Abstract
The spindle assembly checkpoint monitors the integrity of the spindle microtubules, which attach to sister chromatids at kinetochores and play a vital role in preserving genome stability by preventing missegregation. A key target of the spindle assembly checkpoint is securin, the separase inhibitor. In budding yeast, loss of securin results in precocious sister chromatid separation when the microtubule spindle is disrupted. However, in contrast to budding yeast, mammalian securin is not required for spindle checkpoint, suggesting that there are redundant mechanisms controlling the dissolution of sister chromatid cohesion in the absence of securin. One candidate mechanism is the inhibitory phosphorylation of separase. We generated a nonphosphorylable point mutant (S1121A) separase allele in securin-/- mouse embryonic stem cells. Securin-/-separase+/S1121A cells are viable but fail to maintain sister chromatid cohesion in response to the disruption of spindle microtubules, show enhanced sensitivity to nocodazole, and cannot recover from prometaphase arrest.
INTRODUCTION
In mitosis, chromosomes are attached by bipolar spindle microtubules via a structure at the centromere region of chromosomes called kinetochore, and aligned half-way between two centrosomes at the metaphase plate. The two sister chromatids are held together by cohesion complexes before anaphase. The abrupt separation of sister chromatids in anaphase is thought to be caused, at least in part, by loss of cohesion, an event dependent on the activity of the anaphase promoting complex (APC) (Fang et al., 1999; Dej and Orr-Weaver, 2000; Koshland and Guacci, 2000; Nasmyth et al., 2000; Peters, 2002). The APC is an E3 ubiquitin ligase mediating targeted proteolysis through ubiquitination of protein substrates (King et al., 1996). A key event regulated by the APC at the onset of anaphase is the activation of separase, a CD Clan protease of caspase family (Uhlmann et al., 2000). Separase-mediated cleavage of the cohesin component Scc1 is essential for sister separation (Uhlmann et al., 1999; Hauf et al., 2001). Before anaphase, separase is inhibited by its inhibitor securin, and the inhibition is released when securin is ubiquitinated by the APC and degraded (Ciosk et al., 1998; Yanagida, 2000).
Securin was first identified in budding yeast as PDS1 (Yamamoto et al., 1996a,b), and later functional homologues with divergent sequences were found in higher eukaryotes (Zou et al., 1999; Jager et al., 2001). Human securin is an oncogene called PTTG for pituitary tumor transforming gene that is overexpressed in a number of tumors of different tissue origins (Pei and Melmed, 1997; Kakar, 1999; Zhang et al., 1999; Puri et al., 2001; Boelaert et al., 2003). The budding yeast securin PDS1 is not essential for viability, but loss of PDS1 causes genome instability (Yamamoto et al., 1996a,b). Precocious separation of sister chromatids as well as rapid loss of viability is observed when pds1 cells are exposed to nocodazole (Yamamoto et al., 1996b), demonstrating that PDS1 is an essential component of the budding yeast spindle checkpoint pathway. However, mammalian securin is not essential in that respect, because securin-/- murine or human cells did not show premature sister separation when the spindle was disrupted (Jallepalli et al., 2001; Mei et al., 2001). These results indicate that there are other mechanisms to block the activity of separase besides the inhibition by securin.
Recently, it has been demonstrated that vertebrate separase is subjected to another layer of regulation: inhibitory phosphorylation (Stemmann et al., 2001). Phosphorylation of Ser1126 and Thr1346 of human separase inhibits its activity. Phosphorylation at Ser1126 contributes most to the inhibition. The inhibitory phosphorylation is a candidate mechanism that could function in the absence of securin. Here, we report that securin and separase phosphorylation act redundantly to prevent sister chromatid separation when the microtubule spindle is disrupted.
MATERIALS AND METHODS
Generation of S1121A Point Mutation in separase
Mouse separase genomic clones containing exon 18 were isolated from the λKO2 library via homologous recombination using homologies 5′ of exon 18 (in the form of synthetic oligos) (Zhang et al., 2002). One clone, pZ218, was chosen for manipulation. The floxed tetracycline resistant gene (TcR) used in the library screening was looped out with Cre recombinase in vitro to generate pZ218ΔTcR, which contains a loxP site 244 base pairs 5′ of exon 18. S1121A mutation was introduced into pZ218ΔTcR via homologous recombination in Escherichia coli. Briefly, oligos (70 nt) containing the desired mutation (including an Age I site) and homologous to the sequences flanking S1121 were synthesized and annealed to generate two linkers. The linkers were ligated to TcR, and the resulting DNA fragment was gel purified and electroporated together with pZ218ΔTcR into a recombinogenic strain of E. coli (DH10β/pML104) (Zhang et al., 2002). Recombinant clones were selected and identified via restriction digestion. One correct clone, pZ226, was sequenced to ensure the presence of the mutation. TcR was removed from pZ226 via partial Age I digestion and religation, producing pZ226ΔTcR. pZ226ΔTcR was fused via the loxP site 5′ of exon 18 by Cre in vitro with pKOEZ-20, a conditional plasmid (afforded by the OriR6Kγ) containing one loxP site, PGK-Puro and KnR, to generate the targeting construct pZ228.
