Abstract
In the oocytes of many species, bipolar spindles form in the absence of centrosomes. Drosophila melanogaster oocyte chromosomes have a major role in nucleating microtubules, which precedes the bundling and assembly of these microtubules into a bipolar spindle. Here we present evidence that a region similar to the anaphase central spindle functions to organize acentrosomal spindles. Subito mutants are characterized by the formation of tripolar or monopolar spindles and nondisjunction of homologous chromosomes at meiosis I. Subito encodes a kinesinlike protein and associates with the meiotic central spindle, consistent with its classification in the Kinesin 6/MKLP1 family. This class of proteins is known to be required for cytokinesis, but our results suggest a new function in spindle formation. The meiotic central spindle appears during prometaphase and includes passenger complex proteins such as AurB and Incenp. Unlike mitotic cells, the passenger proteins do not associate with centromeres before anaphase. In the absence of Subito, central spindle formation is defective and AurB and Incenp fail to properly localize. We propose that Subito is required for establishing and/or maintaining the central spindle in Drosophila oocytes, and this substitutes for the role of centrosomes in organizing the bipolar spindle.
INTRODUCTION
In the oocytes of many animals, bipolar spindles form in the absence of centrosomes (Compton, 2000; Karsenti and Vernos, 2001). In the acentrosomal pathway for spindle formation, the chromosomes trigger spindle formation by capturing free microtubules that are present in the cytoplasm (Theurkauf and Hawley, 1992; McKim and Hawley, 1995). These microtubules are then bundled and sorted to generate two poles in a process that involves a variety of motor protein–microtubule interactions (Matthies et al., 1996; Walczak et al., 1998). Plus-end-directed motors of the BimC class such as Eg5 are proposed to generate bundles of antiparallel microtubules, an activity that could be important for promoting the formation of bipolar instead of monopolar spindles (Karsenti and Vernos, 2001). Minus-end-directed motors such as kinesins in the C-terminal motor class or dynein have been proposed to bundle parallel microtubules and taper them into defined poles (Matthies et al., 1996; Walczak et al., 1998).
Although the activities of a variety of motors has been studied in such systems as Xenopus extracts, the formation of acentrosomal spindles in vivo is still poorly understood. Although Drosophila female meiosis is an excellent system to study acentrosomal spindle formation, the only motor protein with a role in spindle assembly that has been extensively studied is NCD, a C-terminal motor kinesin. Consistent with a role in focusing the poles, ncd mutant spindles are frequently multipolar or apolar (Hatsumi and Endow, 1992; Matthies et al., 1996). Nonmotor proteins have also been shown to make important contributions to Drosophila acentrosomal spindle organization. For example, spindle pole-associated proteins TACC and MSPS have a role in bipolar spindle pole formation (Cullen and Ohkura, 2001). The AXS protein is present within a structure ensheathing the meiotic spindle and has a role in meiotic spindle assembly (Kramer and Hawley, 2003). These studies suggest there are important proteins or structures that modulate the interaction of motors and microtubules in acentrosomal spindle assembly.
Previous studies in Drosophila oocytes have suggested that the process of acentrosomal spindle formation is initiated by the capture of free microtubules by the chromosomes followed by bundling and sorting of microtubules by minus-end-directed motors and the accumulation of certain proteins at the spindle poles (Matthies et al., 1996; Cullen and Ohkura, 2001). In this article we have built on this model by investigating the role of the subito (sub) gene in acentrosomal spindle formation. sub encodes a kinesinlike protein whose sequence is most similar to the MKLP1 (mitotic kinesin like protein 1) family (Giunta et al., 2002) (now Kinesin 6, Dagenbach and Endow, 2004). Mutants in sub have a phenotype consistent with a role in organizing the bipolar spindle. Female meiosis in sub mutants is characterized by the formation of monopolar and tripolar spindles and the nondisjunction of homologous chromosomes during the first meiotic division (Giunta et al., 2002).
Here we show that SUB protein is bound to the meiotic central spindle, a pattern that is consistent with its assignment to the MKLP1 class of proteins. SUB and the central spindle appear at the earliest stages of prometaphase, before bipolar spindle formation. Indeed, SUB is required for assembly of the central spindle. We suggest that the precocious assembly of the central spindle has a primary role in organizing the meiotic spindle in the absence of centrosomes.
MATERIALS AND METHODS
Genetics and Sequencing of sub alleles
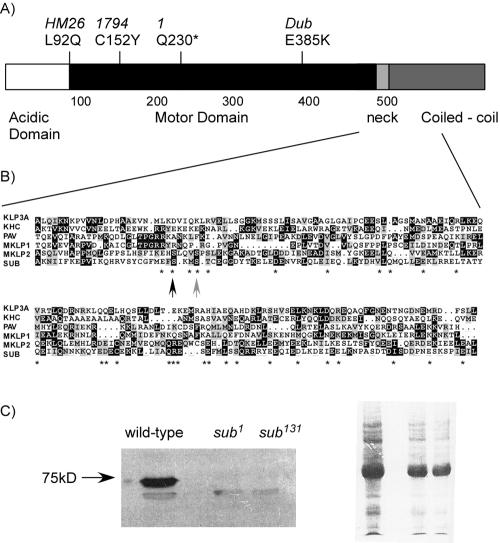
The isolation and genetic analysis of most sub alleles has been described previously (Giunta et al., 2002), including two alleles of sub that were identified in a screen for female sterile mutations (Schupbach and Wieschaus, 1989) and one dominant allele (Moore et al., 1994). sub1794 is a fertile hypomorph, whereas sub131, sub202, sub1, and subHM26 are female sterile alleles. As described previously (Giunta et al., 2002), sub131 and sub202 were generated by excision of a P-element and delete most of the sub coding region. Sequencing demonstrated that sub1 creates a stop codon and subHM26 causes a missense mutation (Figure 1). Sequencing of these sub mutations was performed by PCR amplification from sub mutant homozygotes followed by blunt-end cloning into the pT7Blue vector (Perfectly Blunt cloning system, Novagen, Madison, WI). At least two mutant DNA clones and another from a strain of the same genetic background were sequenced at the University of Medicine and Dentistry of New Jersey sequencing facility. Sequences were analyzed using the Wisconsin Package Version 10.0 (Genetics Computer Group).
Figure 1.
Subito (SUB) is a kinesinlike protein in the MKLP1 family. (A) Schematic of SUB protein organization showing the amino acid changes in the known mutants. sub131 deletes most of the subcoding region (Giunta et al., 2002) and sub1 is a nonsense mutation. (B) Sequence alignment of the neck-linker region in the Drosophila and human MKLP1 group along with two other Drosophila proteins, conventional kinesin heavy chain and KLP3A. The black arrow indicates the Polo kinase site identified by Neef et al. (2003), the gray arrow indicates a second conserved serine, and the stars denote identical or similar amino acids unique to MKLP2 and SUB. (C) Western blot of ovary protein using anti-SUB antibodies. A smaller nonspecific band and a Ponceau S-stained membrane serve as loading controls.
ncd1 is a deletion of the coding region and is fertile (Yamamoto et al., 1989), taccstella592 is a female sterile mutant (Lee et al., 2001), and polo1 is a hypomorphic female sterile mutant (Riparbelli et al., 2000). γ-Tub37C1 and γ-Tub37C3 are female sterile alleles in one of the two Drosophila γ-tubulin genes (Tavosanis et al., 1997) and α-Tub67C1 and α-Tub67C2 are females sterile alleles of a specialized α-tubulin gene (Matthews et al., 1993).
