang et al., 2001). These mutants bind more avidly to MTs and increase the cellular MT stability (Chang et al., 2001). We now show that cell division defects induced by septin depletion can be overcome by the concomitant silencing of MAP4 expression (Figure 9). These results suggest a plausible mechanism by which septins might affect cell division. By binding and sequestering MAP4, septins contribute to the dynamic instability of the MT cytoskeleton during mitosis and cytokinesis. Loss of septins will increase the availability of MAP4, which will cause an inappropriate stabilization of the MT cytoskeleton (Figure 7A) and hinder cell cycle progression. Depleting both septins and MAP4 overcomes this stabilization, and cells are consequently able to proceed through mitosis and cytokinesis. Interestingly, the surface area of the septin-depleted cells was also reduced by knockdown of MAP4, suggesting that the MAP4-mediated regulation of MT stability by septins might be an important factor, even in interphase, for cell morphology. It will be important to identify further molecular functions of this regulatory pathway and to determine whether there is cross-talk between septins and the protein kinases that also participate in the control of MAP4 interactions with MTs.
Supplementary Material
Acknowledgments
We thank Janet Olmsted (University of Rochester, Rochester, NY) for the GFP-MAP4 construct and the Lymphocyte Culture Facility (University of Virginia) for development of mAb 9E7. We also thank Claudia Low (University of Virginia) for providing recombinant septin trimer, Laura Adang (University of Virginia) for blinded analysis of immunofluorescence data, and Anne Spang (FML, Tübingen, Germany) for critical reading of the manuscript. This work was supported by Grant GM-66306 from the National Institutes of Health, Department of Health and Human Services.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-03-0267) on August 10, 2005.
Abbreviations used: AD, affinity domain; MAP4, microtubule-associated protein 4; MT, microtubule; PRD, proline-rich domain; ROI, region of interest.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Aizawa, H., Emori, Y., Mori, A., Murofushi, H., Sakai, H., and Suzuki, K. (1991). Functional analyses of the domain structure of microtubule-associated protein-4 (MAP-U). J. Biol. Chem. 266, 9841-9846. [PubMed] [Google Scholar]
- Andersen, S. S. (2000). Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18. Trends Cell Biol. 10, 261-267. [DOI] [PubMed] [Google Scholar]
- Barral, Y., Mermall, V., Mooseker, M. S., and Snyder, M. (2000). Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell 5, 841-851. [DOI] [PubMed] [Google Scholar]
- Castillon, G. A., Adames, N. R., Rosello, C. H., Seidel, H. S., Longtine, M. S., Cooper, J. A., and Heil-Chapdelaine, R. A. (2003). Septins have a dual role in controlling mitotic exit in budding yeast. Curr. Biol. 13, 654-658. [DOI] [PubMed] [Google Scholar]
- Caviston, J. P., Longtine, M., Pringle, J. R., and Bi, E. (2003). The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol. Biol. Cell 14, 4051-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, W., Gruber, D., Chari, S., Kitazawa, H., Hamazumi, Y., Hisanaga, S., and Bulinski, J. C. (2001). Phosphorylation of MAP4 affects microtubule properties and cell cycle progression. J. Cell Sci. 114, 2879-2887. [DOI] [PubMed] [Google Scholar]
- Chant, J. (1996). Septin scaffolds and cleavage planes in Saccharomyces. Cell 84, 187-190. [DOI] [PubMed] [Google Scholar]
