Abstract
The homeobox-containing aristaless-related protein ARX has been directly linked to the development of a number of human disorders involving mental retardation and epilepsy and clearly plays a critical role in development of the vertebrate central nervous system. In this work, we investigate the role of ALR-1, the Caenorhabditis elegans aristaless orthologue, in amphid sensory function. Our studies indicate that ALR-1 is required for maintenance of the amphid organ structure throughout larval development. Mutant analysis indicates a progressive loss in the amphid neurons' ability to fill with lipophilic dyes as well as a declining chemotactic response. The degeneration in amphid function corresponds with a failure of the glial-like amphid socket cell to maintain its specific cell shape and cell–cell contacts. Consistent with ALR-1 expression within the amphid socket cell, our results indicate a cell autonomous role for ALR-1 in maintaining cell shape. Furthermore, we demonstrate a role for ALR-1 in the proper morphogenesis of the anterior hypodermis. Genetic interaction tests also suggest that ALR-1 may function cooperatively with the cell adhesion processes in maintaining the amphid sensory organs.
INTRODUCTION
Mutations in the human aristaless-related gene ARX have been implicated in a surprising diversity of X-linked disorders involving mental retardation and epilepsy. X-linked West syndrome, Partington syndrome, X-linked myoclonic epilepsy with spasticity and intellectual disability, and nonsyndromic forms of X-linked mental retardation are all derived by mutations in ARX (Bienvenu et al., 2002; Scheffer et al., 2002; Stromme et al., 2002a,b; Kato et al., 2004). Each of these disorders displays severe deficits in cognitive function; however, physical abnormalities of the brain are typically absent. In contrast, X-linked lissencephaly with abnormal genitalia (XLAG), also etiologically linked to ARX mutations (Kitamura et al., 2002; Kato et al., 2004), is characterized by distinct and severe brain malformations as well as intractable epilepsy. The diversity of phenotypes associated with mutations in ARX suggests a complex condition with a number of influencing factors.
ARX knockout mice exhibit defects similar to the clinical indications seen in XLAG individuals (Kitamura et al., 2002). At the cellular level, ARX knockout mice have clear defects in proliferation, differentiation, and migration of neurons within specific areas of the forebrain (Kitamura et al., 2002). These areas correlate well with the expression patterns for ARX in both mice and humans (Miura et al., 1997; Bienvenu et al., 2002; Ohira et al., 2002; Poirier et al., 2004). These observations support the conclusion that ARX plays a fundamental role in the development and function of the forebrain. An important step in understanding the mechanisms by which ARX mutations exert their deleterious effects will be the identification and analysis of subcellular processes controlled by ARX-related proteins.
ARX is orthologous to the paired class homeobox transcription factor aristaless of Drosophila (Galliot et al., 1999). At the developmental level, Aristaless expression defines the presumptive tip within the larval imaginal disks (Campbell et al., 1993; Schneitz et al., 1993; Campbell and Tomlinson, 1998). Loss of Aristaless function results in a failure to develop the distal-most leg segments, the pretarsus, and the distal-most antennal sensory structures, the arista (Schneitz et al., 1993; Campbell and Tomlinson, 1998). More specifically, Aristaless expression has been implicated in defining the distal-most segment boundary by controlling Fasciclin 2 expression (Tsuji et al., 2000). Fasciclin 2 is an immunoglobulin superfamily protein implicated in cell adhesion, suggesting a direct role for Aristaless in demarcating cell adhesion properties for those cells specific to the distal regions of the developing appendages.
In this study, we examined the role of ALR-1, the C. elegans aristaless orthologue (Galliot et al., 1999), in amphid organ function. The C. elegans chemotactic responses are primarily mediated by the amphid neurons (reviewed in Mori, 1999). The amphids are bilateral chemosensory organs, located in the nose of the animal, that are composed of 12 sensory neurons and their associated glial-like support cells, the sheath and socket cells (Ward et al., 1975). The sheath cell associates with all 12 neurons by surrounding their dendrite endings, isolating them from surrounding tissues. The amphid socket cell bridges the gap between the sheath cell and the external cuticle, creating a pore through which the exposed sensory cilia of six amphid neurons contact the environment. The intimate association between the sheath and socket cell serves to create an exclusive environment for the sensory cilia. An analogous structure in the tail of the animal, composed of one sheath and two socket cells, supports the two phasmid neurons on either side of the worm (Hall and Russell, 1991). The integrity of these organ structures is required to mediate responses to a wide range of compounds and stimuli (Lewis and Hodgkin, 1977; Albert et al., 1981; Perkins et al., 1986; Hilliard et al., 2002). Our studies reveal that the alr-1 gene product is required to maintain the functional and structural integrity of the amphid organs during larval development. Furthermore, we demonstrate that expression of ALR-1 is specifically required in the amphid socket cells to maintain their unique cell shape and cell–cell contacts. Finally, we also define a role for ALR-1 during embryogenesis involving morphogenesis of the hypodermal cells that enclose the anterior of the embryo.
MATERIALS AND METHODS
Strains
All C. elegans strains were maintained according to the standard protocol (Brenner, 1974). All strain analyses were performed at 20°C. The alleles used in this study were as follows: hmp-2(qm39) I, osm-3(p802) IV, hmp-1(fe4) V, alr-1(ok545) X, and ctDf19 X. A transgenic array expressing alr-1p::GFP (kuEx146; see below), marked with a dominant Roller gene, pRF4 containing rol-6(su1006), was used to visualize alr-1 promoter activity. A transgenic array expressing unc-53pB::GFP (bgEx 21) (Stringham et al., 2002) marked with a dominant Roller gene, pRF4 containing rol-6(su1006), was used to visualize the amphid socket cells. An integrated transgenic array, itr-1pb::alr-1 (kuIs67; see below) was used to assay ALR-1 expression in the amphid socket cells. An integrated transgenic array, ajm-1::GFP (kuIs46 X) (Chen and Han, 2001), was used to visualize epithelial cell boundaries (Koppen et al., 2001).
Characterization of alr-1(ok545) cDNA
The alr-1(ok545) lesion was sequenced directly from genomic DNA and was identical to that reported by the C. elegans Gene Knockout Consortium (http://celeganskoconsortium.omrf.org) for the corresponding gene, R08B4.2. To determine the nature of the mRNA produced from this allele, we generated cDNAs with alr-1 gene-specific primers (atgcccgagttgaagaaagaagac and gcatgaactttcttcttttggcttcac) and the SuperScript One-Step RT-PCR kit (Invitrogen, Carlsbad, CA). These PCR products were cloned and sequenced. A single class of alr-1 cDNA from the ok545 strain was identified, which revealed an aberrantly spliced transcript joining exons 3 and 5. This procedure would not identify mRNA products that arise from alternative splicing occurring outside of the primer sequences.
Expression Constructs
The alr-1p::GFP reporter construct was generated by inserting 1 kb of upstream regulatory sequence into the pPD95.75 vector (a gift from A. Fire), which contains the green fluorescent protein (GFP) coding sequence (Chalfie et al., 1994) and the 3′ untranslated region of unc-54 (Fire et al., 1990) at the 3′ end. This construct was injected into N2 worms at 10 ng/μl along with a PCR product corresponding to 6 kb of overlapping alr-1 upstream regulatory sequence and a dominant Roller marker, pRF4 containing rol-6 (su1006),at100 ng/μl to generate kuEx146. The itr-1pB::alr-1 rescuing construct was generated by inserting 2.2 kb of the itr-1 promoter B sequence (Gower et al., 2001) upstream of the full-length alr-1 cDNA (yk391d5) in the pBS SK(-) vector. This construct was injected into N2 worms at 1 ng/μl along with a sur-5::GFP marker (pTG96.2) (Yochem et al., 1998) at 50 ng/μl. This construct was integrated into the genome by using standard methods (Epstein, 1995) to generate kuIs67.