Germ-line Clone Analysis of Incenp
Drosophila Incenp mutations cause early larval lethality (M. Carmena, personal communication). To study the role of Incenp during female meiosis, we generated mutant germline clones in P{hsFLP}12/+; P{FRT(whs)}G13 Incen-pEP2340/ P{FRT(whs)}G13 P{ovoD1–18}2R females. These females were generated by crossing P{hsFLP}12; P{FRT(whs)}G13 IncenpEP2340/CyO females to +/Y; P{FRT(whs)}G13 P{ovoD1–18}2R/CyO males for 2 d,followed by heat shock for 1 h at 37°C on days 3 and 4. The P{hsFLP}12/+; P{FRT(whs)}G13 IncenpEP2340/ P{FRT(whs)}G13 P{ovoD1–18}2R females were expected to lack mature oocytes because of the dominant ovoD1 mutation. If a mitotic recombination event occurred, germline cells homozygous for incenpEP2340 would be produced. However, in no cases were oocytes observed from ∼50 heat shocked females. In contrast, most of the 23 females in a control experiment without the IncenpEP2340 mutation produced mature oocytes. These results suggest that Incenp is required for early stages of oocyte development.
Generation of the SUB Antibody
A carboxy-terminal fragment of sub encoding amino acids 498–628 was cloned from a cDNA (Stapleton et al., 2002) as an EcoRI-XhoI fragment into pET30A (Novagen) and expressed in E. coli BL(21)DE3. This fragment contains the poorly conserved C-terminal domain of sub that follows the motor domain. The fusion proteins were purified using His-binding Ni2+ binding resin columns (Novagen) under denaturing conditions. After electroelution, the proteins were concentrated, dialyzed into 50 mM HEPES pH 7.5 and used to raise antibodies in rats (Covance, Denver, PA).
Western Blotting and Immunofluorescence Microscopy
Total ovary protein was isolated by dissecting whole ovaries from yeasted females in phosphate-buffered saline and then grinding and boiling them in SDS gel loading buffer. Protein from ∼2–3 ovaries was loaded per lane. The rat anti-SUB primary antibody was used at 1:5000 and the secondary anti-rat HRP antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) was used at 1:5000. The secondary was detected using ECL reagents (Amersham, Piscataway, NJ).
For immunofluorescence, stage 14 oocytes were collected from 3- to 7-d-old yeast-fed females and fixed as described previously and mounted in Vectashield (Vector Laboratories, Burlingame, CA; Theurkauf and Hawley, 1992; McKim et al., 1993). Anaphase oocytes were collected by allowing females to lay eggs on grape juice agar plates for 20 min after a short precollection period. The embryos were dechorionated in 50% bleach for 2 min and then fixed in cold heptane/methanol (Rothwell and Sullivan, 2000). Oocytes were stained for DNA with Hoescht and for microtubules with anti-tubulin monoclonal antibody DM1A (at 1:50) directly conjugated to FITC (Sigma, St. Louis, MO). When examining sub mutant oocytes, heterozygotes for protein null alleles, either sub1/sub131 or sub1/sub202, were often used. Heterozygotes were used to eliminate potential genetic background effects but the same results were observed in sub1, sub131, or sub202 homozygotes. The rat anti-SUB antibody was used at 1:75 combined with either a Cy3 or Cy5 anti-rat secondary antibody adsorbed against a range of mammalian serum proteins including mouse and rabbit (Jackson Laboratories, 112-165-167 and 112-175-167). Additional primary antibodies were TACC (1:75), AurB (1:250), INCENP (1:250), RACGAP50C (1:100), POLO (1:15), and MEI-S332 (1:1000) with Cy3-conjugated secondary antibodies (Jackson Laboratories, West Grove, PA). NCD was observed using a GFP fusion protein (Endow and Komma, 1997). Images were collected on a Leica TCS SP confocal microscope (Deerfield, IL) with a 63×, NA 1.3 lens. Images are shown as maximum projections of complete image stacks followed by cropping in Adobe Photoshop (San Jose, CA).
RESULTS
SUB Is a Kinesinlike Protein with Similarities to the MKLP1 Family
We previously suggested that SUB (Giunta et al., 2002) and Pavarotti (PAV; Adams et al., 1998) are two Drosophila kinesinlike proteins in the MKLP1 (or Kinesin 6) family (Nislow et al., 1992). Although this family was originally defined by MKLP1, PAV, and their orthologues (Dagenbach and Endow, 2004), sequence and functional studies suggest it could also include paralogs such as MKLP2 (formerly RabK6; Neef et al., 2003). In addition, SUB (referred to as DmKlp54E) has been placed on a branch close to the MKLP1 group in a phylogenetic tree derived from the alignment of kinesin motor domain sequences (Dagenbach and Endow, 2004), and there are several conserved amino acids in the neck-linker region of all four proteins that are not present in other kinesins (Figure 1). PAV and MKLP1 have the highest level of amino acid identity or similarity and are probably orthologues. Similarly, there are several identical or similar amino acids in the neck-linker regions of SUB and MKLP2 that are not found in other kinesins. This includes the serine residue in MKLP2 that is phosphorylated by Polo kinase (Neef et al., 2003) and the corresponding acidic residue at -2 that is often found at Polo kinase sites. Furthermore, although all four of these MKLP1 homologues have nonconserved N-terminal domains of ∼100 amino acids, this domain is basic in PAV and MKLP1 but acidic in SUB and MKLP2. These sequence comparisons and the functional studies described here and in Neef et al. (2003) raise the possibility that SUB is the Drosophila ortholog of MKLP2.
SUB Localizes to the Central Spindle during Female Meiosis I
Antibodies were raised against the poorly conserved C-terminus of the SUB protein that follows the motor domain (Figure 1). These antibodies recognize an ∼75-kDa band on a Western blot that is absent in homozygotes of the null alleles sub1 and sub131 (Figure 1C). To examine the localization of SUB during meiosis, we stained mature Drosophila oocytes (stage 14), which in wild type, are arrested at metaphase I.
