Abstract
Using on-line microdialysis, we have characterized in vivo dynamics of pineal 5-hydroxytryptamine (5-HT; serotonin) release. Daily pineal 5-HT output is triphasic: (i) 5-HT levels are constant and high during the day; (ii) early in the night, there is a novel sharp rise in 5-HT synthesis and release, which precedes the nocturnal rise in melatonin synthesis; and (iii) late in the night, levels are low. This triphasic 5-HT production persists in constant darkness and is influenced strongly by intrusion of light at night. We demonstrate that both diurnal 5-HT synthesis and 5-HT release are activated by sympathetic innervation from the superior cervical ganglion and show that these processes are controlled by distinct receptors. The increase in 5-HT synthesis is controlled by β-adrenergic receptors, whereas the increase in 5-HT release is mediated by α-adrenergic signaling. On the other hand, the marked decrease in 5-HT content and release late at night is a passive process, influenced by the extent of melatonin synthesis. In the absence of melatonin synthesis, the late-night decline in 5-HT release is prevented, reaching levels roughly twice as high as that of the day value. In summary, our results demonstrate that 5-HT levels display marked circadian rhythms that depend on adrenergic signaling.
Keywords: melatonin‖circadian rhythm‖in vivo microdialysis‖pineal gland
The neurotransmitter 5-hydroxytryptamine (5-HT) plays diverse roles in a number of central and peripheral processes (1). It is essential for the normal function of the pineal, where its content is higher than in any other part of the body. In the pineal, 5-HT concentration displays a remarkable diurnal pattern (2–4), with day levels much higher than night levels. The diurnal fluctuation of pineal 5-HT is abolished by surgical removal of superior cervical ganglion (SCG) and persists in constant darkness (5, 6). 5-HT synthetic enzymes tryptophan hydroxylase (TPH), the rate-limiting enzyme of 5-HT production, and aromatic amino acid decarboxylase, both are found in large quantities in the pineal gland. In the pineal, 5-HT is a metabolic precursor to melatonin, a nocturnal hormone postulated to function in sleep, clock resetting, and seasonal reproduction (7, 8). Specifically, 5-HT is metabolized by the melatonin synthetic enzyme serotonin N-acetyltransferase (NAT) to form N-acetylserotonin (NAS) (9). In the rat pineal, the rate of NAT RNA synthesis, which increases dramatically at night (10, 11), controls 5-HT metabolism and melatonin production. Although 5-HT levels display marked diurnal variations, the process is thought to be controlled entirely by NAT activity, and no active regulation of 5-HT production has ever been documented in the pineal of any model organisms.
Several pieces of evidence, however, suggest that 5-HT synthesis is dynamically regulated in the pineal. Activities of TPH, the rate-limiting enzyme of 5-HT synthesis, display a 2-fold increase at night in the rat pineal (12, 13). TPH mRNA oscillates in the chick pineal gland (14, 15), where the night levels are much higher than their daytime trough. In addition, TPH mRNA levels are higher at night in Xenopus larvae (16) and chick retina (17), which also express elevated nocturnal melatonin synthesis. Despite these data, an increased production of 5-HT at night has never been reported in vivo.
Because pineal glands synthesize, metabolize, and secrete 5-HT, Walker and Aloyo (18) have proposed to use the rat pineal as an in vitro model for studying mechanisms of 5-HT secretion. In the following years, however, this notion has not gained wide support, perhaps partially because of the lack of evidence that a similar mechanism for regulating 5-HT release exists in vivo. In fact, little is known in regard to the in vivo regulation of endogenous pineal 5-HT release. In this study, using an improved pineal microdialysis system, we characterized 5-HT synthesis and release profiles and investigated the regulatory mechanisms that govern pineal 5-HT production in freely moving rats.
Materials and Methods
Animals.
All animal protocols were conducted in accordance with the institutional animal care and use committee. Adult (200–300 g) male Sprague–Dawley rats were obtained from Harlan Sprague–Dawley and housed in a temperature-controlled room under a 14:10 h light/dark cycle (600 lux illuminance; lights on at 11 a.m.) for at least 10 days before experimentation.
Drugs.
Norepinephrine and propranolol were purchased from Sigma, and actinomycin D (ActD) was from Calbiochem.
Surgery.
Surgical techniques developed for placement of pineal microdialysis probes will be described in detail elsewhere. However, in brief, animals were deeply anesthetized with a combination of ketamine (10 mg/ml, 0.5 ml/100 g weight, i.p.) and xylazine (2 mg/ml, 0.5 ml/100 g weight, i.p.). A circular opening was created in the skull by using a dental burr drill equipped with a shank diamond wheel point (Dremel, Racine, WI). The pia matter that covers the surface of the pineal was carefully removed to expose the pineal, which is connected to the confluence of the sinuses via the pineal vein. The tip of a guide cannula (CMA Microdialysis, North Chelmsford, MA) was positioned directly adjacent to the exposed pineal gland and fixed to the skull with dental cement. The complete surgical procedure lasted about 1 h for each rat. After surgery, each animal was housed in an individual cage in a light-controlled animal room described above and allowed to recover for 24 h.