Linearized pZ228 was introduced to AB2.2 mouse embryonic stem cells or cells with both securin alleles deleted (Mei et al., 2001). The targeted clones were identified through Southern blot analysis. To remove the selection marker PGK-Puro (and the rest of pKOEZ-20), Cre was expressed via transient transfecting the targeted clones. The loopout clones were identified with Southern blot analysis and PCR. For PCR identification of the point mutant, genomic DNA from nontargeted embryonic stem (ES) cells and from S1121A mutant cells were used as templates to amplify a 143-base pair fragment with forward primer 5′-tggtcccatccagtcctctg-3′ and reverse primer 5′-acccagcgcagacagactgc-3′. The PCR products were subjected to Age I restriction digestion. Because of the presence of the mutant allele, the PCR products from the mutant cells were composed of two products, one wild-type and the other mutant. The mutant product could be cleaved, producing two fragments of 131 base pairs and 32 base pairs. The smaller (32-base pair) fragment often ran off the gel.
Cell Culture and Cell Cycle Analysis
ES cells were cultured on a layer of feeder cells in DMEM containing 15% fetal bovine serum supplemented with penicillin and streptomycin (growth medium). Cell cycle distribution was analyzed using flow cytometry analysis with standard protocols. The percentage of G1, S, and G2 population was measured with the Coulter (II) software (Beckman Coulter, Fullerton, CA). For growth curve analysis, 1 × 105 (for 0 and 0.1× nocodazole) and 5 × 105 (for 0.4 and 0.5× nocodazole) cells were seeded in a 35-mm tissue culture dish coated with nondividing feeder cells. Twenty-four hours later, nocodazole was added to the culture media and the time was counted as day 0. Cells were harvested every day for 5 d. Each time point was an average of three dishes.
To synchronize ES cells in prometaphase, cells were incubated with growth media containing 65 ng/ml nocodazole for 6 h first, trypsinized, and replated in fresh media containing nocodazole. The unattached mitotic cells were collected 6 h later (a total of 12 h of nocodazole treatment), washed twice with fresh growth media, plated, and harvested for analyses at different time points. With this selective detachment method, >80% pure mitotic calls could be prepared as determined by mitotic index analysis. For immunostaining of the nocodazole arrested and released cells, ES cells were grown on chamber slides, treated with nocodazole for 12 h, washed, and incubated with fresh growth media. Metaphase spread and mitotic index analysis of ES cells were performed as described previously (Mei et al., 2001).
Western Blot Analysis and Immunostaining
ES cells were collected and lysed on ice in lysis buffer (1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 0.15 M sodium chloride, 2 mM EDTA, 0.01 M sodium phosphate, pH 7.2) supplemented with protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and 1 mM phenylmethylsulfonyl fluoride. Protein concentration was determined with Bio-Rad DC protein assay (Bio-Rad, Hercules, CA). Equal amounts of total protein were separated on denaturing polyacrylamide gel and transferred to polyvinylidene difluoride transfer membrane (Bio-Rad). Blots were probed with the indicated primary and appropriate secondary antibodies and detected using ECL chemiluminescence (GE Healthcare, Piscataway, NJ). Primary antibodies used were affinity purified anti-mouse separase C-terminal peptide antibody, anti-cyclinB1 (GNS1; Santa Cruz Biotechnology, Santa Cruz, CA), anti-securin (clone DCS-280; NeoMarkers, Fremont, CA), and monoclonal anti-β-tubulin (clone E7; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA).
For immunostaining, cells cultured on chamber slides (Nalge Nunc International, Naperville, IL) were fixed with 4% paraformaldehyde for 1 h at 4°C and permeabilized with 0.4% Triton X-100 in phosphate-buffered saline (PBS) for 10 min at room temperature. The cells were then covered with blocking solution (5% goat serum in PBS) for 1 h at 37°C and incubated with anti-β-tubulin polyclonal antibody (Abcam, Cambridge, United Kingdom) (1:200 dilution) and anti-Aurora B monoclonal antibody (BD Biosciences PharMingen, San Diego, CA) (1:200 dilution) in blocking solution for 1 h at 37°C. Primary antibodies were stained with goat anti-mouse secondary antibodies conjugated to fluorescein isothiocyanate (1:200 dilution) and goat anti-rabbit secondary antibodies conjugated to Cy3 (1:500 dilution) for 60 min at 37°C, stained with 4,6-diamidino-2-phenylindole, and visualized with a Nikon ECLIPSE E800 microscope.
RESULTS
Generation of a Nonphosphorylable separase Allele in ES Cells
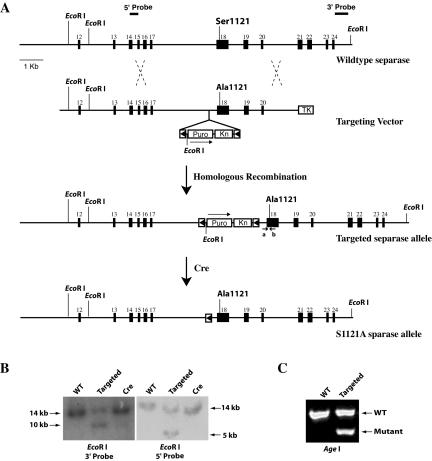
To determine the functional significance of the inhibitory phosphorylation of separase in vivo, we decided to create mouse embryonic stem cell lines that carry a point mutation at the separase locus to block the phosphorylation. Mouse separase is encoded in 31 exons spanning ∼90 kb on chromosome 15 near the telomere region. The critical residue for its protease activity, histidine, in exon 30, and the two inhibitory phosphorylation sites Ser1121 (Ser1126 in human) and Thr1341 (Thr1346 in human) are located in exon 18. Because Ser1121 is the major inhibitory phosphorylation site, we decided to mutate this site first to determine its role in the spindle assembly checkpoint. We isolated mouse separase genomic clones through a novel library screening method (Zhang et al., 2002). Using one of the isolated genomic clones, we changed the Ser residue at position 1121 to Ala via homologous recombination. The mutation generated an Age I site. With additional manipulations, a targeting vector was constructed and introduced into both wild-type and securin-/- ES cells (Mei et al., 2001) (Figure 1A). ES cell clones containing the targeted allele (separaseS1121A_Puro) were identified with Southern blot analysis (Figure 1B). The presence of the Ser-to-Ala mutation was confirmed by the Age I restriction digest of the PCR product of the region encompassing the mutation (Figure 1C) and sequencing of the PCR product (our unpublished data). This targeted allele is expected to be nonfunctional owing to the insertion of the selection maker as well as other DNA sequences of the targeting construct, and thus the point mutation is conditional, becoming expressed only after the Cre-mediated loopout of the Puro selection marker (Figure 1A). Furthermore, the point mutation is expected to be dominant.