Our identification of prometaphase and metaphase spindles was based on previous studies of fixed (Theurkauf and Hawley, 1992) and living oocytes (Matthies et al., 1996; Skold et al., 2005). There is no congression to a metaphase plate in Drosophila oocytes. Instead, the chromosomes come together and condense into a ball to form the karyosome much earlier in oogenesis. After nuclear envelope breakdown (NEB), the chromosomes in the karyosome initiate spindle formation. The initial phases of spindle assembly are characterized by disorganized arrays of microtubules emanating from the karyosome. Once a bipolar spindle forms, it remains stable until the onset of anaphase when the oocyte is activated by passage through the oviduct. As in previous studies (Theurkauf and Hawley, 1992; McKim et al., 1993), staining for tubulin in wild-type oocytes revealed that metaphase I spindles have a prominent band of microtubules that run pole-to-pole and do not terminate at the chromosomes. The bright staining in the middle of this region probably represents the antiparallel overlap of microtubules. We will refer to this region as the meiotic metaphase central spindle (MMCS) in order to distinguish it from the central spindle or midzone present in anaphase of mitotic cells.
Staining of wild-type oocytes with SUB antibodies revealed that SUB protein was found exclusively in the MMCS (Figure 2, A and B). Although SUB always colocalized with tubulin staining, it was also closely associated with the chromosomes. In many spindles, 3D reconstruction revealed that SUB staining appeared more concentrated on one side or in some cases in two clusters on either side of the karyosome. Whether this pattern reflects intrinsic features of the spindle, such as asymmetry within the karyosome, or stochastic properties of central spindle formation, remains to be determined. SUB staining was not detected in the null alleles sub1 and sub131 at prometaphase (see below) or metaphase (Figure 2, C and D), confirming the specificity of the antibody.
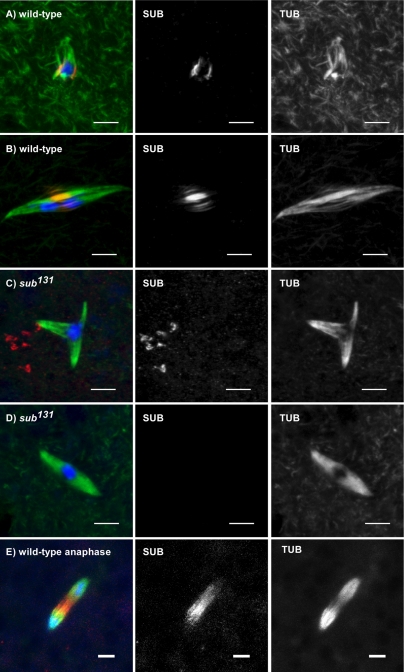
Figure 2.
SUB localizes to the meiotic metaphase central spindle in wild-type female meiosis I. SUB staining is in red, tubulin in green, and DNA in blue in addition to gray scale images of the separate SUB and tubulin channels. Before NEB, SUB is excluded from the nucleus (unpublished data). (A) Prometaphase oocyte: SUB localizes to the presumptive central spindle, even though a mature bipolar spindle has not yet formed. (B) Metaphase I oocyte: A bipolar spindle has formed and SUB staining is associated with the central spindle, which is typically visible as bright bundles of pole-to-pole microtubules. The chromosomes are under tension because they are being pulled toward to the poles but homologues are still connected by chiasmata (visible as thinner DNA staining between the two main masses). (C and D) SUB staining is absent in sub null mutant metaphase I oocyte spindles. Although most sub mutants spindles have polarity defects (C), some have relatively normal structure (D) (see text). In some cases, nonspecific signals are observed. In either case, however, the bright microtubule staining in the central spindle is greatly reduced in sub mutants. (E) SUB localization during meiotic anaphase I. SUB staining remains in the region between the chromosomes as they move toward the poles. Scale bars, 5 μm.
Because genetic and cytogenetic studies (Giunta et al., 2002) have suggested that SUB has an important role in spindle assembly, we investigated when SUB first appears on the meiotic spindle. SUB appeared from the earliest time points after NEB, even on early prometaphase spindles that were characterized by a disorganized array of microtubules (Figure 2A). SUB staining at this stage colocalized with microtubules in the region that will become the MMCS. Thus, SUB localizes to the central spindle before bipolar spindle formation.
Stage 14 oocytes arrest at meiotic metaphase I; therefore, we used two methods to observe anaphase I spindles. First, we looked at mei-218 mutants in which the metaphase arrest is bypassed (McKim et al., 1993). Second, we collected embryos after short periods of egg laying, which allows for the isolation of oocytes undergoing the early meiotic divisions. In both of these experiments, SUB remained in the spindle midzone as the chromosomes moved toward the poles (unpublished data and Figure 2E, respectively).
SUB Is a Mitotic Protein
Because sub mutants are viable, it is possible that sub has a meiosis-specific function and is only required for the unique situation of assembling acentrosomal spindles during female meiosis. There is, however, genetic evidence that sub is expressed in mitotically dividing cells (Moore et al., 1994; Giunta et al., 2002). Consistent with these genetic observations, we observed SUB staining in mitotically dividing cells of the embryo (Supplementary Figure 1). Similar to the metaphase oocytes, SUB protein was observed at the middle of the spindle. SUB protein has also been observed at metaphase of larval neuroblast cells (B. Redding and K. McKim, unpublished results). Thus, SUB protein may be a component of most or all metaphase spindles in Drosophila. Although SUB has an important function in pronuclear fusion, some sub mutant embryos commence but never complete the early embryonic divisions (Giunta et al., 2002). Analysis of these embryos shows evidence of spindle assembly defects and aneuploidy, indicating that sub also has a role in mitotic spindle function (Supplementary Figure 1).
SUB Is Required for Central Spindle Formation
We previously reported that sub null mutant females develop monopolar and tripolar spindles in a majority of meiosis I figures (see Table 4 in Giunta et al., 2002) and are sterile because of a requirement during early embryogenesis. In addition, sub hypomorphic mutants exhibit a high frequency of homologous chromosome nondisjunction at meiosis I (Giunta et al., 2002). Considered along with the SUB staining pattern, these results suggest that the MMCS may have an important role for in bipolar spindle formation and chromosome segregation. For this study, we reexamined the effects on central spindle formation with a new sub mutant data set. Similar to our previous report, 14/17 sub1/sub131 mutant oocytes had abnormal spindle organization compared with only 4/36 in wild-type oocytes. Among the 14 abnormal sub1/sub131 spindles, 3 were monopolar, 9 were tripolar, and 2 had other problems such as fraying of the microtubules. Thus, sub mutant spindles have a defect in maintaining or establishing bipolarity (Figure 2C), although in a minority of cases, relatively normal bipolar spindles were observed (Figure 2D). Notwithstanding these defects, the ability to taper microtubules into poles is not usually affected in sub mutants.
Given the localization of SUB, we examined the MMCS in sub mutants. Strikingly, all sub mutant spindles, even those that were bipolar, lacked metaphase central spindle tubulin staining (Figure 2, C and D, Supplementary Figure 2). Although the amount of central spindle microtubules was variable in wild type, the prominent bundles of central spindle microtubules often observed in wild-type oocytes were never observed in sub mutant oocytes. Instead, there were often small gaps of microtubules staining in the middle of the spindle or only limited evidence of antiparallel microtubule overlap. Additional evidence that the central spindle fails to form in sub mutants is that midzone proteins such as Incenp and AurB did not localize to this region in sub mutants (see below). These results suggest that the MMCS is normally organized or maintained by SUB.