For SCG removal during the microdialysis, rats were removed from the microdialysis setup during the daytime and were deeply anesthetized with a combination of ketamine and xylazine, as above. The experimental group of animals was sympathectomized by removal of both left and right SCG by surgical incisions between the sternohyoideus and omohyoideus muscles. Sympathectomy was confirmed by the observation of Horner's syndrome (constriction of pupil). The control group of animals was sham-operated, by performing the same incision without removing the ganglion. The entire procedure lasted about 30 min for each rat. The rats were allowed to recover for 4 h before resuming microdialysis on-line.
Microdialysis.
Pineal microdialysis was carried out as follows: Just before sampling, rats were briefly anaesthetized with halothane, and the stylet (or dummy probe) was replaced with a microdialysis probe (CMV12, 20-kDa cut-off, 3 or 4 mm length) (CMA Microdialysis) and fixed in place with plastic glue. Both inlet and outlet of dialysis probe were connected to the microbore PEEK tubing (inner diameter 0.12 mm, outer diameter 0.65 mm). The inlet tubing was continuously perfused at a flow rate of 2 μl/min with artificial cerebral spinal fluid (CSF) (Harvard, Holliston, MA) that was delivered by a CMA/102 microdialysis pump. Samples were collected every 10 min by means of the PEEK tube into the 20 μl loop of an automatic injector (BAS, West Lafayette, IN), which was on-line with a HPLC system (see below). The sample loop was set to be retained in the load (or collect) position during a 10-min period and was automatically switched to the injection position very briefly, after which the 10-min cycle was repeated. The rats were linked to the dialysis apparatus through a quartz dual channel swivel (Harvard/Instech, Boston) to prevent the tubing from entanglement. All pharmacological agents with molecular masses below 1,000 Da were delivered to the pineal through the microdialysis tubing. All drugs were dissolved in artificial CSF solution at the final concentrations indicated and perfused into the pineal for the indicated duration times.
HPLC.
The analytical conditions for the simultaneous detection of 5-HT/NAS/melatonin are based on Drijfhout et al. (19) with some minor modifications. A Shimadzu pump was used in conjunction with a Shimadzu fluorescence detector (FD, excitation: 280 nm, emission: 345 nm). Microdialysis samples were injected into the HPLC system through a Valco Instruments (Houston) injection valve with BAS pollen 8 controller and subsequently separated on a reversed-phase C18 column (250 × 4.6 mm, Supelco), set at a constant temperature of 30°C with a Shimadzu column heater and system controller.
To measure total content of 5-HT, NAS, and melatonin, the pineal gland was sonicated in 200 μl of cold 0.1 M perchloric acid. After centrifugation at 10,000 g for 10 min, 20 μl of the clear supernatant was injected into a HPLC system with a Shimadzu autosampler and analyzed by the FD, as above.
Northern Blotting.
Total pineal RNA was prepared by using the RNeasy kit (Qiagen, Valencia, CA). RNA equivalent to half of a pineal gland was loaded in each lane, electrophoresed, transferred onto a nylon membrane, and probed sequentially with radiolabeled NAT, TPH, and glyceraldehyde-3-phosphate dehydrogenase probes by using standard procedures (20).
Results
Pineal 5-HT Secretion and Content in Vivo Follow Circadian Rhythm.
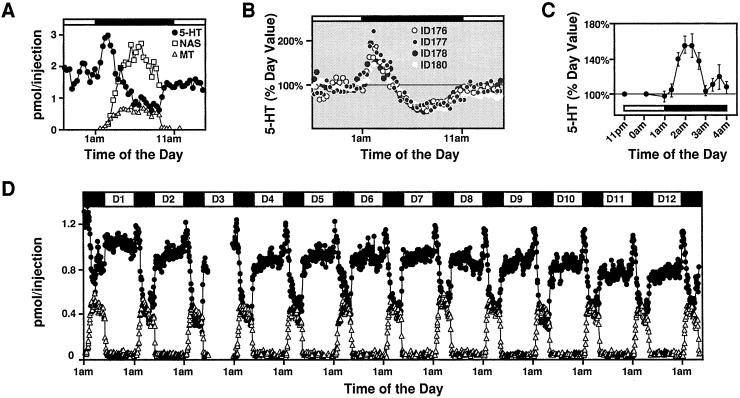
We monitored circadian profile of pineal secretory products in freely moving rats by using an improved in vivo pineal microdialysis technique. The pineal dialysates were analyzed for indoles (5-HT, NAS, and melatonin) at 10-min intervals by using on-line HPLC with an FD. The resulting temporal profile is a highly reproducible and quantitative assessment of the dynamics of pineal indole release. Unlike NAS and melatonin release, which are both markedly increased at night, the 5-HT release profile exhibits a clear circadian rhythm composed of three distinct phases (Fig. 1A). First, during the daytime, 5-HT is relatively constant; second, in the early night, just after the lights are off, 5-HT increases sharply; and third, just as melatonin begins to increase during the night, 5-HT output drops precipitously to low levels. The triphasic 5-HT release is seen in 100% of the animals studied (four of 114 rats analyzed so far are shown in Fig. 1B) and is consistently detected night after night within the same animal for more than 2 weeks (Fig. 1D). The early night rise of 5-HT release is 1.5- to 2-fold greater than the daytime levels, and is found in all strains of rats examined, which include Sprague–Dawley, Long Evans, PVG, and Wistar rats (data not shown).