Analysis of alr-1 Mutant Phenotypes
Dye-filling assays were performed using 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanin (DiI), at 20 μm/ml as described previously (Perkins et al., 1986; Starich et al., 1995). Osmotic avoidance assays were performed on individual worms as described previously (Culotti and Russell, 1978) with some modifications: Adult worms were encircled within a 1.6-mm barrier of 4 M NaCl, and L2 larval worms were encircled within a 0.8-mm barrier of 4 M NaCl. Assays were conducted over a period of 15 min.
Electron Micrographs
Samples were cryoimmobilized using a BAL-TEC HPM 010 high-pressure freezer, followed by freeze-substitution fixation and epoxy embedding as described previously (McDonald and Muller-Reichert, 2002). Then, 100-nm serial sections (90 nm in L1 larva) were examined at 80 keV by using a Philips CM10 electron microscope.
Immunofluorescence
Images were collected using an Axioplan2 microscope (Carl Zeiss, Thorton, NY) and a Hamamatsu C4742-95 charge-coupled device camera (Hamamatsu Photonics, Bridgewater, NJ). Images were analyzed using Openlab 3.1.7 (Improvision, Lexington, MA) software, and figures were compiled using Photoshop 8.0 and Illustrator 11.0 (Adobe Systems, San Jose, CA).
RESULTS
ALR-1 Is Expressed in Hypodermal, Neuronal, and Glial-like Support Cells
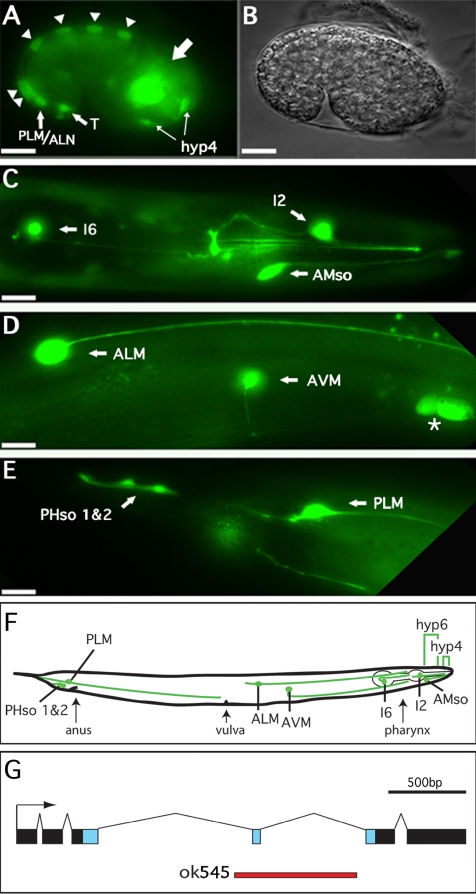
Previous experiments, using antibodies directed against the endogenous ALR-1 protein indicate it is localized exclusively to the nucleus and present in multiple tissues (Melkman and Sengupta, 2005). To characterize the dynamics of gene expression of the alr-1 promoter in live worms, we performed reporter analysis by using an integrated alr-1p::GFP fusion transgene. Containing ∼6 kb of upstream promoter sequence driving the GFP open reading frame, this reporter allowed us to determine transcriptional expression at different times during developmental and adult stages. The GFP protein did not contain a nuclear localization signal, facilitating cell identification based on both cell location and cell morphology. Embryonic analysis indicated an early expression pattern just after the 28-cell stage. The strongest expression at this point was seen in descendants of the C linage as well as less prominent expression within a subset of the AB lineage (our unpublished data). By the comma stage (∼400 cells), GFP expression was apparent in alternating dorsal hypodermal cells before the onset of cell fusions (Figure 1A, triangles). After the onset of the hypodermal cell fusions, GFP was apparent throughout the hyp7 hypodermal syncytium (our unpublished data). At the comma stage, GFP was also strongly expressed in the precursors to the PLM and ALN neurons, the T-cells (precursors to the phasmid socket cells), and the cells that would comprise the hyp4 anterior hypodermal syncytium (Figure 1A). These cells were tentatively identified based on cell position (Sulston et al., 1983) and the strong expression seen within the adult structures derived from these cells (see below). A number of GFP-expressing cells within the head region of the embryo remained unidentified (Figure 1A, large arrow). These cells likely include a number of head and pharyngeal neurons, although other cells types are not ruled out.
Figure 1.
alr-1p::GFP expression in embryonic and adult tissues. (A) Fluorescence and (B) differential interference contrast images of an alr-1p::GFP transgenic embryo at the late comma stage (400 min) showing expression in dorsal hypodermal cells (triangles), T-cells, PLM/ALM neurons, and hyp-4 precursor cells as well as unidentified cell bodies in the head region (large arrow). (C–E) Fluorescence images of alr-1p::GFP transgenic adults. (C) View of the head region revealing expression in the I6 and I2 neurons as well as the amphid socket cells (AMso). (D) Body midsection showing expression in the ALM and AVM neurons as well as nearby ceolomcytes (asterisk). (E) Tail of adult hermaphrodite with GFP expression in the PLM neurons as well as both of the phasmid socket cells (PHso 1 and 2). In all cases, partners of paired cells (left and right) expressed GFP, although they are not shown here because they are out of the plane of focus. (F) Schematic representation of an adult hermaphrodite indicating the relative positions of the alr-1::GFP-expressing cells. Brackets indicate the hyp6 and hyp4 hypodermal syncytia. Anterior is to the right, posterior to the left. Bars, 10 μm. (G) Exon/intron structure of alr-1. Blue boxes represent sequences encoding the homeodomain. The red line represents sequences deleted in alr-1(ok545).
Expression in the larval and adult hermaphrodite was primarily restricted to a subset of neurons and neuronal support cells. The PLM, ALM, and AVM touch neurons and the intrinsic pharyngeal neurons I2 and I6 all showed very strong expression throughout all larval stages and adulthood (Figure 1, C–E). The RIS neuron and one other unidentified, unpaired neuronal cell body located in the retrovesicular ganglion occasionally showed faint expression (our unpublished data). Strong expression was also seen in the glial-like amphid and phasmid socket cells (AMso and PHso 1 and 2) throughout larval development and adulthood (Figure 1, C and E). Strong GFP expression was also observed within the hyp6 and hyp4 hypodermal syncytia (see below), the distal most segments of the intestine and the coelomocytes, the scavenger cells located within the pseudocoelom (Figure 1D, asterisk). Variable GFP expression was also seen in larval and adult worms within the hyp7 hypodermal syncytium (our unpublished data). Importantly, GFP expression was not observed in 23 of the 24 GABAergic neurons (the sole exception being the RIS neuron) that have been shown previously to stain with anti-ALR-1 antibodies (Melkman and Sengupta, 2005). This suggested that the sequences required for ALR-1 expression in these neurons may reside outside of the 6 kb of promoter sequence driving our GFP reporter. Alternatively, GFP expression from our construct may not be readily detectable above background fluorescence in these specific neurons.