SUB Localization Does Not Require Bipolar Spindle Formation
To address whether SUB localization was dependent on bipolar spindle formation, SUB localization was examined in mutants with disrupted spindle organization, including ncd, tacc, α -tub67C, and γ -tub37C (Figure 3). Like sub, ncd encodes a kinesin required for bipolar spindle formation (Hatsumi and Endow, 1992; Matthies et al., 1996). Double mutants with the hypomorph sub1794 have a meiotic phenotype similar to that of the single mutants (Giunta et al., 2002). tacc has an important role in meiotic bipolar spindle formation with some similar phenotypes to sub mutants (Cullen and Ohkura, 2001). γ-Tub37C is one of two Drosophila γ-tubulin isoforms and has previously been shown to have a role in female meiotic spindle formation (Tavosanis et al., 1997). Mutants of the female-specific isoform α-Tub67C are sterile, although defects in spindle formation have not previously been shown (Matthews et al., 1993). Despite severe defects in bipolar spindle formation, these mutants exhibited SUB staining in association with the central spindle. In addition, we have documented meiotic spindle defects in α -tub67C mutant females for the first time. These results suggest that the MMCS and SUB staining are not dependent on bipolar spindle formation. Instead, SUB most likely localizes and functions before the formation of a bipolar spindle. SUB may simply localize to any region of the spindle containing antiparallel microtubules.
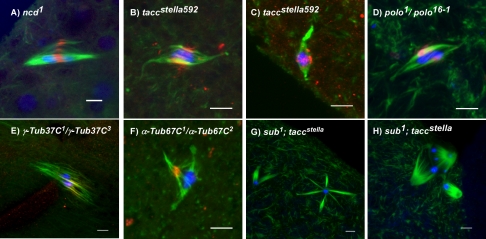
Figure 3.
SUB staining in spindle mutants. SUB is still found in the central spindle in a variety of mutants with disorganized meiotic spindles, such as (A) ncd1, (B and C) taccstella592, (D) polo16–1/polo1, (E) γ-Tub37C1/γ-Tub37C3 and (F) α-Tub67C1/α-Tub67C2. (G and H) Synergistic effects on spindle formation in sub; tacc double mutant oocytes. SUB staining is in red, tubulin in green, and DNA in blue. Scale bars, 5 μm.
Polo Localization during Female Meiosis
One candidate for regulating SUB localization is Polo kinase because, as described above, the SUB ortholog MKLP2 is phosphorylated by Polo in human cells. Observing the effects of polo null mutants is problematic because the homozygotes are lethal. We were, however, able to examine females with a viable but female sterile allele heterozygous to a null allele and found that SUB staining was normal (Figure 3). Indeed, these mutants did not have gross defects in meiotic spindle formation, except for a possible reduction in kinetochore microtubules. The absence of an effect on SUB staining in polo mutants is consistent with the results that in HeLa-S3 cells, MKLP2 is a target for Polo phosphorylation but this is not required for localization (Neef et al., 2003).
Because MKLP2 is required for the localization of Polo kinase to the midzone in HeLa-S3 cells (Neef et al., 2003), we examined Polo localization during Drosophila meiosis. In addition, Drosophila Polo has been shown to have a localization pattern similar in mitotic cells to proteins such as AurB and Incenp (Logarinho and Sunkel, 1998), which as described below, localize to the MMCS in Drosophila oocytes and depend on SUB activity. Polo antibody staining in wild-type metaphase Drosophila oocytes appeared weakly in the MMCS and was not visible in all images. In contrast, it was stronger in foci that colocalized with the DNA (Figure 4A). These Polo foci were probably the kinetochores, consistent with previous studies of larval neuroblasts. In these mitotically dividing cells, Polo was localized to kinetochores during metaphase, and midzone staining was strong only during anaphase (Logarinho and Sunkel, 1998). In sub mutant oocytes, the foci of Polo staining were still observed, which is consistent with the absence of SUB protein at kinetochores (Figure 4B). However, the level of Polo staining was variable and may be dependent on spindle structure, because in some sub mutant oocytes, particularly those with the most disorganized spindles, Polo staining was weak (Figure 4C).
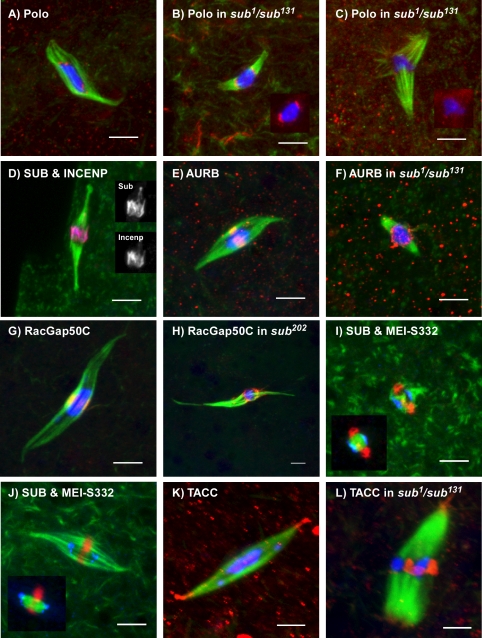
Figure 4.
The meiotic metaphase central spindle contains proteins typically found on mitotic midzones. In these wild-type (A, D, E, G, I, J, and K) or sub protein null mutant (B, C, F, E,and L) oocytes, microtubules are shown in green and the DNA is i nblue, except in D, I, and J, where blue is another protein. In some cases, images of the less frequentbipolar sub mutant spindles were selected to more clearly show the abnormal localization of a midzone protein and that this was due to the absence of SUB rather than spindle structure. (A) POLO (red) localizes to spots on the chromosomes, which are most likely the kinetochores. (B) Polo staining can still be observed in some sub mutant oocytes. (C) In other sub mutant oocytes with more disorganized spindles, however, Polo staining was reduced. The insets in B and C have the microtubule staining removed in order to see Polo staining clearly. (D) Incenp (red) colocalizes with SUB (blue) in the central spindle. The inset images show the separate channels for SUB and Incenp. (E) AurB (red) localizes to the meiotic central spindle. (G) Similar results were obtained using antibodies against RacGap50C. (F and H) In the absence of SUB, midzone proteins (AurB and RacGap50C are shown) accumulate around the karyosome instead of the MMCS. (I and J) MEI-S332 (blue) is a marker for the meiotic centromeres and this staining does not overlap with SUB (red). The inset shows SUB and MEI-S332 with DNA in green. (K) TACC (red) normally localizes to the spindle poles. (L) In sub mutant oocytes, there is less staining at the poles and in some cases TACC it is observed near the center of the spindle. Scale bars, 5 μm.