Figure 1.
Generation of S1121A point mutant separase in mouse ES cells. (A) Diagram of the strategy. (B) Southern blot analyses of genomic DNA isolated from wild-type, targeted, and Cre-mediated loopout ES cells. EcoR I was used to digest the DNA. (C) PCR analysis of genomic DNA isolated from the wild-type and targeted ES cells. PCR products obtained with primers a and b as indicated in A were digested with Age I.
In a test to see whether the loss of securin in combination with this Ser-to-Ala point mutation in separase is a lethal event, we transiently expressed Cre in securin-/-separase+/S1121A_Puro cells. The efficiency of the Cre-mediated loopout in other loci is ∼10% in our hands. We did recover loopout clones from securin-/-separase+/S1121A_Puro cells with a similar efficiency, indicating that the point mutation plus securin null does not cause loss of viability. However, it does cause precocious sister separation (see below). The nonloopout clones were essentially isogenic to the loopout clones and were used as controls. We repeated the targeting and loopout in another independent securin-/- ES cell line (Mei et al., 2001), and similar results were obtained. We used cells with the following four different genotypes in our experiments: wild type (WT), securin+/+ separase+/S1121A_Puro; S1121A separase (SA), securin+/+ separase+/S1121A; securin null (SE), securin-/-separase+/S1121A_Puro; and double mutant (DM), securin-/- separase+/S1121A.
Precocious Separation of Sister Chromatids in the Double Mutant Cells
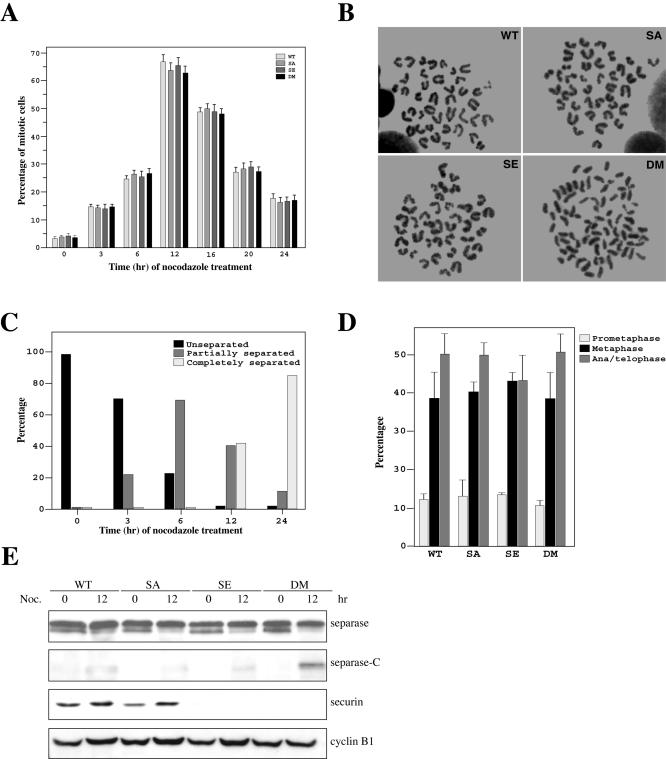
A characteristic response of cells to microtubule poisons is an arrest at prometaphase with condensed mitotic chromosomes composed of unseparated sister chromatids. We treated asynchronously growing WT, SA, SE, and DM cells with nocodazole for various lengths of time and analyzed them by fluorescence-activated cell sorting (FACS). All four types of cells were arrested in G2/M with similar efficiencies (our unpublished data). Mitotic index (percentage of cells with condensed mitotic chromosomes) analysis indicated that all cells showed similar increases in the percentage of mitotic cells in response to nocodazole treatment (Figure 2A), indicating an intact spindle assembly checkpoint in all four types of cells. However, when sister chromatid cohesion was examined, it became apparent that sister chromatids were separated in the double mutant cells (Figure 2B). Sister separation was neither seen in wild-type cells nor in mutant separase cells (Figure 2B). As reported previously (Mei et al., 2001), no sister chromatid separation was observed in securin null cells (Figure 2B; Jallepalli et al., 2001; Mei et al., 2001). In the double mutant cells, a short exposure (3 h) to the microtubule disrupting agent caused ∼20% of the mitotic cells to partially lose sister cohesion, that is, in one cell, there were separated as well as unseparated sister chromatids. After 12 h of treatment, almost all mitotic DM cells had either partially or completely separated their sister chromatids. By 24 h of the treatment, >80% of the mitotic DM cells displayed complete loss of sister cohesion (Figure 2C). These data strongly support the hypothesis that securin and the inhibitory phosphorylation of separase act redundantly to maintain sister chromatid cohesion.