alr-1 Mutants Display a Progressive Dye-filling Defect in the Amphid and Phasmid Neurons
To determine the functional role of ALR-1, we obtained a deletion allele from the C. elegans Knockout Consortium corresponding to the alr-1 open reading frame. Sequence analysis indicated that this strain, alr-1(ok545), contained a 793-bp deletion resulting in the removal of the entire fourth exon of alr-1 (Figure 1G). Sequencing of alr-1 cDNAs isolated from this strain revealed an mRNA product with an aberrant splice pattern joining exons 3 and 5, thereby resulting in a frame shift within the homeodomain. The predicted encoded protein is truncated in the last 30 residues of the 61-amino acid homeodomain and also produces a novel 25 amino acid C terminus. Phenotypically, the alr-1(ok545) strain was superficially indistinguishable from wild type, displaying only an occasional (17% penetrance, n > 200) displaced mouth opening (see below). However, adult worms did show a tendency to crawl up the sides of the culture plate and desiccate. Greater than 15% of adult alr-1(ok545) worms demonstrate this behavior (n > 500), compared with <1% in wild type (n > 500). This behavior is reminiscent of that described previously for strains defective in chemotaxis mediated by the amphid neurons (Starich et al., 1995).
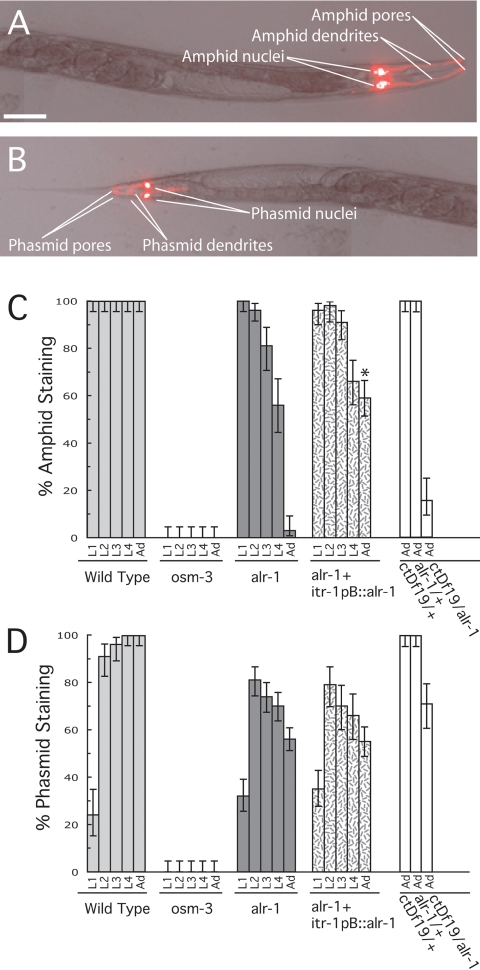
Wild-type animals readily take up fluorescent dyes, such as DiI, into the exposed ciliated endings of the amphid (Figure 2A) and phasmid (Figure 2B) neurons (Perkins et al., 1986; Starich et al., 1995). We used this assay as a preliminary evaluation of amphid function in alr-1(ok545). In wild-type animals, six of the 12 amphid neurons and both phasmid neurons on each side of the animal characteristically stained with DiI throughout larval development and adulthood (Figure 2, A–D). Mutations in genes that affect amphid structure, such as osm-3(p802), result in dye-filling defects (Perkins et al., 1986; Starich et al., 1995). The osm-3 gene encodes a kinesin-like protein required for the development of the distal-most segment of exposed ciliated sensory endings and subsequently has a strong defect in dye filling, as well as a defect in chemotaxis toward soluble compounds (Shakir et al., 1993; Tabish et al., 1995). Typical of other dye-filling mutants, osm-3(p802) showed a complete absence of dye-filled amphid and phasmid neurons throughout larval development and adulthood in our assays (Figure 2, C and D).
Figure 2.
Progressive amphid and phasmid dye-filling defects in alr-1 mutant worms. Worms were stained with DiI as described in Materials and Methods. (A and B) Differential interference contrast/fluorescence merge of a wild-type adult hermaphrodite indicating amphid and phasmid staining via DiI. Dorsal view; anterior is to the right, posterior to the left. Bar, 50 μm. (C) Amphids were considered stained if two or more of the six dye-filling neurons stained. (D) Phasmids were considered stained if at least one of the two dye-filling neurons stained. Strains used were N2 (wild type), osm-3(p802), alr-1(ok545), alr-1(ok545) containing an integrated transgene expressing the wild-type alr-1 cDNA via the itr-1pB promoter (Gower et al., 2001), ctDf19 heterozygote, alr-1(ok545) heterozygote and ctDf19/alr-1(ok545) hemizygote. More than 40 worms were examined for each developmental stage with L1, L2, L3, L4, and Ad representing the different larval stages and adulthood, respectively. Asterisks indicate a significant difference (p < 0.005, via chi-square test) from that of the alr-1(ok545) strain. Error bars represent 95% confidence intervals.
In contrast to both wild type and osm-3(p802), alr-1(ok545) animals showed a progressive defect in amphid and phasmid neuron dye filling. Beginning at the earliest larval stage, L1, essentially 100% of alr-1(ok545) larvae showed wild-type dye filling of the amphid neurons (Figure 2C). However, this phenotype was progressively lost during subsequent larval stages. After the final larval molt, the amphid neurons in alr-1(ok545) adults showed a severe defect in dye filling, with only 5% of the amphid organs showing any significant staining (Figure 2C). Similarly, the phasmid neurons showed a progressive loss in their ability to dye fill, albeit less severe than that of the amphid neurons (Figure 2D). Placing the alr-1(ok545) allele over a genomic deficiency in this region (ctDf19) produced a similar dye-filling phenotype as that of the alr-1(ok545) homozygous strain (Figure 2, C and D), suggesting that the alr-1(ok545) lesion likely results in a loss of function of ALR-1.
These observations suggest that the alr-1(ok545) mutation has a progressive, degenerative effect on the ability of the amphid and phasmid neurons to take up dye. This phenotype and the expression of the alr-1p::GFP transgene in both the amphid and phasmid socket cells (Figure 1, C and E) have led us to propose that ALR-1 might be required for socket cell function. To address this possibility, we attempted to rescue the dye-filling defect of the alr-1(ok545) strain using a promoter sequence specific for the amphid socket cells to drive expression of the wild-type alr-1 cDNA. The itr-1pB promoter has previously been shown to drive expression of reporter constructs specifically in the amphid socket cells, the excretory cell, and the spermatheca (Gower et al., 2001). Expression of the wild-type alr-1 cDNA from the itr-1pB promoter was capable of a partial rescue of the amphid dye-filling defect of alr-1(ok545). Most strikingly, comparison of alr-1(ok545) adults to those harboring the itr-1pB::alr-1 construct indicated an increase in dye-filling efficiency from 5 to 59% (Figure 2C). The itr-1pB promoter is not expressed in the phasmid socket cells, and consequently, this construct did not rescue the phasmid dye-filling defect in this strain (Figure 2D).