Midzone Proteins Depend on Subito
Our results suggest that the MMCS has an important function in organizing the meiotic spindle. This led us to examine what other proteins are located in this region of the meiotic spindle and could contribute to microtubule assembly. Several proteins have been localized to the spindle midzone at anaphase of mitotic cells including the passenger proteins AurB/Ial and Incenp. In mitotic cells, the localization pattern of the passenger proteins depends on the mitotic stage. They localize to the centromeres during metaphase and then move to the spindle midzone at anaphase (Adams et al., 2001; Giet and Glover, 2001). We examined the metaphase I localization pattern of AurB and Incenp by antibody staining to determine if the MMCS also contains these proteins and whether they show an early (mitotic metaphase) or late (mitotic anaphase) staining pattern. We also examined RacGap50C, another mitotic midzone component that forms a complex with the SUB paralog PAV (Somers and Saint, 2003).
For Incenp (Figure 4D), AurB (Figure 4E), and RacGap50C (Figure 4G), we found staining similar or identical to SUB. For example, Incenp perfectly colocalized with SUB in the MMCS (Figure 4D), including early prometaphase staining before a bipolar spindle had formed. To determine the relationship of these proteins to the centromeres, we stained with an antibody to MEI-S332 (Moore et al., 1998). SUB and MEI-S332 always occupied distinct regions around the karyosome in both disorganized prometaphase and bipolar metaphase spindles (Figure 4, I and J). By extension, because the midzone proteins and SUB always colocalize, Incenp and AurB do not localize to the centromere regions during meiotic metaphase I. Indeed, the centromeres and MMCS appear to be properly organized and oriented before a bipolar spindle forms.
The localization of these proteins to the MMCS was dependent on sub activity. In sub mutants, AurB accumulated in a region surrounding the karyosome but not in the region where the MMCS would have been (Figure 4F). Almost identical localization defects were observed with RacGap50C (Figure 4H) and Incenp (unpublished data). This pattern appears to be more extensive than just centromere staining, at least when compared with the MEI-S332 staining and Polo staining described above. Instead, these proteins concentrated in the region where the microtubules are in close proximity to the karyosome. This could occur if, in the absence of a central spindle in sub mutants, these proteins concentrate near the plus-ends of microtubules. A similar effect of midzone disruption on AurB and MKLP1 staining has been observed in HeLa cells (Kurasawa et al., 2004). It was suggested that localization toward the plus ends was an intermediate stage in development of the midzone. It is possible these proteins have an affinity for the plus ends of microtubules or other motors, such as the Drosophila MKLP1 ortholog Pavarotti, that actively transport them there (Matuliene and Kuriyama, 2002). Unfortunately, we have been unable to test the role of the passenger proteins in acentrosomal spindle formation because Incenp appears to be required during early stages of oogenesis (see Materials and Methods).
Two Pathways for Meiotic Spindle Formation
Although SUB has an important role in spindle formation, most sub mutant spindles retain the ability for form poles. Therefore, SUB-independent activities must be functioning to organize spindle poles. TACC and MSPS may have a role in this function because these proteins localize to meiotic spindle poles. Previous genetic and cytological studies have shown that tacc and msps have an important role in meiotic bipolar spindle formation (Cullen and Ohkura, 2001), and the mutants have been reported to have phenotypes similar to sub mutants. To investigate the relationship between TACC and SUB, we examined TACC localization in sub mutants and examined the phenotype of double mutant combinations. As reported previously, TACC localized to the acentrosomal spindle poles in wild-type oocytes (Figure 4K). In sub mutants, however, the spindle pole staining was weaker and in some cases accumulated near the chromosomes (Figure 4L). Thus, it is possible that the phenotype of SUB mutants may be related to defects in TACC localization.
To test if SUB and TACC function in distinct spindle forming activities or have similar functions during spindle assembly, we constructed sub; tacc double mutants. The sub1; taccstella592 double mutant had severe spindle formation defects, more dramatic than either single mutant (compare Figure 2C and Figure 3B with Figure 3, G and H). Unlike the single mutants, there were often multiple bundles of microtubules, some associating with chromosomes. Although microtubules were still associating with the chromosomes, most bundles of microtubules were randomly organized. Similar defects were also observed in sub1794/sub1; taccstella592 but not sub1794/sub1794; taccstella592 females, demonstrating this phenotype was dependent on severe loss of sub function. The double mutants appear to retain the ability to assemble kinetochore microtubules. This phenotype could be explained by a combination of tacc and sub mutant defects: a failure to stabilize the spindle poles (tacc) and a failure to organize the microtubules around the chromosomes (sub). These results suggest that sub and tacc contribute to different pathways that function to organize the microtubules of acentrosomal spindles. This is consistent with the observation that SUB and TACC associate with distinct structures or populations of microtubules.
DISCUSSION
Evidence that Subito Belongs to the MKLP1 Family of Kinesinlike Proteins
Meiotic spindle microtubules in most oocytes must be organized without centrosomes to organize the poles. Although the chromosomes play a critical role in spindle formation by capturing free microtubules (replacing the nucleation step of centrosomes), it is not clear what organizes the bundling and elongation of microtubules into a bipolar spindle. Our results suggest that the kinesinlike protein Subito has an important role in Drosophila acentrosomal spindle formation, possibly by organizing the prominent central spindle that assembles at meiotic prometaphase. Interestingly, SUB has several characteristics similar to MKLP2: SUB localizes to a region of antiparallel microtubules, in this case the meiotic metaphase central spindle; it is required for central spindle formation; it is required for the localization of other central spindle proteins, and it has nonmotor domain sequence similarity including amino acids that could be phosphorylated by Polo kinase. Similarly, a phylogenetic tree made from the alignment of kinesin motor domain sequences has SUB in a cluster close to the MKLP1 group (Dagenbach and Endow, 2004). As described below, we suggest these features allow SUB to contribute to the organizing of Drosophila acentrosomal spindles by establishing or maintaining the central spindle at prometaphase and metaphase.
Mammalian MKLP1 (Matuliene and Kuriyama, 2002), Drosophila PAV (Adams et al., 1998), and the C. elegans ortholog ZEN-4 (Raich et al., 1998; Mishima et al., 2002) have been found in the spindle midzone during anaphase and have an important function in cytokinesis. The midzone has been implicated in establishing the placement of the cytoplasmic furrow, although there are exceptions (D'Avino et al., 2005). Furthermore, MKLP1 was found to bundle microtubules and to promote anti-parallel sliding in vitro (Nislow et al., 1992). This is consistent with its localization in the spindle midzone, where microtubules overlap in antiparallel orientation. In addition, from the direction of the antiparallel sliding of microtubules it was concluded that MKLP1 is a plus-end-directed motor. Although less is known about MKLP2, it is also required for the spindle midzone and cytokinesis, and like other MKLP1 family members, the protein accumulates at the midzone (Hill et al., 2000; Fontijn et al., 2001; Neef et al., 2003). Our characterization of SUB suggests that organisms with two MKLP1-like proteins are not restricted to vertebrates.