- Dent, J., Kato, K., Peng, X. R., Martinez, C., Cattaneo, M., Poujol, C., Nurden, P., Nurden, A., Trimble, W. S., and Ware, J. (2002). A prototypic platelet septin and its participation in secretion. Proc. Natl. Acad. Sci. USA 99, 3064-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes, G., Ebneth, A., and Mandelkow, E. M. (1998). MAPs, MARKs and microtubule dynamics. Trends Biochem. Sci. 23, 307-311. [DOI] [PubMed] [Google Scholar]
- Drewes, G., Ebneth, A., Preuss, U., Mandelkow, E. M., and Mandelkow, E. (1997). MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89, 297-308. [DOI] [PubMed] [Google Scholar]
- Ebneth, A., Drewes, G., Mandelkow, E. M., and Mandelkow, E. (1999). Phosphorylation of MAP2c and MAP4 by MARK kinases leads to the destabilization of microtubules in cells. Cell Motil. Cytoskeleton 44, 209-224. [DOI] [PubMed] [Google Scholar]
- Ebneth, A., Godemann, R., Stamer, K., Illenberger, S., Trinczek, B., and Mandelkow, E. (1998). Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J. Cell Biol. 143, 777-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, C. M., and Kellogg, D. (1999). Septins: cytoskeletal polymers or signalling GTPases? Trends Cell Biol. 9, 387-394. [DOI] [PubMed] [Google Scholar]
- Gupton, S. L., Salmon, W. C., and Waterman-Storer, C. M. (2002). Converging populations of f-actin promote breakage of associated microtubules to spatially regulate microtubule turnover in migrating cells. Curr. Biol. 12, 1891-1899. [DOI] [PubMed] [Google Scholar]
- Hartwell, L. H. (1971). Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp. Cell Res. 69, 265-276. [DOI] [PubMed] [Google Scholar]
- Iida, J., Itoh, T. J., Hotani, H., Nishiyama, K., Murofushi, H., Bulinski, J. C., and Hisanaga, S. (2002). The projection domain of MAP4 suppresses the microtubule-bundling activity of the microtubule-binding domain. J. Mol. Biol. 320, 97-106. [DOI] [PubMed] [Google Scholar]
- Joberty, G., Perlungher, R. R., Sheffield, P. J., Kinoshita, M., Noda, M., Haystead, T., and Macara, I. G. (2001). Borg proteins control septin organization and are negatively regulated by Cdc42. Nat. Cell Biol. 3, 861-866. [DOI] [PubMed] [Google Scholar]
- Joberty, G., Petersen, C., Gao, L., and Macara, I. G. (2000). The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2, 531-539. [DOI] [PubMed] [Google Scholar]
- Kartmann, B., and Roth, D. (2001). Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J. Cell Sci. 114, 839-844. [DOI] [PubMed] [Google Scholar]
- Katsuki, M., Tokuraku, K., Murofushi, H., and Kotani, S. (1999). Functional analysis of microtubule-binding domain of bovine MAP4. Cell Struct. Funct. 24, 337-344. [DOI] [PubMed] [Google Scholar]
- Kinoshita, A., Noda, M., and Kinoshita, M. (2000). Differential localization of septins in the mouse brain. J. Comp. Neurol. 428, 223-239. [DOI] [PubMed] [Google Scholar]
- Kinoshita, M., Field, C. M., Coughlin, M. L., Straight, A. F., and Mitchison, T. J. (2002). Self- and actin-templated assembly of mammalian septins. Dev. Cell 3, 791-802. [DOI] [PubMed] [Google Scholar]
- Kinoshita, M., Kumar, S., Mizoguchi, A., Ide, C., Kinoshita, A., Haraguchi, T., Hiraoka, Y., and Noda, M. (1997). Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 11, 1535-1547. [DOI] [PubMed] [Google Scholar]
- Kitazawa, H., et al. (2000). Ser787 in the proline-rich region of human MAP4 is a critical phosphorylation site that reduces its activity to promote tubulin polymerization. Cell Struct. Funct. 25, 33-39. [DOI] [PubMed] [Google Scholar]
- Longtine, M. S., and Bi, E. (2003). Regulation of septin organization and function in yeast. Trends Cell Biol. 13, 403-409. [DOI] [PubMed] [Google Scholar]
- Longtine, M. S., DeMarini, D. J., Valencik, M. L., Al-Awar, O. S., Fares, H., De Virgilio, C., and Pringle, J. R. (1996). The septins: roles in cytokinesis and other processes. Curr. Opin. Cell Biol. 8, 106-119. [DOI] [PubMed] [Google Scholar]