Figure 1.
5-HT production in the pineal displays triphasic circadian rhythm. (A) Typical diurnal profiles of 5-HT, NAS, and melatonin secretion measured by in vivo pineal microdialysis. In addition to the well-known nighttime drop and the relatively high daytime levels of 5-HT, a sharp rise in 5-HT release is observed in the early night. Each data point represents pineal indoles contained in a 10-min sample collected at the indicated times. In all cases, the black bar at the top indicates the dark period (1 a.m. to 11 a.m.). (B) Diurnal rhythms of 5-HT secretion in multiple rats. Although only four rats are shown, the early nocturnal rise in 5-HT secretion is consistently found in all animals tested (n = 114). 5-HT levels are calculated as the percentage of basal daytime production for each animal. (C) The early increase in total pineal content of 5-HT. Pineal glands of adult rats killed at the indicated times are measured for their 5-HT concentrations by using HPLC and FD (n = 5). (D) Profiles of pineal indole release in a single rat over a 13-day period. Day 1 (D1) indicates the day 24 h after the probe insertion. 5-HT, NAS (not shown), and melatonin levels are remarkably stable and consistent night after night. Notice that the early nocturnal rise in 5-HT secretion is detected in every diurnal cycle monitored.
Total pineal 5-HT content from isolated pineal glands was assayed at 20-min intervals at early night when 5-HT release is increased. As shown in Fig. 1C, there is a corresponding increase (1.5-fold over day level) in 5-HT total concentration that coincides with the elevated 5-HT release.
Circadian 5-HT Release Is Clock-Controlled and Depends on SCG Innervation.
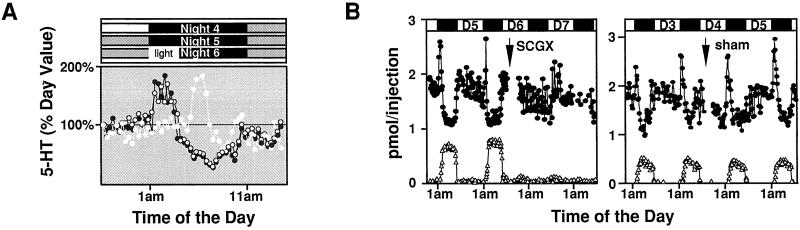
To test whether the peak in 5-HT is driven by an endogenous clock, we measured 5-HT release under constant dark and tested the response of individual rats to light stimulation. The early 5-HT peak persists in constant dark and can be phase-shifted by a 3-h delay of darkness onset in the beginning of the dark phase (Fig. 2A), indicating that it is clearly controlled by a clock. Although the SCG is the most likely relay of the clock signals, there are other inputs to the pineal such as direct brain innervation to the pineal through the pineal stalk (21), and numerous humoral inputs (J.B., unpublished data), which also could serve as relays for the clock signals. To ascertain the role of the SCG in induction of 5-HT rhythms, we monitored 5-HT release before and after surgical removal of the SCG. As shown in Fig. 2B, the nocturnal rise in serotonin release is abolished after extirpation of the SCG.
Figure 2.
The influence of lighting and SCG on triphasic 5-HT secretion. (A) 5-HT secretion profiles under constant darkness, and after a 3-h delay of dark onset. Pineal dialysates are monitored in a freely moving rat for 5-HT secretion for 3 days under normal lighting (light/dark = 14:10, lights off at 1 a.m.). On day 4 (○), the animal is placed in constant darkness until 1 a.m. on day 6, when 3 h of light is given between 1 a.m. and 4 a.m., as indicated at the top. 5-HT levels for days 4 (○), 5 (●), and 6 (white triangle) are calculated as the percentage of basal daytime production for each cycle. Rats placed in constant darkness for one cycle (this study) to six cycles (data not shown) display identical triphasic patterns of 5-HT release. The light-induced shift in 5-HT secretion is seen all of the animals tested (n = 5). (B) 5-HT (●) and melatonin (▵) release patterns before and after surgical removal of SCG. On day 6 (3 p.m.) of in vivo microdialysis monitoring, SCG is removed bilaterally and microdialysis is continued on-line afterward. The diurnal rhythms in 5-HT secretion are abolished in SCG-removed but intact in sham-operated rat. Identical results are obtained in all animals tested (n = 4).
The Nocturnal Drop in 5-HT Content and Release Is Driven by Melatonin Synthesis.