Figure 2.
Responses of the double mutant ES cells to spindle microtubule disruption. (A) Mitotic index analysis. Asynchronously growing wild-type, separase single mutant, securin null, and the double mutant ES cells were treated with nocodazole and analyzed. (B). Loss of sister chromatid cohesion in the securin and separase double mutant cells. Chromosome spreads were prepared using cells treated with nocodazole for 12 h. (C) Gradual loss of sister cohesion in the double mutant cells. Percentage of the double mutant cells with different degrees of sister separation were determined at different time points after nocodazole treatment. (D) Normal timing of mitosis in DM cells. Asynchronously growing cells were fixed and stained for DNA and tubulin. The number of cells in different phases of mitosis was counted and the percentage in each phase was calculated and graphed. (E) Western blot analysis of separase, securin, and cyclin B1 in asynchronously growing and 12-h nocodazole-treated cells.
These results prompted us to ask whether the DM cells could maintain sister cohesion in an undisturbed cell cycle, in other words, whether anaphase in these cells is advanced. To address that question, we determined the percentage of metaphase as well as ana/telophase cells out of the mitotic cells in an asynchronous population. If the double mutant cells enter anaphase prematurely, one would expect to see a decrease in the percentage of metaphase cells and an increase in the percentage of ana/telophase cells. Asynchronously growing cells were fixed in paraformaldehyde and stained for DNA and microtubules. We counted the number of cells in prometaphase (with condensed DNA but no spindle), metaphase (with a spindle but no visible separation of sister chromatids), and ana/telophase (with a spindle and clearly separated sisters). The percentage of each phase was calculated and presented in Figure 2D. There were no differences in the percentages of metaphase cells among the four types of cells and no increased accumulation of ana/telophase cells was observed in the DM cell population, indicating that normal timing of mitosis is maintained in the double mutant cells.
Premature Activation of Separase in the Double Mutant Cells
The precocious sister chromatid separation in DM cells suggests that separase is prematurely activated in the absence of a functional spindle. The activation of separase is accompanied by its autocleavage, which can be visualized through Western blot analysis (Waizenegger et al., 2002; Zou et al., 2002). As shown in Figure 2E, although prolonged nocodazole arrest caused some activation of separase in all four types of cells, the activation was much stronger in DM cells than in other three types of cells. Apparently, the limited activation of separase in WT, SA, and SE cells was not sufficient to cause sister separation. Interestingly, there was little activation of separase in asynchronously growing double mutant cells (Figure 2E, time 0), suggesting the existence of other unidentified mechanisms that limit the activation of separase even when securin and the inhibitory phosphorylation are eliminated. Thus, although the activation of separase became substantial after prolonged prometaphase arrest, the activation probably did not reach the same degree as in a normal anaphase. Therefore, the loss of sister cohesion in nocodazole-treated DM cells is not abrupt, but gradual (Figure 1C). The smaller and minor form of separase detected by the Western blot analysis (Figure 2E) may be a result of alternative splicing or alternative translation. It was reported that securin is required to maintain the level of separase by acting as its chaperon (Jallepalli et al., 2001). We observed little change, however, in the level of separase in ES cells lacking securin. Mouse embryonic stem cells may have other mechanisms to stabilize separase in the absence of securin.
As cells accumulate in prometaphase in response to nocodazole treatment, there were modest increases in the levels of securin in both WT and SA cells (Figure 2E). There was no securin in SE and DM cells (Figure 2E). Furthermore, little change in the levels of cyclin B1 was observed in all of these cells between nocodazole-treated and untreated samples (Figure 2E). These results are consistent with the mitotic index analysis (Figure 2A), indicating mitotic arrests induced by nocodazole in all four types of cells.
The Double Mutant Cells Are More Sensitive to Spindle Poison
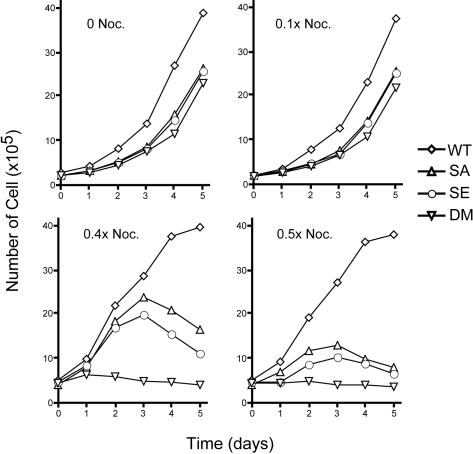
Mad2-deficiency causes lethality due to the failure of spindle assembly checkpoint, whereas our double mutant cells grow more or less normally (see below), suggesting that under normal growth conditions neither securin nor inhibitory phosphorylation of separase is required for the coordination of events leading to anaphase. However, they may be required when the microtubule spindle is under stress. To test that hypothesis, we grew the cells in the presence of sublethal concentrations of nocodazole and determined their growth rates. In the absence of spindle poison, the double mutant cells grew slower than WT cells, and so did SA and SE cells (Figure 3). It is unclear whether the slow growth is related to defects in the control of separase. However, there were no observable changes in the cell cycle profiles of the mutant cells (our unpublished data), indicating that these cells do not slow down at a particular cell cycle phase. Furthermore, all four cell types accumulated mitotic cells at the same rate when treated with nocodazole (Figure 2A), strongly supporting the notion that there were no inherent defects in the cell cycle itself in the mutant cells. However, the three mutant cell types did display reduced plating efficiencies (our unpublished data), which explains, at least in part, the slow growth.