Two observations suggest that the MMCS is mostly or entirely absent in sub mutants. First, sub mutant spindles lack the prominent band of antiparallel microtubules arising from the overlap of pole to pole spindle fibers. Second, proteins that normally associate with this region, such as Incenp, AurB, and RacGap50C, are absent in sub mutant oocytes. Nonetheless, it is difficult to rule out if other proteins are able to promote formation of a thin and fragile central spindle in sub mutants. A candidate with this function could be PAV but, because of its lethal phenotype and because pav mutant germlines do not make oocytes (Minestrini et al., 2002), we could not determine if PAV contributes to the meiotic spindle assembly. However, the severe defect in meiotic central spindle formation in sub mutants suggests that PAV cannot compensate in a significant way for the absence of SUB.
Passenger Proteins Appear at the Midzone during Female Meiotic Metaphase I
Our studies suggest a new role for the central spindle in bipolar spindle formation and chromosome segregation. The localization pattern of central spindle components, such as members of the passenger protein complex AurB and Incenp, is consistent with the idea that a central spindle is forming precociously in oocytes. Although it is typical in Drosophila and human mitotic cells for AurB and Incenp to initially associate with centromeres and then move to the midzone at anaphase (Adams et al., 2001; Giet and Glover, 2001; Gruneberg et al., 2004), in Drosophila oocytes, these proteins appear on the central spindle much earlier in prometaphase. Furthermore, the meiotic division of Drosophila oocytes appears to skip the stage in mitotic cells (metaphase) where passenger proteins associate with centromeres. We have not observed SUB, AurB, or Incenp at the centromeres during female meiosis; they appear to be associated only with the nonkinetochore microtubules. This appears to be specific only to a subset of midzone proteins. Polo exhibited kinetochore staining typical of mitotic metaphase at meiotic metaphase I. In addition, KLP3A, a kinesinlike protein that associates with the anaphase midzone in mitotic cells, has been reported to stain along the length of female meiotic spindles and only moves to the midzone at anaphase (Williams et al., 1997).
AurB and Incenp localization to the oocyte MMCS depends on SUB. Similarly, Incenp and AurB midzone localization depends on MKLP2 in mammalian mitotic cells, and MKLP2 may even have a direct interaction with AurB (Gruneberg et al., 2004). An important aspect of SUB function could be to recruit proteins like AurB in order to stimulate chromosome–microtubule interactions (Gassmann et al., 2004; Sampath et al., 2004). Consistent with this model, phosphorylation of the microtubule-destabilizing kinesin MCAK by AurB stimulates chromatin induced spindle assembly in Xenopus extracts (Ohi et al., 2004). We have not, however, been able to determine the role, if any, of the passenger proteins in meiotic spindle formation.
Antiparallel Microtubules Organize the Meiotic Acentrosomal Spindle
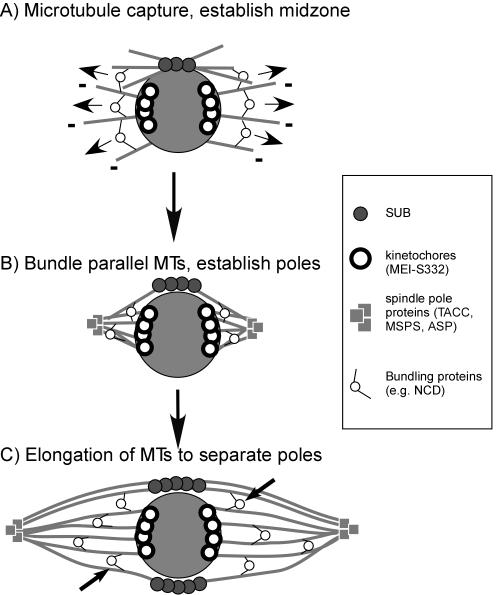
Previous models for acentrosomal spindle formation suggested that the process was initiated by the capture of free microtubules by the chromosomes followed by bundling and sorting of microtubules by minus-end-directed motors to form the poles (Matthies et al., 1996; Skold et al., 2005). However, these models lack a mechanism to ensure that the kinetochore microtubules are oriented toward only one of two poles. For example, how are the two half spindles oriented relative to each other and what limits the spindle to have only two poles? On the basis of the localization pattern of SUB and the phenotype of sub mutants, we present a model for acentrosomal spindle formation in Drosophila oocytes that addresses these questions (Figure 5). We propose that a structure composed of antiparallel microtubules is organized during prometaphase. The axis of the spindle is defined by this structure, the MMCS, which provides the scaffold on which to build a bipolar spindle during prometaphase and metaphase. Proteins that localize to the spindle poles have a separate function in spindle pole formation and the functions of the central spindle or spindle-poles are partially redundant for maintaining spindle integrity and establishing poles. As the sub; tacc double mutant phenotype demonstrates, in the absence of these structures, the spindle loses all organization.
Figure 5.
Model for acentrosomal spindle formation in Drosophila oocytes. (A) The chromosomes enter prometaphase clustered together in a ball, the karyosome, and capture microtubules that are not organized into a bipolar array. At this time, SUB protein accumulates on microtubules adjacent to the karyosome. (B) Motor proteins, possibly involving minus-end-directed motor proteins such as NCD, bundle parallel microtubules, and taper them into defined poles. In parallel with this process, the spindle is stabilized by proteins that accumulate at the poles. Proteins that localize to the female meiotic spindle poles include Asp (Riparbelli et al., 2002), MSPS and TACC (Cullen and Ohkura, 2001). (C) The direction of elongation/bundling/sliding is dictated by the metaphase central spindle. Critical to the model is that the orientation of the kinetochore microtubules is established through interactions (via cross-linking) with the central spindle. Examples of these interactions are shown with by the arrows. The “backbone” structure provided by the central spindle defines the long axis of the spindle, ensuring that the two poles form on opposite sides of the chromosomes and prevents additional poles from forming. In fixed images, the SUB-staining region appears localized to one side of the karyosome, but in other images there are two or more clusters of SUB staining. Elongation of the spindle could involve the capture of additional microtubules to lengthen the spindle. Thus, spindle elongation could occur via microtubule capture and bundling with or without motor activity, microtubule sliding or growth at the plus ends.