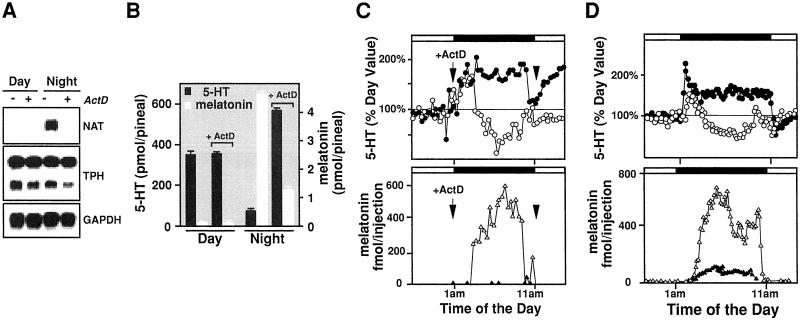
If the melatonin production is the driving force for the reduced production late at night, the pineal 5-HT content and release in the absence of melatonin synthesis should be constitutively elevated throughout the dark period. We tested this idea by suppressing melatonin formation through transient transcription block. Previously we showed that the rate of NAT transcription regulates melatonin synthesis in rat (10). ActD, an inhibitor of RNA synthesis, effectively reduces or abolishes pineal melatonin production (22, 23). Rat pineals treated with ActD contained mRNA for three enzymes involved in melatonin synthesis [TPH, aromatic amino acid decarboxylase (not shown), and hydroxyindole-O-methyltransferase (not shown)] but not for NAT (Fig. 3A). NAT protein is accordingly undetectable in the presence of ActD, and there were no changes seen in the size or quantity of other enzymes (data not shown). To determine the effect of ActD on pineal 5-HT and melatonin synthesis, we tested cohorts of animals killed at various time points with or without ActD injection. As shown in Fig. 3B, the intracellular 5-HT production is elevated dramatically when NAS (not shown) and melatonin production is suppressed. Importantly, ActD has no detectable effect in 5-HT production itself during the day.
Figure 3.
The nocturnal 5-HT content and release are elevated throughout the night period in the absence of melatonin production. (A) ActD treatment of night pineal gland effectively and specifically abolishes NAT transcription. Saline and ActD (1 mg/kg, i.p.) injected rats were killed 8 h after the injection at 8 p.m. during the day and at 8:30 a.m. at night, analyzed for their pineal RNA content of NAT, TPH, aromatic amino acid decarboxylase (not shown), hydroxyindole-O-methyltransferase (not shown), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Each lane contains 10% of total RNA pooled from five treated glands. (B) ActD treatment of night pineals resulted in increased 5-HT production and reduced melatonin formation. ActD-treated rats were killed identically as in A, and their pineals were analyzed for 5-HT and melatonin content by using HPLC-FD. Each bar represents the mean of five individual rats. (C) 5-HT release remains increased over the night period after i.p. ActD injection. ActD (1 mg/kg) was injected into adult rat 30 min before the lights were off. Open symbols represent profiles of 5-HT (○, Upper) and melatonin (▵, Lower) secretion before the ActD treatment, and the solid symbols indicate the release patterns of 5-HT (●, Upper) and melatonin (▴, Lower) after the ActD injection. 5-HT levels (Upper) are calculated as the percentage of basal daytime production (n = 4). (D) 5-HT secretion remains elevated after direct ActD treatment of the pineal in vivo. ActD (100 μg/ml) is infused through a microdialysis probe directly into the pineal from 11 p.m. to 1 p.m. 5-HT and melatonin release was monitored by using on-line microdialysis with HPLC-FD before (○ for 5 HT, Upper; ▵ for melatonin, Lower), and during (● for 5-HT, Upper; ▴ for melatonin, Lower) the drug treatments. Identical results were obtained with all of the animals treated (n = 5).
Effect of ActD on pineal 5-HT and melatonin secretion was tested by using in vivo pineal microdialysis of rats injected with ActD. As shown in Fig. 3C, a single dose of ActD injected 30 min before the onset of darkness dramatically increased 5-HT release by 2-fold throughout the night while eliminating melatonin production. This finding is in contrast with the ActD injected during the day, which has no effect on 5-HT release (data not shown). Similar results are obtained when ActD is infused directly into the pineal through microdialysis probes for the entire night period (Fig. 3D).
β-Adrenergic Signaling Increases 5-HT Synthesis at Night.