Figure 3.
Double mutant cells are sensitive to nocodazole. Cells were grown in the presence of different concentrations of nocodazole. The 1× nocodazole equals 65 ng/ml, which would completely arrest cells at prometaphase. Notice that the number of cells seeded for higher doses of nocodazole was different from that for lower or no nocodazole because of the increased loss of cells at higher drug concentrations.
Adding 0.065 ng/ml nocodazole (0.1×, 1/10 of the concentration that would arrest the cells completely) had little effect on the growth of these cells (Figure 3). When the concentration of the drug was increased to 0.4× or 0.5×, although the WT cells continued to grow, both SA and SE cells grew significantly slower than the WT cells, and the double mutant cells were unable to grow at all (Figure 3). Interestingly, at 0.4× nocodazole, SA and SE cells grew for the initial 3 d, but they died off afterward, probably due to the accumulation of mitotic errors. These results indicate that both securin and separase phosphorylation protect the cells when microtubules are under stress; loss of both makes cells extremely sensitive to the spindle poison.
The Double Mutant Cells Cannot Recover from Nocodazole Treatment
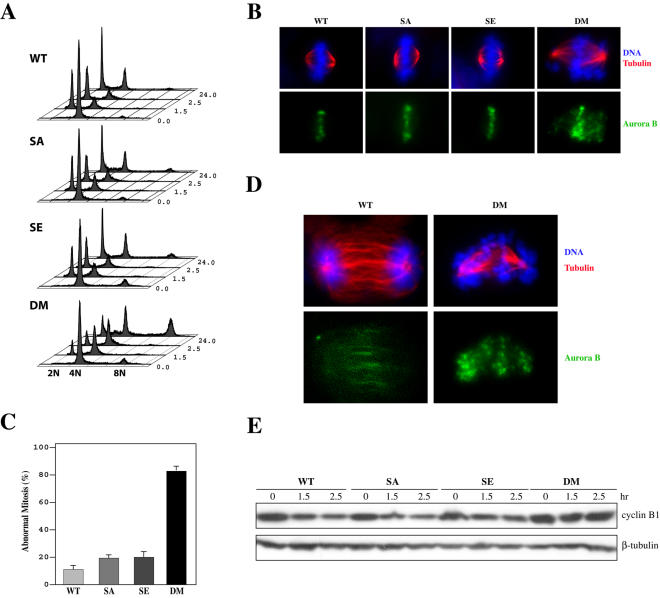
The mitotic index analyses demonstrated that the double mutant cells (Figure 2A) could not exit mitosis in the absence of a functional spindle, indicating that the mere activation of separase is unable to turn off the spindle assembly checkpoint, even though the sister chromatids may have already separated. When these cells are released from the arrest, they are predicted to be unable to proceed through mitosis normally, or unable to exit mitosis at all, because the already separated sister chromatids might activate the spindle checkpoint again due to their inability to establish bipolar attachment. To demonstrate that, we arrested the four types of cells with nocodazole for 12 h, collected mitotic cells via selective detachment (see Materials and Methods for details), released them from the arrest, and monitored their cell cycle progression with FACS. Whereas WT, SE, and SA cells readily exited mitosis and entered next G1 upon the release, a large proportion of DM cells could not exit (Figure 4A). Eventually, these cells entered the cell cycle again without cell division (Figure 4A, notice the 8N peak at 24 h). This result indicated that the double mutant cells were prevented from completing mitosis.
Figure 4.
Securin and separase double mutant cells fail to recover from prometaphase arrest. (A) Cell cycle profiles of cells that were arrested 12 h with nocodazole, collected through selective detachment, plated in fresh media, and harvested at different time points for analysis. (B) Immunostaining for β-tubulin and Aurora B. Cells were arrested with nocodazole for 12 h and released for 1.0 h before being fixed and stained. (C) Percentages of abnormal mitotic configurations as seen in B. (D) Sustained Aurora B staining on the DNA of securin and separase double mutant cells. Cells were treated and analyzed the same way as in B except that the release was 2 h. (E) Western blot analysis of cyclin B1. Twelve-hour nocodazole-arrested mitotic cells were collected via selective detachment, plated in fresh media, and harvested for analysis.
Microscopic examination of the released cells revealed aberrant mitotic figures in DM cells (Figure 4B). Chromosomes in DM cells could not align at the metaphase plate, and there was DNA at spindle poles. The chromosomes at the poles are likely those that had separated from their sisters. They could only form monoattachments by the spindle and thereby were pulled over to the poles instead of the metaphase plate. About 80% of DM cells displayed aberrant mitotic figures, whereas it was 10% in WT cells and ∼20% in SA and SE cells (Figure 4C). When WT cells entered anaphase and telophase, they lost chromosomal Aurora B staining as a result of the relaxation of spindle assembly checkpoint (Figure 4, B and D). However, the double mutant cells seemed unable to perform anaphase and Aurora B stayed on their chromosomes for long periods of time after the release (Figure 4D). SA and SE cells behaved the same way as WT cells (our unpublished data). Because Aurora B is an essential component of the spindle assembly checkpoint (Shannon and Salmon, 2002; Ditchfield et al., 2003; Lens and Medema, 2003), sustained chromosomal staining of Aurora B indicates the activation of the checkpoint in DM cells. Furthermore, when the levels of cyclin B1 were analyzed, we found that the level decreased in WT, SA, and SE cells as they exited mitosis, but the cyclin B1 level in DM cells experienced little change after the release, again indicating spindle checkpoint activation. Together, these results demonstrate that the double mutant cells cannot recover from the mitotic arrest induced by spindle disruption because the prematurely separated sister chromatids keep the spindle checkpoint active. Eventually, the cells adapted to the checkpoint and entered the cell cycle without division (Figure 4A).