SUB and the MMCS could be required at several points in spindles assembly. The MMCS may have a role in the transition from prometaphase, with its disorganized microtubules around the karyosome, to metaphase with a bipolar spindle. The interaction of kinetochore microtubules with pole-to-pole microtubules of the MMCS via parallel microtubule bundling could determine the formation and relative orientation of only two poles (Figure 5). In addition, SUB probably has a role in maintaining spindle bipolarity. By maintaining the MMCS, SUB could attenuate the activity that is active to establish poles during prometaphase but must be inactive during metaphase. Repeated attempts at new spindle pole formation could generate extra poles in sub mutants. Indeed, we observed what appears to be newly formed short half spindles and the ectopic appearance of TACC in the middle of the spindle of sub mutants, suggesting that de novo pole formation can occur at metaphase. The presence of monopolar spindles could occur if the MMCS has a role in maintaining half spindles, resulting in the collapse of half spindles at metaphase in sub mutants. This dynamic portrayal of the meiotic spindle in sub mutants is consistent with real time observations in ncd mutants (Matthies et al., 1996). Although wild-type spindles appear to be stable structures over long periods of time, ncd mutant spindles are dynamic structures, where bipolar spindles will form only to lose their organization to become apolar, monopolar, or even completely disassemble and then reform again.
The role for the MMCS described above in coordinating spindle pole formation can explain the sub genetic and cytological mutant phenotypes. However, we have not ruled out other roles for SUB in chromosomes segregation. An alternative is that SUB contributes to a balance of forces between pushing apart or pulling together the spindle poles (Sharp et al., 1999). In sub mutants, this could lead to a defect in spindle pole positioning. Two observations argue against this hypothesis. First, the sub mutant phenotype is not alleviated by defects in ncd (Giunta et al., 2002), in contrast to Klp61F (Wilson et al., 2004), which has this role in mitotic cells. Second, this function does not easily explain why sub mutants often have multiple poles, whereas the length of the half spindles are not dramatically shorter than wild-type. We also cannot rule out a role for SUB in facilitating interactions between the chromosomes and the microtubules. This could have a role in aligning the homolog pairs at metaphase I, similar to what has been proposed for the chromokinesin NOD (Zhang et al., 1990; Theurkauf and Hawley, 1992). Although in nod mutants, the nondisjunction phenotype is not associated with defects in spindle organization.
We thus favor a model in which SUB directly contributes to bipolar spindle formation by organizing and/or stabilizing the MMCS. An important implication of this model is that, to compensate for the absence of centrosomes, the oocyte has modified the regulation of the central spindle so that it appears earlier in order to direct spindle formation. This is a novel function for the central spindle and contrasts with the suggestion for mitotic cells that the midzone accumulation of Incenp and AurB needs to be inhibited until anaphase (Pereira and Schiebel, 2003; Mishima et al., 2004). An important question that we are currently investigating is what controls SUB localization. One possibility is that the concentration of a factor that promotes microtubule assembly, such as ran-GTP (Kahana and Cleveland, 1999), is greatest in one region of the karyosome. Given the SUB/MKLP2 conservation of sequence and function, it will be interesting to determine if the central spindle has an important role in organizing the acentrosomal spindles of oocytes in mammals and other animals or in plants. Furthermore, as described here for embryos and will be described elsewhere for other mitotic cells (B. Redding and K. McKim, unpublished results), SUB also has a role in spindle assembly of mitotic cells. This is consistent with the hypothesis that acentrosomal spindle assembly occurs through the modification of functions already present in mitotic cells.
Supplementary Material
Acknowledgments
We are grateful to Li Nguyen for technical assistance and R. Scott Hawley, who initially suggested that the central spindle might be important for organizing the meiotic spindle. We also thank Mar Carmena, Terry Orr-Weaver, Jordan Raff, Robert Saint, and Claudio Sunkel for providing antibodies and R. Scott Hawley and Cordelia Rauskolb for comments on the manuscript. Some stocks used in this study were obtained from the Bloomington Stock Center. This work was supported by a grant from the National Institutes of Health (GM 067142) to K.M.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-11-0964) on July 29, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Adams, R. R., Maiato, H., Earnshaw, W. C., and Carmena, M. (2001). Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 153, 865-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, R. R., Tavares, A. A., Salzberg, A., Bellen, H. J., and Glover, D. M. (1998). pavarotti encodes a kinesinlike protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 12, 1483-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton, D. A. (2000). Spindle assembly in animal cells. Annu. Rev. Biochem. 69, 95-114. [DOI] [PubMed] [Google Scholar]
- Cullen, C. F., and Ohkura, H. (2001). Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat. Cell Biol. 3, 637-642. [DOI] [PubMed] [Google Scholar]
- Dagenbach, E. M., and Endow, S. A. (2004). A new kinesin tree. J. Cell Sci. 117, 3-7. [DOI] [PubMed] [Google Scholar]
- D'Avino, P. P., Savoian, M. S., and Glover, D. M. (2005). Cleavage furrow formation and ingression during animal cytokinesis: a microtubule legacy. J. Cell Sci. 118, 1549-1558. [DOI] [PubMed] [Google Scholar]
- Endow, S. A., and Komma, D. J. (1997). Spindle dynamics during meiosis in Drosophila oocytes. J. Cell Biol. 137, 1321-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontijn, R. D., Goud, B., Echard, A., Jollivet, F., van Marle, J., Pannekoek, H., and Horrevoets, A. J. (2001). The human kinesin-like protein RB6K is under tight cell cycle control and is essential for cytokinesis. Mol. Cell. Biol. 21, 2944-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann, R., Carvalho, A., Henzing, A. J., Ruchaud, S., Hudson, D. F., Honda, R., Nigg, E. A., Gerloff, D. L., and Earnshaw, W. C. (2004). Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 166, 179-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet, R., and Glover, D. M. (2001). Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152, 669-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta, K. L., Jang, J. K., Manheim, E. M., Subramanian, G., and McKim, K. S. (2002). subito encodes a kinesin-like protein required for meiotic spindle pole formation in Drosophila melanogaster. Genetics 160, 1489-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneberg, U., Neef, R., Honda, R., Nigg, E. A., and Barr, F. A. (2004). Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell Biol. 166, 167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsumi, M., and Endow, S. A. (1992). Mutants of the microtubule motor protein, nonclaret disjunctional, affect spindle structure and chromosome movement in meiosis and mitosis. J. Cell Sci. 101, 547-559. [DOI] [PubMed] [Google Scholar]