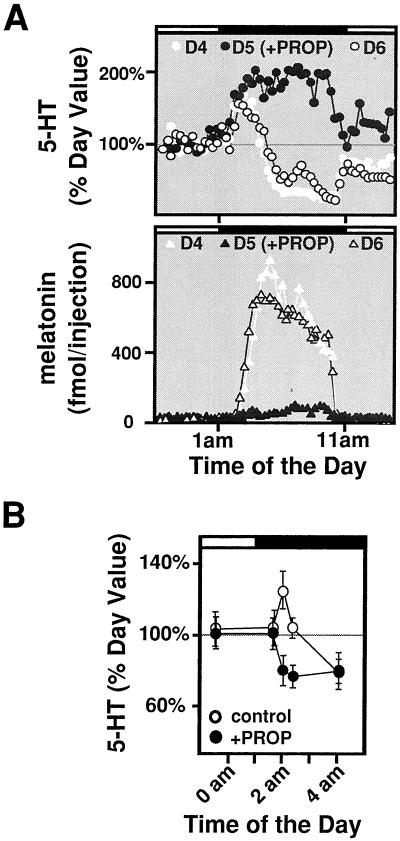
A number of reports have described elevated levels of pineal TPH activity at night (12, 13), which is abolished in the presence of the β-adrenergic blocker propranolol (12). We therefore investigated the role of β-adrenergic receptors in circadian 5-HT content and release. We monitored 5-HT release in rat pineal infused with propranolol (10 μM, 11 p.m. to 1 p.m.) to block β-adrenergic signaling and found that nighttime 5-HT release is markedly elevated during the entire dark phase, while melatonin release (and synthesis, not shown) is completely blocked (Fig. 4A). We measured total pineal 5-HT content at various times in cohorts of animals with or without propranolol treatment and found that the transient increase in 5-HT production at early night is completely eliminated by propranolol (Fig. 4B). Interestingly, in the presence of propranolol, the intracellular 5-HT level seems to be lower than the daytime value, perhaps as a reflection of the increased release shown in Fig. 4A.
Figure 4.
Effect of β-adrenergic signaling on nocturnal 5-HT synthesis and release. (A) Blockade of β-adrenergic receptors leads to elevated 5-HT secretion. β-Adrenergic receptor blocker propranolol (PROP, 10 μM) was infused directly into the pineal through the microdialysis probe between 11 p.m. and 1 p.m. at day 5 (D5). Secretion profiles of 5-HT (Upper) and melatonin (Lower) were measured before, during, and after the drug treatment with in vivo pineal microdialysis. Identical results were seen in all rats tested (n = 4). (B) 5-HT content during early night is reduced in the absence of β-adrenergic signaling. Rats injected with propranolol (PROP, 30 mg/kg, i.p.) were killed at the indicated times 4 h after the injections and analyzed for their pineal 5-HT levels by using HPLC-FD. Each data point represents the mean of five rats at the indicated times.
α-Adrenergic Signaling Controls 5-HT Release.
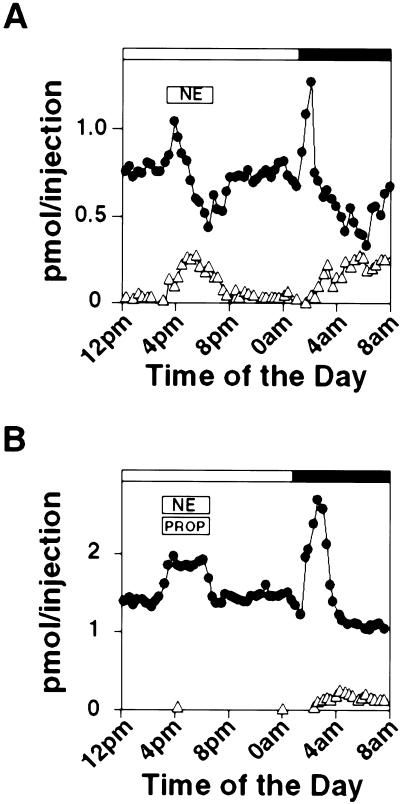
The possibility that 5-HT release could be controlled by α-adrenergic receptors was tested. We sought to determine directly whether α-adrenergic signaling is sufficient in mediating the elevated 5-HT release in the day pineals by infusing norepinephrine alone or in combination with propranolol directly into the pineal by microdialysis tube. As shown in Fig. 5A, norepinephrine infusion during the day resulted in a transient increase of 5-HT release within 20 min of drug application and a delayed melatonin release, both of which disappeared soon after the termination of the norepinephrine infusion. In the presence of propranolol that completely abolished melatonin release, however, 5-HT release is still elevated throughout the period of norepinephrine treatment (Fig. 5B).
Figure 5.
α-Adrenergic signaling promotes 5-HT release in vivo. (A) 5-HT release and melatonin (MT) output both are elevated by norepinephrine (NE, 20 μM) between 3 p.m. and 7 p.m., and 5-HT and melatonin levels were monitored before, during, and after the drug infusion by using microdialysis on-line with HPLC-FD. (B) Norepinephrine in the presence of β-blocker elevates 5-HT secretion. β-Adrenergic blocker propranolol (PROP, 20 μM) and norepinephrine (NE, 20 μM) were infused directly into the pineal between 3 p.m. and 7 p.m. 5-HT and melatonin levels were assayed as described in A. Similar results were obtained with other tested rats (n = 4).