No Increased Genome Instability in the Double Mutant ES Cells
Given the defects in maintaining sister cohesion in DM cells, we next examined whether these cells display genome instability in the form of aneuploidy. To that end, we karyotyped cells with all four kinds of genotypes. We counted ∼70 metaphase spreads for each type of cells. As shown in Table 1, there were no significant differences in the distribution of cells with different karyotypes among the cells with different genotypes. About 80% of the cells display the normal karyotype of 40 chromosomes. This result indicates that there are no grossly increased rates of gains or losses of chromosomes in DM cells. This is in contrast to the previous report that the colon cancer cell line HCT116 displays genome instability when securin is deleted (Jallepalli et al., 2001). It is possible that mouse embryonic stem cells have additional mechanisms to ensure genome stability to maintain their pluripotency. For example, the grossly aneuploid cells may be eliminated from the population via apoptosis.
Table 1.
Karyotyping of the four types of cells
| Karyotype | 38 | 39 | 40 | 41 | 42 |
|---|---|---|---|---|---|
| WT | 1 | 4 | 51 | 3 | 2 |
| SA | 2 | 4 | 57 | 6 | 1 |
| SE | 2 | 5 | 63 | 5 | 4 |
| DM | 2 | 8 | 61 | 9 | 1 |
DISCUSSION
Spindle assembly checkpoint prevents sister chromatid separation when the microtubule spindle is compromised or when there are mistakes in the attachment of microtubules to sister chromatids (Shah and Cleveland, 2000; Gillett and Sorger, 2001; Yu, 2002; Zhou et al., 2002; Chan and Yen, 2003; Lens and Medema, 2003). This is accomplished through inhibiting the ubiquitination activity of APC/Cdc20 by Mad2 and BubR1 (Yu, 2002). In this report, we demonstrated that securin is redundant with the inhibitory phosphorylation of separase in preventing precocious sister chromatid separation. Thus, the mammalian spindle assembly checkpoint maintains sister chromatids cohesion through stabilizing securin to directly inhibit separase and through maintaining high cyclin B1/Cdk1 kinase activity to keep separase phosphorylated.
Loss of either Mad2 or BubR1 is incompatible with the viability of mammalian cells (Dobles et al., 2000; Baker et al., 2004; Michel et al., 2004; Wang et al., 2004), suggesting that the spindle assembly checkpoint might be required in every cell cycle to prevent premature sister separation, to coordinate events leading to anaphase, or to control the timing of anaphase. In the absence of these two checkpoint genes, cells may accumulate unbearable mitotic errors and eventually die. However, our securin and separase double mutant cells are viable and grow more or less normally, and maintain normal timing of mitosis (Figure 2D). One reason could be that the activation of separase caused by the loss of securin and the inhibitory phosphorylation is limited by still unidentified mechanisms that inhibit separase. In agreement, we found little activation of separase in asynchronously growing double mutant cells (Figure 2E). Another reason could be that the spindle assembly checkpoint still has other targets that function to protect sister cohesion in the absence of securin and in the presence of a dominant nonphosphorylable mutant separase. One candidate target is shugoshin (Sgo) (Kitajima et al., 2004; Watanabe, 2004). It has recently been shown that the vertebrate shugoshin is a substrate of the APC and is essential for sister chromatid cohesion (Salic et al., 2004; Tang et al., 2004). It was thought that shugoshin maintains the integrity of the sister cohesin complex (Salic et al., 2004; Tang et al., 2004). Shugoshin also could shield Scc1 from being attacked by separase. Thus, even though separase is prematurely activated in our double mutant cells, it would not gain the access to Scc1 immediately. As such, the timing of sister separation is not grossly disrupted, and bipolar attachment as well as alignment of sister chromatids at the metaphase plate can still happen on time in a normal undisturbed cell cycle. Thereby, cell viability is preserved. However, shugoshin apparently could not protect the cohesin complex for prolonged prometaphase arrest, because the nocodazole treatment eventually led to sister chromatid separation in securin and separase double mutant cells. It is possible that Sgo may dissociate from the cohesion complex after a prolonged prometaphase arrest, allowing the cleavage of Scc1 by separase. Alternatively, Sgo is unable to block the cleavage completely but merely makes it more difficult for separase. Thus, with time, the prematurely activated separase gradually disintegrates the cohesin complex. In agreement with the latter, we found that the long-term arrest of HeLa cells with nocodazole (up to 18 h) does not dissociate Sgo from chromosomes (Huang and Zhang, unpublished observation).
The viability in DM cells may be maintained by another negative phosphorylation site of separase (Thr1346 in human). However, we found that it is not the case. We have generated another separase allele that lacks both inhibitory phosphorylation sites (S1121A and T1341A). When combined with securin null, this double point mutant does not cause lethality but causes defects in the maintenance of sister cohesion, however, to the same degree as S1121A does (Huang and Zhang, unpublished observation), thus demonstrating that the simultaneous loss of securin and the two inhibitory phosphorylation sites of separase is still compatible with cellular viability.
It is also possible that the wild-type separase allele in the double mutant cells is somehow interfering with the activation or function of the nonphosphorylable mutant allele, and thereby preserves the viability of the cells. However, when the wild-type allele of separase was inactivated through targeting in the double mutant cells, the resultant cells were still viable (Tran and Zhang, unpublished observation), thus eliminating this possibility.