alr-1 Mutants Display Defects in Osmotic Avoidance
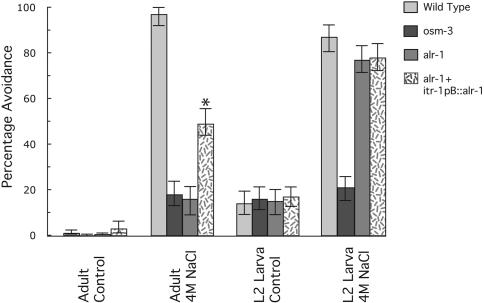
The ASH amphid neurons mediate avoidance of areas of high osmolarity (Bargmann et al., 1990). Assays for defects in osmotic avoidance are performed by encircling individual worms with high concentrations of NaCl and then scoring whether the worm crosses this barrier within a defined time (Culotti and Russell, 1978). We used these assays as a means of measuring amphid function in alr-1(ok545). In our assays, wild-type adult worms typically did not cross a barrier of 4M NaCl (97% avoidance; Figure 3). Adult alr-1(ok545) animals, in contrast, showed a very strong defect in osmotic avoidance, comparable with that of osm-3(p802) worms (18 and 16% avoidance, respectively; Figure 3). Consistent with our dye-filling assays, alr-1(ok545) adults harboring the itr-1pB::alr-1 construct showed a greater than threefold increase in their ability to avoid areas of high osmolarity compared with alr-1(ok545) alone (49 vs. 16% avoidance, respectively; Figure 3), thus suggesting that ALR-1function within the amphid socket cells likely underlies the osmotic avoidance defects of alr-1(ok545). Assaying worms in the absence of high osmolarity produced a similar result among the different strains (Figure 3), indicating that defects in motility were not responsible for the differences between these strains.
Figure 3.
Osmotic defects of alr-1 mutant worms. Worms were assayed by placing individuals within an encircling barrier of 4 M NaCl (diameter of 1.6 mm for adults, 0.8 mm for L2 larva) as described previously (Culotti and Russell, 1978). Avoidance was scored as the inability of worms to cross the barrier within 15 min. Controls were assayed in the absence of 4 M NaCl. Strains used were N2 (wild type), osm-3(p802), alr-1(ok545), and alr-1(ok545) containing an integrated transgene expressing the wild-type alr-1 cDNA via the itr-1pB promoter (Gower et al., 2001). At least 200 individuals were assayed for each strain and stage. Asterisks indicate a significant difference (p < 0.005, via chi-square test) from that of the alr-1(ok545) strain. Error bars represent 95% confidence intervals.
To determine whether the defects in amphid function were degenerative as with the dye-filling defect, we performed osmotic avoidance assays on early larval stages. Assaying the earliest larval stage, L1, was problematic due to limited larval mobility and sensitivity to relatively low concentrations of NaCl. Instead, we performed assays on worms at the L2 larval stage. To accurately compare osmotic avoidance assays between adult and L2 larval worms, the diameter of the encircling barrier was decreased from 1.6 to 0.8 mm in an effort to compensate for the decreased mobility of the L2 larvae. In these assays, both the L2 larval alr-1(ok545) animals and those harboring the itr-1pB::alr-1 construct showed a nearly wild-type ability to avoid high osmolarity (77 and 78% vs. 87% avoidance, respectively; Figure 3). Compared with the severe defect seen in L2 larvae of osm-3(p802) animals (21% avoidance; Figure 3), this observation suggested that L2 larval alr-1(ok545) animals retained a significant portion of their ability to perceive and avoid areas of high osmolarity. Similar to adults, assaying L2 larvae in the absence of high osmolarity indicated similar motility rates between strains (Figure 3).
Ultrastructural Defects of the Amphid Organs in Adult alr-1 Mutants
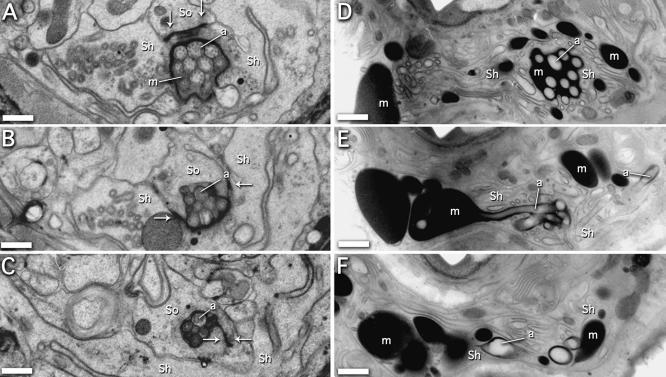
To better determine the underlying cause of the dye filling and osmotic avoidance defects seen in alr-1(ok545) worms, we reconstructed the anterior-most portions of the amphid structures using serial section electron micrographs and compared both wild-type and mutant worms. In total, two adults and two L1 larvae were reconstructed for each strain. In wild-type adults, the amphid cilia passed through amphid pocket ∼4 μm from the tip of the nose (Figure 4A). The amphid pocket is a deep invagination of the sheath cell filled with a sheath cell-secreted matrix material (Ward et al., 1975). The matrix material was contained within this exclusive extracellular space by the close association of the amphid sheath and socket cells. The cilia that were exposed to the environment passed from the amphid pocket into the socket cell (at 2.5 μm; Figure 4B) and were bundled tightly within the cuticle-lined pore at ∼1 μm (Figure 4C). The intersection between the sheath and socket cells as well as the socket cell self-junction were marked by the presence of electron-dense junctions (Figure 4, A–C, arrows), consistent with previous findings (Ward et al., 1975; Perkins et al., 1986).
Figure 4.
Ultrastructure defects of the amphid organs in alr-1 mutant adults. (A–C) Transverse sections through the left amphid of a wild-type adult at ∼4, 2.5, and 1 μm from the tip of the head, respectively. (A) The channel cilia are present within the amphid pocket formed by the sheath cell (Sh) and are surrounded by matrix material (m). Electron-dense junctions between the sheath cell and the socket cell (So) are indicated (arrows). (B) Section through the sheath cell–socket cell junction where the socket cell begins to contact the amphid cilia. (C) Section through the socket cell self-junction. (D–F) Comparable sections of an alr-1(ok545) adult. (D) The channel cilia are present within the amphid pocket formed by the sheath cell, although the matrix material is considerably darker and occurs throughout the tip of the worm. (E) The amphid socket cell is not apparent, fails to make contact with the amphid cilia, and the characteristic electron-dense junctions are not present. (F) The amphid cilia have fallen into disarray, and the presence of the socket cell is not evident. Representative amphid cilia are indicated (a) in each panel. Bars, 0.5 μm.
Equivalent sections through alr-1(ok545) adult animals indicated gross structural defects at the anterior-most ends of the amphid organs. At 4 μm, the amphid cilia passed through the amphid pocket similar to wild type, but the matrix material within the amphid pocket seemed unusually dark (Figure 4D). At 2.5 and 1 μm, the amphid cilia fell into disarray by spreading throughout the nose of the worm, often ending within large pockets of matrix material (Figure 4, E and F). The presence of the matrix material secreted by the sheath cell, found throughout the nose of the worm, suggests that the structural integrity of the amphid organ was impaired. Strikingly, the amphid socket cell was not apparent; the characteristic electron-dense junctions between the sheath and socket cells were not present. Careful analysis of the sheath cells in all of our adult sections (to a depth >20 μm) did not reveal any apparent junctions with a presumptive socket cell. However, the characteristic electron-dense junctions between the sheath cell and the amphid cilla (Ward et al., 1975; Perkins et al., 1986) were present (our unpublished data). Any structure, based on morphology and position, that would have been readily identifiable as the socket cell was not apparent in our sections. The amphid neurons not normally associated with the socket cell (AWA, AWB, and AWC neurons) were present in these sections, but their exact morphology was difficult to determine because of the general disorder of the amphid organs. Other structures in these sections, including the AFD amphid neurons, seemed intact, although slightly askew due to the presence of the abundant matrix material. Unfortunately, the poor preservation of microtubule structures, in both the wild type and alr-1(ok545) samples, did not allow direct comparison of the intracellular architecture of the amphid cilia.