Discussion
Using an improved in vivo microdialysis system, we demonstrate that 5-HT production in the rat pineal gland is circadianly regulated in vivo, which depends on adrenergic signaling provided by the SCG innervation, and independent of melatonin synthesis.
Circadian Rhythms of 5-HT Synthesis and Release.
The remarkable increase of 5-HT release during the early dark period consistently identified in our pineal microdialysis experiments was the starting point for our investigation. The elevated nocturnal increase in 5-HT release was reported earlier by Azekawa and his colleagues (24), but because of technical limitations, this release could not be detected for more than one cycle. In fact, one of the major factors affecting in vivo pineal recovery of 5-HT release appears to be the degree of tissue injury from the surgical implantation of the probes. Other pineal microdialysis studies likely failed to uncover a consistent rhythm in 5-HT secretion because of traumatic procedures for probe implantation. In contrast, our studies used a surgical approach that causes minimal damage to the surrounding brain. In addition, our improvements allowed us to monitor the release of pineal indoles in a single rat continuously for more than 2 weeks without a decline in signal. The pineal secretory rhythm is highly reproducible and robust for the entire monitoring period; in a given individual the previous day's recordings are highly reproducible and serve as excellent internal controls, enabling the investigator to precisely measure changes in pineal physiology. The system is therefore ideal for monitoring the effect of light and pharmacological agents on pineal physiology in vivo, as demonstrated in our light interference experiments.
In addition to increased night release of 5-HT, we demonstrate that 5-HT content is transiently elevated soon after the dark onset. The extremely short-lived increase in 5-HT content may be the reason for failed attempts to establish increase in 5-HT synthesis in night pineal glands in other studies. Both 5-HT release and total content decrease dramatically later at night as soon as melatonin synthesis is activated. We reasoned that the nocturnal reduction in 5-HT levels may be caused by the increased melatonin synthesis and that 5-HT production can be restored to the early night levels if melatonin synthesis is eliminated. When melatonin production is suppressed, we indeed observed elevated 5-HT content and release that persists throughout the dark period. These data demonstrate that 5-HT synthesis and secretion both are activated at night and the later drop of 5-HT is merely a refection of increased consumption by NAT.
We reach this conclusion by inhibiting NAT production with the RNA synthesis inhibitor ActD. We believe that among the melatonin synthetic enzymes, NAT is affected most readily by the ActD treatment, based on several lines of evidence. First, in our Northern blot analysis, NAT is the only molecule affected after the transient ActD treatment at night. In addition, ActD treatment does not affect the daytime 5-HT production, which suggests that 5-HT synthetic enzymes are not targeted by the acute inhibition of RNA synthesis.
Mechanisms of Diurnal 5-HT Synthesis and Release.
We demonstrate that 5-HT synthesis and release both are elevated in the night pineal gland by increased adrenergic signaling in vivo, which is driven by the SCG innervation. Our data indicate that the increased 5-HT synthesis is mediated by activation of β-adrenergic receptor, whereas the elevated 5-HT release is controlled by α-adrenergic signaling. Our studies fit well with previous reports showing that TPH, the rate-limiting enzyme in 5-HT synthesis, displays a 2-fold increase in enzyme activity in the night pineal gland (12, 13). Consistent with the reports that β-adrenergic signaling controls diurnal pineal TPH activity (12, 25, 26), increased 5-HT synthesis is abolished when β-adrenergic receptor is blocked in vivo in our experiments.
It is well established that β-adrenergic signaling leads to an increase in intracellular concentration of cAMP, a key messenger for generating rhythms in melatonin synthesis and release in the pineal (27). A number of studies indicate that cAMP signaling is also important in regulating the activities of TPH, the rate-limiting enzyme in 5-HT synthesis. TPH is a target of cAMP-dependent protein kinase A (PKA) in vitro (28), and cAMP signaling leads to increased TPH activity and protein level (29, 30). Whether cAMP is the determining factor in the nocturnal increase in 5-HT synthesis and if cAMP's action is mediated through PKA in vivo remains to be determined.
In vitro studies using pineal organ cultures reveal that 5-HT can be released into the media with agents activating α-adrenergic receptors (31, 32). Our studies demonstrate that even in the absence of β-adrenergic signaling, the diurnal 5-HT release is still preserved in vivo, although melatonin synthesis is completely inhibited, implicating α-adrenergic control of the nocturnal 5-HT release. To establish the role of α-adrenergic receptors in 5-HT release in vivo, we infused norepinephrine and propranolol directly into the pineal during the day and observed large increases in 5-HT secretion. These experiments demonstrate that α-adrenergic signaling regulate the increase in nocturnal 5-HT secretion in vivo. Clearly, the subtype of α-adrenoceptors involved in regulation of 5-HT release remains to be determined.