Although neither securin nor separase phosphorylation is required to block sister chromatid separation in response to nocodazole treatment (Figure 2B), both are required for optimal growth when cells were cultured with sublethal doses of nocodazole (Figure 3). Under such conditions, it is expected that cells would need extra time to align chromosomes to the metaphase plate; therefore, premature activation of separase would not be tolerated as it is under normal growth conditions. Not unexpectedly, the double mutant cells are extremely sensitive to the presence of nocodazole in culture media.
Consistent with the fact that the inhibition of separase is only one of the end effects of spindle assembly checkpoint, preventing the inhibition is not sufficient to escape the checkpoint. Thus, securin and separase double mutant cells could still be arrested by nocodazole (Figure 2A), although the sister chromatids had become separated (Figure 2, B and C). When these cells were released from the arrest, they could not proceed through mitosis normally because the separated sister chromatids kept spindle checkpoint active. This phenotype is very similar to that observed in DT40 chicken cells deficient in Scc1 (Sonoda et al., 2001).
We have recently obtained mice carrying the separaseS1121A allele. These animals are grossly normal (Huang and Zhang, unpublished data). They are being analyzed to see whether securin-/-separase+/S1121A or securin-/-separaseS1121A/S1121A mice display defects in embryogenesis, have reduced fertility, or develop spontaneous tumors, to reveal the function of these two spindle checkpoint mechanisms in a whole organism.
Acknowledgments
We thank Drs. Hui Zou and Hongtao Yu at UT Southwestern Medical Center (Dallas, TX) for sharing reagents and unpublished results. We thank other members of the Zhang laboratory for helpful discussion and reading of the manuscript. P. Z. is supported by grants from National Institutes of Health.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-03-0190) on July 19, 2005.
References
- Baker, D. J., et al. (2004). BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 36, 744-749. [DOI] [PubMed] [Google Scholar]
- Boelaert, K., McCabe, C. J., Tannahill, L. A., Gittoes, N. J., Holder, R. L., Watkinson, J. C., Bradwell, A. R., Sheppard, M. C., and Franklyn, J. A. (2003). Pituitary tumor transforming gene and fibroblast growth factor-2 expression: potential prognostic indicators in differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 88, 2341-2347. [DOI] [PubMed] [Google Scholar]
- Chan, G. K., and Yen, T. J. (2003). The mitotic checkpoint: a signaling pathway that allows a single unattached kinetochore to inhibit mitotic exit. Prog. Cell Cycle Res. 5, 431-439. [PubMed] [Google Scholar]
- Ciosk, R., Zachariae, W., Michaelis, C., Shevchenko, A., Mann, M., and Nasmyth, K. (1998). An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell 93, 1067-1076. [DOI] [PubMed] [Google Scholar]
- Dej, K. J., and Orr-Weaver, T. L. (2000). Separation anxiety at the centromere. Trends Cell Biol. 10, 392-399. [DOI] [PubMed] [Google Scholar]
- Ditchfield, C., Johnson, V. L., Tighe, A., Ellston, R., Haworth, C., Johnson, T., Mortlock, A., Keen, N., and Taylor, S. S. (2003). Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161, 267-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobles, M., Liberal, V., Scott, M. L., Benezra, R., and Sorger, P. K. (2000). Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell 101, 635-645. [DOI] [PubMed] [Google Scholar]
- Fang, G., Yu, H., and Kirschner, M. W. (1999). Control of mitotic transitions by the anaphase-promoting complex. Phil. Trans. R. Soc. Lond. B Biol. Sci. 354, 1583-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett, E. S., and Sorger, P. K. (2001). Tracing the pathway of spindle assembly checkpoint signaling. Dev. Cell 1, 162-164. [DOI] [PubMed] [Google Scholar]
- Hauf, S., Waizenegger, I. C., and Peters, J. M. (2001). Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science 293, 1320-1323. [DOI] [PubMed] [Google Scholar]
- Jager, H., Herzig, A., Lehner, C. F., and Heidmann, S. (2001). Drosophila separase is required for sister chromatid separation and binds to PIM and THR. Genes Dev. 15, 2572-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallepalli, P. V., Waizenegger, I. C., Bunz, F., Langer, S., Speicher, M. R., Peters, J. M., Kinzler, K. W., Vogelstein, B., and Lengauer, C. (2001). Securin is required for chromosomal stability in human cells. Cell 105, 445-457. [DOI] [PubMed] [Google Scholar]
- Kakar, S. S. (1999). Molecular cloning, genomic organization, and identification of the promoter for the human pituitary tumor transforming gene (PTTG). Gene 240, 317-324. [DOI] [PubMed] [Google Scholar]
- King, R. W., Deshaies, R. J., Peters, J. M., and Kirschner, M. W. (1996). How proteolysis drives the cell cycle. Science 274, 1652-1659. [DOI] [PubMed] [Google Scholar]
- Kitajima, T. S., Kawashima, S. A., and Watanabe, Y. (2004). The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 427, 510-517. [DOI] [PubMed] [Google Scholar]