EM analysis of L1 larvae indicated that the amphid socket cell was initially formed and intact in alr-1(ok545). In wild-type L1 worms, the socket cell was apparent, forming a cylindrical-shaped pore at its most anterior end via self-junctions (Figure 5A, arrows). The socket cell in alr-1(ok545) was also apparent with the self-junctions and the sheath–socket cell junctions intact (Figure 5B, arrows; our unpublished data). However, the socket cell in alr-1(ok545) did seem significantly less substantial than that of wild type.
Figure 5.
Socket cell ultrastructure in alr-1 mutant L1 larvae. (A) Transverse section through the amphid socket cell (So) in wild-type L1 larvae ∼0.5 μm from the tip of the head. Electron-dense socket cell self-junctions are indicated (arrows). (B) Comparable section in alr-1(ok545) L1 larvae. Although the socket cell looks similar to that of wild type in morphology, the overall size seems somewhat diminished. Bars, 0.5 μm.
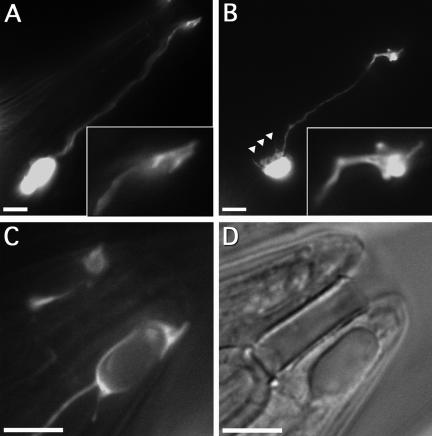
The Socket Cell Shape Is Changed in alr-1 Mutants
To determine the fate of the amphid socket cell, we used a GFP reporter driven by the unc-53pB promoter. The unc-53pB promoter has been shown previously to express very strongly throughout the larval and adult stages in a number of tissues, including the amphid socket cells (Stringham et al., 2002). The unc-53pB::GFP reporter allowed us to accurately record the presence of the amphid socket cell as well as its cell morphology, from the earliest larval stages throughout adulthood.
In wild type, the amphid socket cell had a variable cell body position close to the anterior bulb of the pharynx with a single slender appendage extending toward the nose of the animal (Figure 6A). The distal end of the appendage formed a distinct cylindrical “socket” shape (Figure 6A, inset) that lay in close contact with the cuticle. This morphology is typically invariant and consistent throughout all stages. In contrast, the amphid socket cell morphology in alr-1(ok545) worms underwent significant changes toward the end of larval development. At the earliest larval stages, L1 through L3, the socket cell seemed typical of wild-type animals. However, beginning in the L4 larval stage and becoming predominant in adults, the distal end of the appendage began to look misshapen (Figure 6B). The cylindrical-shaped ending was typically no longer apparent, becoming amorphous with variable projections and membrane excrescences (Figure 6B, inset). The distal end also no longer made contact with the cuticle and often had retracted a significant distance (on average, >5 μm). Furthermore, the boundary defining the cell body was typically more disordered than wild type and was often seen to display numerous membrane projections (Figure 6B, triangles). The distal-most ending was also frequently deformed by large, intracellular vacuoles (Figure 6, C and D). These vacuoles were most prominent in older adults, perhaps implicating late-stage processes in the morphological changes at the distal end of the amphid socket cell.
Figure 6.
Changes in the socket cell morphology in alr-1 mutant worms. (A–C) Fluorescence and (D) differential interference contrast images of unc53pB::GFP transgenic adults showing expression in the amphid socket cells. (A) Wild-type amphid socket cell morphology displaying the characteristic cylindrical distal ending (inset). (B) The amphid socket cell in alr-1(ok545) showing a typically misshapen distal ending (inset) and membrane projections near the cell body (triangles). (C and D) The distal end of an alr-1(ok545) amphid socket cell exhibiting a large intracellular vesicle. Anterior is to the upper right, posterior to the lower left. Bars, 10 μm.
The Dye-filling Defect of the alr-1 Mutation Is Enhanced by Mutations in the Cadherin–Catenin Complex
The dramatic changes in cell shape that were observed in the alr-1(ok545) amphid socket cells as well as the apparent lack of electron-dense junctions between the socket and sheath cells led to the hypothesis that changes in cell adhesion properties may be responsible for the observed phenotypes. To test this hypothesis, genetic interactions between alr-1(ok545) and components associated with the C. elegans adherens junction complex were assayed. The highly conserved cadherin–catenin complex is known to mediate cell adhesion in diverse organisms (reviewed in Tepass, 1999; Wheelock and Johnson, 2003) and localizes to the C. elegans adherens junction (Costa et al., 1998; Raich et al., 1999). In C. elegans, the core components of the cadherin–catenin complex include hmr-1, hmp-1, and hmp-2, which encode classical cadherin, α-catenin, and β-catenin/Armadillo, respectively (Costa et al., 1998). The hmr-1, hmp-1, and hmp-2 genes are all required for early embryonic development; strong loss-offunction mutations are nearly 100% lethal (Costa et al., 1998).
To determine whether the cadherin–catenin complex might enhance the dye-filling phenotype associated with alr-1(ok545), we took advantage of the hypomorphic alleles hmp-1(fe4) (Pettitt et al., 2003) and hmp-2(qm39) (Costa, unpublished observations), previously known as mad-1(qm39) (Hekimi et al., 1995). The hmp-1(fe4) and hmp-2(qm39) mutations alone had completely wild-type levels of amphid dye filling at all developmental stages (Figure 7). In contrast, the alr-1(ok545); hmp-1(fe4) and alr-1(ok545); hmp-2(qm39) double-mutants showed a significant and reproducible earlier onset of the dye-filling defect compared with alr-1(ok545) alone (Figure 7). Specifically, the number of amphid organs that dye filled in the L2, L3, and L4 larval stages decreased by 7, 17, and 21% (p < 0.005), respectively, in the alr-1(ok545); hmp-1(fe4) strain and by 17, 17, and 15% (p < 0.005), respectively, in the alr-1(ok545); hmp-1(qm39) strain compared with alr-1(ok545) alone. These observations indicated that impaired function of the cadherin–catenin complex could enhance the dye-filling defect of alr-1(ok545).
Figure 7.