The α receptor-promoted 5-HT release in the pineal gland has been reported in pineal organ cultures in vitro (18) and in median raphe nucleus in vivo (33). Furthermore, α-adrenergic receptor is shown to be present in serotonergic cell bodies (34, 35), controlling the firing rate of serotonergic neurons in the dorsal raphe nucleus (36, 37); and α-adrenoreceptor antagonists are shown to decrease the release of 5-HT in nerve endings (38–40). Our study of regulation of 5-HT release in the pineal gland confirms and expands the current knowledge of mechanisms of 5-HT secretion and establishes the pineal as a valid system for understanding the regulation of 5-HT synthesis and release.
Significance of the Rhythmic 5-HT Synthesis and Release.
Our data suggest that in response to the nocturnal adrenergic signaling 5-HT synthesis and release both are activated. In the absence of increased nocturnal 5-HT synthesis, however, the elevated release of 5-HT leads to a proportional decrease in intracellular 5-HT levels. In the absence of increased 5-HT synthesis, melatonin synthetic enzymes would have to deal with a large decrease in the substrate concentration, which could lead to a severe reduction in levels of melatonin available to the rest of the body. These data suggest that increased 5-HT synthesis may serve to compensate for the loss of intracellular 5-HT caused by increased export and to meet the need of accelerated melatonin synthesis.
Another proposed possibility is that released 5-HT may prime melatonin synthesis. In addition to its intracellular role as precursor of melatonin synthetic machinery, in vitro evidence provided by a number of studies suggest that the extracellular 5-HT enhances melatonin synthesis in an autocrine manner (41–43). In our experiments, however, even when the extracellular 5-HT levels display more than 2-fold changes we still could not observe any influence in the level of melatonin released into the extracellular space. In fact, melatonin is the most stable indole compared with 5-HT and NAS when more than one cycle of release is followed in the same animal in our experiment. These data, however, do not exclude the possibility that the enhanced melatonin is not reflected by its extracellular concentration in these experiments.
In conclusion, the present data demonstrate that 5-HT synthesis and secretion in the pineal is nocturnally stimulated in vivo through the activation of adrenergic signaling, which is controlled by a central clock. The pineal may serve as a model system for investigating the mechanisms of 5-HT production.
Acknowledgments
We thank Drs. Michael M. Wang and Marnie Halpern for critical reading of this manuscript. This work was supported by National Institutes of Health Grant RO1 NS41971–01 (to J.B.). J.B. is a recipient of the John Merck Scholars Award.
Abbreviations
- 5-HT
5-hydroxytryptamine
- SCG
superior cervical ganglion
- TPH
tryptophan hydroxylase
- NAT
N-acetyltransferase
- NAS
N-acetylserotonin
- FD
fluorescence detector
- ActD
actinomycin D
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mockus S M, Vrana K E. J Mol Neurosci. 1998;10:163–179. doi: 10.1007/BF02761772. [DOI] [PubMed] [Google Scholar]
- 2.Quay W B. Gen Comp Endocrinol. 1963;3:473–479. doi: 10.1016/0016-6480(63)90079-0. [DOI] [PubMed] [Google Scholar]
- 3.Snyder S H, Axelrod J. Federation Proc. 1964;23:206. [Google Scholar]
- 4.Snyder S H, Axelrod J, Zweig M. Biochem Pharmacol. 1965;14:831–835. doi: 10.1016/0006-2952(65)90102-4. [DOI] [PubMed] [Google Scholar]
- 5.Snyder S H, Zweig M, Axelrod J, Fischer J E. Proc Nat Acad Sci USA. 1965;53:301–305. doi: 10.1073/pnas.53.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder S H, Axelrod J. Science. 1965;149:542–544. doi: 10.1126/science.149.3683.542. [DOI] [PubMed] [Google Scholar]
- 7.Borjigin J, Li X, Snyder S H. Annu Rev Pharmacol Toxicol. 1999;39:53–65. doi: 10.1146/annurev.pharmtox.39.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Masana M I, Dubocovich M L. Science's STKE. 2001. http://stke.sciencemag.org/cgi/content/full/OC_sigtrans , http://stke.sciencemag.org/cgi/content/full/OC_sigtrans;2001/107/pe39. ;2001/107/pe39. [DOI] [PubMed] [Google Scholar]