- Koshland, D. E., and Guacci, V. (2000). Sister chromatid cohesion: the beginning of a long and beautiful relationship. Curr. Opin. Cell Biol. 12, 297-301. [DOI] [PubMed] [Google Scholar]
- Lens, S. M., and Medema, R. H. (2003). The survivin/Aurora B complex: its role in coordinating tension and attachment. Cell Cycle 2, 507-510. [DOI] [PubMed] [Google Scholar]
- Mei, J., Huang, X., and Zhang, P. (2001). Securin is not required for cellular viability, but is required for normal growth of mouse embryonic fibroblasts. Curr. Biol. 11, 1197-1201. [DOI] [PubMed] [Google Scholar]
- Michel, L., Diaz-Rodriguez, E., Narayan, G., Hernando, E., Murty, V. V., and Benezra, R. (2004). Complete loss of the tumor suppressor MAD2 causes premature cyclin B degradation and mitotic failure in human somatic cells. Proc. Natl. Acad. Sci. USA 101, 4459-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth, K., Peters, J. M., and Uhlmann, F. (2000). Splitting the chromosome: cutting the ties that bind sister chromatids. Science 288, 1379-1385. [DOI] [PubMed] [Google Scholar]
- Pei, L., and Melmed, S. (1997). Isolation and characterization of a pituitary tumor-transforming gene (PTTG). Mol. Endocrinol. 11, 433-441. [DOI] [PubMed] [Google Scholar]
- Peters, J. M. (2002). The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9, 931-943. [DOI] [PubMed] [Google Scholar]
- Puri, R., Tousson, A., Chen, L., and Kakar, S. S. (2001). Molecular cloning of pituitary tumor transforming gene 1 from ovarian tumors and its expression in tumors. Cancer Lett. 163, 131-139. [DOI] [PubMed] [Google Scholar]
- Salic, A., Waters, J. C., and Mitchison, T. J. (2004). Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118, 567-578. [DOI] [PubMed] [Google Scholar]
- Shah, J. V., and Cleveland, D. W. (2000). Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell 103, 997-1000. [DOI] [PubMed] [Google Scholar]
- Shannon, K. B., and Salmon, E. D. (2002). Chromosome dynamics: new light on Aurora B kinase function. Curr. Biol. 12, R458-R460. [DOI] [PubMed] [Google Scholar]
- Sonoda, E., et al. (2001). Scc1/Rad21/Mcd1 is required for sister chromatid cohesion and kinetochore function in vertebrate cells. Dev. Cell 1, 759-770. [DOI] [PubMed] [Google Scholar]
- Stemmann, O., Zou, H., Gerber, S. A., Gygi, S. P., and Kirschner, M. W. (2001). Dual inhibition of sister chromatid separation at metaphase. Cell 107, 715-726. [DOI] [PubMed] [Google Scholar]
- Tang, Z., Sun, Y., Harley, S. E., Zou, H., and Yu, H. (2004). Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc. Natl. Acad. Sci. USA 101, 18012-18017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann, F., Lottspeich, F., and Nasmyth, K. (1999). Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400, 37-42. [DOI] [PubMed] [Google Scholar]
- Uhlmann, F., Wernic, D., Poupart, M. A., Koonin, E. V., and Nasmyth, K. (2000). Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103, 375-386. [DOI] [PubMed] [Google Scholar]
- Waizenegger, I., Gimenez-Abian, J. F., Wernic, D., and Peters, J. M. (2002). Regulation of human separase by securin binding and autocleavage. Curr. Biol. 12, 1368-1378. [DOI] [PubMed] [Google Scholar]
- Wang, Q., Liu, T., Fang, Y., Xie, S., Huang, X., Mahmood, R., Ramaswamy, G., Sakamoto, K. M., Darzynkiewicz, Z., Xu, M., and Dai, W. (2004). BUBR1 deficiency results in abnormal megakaryopoiesis. Blood 103, 1278-1285. [DOI] [PubMed] [Google Scholar]
- Watanabe, Y. (2004). Modifying sister chromatid cohesion for meiosis. J. Cell Sci. 117, 4017-4023. [DOI] [PubMed] [Google Scholar]
- Yamamoto, A., Guacci, V., and Koshland, D. (1996a). Pds1p is required for faithful execution of anaphase in the yeast, Saccharomyces cerevisiae. J. Cell Biol. 133, 85-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, A., Guacci, V., and Koshland, D. (1996b). Pds1p, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s). J. Cell Biol. 133, 99-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida, M. (2000). Cell cycle mechanisms of sister chromatid separation; roles of Cut1/separin and Cut2/securin. Genes Cells 5, 1-8. [DOI] [PubMed] [Google Scholar]
- Yu, H. (2002). Regulation of APC-Cdc20 by the spindle checkpoint. Curr. Opin. Cell Biol. 14, 706-714. [DOI] [PubMed] [Google Scholar]
- Zhang, P., Li, M. Z., and Elledge, S. J. (2002). Towards genetic genome projects: genomic library screening and gene-targeting vector construction in a single step. Nat. Genet. 30, 31-39. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Horwitz, G. A., Prezant, T. R., Valentini, A., Nakashima, M., Bronstein, M. D., and Melmed, S. (1999). Structure, expression, and function of human pituitary tumor-transforming gene (PTTG). Mol. Endocrinol. 13, 156-166. [DOI] [PubMed] [Google Scholar]
- Zhou, J., Yao, J., and Joshi, H. C. (2002). Attachment and tension in the spindle assembly checkpoint. J. Cell Sci. 115, 3547-3555. [DOI] [PubMed] [Google Scholar]
- Zou, H., McGarry, T. J., Bernal, T., and Kirschner, M. W. (1999). Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science 285, 418-422. [DOI] [PubMed] [Google Scholar]
- Zou, H., Stemman, O., Anderson, J. S., Mann, M., and Kirschner, M. W. (2002). Anaphase specific auto-cleavage of separase. FEBS Lett. 528, 246-250. [DOI] [PubMed] [Google Scholar]