Genetic interactions between alr-1 mutant worms and components of the cadherin–catenin complex. Amphid dye filling assayed as in Figure 2. Strains assayed were hmp-1(fe4), hmp-2(qu39), and alr-1(ok545) and double mutants alr-1(ok545); hmp-1(fe4) and alr-1(ok545); hmp-2(qu39). More than 55 worms were examined for each developmental stage. Asterisks indicate a significant difference (p < 0.005, via chi-square test) from that of the alr-1(ok545) strain. Error bars represent 95% confidence intervals.
alr-1 Mutants Display Defects in Anterior Hypodermal Morphogenesis
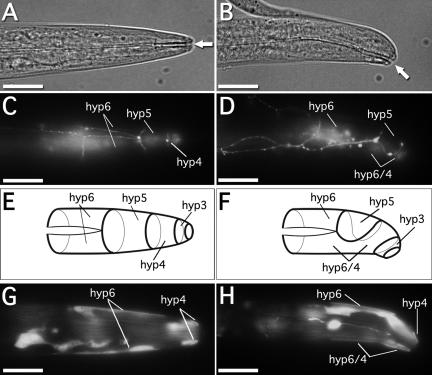
The body morphology of the alr-1(ok545) strain was superficially indistinguishable from wild type; however, worms did show an occasionally displaced mouth opening (Figure 8, A and B), typically to the ventral side of the worm. Aproximatly 17% of alr-1(ok545) worms (36 of 210 animals assayed) demonstrated this phenotype, compared with <1% in wild type (2 of 227 animals assayed). This phenotype is reminiscent of that described previously for strains defective in morphogenesis of the anterior hypodermis (Brenner, 1974; Wang et al., 1999; Ginzburg et al., 2002). The morphogenesis of the C. elegans hypodermis occurs during embryogenesis (reviewed in Shemer and Podbilewicz, 2000; Piekny and Mains, 2003). The hypodermal precursors are initially positioned as a sheet of cells resting on the dorsal surface of the embryo. During morphogenesis of the head hypodermis, the lateral-most anterior cells of the hypodermal sheet migrate ventrally to meet at the ventral midline. These cells encircle the head of the embryo and subsequent cell fusion events produce the four toroidal-shaped cells, hyp3–6. The boundaries of these cells are easily observed in both larvae and adults using the apical junction marker ajm-1::GFP (Mohler et al., 1998; Koppen et al., 2001).
Figure 8.
Anterior hypodermal defects in alr-1 mutant worms. Differential interference contrast images of (A) wild-type and (B) alr-1(ok545) L1 larvae, indicating a displaced mouth opening to the ventral side in mutant worms (arrows). Fluorescence images of (C) wild-type and (D) alr-1(ok545) ajm-1::GFP transgenic L1 larvae depicting the adherens junctions between adjacent hypodermal cells. (E and F) Schematic representations of ajm1::GFP expression observed in C and D, respectively, indicating the relative positions of the anterior hypodermal cells. In alr-1(ok545) worms, hyp5 fails to contact itself at the ventral midline, and hyp4 and hyp6 fuse aberrantly. Fluorescence images of (G) wild-type and (H) alr-1(ok545) alr-1p::GFP transgenic L1 larvae, showing expression in the hyp4 and hyp6 hypodermal syncytial cells. Anterior is to the right, dorsal is up. Bars, 10 μm.
In alr-1(ok545) worms that had a displaced mouth opening, the hyp4, hyp5, and hyp6 hypodermal cells seemed to have formed improperly. Specifically, the hyp5 cell most often failed to traverse the entire circumference of the worm, leaving a gap ventrally between the leading edges of the cell (Figure 8, D and F). Although limited contact between the leading edges of hyp5 was occasionally observed, in these instances, ajm-1::GFP was present at this border thereby indicating that fusion of the hyp5 cell was incomplete (our unpublished data). Furthermore, in those cases in which hyp5 did not make contact with itself at the ventral midline, no cell border was apparent between hyp4 and hyp6 when visualized via ajm-1::GFP (Figure 8, D and F). This observation indicated an ectopic dorsal-ventral fusion between the hyp4 and hyp6 cells. Consistent with a direct role for ALR-1 in morphogenesis of these cells, alr-1p::GFP was observed in hyp4 and hyp6 in wild-type larvae (Figure 8G) and in the hyp4/6 syncytium in alr-1(ok545) (Figure 8H). However, alr-1p::GFP was excluded from the hyp5 cell in both wild-type and alr-1(ok545) worms (Figure 8, G and H).
DISCUSSION
Several lines of evidence indicate that ALR-1 is required to maintain the structural and functional integrity of the amphid sensory organs throughout larval development. First, the alr-1(ok545) strain shows defects in the ability of the amphid neurons to fill with the lipophilic dye DiI (Figure 2C); however, this defect is only apparent in later larval stages and adult worms. Second, alr-1(ok545) adult worms also show severe defects in osmotic avoidance mediated by the ASH amphid neurons (Figure 3). This stands in contrast to alr-1(ok545) worms assayed during the early L2 larval stage, which demonstrate the ability to avoid areas of high osmolarity comparable with wild type (Figure 3). Together, these observations suggest that the amphid organs in alr-1(ok545) animals are undergoing some form of progressive degeneration during larval development. Consistent with this conclusion, ultrastructure analysis of both L1 larval and adult alr-1(ok545) worms indicates that the amphid organs seem structurally intact at hatching but fall into severe disarray by adulthood (Figures 4 and 5).
The progressive degeneration of the amphid organs observed in alr-1 mutants is precipitated by dramatic changes in the cell shape of the amphid socket cells. The most striking difference we observed in our ultrastructure analysis between wild-type and alr-1(ok545) adult worms was the apparent absence of the amphid socket cells (Figure 4). However, analysis using a socket-cell-specific GFP marker indicated that the socket cells were indeed present in adult alr-1 mutant worms but failed to maintain their unique cell shape and cell-cell contacts (Figure 6). Considering the specific expression of our alr-1p::GFP reporter construct in the amphid socket cells (Figure 1C) and the requirement for ALR-1 expression within the socket cells for proper amphid function (Figures 2C and 3), we propose that ALR-1 is intrinsically required for maintaining the shape of the socket cells throughout larval development.
The architecture of a cell is defined by the arrangement of its cytoskeleton and proper adhesion via cell–cell and cell–extracellular matrix interactions. However, the exact molecular mechanisms required for producing and maintaining specific cell morphologies is not well understood. The structure of the amphid socket cell is essential for amphid neuron function and may serve as a unique model for insight into morphogenesis of the amphid organ and the regulation of cell shape. Because cell adhesion complexes are often associated with the cytoskeleton, they represent a critical juncture for the determination of cell shape. Our genetic analysis revealed that reduction-of-function mutations in the C. elegans α-catenin and β-catenin homologues hmp-1 and hmp-2 were capable of exacerbating the dye-filling phenotype associated with alr-1(ok545) (Figure 7). Although the exact molecular basis of these genetic interactions is not clear, these data suggest that defects in ALR-1 and the cadherin–catenin complex may affect related cellular processes.
Although ALR-1 is required to maintain the unique shape and integrity of the amphid socket cells, it is not apparent whether its function is required during larval development or, alternatively, whether it functions at an earlier stage to establish the proper structure of these cells, which may be required to tolerate the mechanical stresses generated during the growth of the organism. Although these models are not mutually exclusive, it is apparent in our ultrastructure analysis of alr-1(ok545) L1 larvae that the size of the socket cell is significantly diminished (Figure 5B), suggesting that the embryonic development of these cells may be impaired. Furthermore, we have observed that the sister cells of the amphid socket cells (CEM neurons), which undergo programmed cell death in hermaphrodites, fail to express the cell specific marker PKD-2::GFP (Barr et al., 2001) in either alr-1(ok545) males or alr-1(ok545);ced-3(n717) hermaphrodites (Tucker and Han, unpublished observations). This suggests that the defects observed in these cells may be due to a partial cell fate transformation of this cell linage. Consistent with this idea, recent work has also implicated ALR-1 as being involved in cell fate specification of the AWA, ASG amphid neurons, and the VD motor neurons (Melkman and Sengupta, 2005). Together, these results implicate ALR-1 as a key regulator of cell determination in multiple neuronal cell lineages.