- Hill, E., Clarke, M., and Barr, F. A. (2000). The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J. 19, 5711-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana, J. A., and Cleveland, D. W. (1999). Beyond nuclear transport. Ran-GTP as a determinant of spindle assembly. J. Cell Biol. 146, 1205-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti, E., and Vernos, I. (2001). The mitotic spindle: a self-made machine. Science 294, 543-547. [DOI] [PubMed] [Google Scholar]
- Kramer, J., and Hawley, R. S. (2003). The spindle-associated transmembrane protein Axs identifies a membranous structure ensheathing the meiotic spindle. Nat. Cell Biol. 5, 261-263. [DOI] [PubMed] [Google Scholar]
- Kurasawa, Y., Earnshaw, W. C., Mochizuki, Y., Dohmae, N., and Todokoro, K. (2004). Essential roles of KIF4 and its binding partner PRC1 in organized central spindle midzone formation. EMBO J. 23, 3237-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M. J., Gergely, F., Jeffers, K., Peak-Chew, S. Y., and Raff, J. W. (2001). Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behavior. Nat. Cell. Biol. 3, 643-649. [DOI] [PubMed] [Google Scholar]
- Logarinho, E., and Sunkel, C. E. (1998). The Drosophila POLO kinase localises to multiple compartments of the mitotic apparatus and is required for the phosphorylation of MPM2 reactive epitopes. J. Cell Sci. 111(Pt 19), 2897-2909. [DOI] [PubMed] [Google Scholar]
- Matthews, K. A., Rees, D., and Kaufman, T. C. (1993). A functionally specialized a-tubulin is required for oocyte meiosis and cleavage mitoses in Drosophila. Development 117, 977-991. [DOI] [PubMed] [Google Scholar]
- Matthies, H. J., McDonald, H. B., Goldstein, L. S., and Theurkauf, W. E. (1996). Anastral meiotic spindle morphogenesis: role of the non-claret disjunctional kinesin-like protein. J. Cell Biol. 134, 455-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuliene, J., and Kuriyama, R. (2002). Kinesin-like protein CHO1 is required for the formation of midbody matrix and the completion of cytokinesis in mammalian cells. Mol. Biol. Cell 13, 1832-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim, K. S., and Hawley, R. S. (1995). Chromosomal control of meiotic cell division. Science 270, 1595-1601. [DOI] [PubMed] [Google Scholar]
- McKim, K. S., Jang, J. K., Theurkauf, W. E., and Hawley, R. S. (1993). Mechanical basis of meiotic metaphase arrest. Nature 362, 364-366. [DOI] [PubMed] [Google Scholar]
- Minestrini, G., Mathe, E., and Glover, D. M. (2002). Domains of the Pavarotti kinesin-like protein that direct its subcellular distribution: effects of mislocalisation on the tubulin and actin cytoskeleton during Drosophila oogenesis. J. Cell Sci. 115, 725-736. [DOI] [PubMed] [Google Scholar]
- Mishima, M., Kaitna, S., and Glotzer, M. (2002). Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell 2, 41-54. [DOI] [PubMed] [Google Scholar]
- Mishima, M., Pavicic, V., Gruneberg, U., Nigg, E. A., and Glotzer, M. (2004). Cell cycle regulation of central spindle assembly. Nature 430, 908-913. [DOI] [PubMed] [Google Scholar]
- Moore, D. P., Miyazaki, W. Y., Tomkiel, J., and Orr-Weaver, T. L. (1994). Double or nothing: a Drosophila mutation affecting meiotic chromosome segregation in both females and males. Genetics 136, 953-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, D. P., Page, A. W., Tang, T. T., Kerrebrock, A. W., and Orr-Weaver, T. L. (1998). The cohesion protein MEI-S332 localizes to condensed meiotic and mitotic centromeres until sister chromatids separate. J. Cell Biol. 140, 1003-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef, R., Preisinger, C., Sutcliffe, J., Kopajtich, R., Nigg, E. A., Mayer, T. U., and Barr, F. A. (2003). Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J. Cell Biol. 162, 863-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nislow, C., Lombillo, V. A., Kuriyama, R., and McIntosh, J. R. (1992). A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature 359, 543-547. [DOI] [PubMed] [Google Scholar]
- Ohi, R., Sapra, T., Howard, J., and Mitchison, T. J. (2004). Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol. Biol. Cell 15, 2895-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, G., and Schiebel, E. (2003). Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science 302, 2120-2124. [DOI] [PubMed] [Google Scholar]
- Raich, W. B., Moran, A. N., Rothman, J. H., and Hardin, J. (1998). Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol. Biol. Cell 9, 2037-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli, M. G., Callaini, G., and Glover, D. M. (2000). Failure of pronuclear migration and repeated divisions of polar body nuclei associated with MTOC defects in polo eggs of Drosophila. J. Cell Sci. 113, 3341-3350. [DOI] [PubMed] [Google Scholar]
- Riparbelli, M. G., Callaini, G., Glover, D. M., and Avides Md Mdo, C. (2002). A requirement for the Abnormal Spindle protein to organise microtubules of the central spindle for cytokinesis in Drosophila. J. Cell Sci. 115, 913-922. [DOI] [PubMed] [Google Scholar]
- Rothwell, W. F., and Sullivan, W. (2000). Fluorescent analysis of Drosophila embryos. In: Drosophila Protocols, ed. W. Sullivan, M. Ashburner, and R. S. Hawley, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 141-157.
- Sampath, S. C., Ohi, R., Leismann, O., Salic, A., Pozniakovski, A., and Funabiki, H. (2004). The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell 118, 187-202. [DOI] [PubMed] [Google Scholar]
- Schupbach, T., and Wieschaus, E. (1989). Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics 121, 101-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, D. J., Yu, K. R., Sisson, J. C., Sullivan, W., and Scholey, J. M. (1999). Antagonistic microtubule-sliding motors position mitotic centrosomes in Drosophila early embryos. Nat. Cell Biol. 1, 51-54. [DOI] [PubMed] [Google Scholar]
- Skold, H. N., Komma, D. J., and Endow, S. A. (2005). Assembly pathway of the anastral Drosophila oocyte meiosis I spindle. J. Cell Sci. 118, 1745-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers, W. G., and Saint, R. (2003). A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell 4, 29-39. [DOI] [PubMed] [Google Scholar]
- Stapleton, M. et al. (2002). The Drosophila gene collection: identification of putative full-length cDNAs for 70% of D. melanogaster genes. Genome Res. 12, 1294-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavosanis, G., Llamazares, S., Goulielmos, G., and Gonzalez, C. (1997). Essential role for gamma-tubulin in the acentriolar female meiotic spindle of Drosophila. EMBO J. 16, 1809-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf, W. E., and Hawley, R. S. (1992). Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 116, 1167-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak, C. E., Vernos, I., Mitchison, T. J., Karsenti, E., and Heald, R. (1998). A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol. 8, 903-913. [DOI] [PubMed] [Google Scholar]
- Williams, B. C., Dernburg, A. F., Puro, J., Nokkala, S., and Goldberg, M. L. (1997). The Drosophila kinesin-like protein KLP3A is required for proper behavior of male and female pronuclei at fertilization. Development 124, 2365-2376. [DOI] [PubMed] [Google Scholar]
- Wilson, P. G., Simmons, R., and Shigali, S. (2004). Novel nuclear defects in KLP61F-deficient mutants in Drosophila are partially suppressed by loss of Ncd function. J. Cell Sci. 117, 4921-4933. [DOI] [PubMed] [Google Scholar]
- Yamamoto, A. H., Komma, D. J., Shaffer, C. D., Pirrotta, V., and Endow, S. A. (1989). The claret locus in Drosophila encodes products required for eye color and for meiotic chromosome segregation. EMBO J. 8, 3543-3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P., Knowles, B. A., Goldstein, L.S.B., and Hawley, R. S. (1990). A kinesin-like protein required for distributive chromosome segregation in Drosophila. Cell 63, 1053-1062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.