- Macara, I. G., et al. (2002). Mammalian septins nomenclature. Mol. Biol. Cell 13, 4111-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey, A. J., Haystead, T. A., and Pearson, W. R. (2002). Getting more from less: algorithms for rapid protein identification with multiple short peptide sequences. Mol. Cell Proteomics 1, 139-147. [DOI] [PubMed] [Google Scholar]
- Mandelkow, E., and Mandelkow, E. M. (1995). Microtubules and microtubule-associated proteins. Curr. Opin. Cell Biol. 7, 72-81. [DOI] [PubMed] [Google Scholar]
- Mandelkow, E. M., Thies, E., Trinczek, B., Biernat, J., and Mandelkow, E. (2004). MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J. Cell Biol. 167, 99-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary, S., Charrasse, S., Meriane, M., Comunale, F., Travo, P., Blangy, A., and Gauthier-Rouviere, C. (2002). Biogenesis of N-cadherin-dependent cell-cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism. Mol. Biol. Cell 13, 285-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata, K., Asano, T., Nozawa, Y., and Inagaki, M. (2004). Biochemical and cell biological analyses of a mammalian septin complex, Sept7/9b/11. J. Biol. Chem. 279, 55895-55904. [DOI] [PubMed] [Google Scholar]
- Nagata, K., et al. (2003). Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J. Biol. Chem. 278, 18538-18543. [DOI] [PubMed] [Google Scholar]
- Nguyen, H. L., Chari, S., Gruber, D., Lue, C. M., Chapin, S. J., and Bulinski, J. C. (1997). Overexpression of full- or partial-length MAP4 stabilizes microtubules and alters cell growth. J. Cell Sci. 110, 281-294. [DOI] [PubMed] [Google Scholar]
- Nguyen, H. L., Gruber, D., and Bulinski, J. C. (1999). Microtubule-associated protein 4 (MAP4) regulates assembly, protomer-polymer partitioning and synthesis of tubulin in cultured cells. J. Cell Sci. 112, 1813-1824. [DOI] [PubMed] [Google Scholar]
- Nguyen, T. Q., Sawa, H., Okano, H., and White, J. G. (2000). The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J. Cell Sci. 113, 3825-3837. [DOI] [PubMed] [Google Scholar]
- Oegema, K., Desai, A., Wong, M. L., Mitchison, T. J., and Field, C. M. (1998). Purification and assay of a septin complex from Drosophila embryos. Methods Enzymol. 298, 279-295. [DOI] [PubMed] [Google Scholar]
- Olmsted, J. B., Stemple, D. L., Saxton, W. M., Neighbors, B. W., and McIntosh, J. R. (1989). Cell cycle-dependent changes in the dynamics of MAP 2 and MAP 4 in cultured cells. J. Cell Biol. 109, 211-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, K. R., and Olmsted, J. B. (1999). Analysis of microtubule organization and dynamics in living cells using green fluorescent protein-microtubule-associated protein 4 chimeras. Methods Enzymol. 302, 103-120. [DOI] [PubMed] [Google Scholar]
- Ookata, K., Hisanaga, S., Bulinski, J. C., Murofushi, H., Aizawa, H., Itoh, T. J., Hotani, H., Okumura, E., Tachibana, K., and Kishimoto, T. (1995). Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2 kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J. Cell Biol. 128, 849-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookata, K., Hisanaga, S., Okano, T., Tachibana, K., and Kishimoto, T. (1992). Relocation and distinct subcellular localization of p34cdc2-cyclin B complex at meiosis reinitiation in starfish oocytes. EMBO J. 11, 1763-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookata, K., Hisanaga, S., Okumura, E., and Kishimoto, T. (1993). Association of p34cdc2/cyclin B complex with microtubules in starfish oocytes. J. Cell Sci. 105, 873-881. [DOI] [PubMed] [Google Scholar]