In this article, we have also demonstrated that ALR-1 is required for proper morphogenesis of the anterior hypodermis and positioning of the mouth opening. The underlying defect in these instances seems to be improper formation of the hyp5 hypodermal syncytium (Figure 8). During morphogenesis of the anterior hypodermis (reviewed in Shemer and Podbilewicz, 2000; Piekny and Mains, 2003), cells from the dorsal side of the embryo extend to meet at the ventral midline. Subsequent fusion between specific cells produces the toroidal-shaped cells that enclose the head of the embryo. Failure of the hyp5 precursor cells to properly migrate and/or make proper contacts at the ventral midline is the most plausible explanation for the defects we have observed in alr-1(ok545) worms. Interestingly, expression of alr-1p::GFP was not observed in hyp5 but in the neighboring hyp4 and hyp6 cells (Figure 8, G and H). Although it is possible that endogenous ALR-1 is expressed in hyp5, the alr-1p::GFP expression pattern suggests that proper formation of hyp5 may require external cues originating from nearby cells. Alternatively, formation of hyp5 may be impaired by aberrant cell-cell contacts between hyp4 and hyp6 that may occur in alr-1(ok545) worms. Consistent with this hypothesis, in wild type, before ventral migration of the hypodermal precursors, it seems that the lateral-most hyp4 and hyp6 precursors share a cell border. In effect, this cell contact excludes the hyp5 precursors from the migrating edge. Consequently, for the hyp5 precursor cells to meet at the ventral midline, the contact between the hyp4 and hyp6 precursors must be disrupted. Failure to disrupt these contacts, possibly stabilized by ectopic fusions between hyp4 and hyp6 precursors, could lead to the phenotypes we have observed in alr-1(ok545) worms.
An important implication of the work presented here is that the aristaless orthologues in other organisms may regulate similar processes to that of ALR-1 in C. elegans. The migration of neurons during vertebrate development often uses cellular substrates, and the structural integrity of these support cells as well as their adhesive properties are fundamental to this process (reviewed in Marin and Rubenstein, 2003). Considering the role of ALR-1 in maintaining the shape of the amphid socket cells and its role in hypodermal morphogenesis, it is conceivable that the neuronal cell migration defects seen in ARX-deficient mice may be a result of cell shape defects or a failure to regulate specific cell–cell contacts during development of the forebrain. However, given the complex nature of the human disorders caused by mutations in ARX, it would not be surprising if the clinical indications were generated by alterations in multiple pathways at the cellular level. Future work identifying the gene products directly regulated by ALR-1 will improve our understanding of the mechanisms underlying the maintenance of cell shape and/or cell adhesion and may provide significant insight into the etiology of the varying disorders caused by mutations in ARX.
Acknowledgments
We thank the C. elegans Gene Knockout Consortium (Oklahoma Medical Research Foundation) for generating the ok545 deletion strain; T. Stiernagle at the C. elegans Genetic Center (University of Minnesota) for various strains used in this work; T. Bogaert for the Unc-53pB::GFP strain; H. Baylis for the Itr-1pB::GFP strain; J. Pettitt for the hmp-1(fe4) strain; Y. Kohara for cDNA clones, A. Fire for GFP vectors; and J. Priess for the HMR-1 and HMP-2 antibodies. We thank M. Costa for sharing unpublished observations. We thank T. Melkman and P. Sengupta (Brandeis University) for communicating data before publication. We also thank W. Johnson, K. Nyberg, H. Smith, D. Starr, and other members of the Han and Wood laboratories for helpful discussions and comments. M. T. is a Damon Runyon Fellow supported by the Damon Runyon Cancer Research Foundation (DRG-#1719-02). This work was supported by the Howard Hughes Medical Institute, of which M. H. is an investigator, and by National Institutes of Health Grant RR00592 to The Boulder Laboratory for 3-D Electron Microscopy of Cells.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-03-0205) on July 29, 2005.
References
- Albert, P. S., Brown, S. J., and Riddle, D. L. (1981). Sensory control of dauer larva formation in Caenorhabditis elegans. J. Comp. Neurol. 198, 435-451. [DOI] [PubMed] [Google Scholar]
- Bargmann, C. I., Thomas, J. H., and Horvitz, H. R. (1990). Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb. Symp. Quant. Biol. 55, 529-538. [DOI] [PubMed] [Google Scholar]
- Barr, M., DeModena, J., Braun, D., Nguyen, C., Hall, D. H., and Sternberg, P. (2001). The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr. Biol. 11, 1341-1346. [DOI] [PubMed] [Google Scholar]
- Bienvenu, T., et al. (2002). ARX, a novel Prd-class-homeobox gene highly expressed in the telencephalon, is mutated in X-linked mental retardation. Human Molecular Genetics 11, 981-991. [DOI] [PubMed] [Google Scholar]
- Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, G., and Tomlinson, A. (1998). The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development 125, 4483-4493. [DOI] [PubMed] [Google Scholar]
- Campbell, G., Weaver, T., and Tomlinson, A. (1993). Axis specification in the developing Drosophila appendage: the role of wingless, decapentaplegic, and the homeobox gene aristaless. Cell 74, 1113-1123. [DOI] [PubMed] [Google Scholar]
- Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W., and Prasher, D. C. (1994). Green fluorescent protein as a marker for gene expression. Science 263, 802-805. [DOI] [PubMed] [Google Scholar]
- Chen, Z., and Han, M. (2001). Role of C. elegans lin-40 MTA in vulval fate specification and morphogenesis. Development 128, 4911-4921. [DOI] [PubMed] [Google Scholar]
- Costa, M., Raich, W., Agbunag, C., Leung, B., Hardin, J., and Priess, J. R. (1998). A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J. Cell Biol. 141, 297-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotti, J. G., and Russell, R. L. (1978). Osmotic avoidance defective mutants of the nematode Caenorhabditis elegans. Genetics 90, 243-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein, H. F., and Shakes, D. C. (ed.) (1995). Caenorhabditis elegans: Modern Biological Analysis of an Organism, San Diego: Academic Press.
- Fire, A., Kondo, K., and Waterston, R. (1990). Vectors for low copy transformation of C. elegans. Nucleic Acids Res. 18, 4269-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliot, B., de Vargas, C., and Miller, D. (1999). Evolution of homeobox genes: Q50 paired-like genes founded the Paired class. Dev. Genes Evol. 209, 186-197. [DOI] [PubMed] [Google Scholar]
- Ginzburg, V. E., Roy, P. J., and Culotti, J. G. (2002). Semaphorin 1a and semaphorin 1b are required for correct epidermal cell positioning and adhesion during morphogenesis in C. elegans. Development 129, 2065-2078. [DOI] [PubMed] [Google Scholar]
- Gower, N. J., Temple, G. R., Schein, J. E., Marra, M., Walker, D. S., and Baylis, H. A. (2001). Dissection of the promoter region of the inositol 1,4,5-trisphosphate receptor gene, itr-1, in C. elegans: a molecular basis for cell-specific expression of IP3R isoforms. J. Mol. Biol. 306, 145-157. [DOI] [PubMed] [Google Scholar]
- Hall, D. H., and Russell, R. L. (1991). The posterior nervous system of the nematode Caenorhabditis elegans: serial reconstruction of identified neurons and complete pattern of synaptic interactions. J. Neurosci. 11, 1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekimi, S., Boutis, P., and Lakowski, B. (1995). Viable maternal-effect mutations that affect the development of the nematode Caenorhabditis elegans. Genetics 141, 1351-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard, M. A., Bargmann, C. I., and Bazzicalupo, P. (2002). C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr. Biol. 12, 730-734. [DOI] [PubMed] [Google Scholar]
- Kato, M., et al. (2004). Mutations of ARX are associated with striking pleiotropy and consistent genotype-phenotype correlation. Hum. Mutat. 23, 147-159. [DOI] [PubMed] [Google Scholar]
- Kitamura, K., et al. (2002). Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat. Genet. 32, 359-369. [DOI] [PubMed] [Google Scholar]
- Koppen, M., Simske, J. S., Sims, P. A., Firestein, B. L., Hall, D. H., Radice, A. D., Rongo, C., and Hardin, J. D. (2001). Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat. Cell Biol. 3, 983-991. [DOI] [PubMed] [Google Scholar]
- Lewis, J. A., and Hodgkin, J. A. (1977). Specific neuroanatomical changes in chemosensory mutants of the nematode Caenorhabditis elegans. J. Comp. Neurol. 172, 489-510. [DOI] [PubMed] [Google Scholar]
- Marin, O., and Rubenstein, J. L. (2003). Cell migration in the forebrain. Annu. Rev. Neurosci. 26, 441-483. [DOI] [PubMed] [Google Scholar]
- McDonald, K., and Muller-Reichert, T. (2002). Cryomethods for thin section electron microscopy. Methods Enzymol 351, 96-123. [DOI] [PubMed] [Google Scholar]
- Melkman, T., and Sengupta, P. (2005). Regulation of chemosensory and GABAergic motoR neuron development by the C. elegans Aristaless/Arx homolog. arl-1. Development 132, 1935-1949. [DOI] [PubMed] [Google Scholar]
- Miura, H., Yanazawa, M., Kato, K., and Kitamura, K. (1997). Expression of a novel aristaless related homeobox gene `Arx' in the vertebrate telencephalon, diencephalon and floor plate. Mech. Dev. 65, 99-109. [DOI] [PubMed] [Google Scholar]
- Mohler, W. A., Simske, J. S., Williams-Masson, E. M., Hardin, J. D., and White, J. G. (1998). Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr. Biol. 8, 1087-1090. [DOI] [PubMed] [Google Scholar]
- Mori, I. (1999). Genetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Annu. Rev. Genet. 33, 399-422. [DOI] [PubMed] [Google Scholar]
- Ohira, R., et al. (2002). Human ARX gene: genomic characterization and expression. Molecular Genetics & Metabolism 77, 179-188. [DOI] [PubMed] [Google Scholar]
- Perkins, L. A., Hedgecock, E. M., Thomson, J. N., and Culotti, J. G. (1986). Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117, 456-487. [DOI] [PubMed] [Google Scholar]
- Pettitt, J., Cox, E. A., Broadbent, I. D., Flett, A., and Hardin, J. (2003). The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J. Cell Biol. 162, 15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekny, A. J., and Mains, P. E. (2003). Squeezing an egg into a worm: C. elegans embryonic morphogenesis. Sci. World J. 3, 1370-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier, K., et al. (2004). Neuroanatomical distribution of ARX in brain and its localisation in GABAergic neurons. Brain Res. Mol. Brain Res. 122, 35-46. [DOI] [PubMed] [Google Scholar]
- Raich, W. B., Agbunag, C., and Hardin, J. (1999). Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr. Biol. 9, 1139-1146. [DOI] [PubMed] [Google Scholar]
- Scheffer, I. E., Wallace, R. H., Phillips, F. L., Hewson, P., Reardon, K., Parasivam, G., Stromme, P., Berkovic, S. F., Gecz, J., and Mulley, J. C. (2002). X-linked myoclonic epilepsy with spasticity and intellectual disability: mutation in the homeobox gene ARX. Neurology 59, 348-356. [DOI] [PubMed] [Google Scholar]
- Schneitz, K., Spielmann, P., and Noll, M. (1993). Molecular genetics of aristaless, a prd-type homeo box gene involved in the morphogenesis of proximal and distal pattern elements in a subset of appendages in Drosophila. Genes Dev. 7, 114-129. [DOI] [PubMed] [Google Scholar]
- Shakir, M. A., Fukushige, T., Yasuda, H., Miwa, J., and Siddiqui, S. S. (1993). C. elegans osm-3 gene mediating osmotic avoidance behaviour encodes a kinesin-like protein. Neuroreport 4, 891-894. [DOI] [PubMed] [Google Scholar]
- Shemer, G., and Podbilewicz, B. (2000). Fusomorphogenesis: cell fusion in organ formation. Dev. Dyn. 218, 30-51. [DOI] [PubMed] [Google Scholar]
- Starich, T. A., Herman, R. K., Kari, C. K., Yeh, W. H., Schackwitz, W. S., Schuyler, M. W., Collet, J., Thomas, J. H., and Riddle, D. L. (1995). Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics 139, 171-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringham, E., Pujol, N., Vandekerckhove, J., and Bogaert, T. (2002). unc-53 controls longitudinal migration in C. elegans. Development 129, 3367-3379. [DOI] [PubMed] [Google Scholar]
- Stromme, P., Mangelsdorf, M. E., Scheffer, I. E., and Gecz, J. (2002a). Infantile spasms, dystonia, and other X-linked phenotypes caused by mutations in Aristaless related homeobox gene, ARX. Brain Dev. 24, 266-268. [DOI] [PubMed] [Google Scholar]
- Stromme, P., et al. (2002b). Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nature Genetics 30, 441-445. [DOI] [PubMed] [Google Scholar]
- Sulston, J. E., Schierenberg, E., White, J. G., and Thomson, J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119. [DOI] [PubMed] [Google Scholar]
- Tabish, M., Siddiqui, Z. K., Nishikawa, K., and Siddiqui, S. S. (1995). Exclusive expression of C. elegans osm-3 kinesin gene in chemosensory neurons open to the external environment. J. Mol. Biol. 247, 377-389. [DOI] [PubMed] [Google Scholar]
- Tepass, U. (1999). Genetic analysis of cadherin function in animal morphogenesis. Curr. Opin. Cell Biol. 11, 540-548. [DOI] [PubMed] [Google Scholar]
- Tsuji, T., Sato, A., Hiratani, I., Taira, M., Saigo, K., and Kojima, T. (2000). Requirements of Lim1, a Drosophila LIM-homeobox gene, for normal leg and antennal development. Development 127, 4315-4323. [DOI] [PubMed] [Google Scholar]
- Wang, X., Roy, P. J., Holland, S. J., Zhang, L. W., Culotti, J. G., and Pawson, T. (1999). Multiple ephrins control cell organization in C. elegans using kinase-dependent and -independent functions of the VAB-1 Eph receptor. Mol. Cell 4, 903-913. [DOI] [PubMed] [Google Scholar]
- Ward, S., Thomson, N., White, J. G., and Brenner, S. (1975). Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans.?2UU. J. Comp. Neurol. 160, 313-337. [DOI] [PubMed] [Google Scholar]
- Wheelock, M. J., and Johnson, K. R. (2003). Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 19, 207-235. [DOI] [PubMed] [Google Scholar]
- Yochem, J., Gu, T., and Han, M. (1998). A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics 149, 1323-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]