- Ookata, K., Hisanaga, S., Sugita, M., Okuyama, A., Murofushi, H., Kitazawa, H., Chari, S., Bulinski, J. C., and Kishimoto, T. (1997). MAP4 is the in vivo substrate for CDC2 kinase in HeLa cells: identification of an M-phase specific and a cell cycle-independent phosphorylation site in MAP4. Biochemistry 36, 15873-15883. [DOI] [PubMed] [Google Scholar]
- Peng, X. R., Jia, Z., Zhang, Y., Ware, J., and Trimble, W. S. (2002). The septin CDCrel-1 is dispensable for normal development and neurotransmitter release. Mol. Cell. Biol. 22, 378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno, G., LeDizet, M., and Chang, X. J. (1987). Microtubules containing acetylated α-tubulin in mammalian cells in culture. J. Cell Biol. 104, 289-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plafker, K., and Macara, I. G. (2002). Fluorescence resonance energy transfer biosensors that detect Ran conformational changes and a Ran x GDP-importin-β-RanBP1 complex in vitro and in intact cells. J. Biol. Chem. 277, 30121-30127. [DOI] [PubMed] [Google Scholar]
- Riederer, B. M., Pellier, V., Antonsson, B., Di Paolo, G., Stimpson, S. A., Lutjens, R., Catsicas, S., and Grenningloh, G. (1997). Regulation of microtubule dynamics by the neuronal growth-associated protein SCG10. Proc. Natl. Acad. Sci. USA 94, 741-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, C. I., and Atweh, G. F. (2004). The role of stathmin in the regulation of the cell cycle. J. Cell. Biochem. 93, 242. [DOI] [PubMed] [Google Scholar]
- Rusan, N. M., Fagerstrom, C. J., Yvon, A. M., and Wadsworth, P. (2001). Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-α tubulin. Mol. Biol. Cell 12, 971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield, P. J., Oliver, C. J., Kremer, B. E., Sheng, S., Shao, Z., and Macara, I. G. (2003). Borg/septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. J. Biol. Chem. 278, 3483-3488. [DOI] [PubMed] [Google Scholar]
- Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. (1996). Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850-858. [DOI] [PubMed] [Google Scholar]
- Shiina, N., and Tsukita, S. (1999a). Mutations at phosphorylation sites of Xenopus microtubule-associated protein 4 affect its microtubule-binding ability and chromosome movement during mitosis. Mol. Biol. Cell 10, 597-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina, N., and Tsukita, S. (1999b). Regulation of microtubule organization during interphase and M phase. Cell Struct. Funct. 24, 385-391. [DOI] [PubMed] [Google Scholar]
- Spiliotis, E. T., Kinoshita, M., and Nelson, W. J. (2005). A mitotic septin scaffold required for Mammalian chromosome congression and segregation. Science 307, 1781-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surka, M. C., Tsang, C. W., and Trimble, W. S. (2002). The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol. Biol. Cell 13, 3532-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter, D. M., Schaefer, A. W., and Forscher, P. (2004). Microtubule dynamics are necessary for SRC family kinase-dependent growth cone steering. Curr. Biol. 14, 1194-1199. [DOI] [PubMed] [Google Scholar]
- Takizawa, P. A., DeRisi, J. L., Wilhelm, J. E., and Vale, R. D. (2000). Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science 290, 341-344. [DOI] [PubMed] [Google Scholar]
- Trinczek, B., Ebneth, A., Mandelkow, E. M., and Mandelkow, E. (1999). Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J. Cell Sci. 112, 2355-2367. [DOI] [PubMed] [Google Scholar]
- Vega, I. E., and Hsu, S. C. (2003). The septin protein Nedd5 associates with both the exocyst complex and microtubules and disruption of its GTPase activity promotes aberrant neurite sprouting in PC12 cells. Neuroreport 14, 31-37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.