- 9.Klein D C, Weller J L. Science. 1970;169:1093–1095. doi: 10.1126/science.169.3950.1093. [DOI] [PubMed] [Google Scholar]
- 10.Borjigin J, Wang M M, Snyder S H. Nature (London) 1995;378:783–785. doi: 10.1038/378783a0. [DOI] [PubMed] [Google Scholar]
- 11.Roseboom P H, Coon S L, Baler R, McCune S K, Weller J L, Klein D C. Endocrinology. 1996;137:3033–3045. doi: 10.1210/endo.137.7.8770929. [DOI] [PubMed] [Google Scholar]
- 12.Shibuya H, Toru M, Watanabe S. Brain Res. 1977;138:364–368. doi: 10.1016/0006-8993(77)90754-5. [DOI] [PubMed] [Google Scholar]
- 13.Sitaram B R, Lees G J. J Neurochem. 1978;31:1021–1026. doi: 10.1111/j.1471-4159.1978.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 14.Florez J C, Seidenman K J, Barrett R K, Sangoram A M, Takahashi J S. Brain Res Mol Brain Res. 1996;42:25–30. doi: 10.1016/s0169-328x(96)00104-0. [DOI] [PubMed] [Google Scholar]
- 15.Green C B, Besharse J C, Zatz M. Brain Res. 1996;738:1–7. doi: 10.1016/0006-8993(96)00743-3. [DOI] [PubMed] [Google Scholar]
- 16.Green C B, Cahill G M, Besharse J C. Brain Res. 1995;677:283–290. doi: 10.1016/0006-8993(95)00166-n. [DOI] [PubMed] [Google Scholar]
- 17.Chong N W, Cassone V M, Bernard M, Klein D C, Iuvone P M. Brain Res Mol Brain Res. 1998;61:243–250. doi: 10.1016/s0169-328x(98)00219-8. [DOI] [PubMed] [Google Scholar]
- 18.Walker R F, Aloyo V J. Adv Exp Med Biol. 1987;221:223–236. doi: 10.1007/978-1-4684-7618-7_17. [DOI] [PubMed] [Google Scholar]
- 19.Drijfhout W J, Grol C J, Westerink B H. J Neurochem. 1993;61:936–942. doi: 10.1111/j.1471-4159.1993.tb03605.x. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 21.Moller M, Hay-Schmidt A. J Pineal Res. 1998;25:19–23. doi: 10.1111/j.1600-079x.1998.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Brito A, Troiani M E, Menendez-Pelaez A, Delgado M J, Reiter R J. J Cell Biochem. 1990;44:55–60. doi: 10.1002/jcb.240440105. [DOI] [PubMed] [Google Scholar]
- 23.Voisin P, Harrington M G, Weller J L, Goldman D, Merril C R, Klein D C. Brain Res. 1990;517:25–34. doi: 10.1016/0006-8993(90)91003-y. [DOI] [PubMed] [Google Scholar]
- 24.Azekawa T, Sano A, Sei H, Morita Y. Neurosci Lett. 1991;132:93–96. doi: 10.1016/0304-3940(91)90441-u. [DOI] [PubMed] [Google Scholar]
- 25.Wurtman R J, Shein H M, Larin F. J Neurochem. 1971;18:1683–1687. doi: 10.1111/j.1471-4159.1971.tb03741.x. [DOI] [PubMed] [Google Scholar]
- 26.Toru M, Watanabe S, Nishikawa T, Semba J, Shibuya H. Adv Biosci. 1978;21:253–255. [PubMed] [Google Scholar]
- 27.Klein D C, Coon S L, Roseboom P H, Weller J L, Bernard M, Gastel J A, Zatz M, Iuvone P M, Rodriguez I R, Begay V, et al. Recent Prog Horm Res. 1997;52:307–357. [PubMed] [Google Scholar]
- 28.Johansen P A, Jennings I, Cotton R G, Kuhn D M. J Neurochem. 1995;65:882–888. doi: 10.1046/j.1471-4159.1995.65020882.x. [DOI] [PubMed] [Google Scholar]
- 29.Ehret M, Pevet P, Maitre M. J Neurochem. 1991;57:1516–1521. doi: 10.1111/j.1471-4159.1991.tb06346.x. [DOI] [PubMed] [Google Scholar]
- 30.Florez J C, Takahashi J S. J Neurochem. 1996;67:242–250. doi: 10.1046/j.1471-4159.1996.67010242.x. [DOI] [PubMed] [Google Scholar]
- 31.Aloyo V J, Walker R F. J Endocrinol. 1987;114:3–9. doi: 10.1677/joe.0.1140003. [DOI] [PubMed] [Google Scholar]
- 32.Aloyo V J, Walker R F. Neuroendocrinology. 1988;48:61–66. doi: 10.1159/000124990. [DOI] [PubMed] [Google Scholar]
- 33.Adell A, Artigas F. Eur J Neurosci. 1999;11:2305–2311. doi: 10.1046/j.1460-9568.1999.00642.x. [DOI] [PubMed] [Google Scholar]
- 34.Day H E, Campeau S, Watson S J, Jr, Akil H. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- 35.Gallager D W, Aghajanian G K. Eur J Pharmacol. 1976;39:341–355. doi: 10.1016/0014-2999(76)90144-8. [DOI] [PubMed] [Google Scholar]
- 36.Baraban J M, Aghajanian G K. Eur J Pharmacol. 1980;66:287–294. doi: 10.1016/0014-2999(80)90461-6. [DOI] [PubMed] [Google Scholar]
- 37.Marwaha J, Aghajanian G K. J Pharmacol Exp Ther. 1982;222:287–293. [PubMed] [Google Scholar]
- 38.Rouquier L, Claustre Y, Benavides J. Eur J Pharmacol. 1994;261:59–64. doi: 10.1016/0014-2999(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 39.Hjorth S, Bengtsson H J, Milano S, Lundberg J F, Sharp T. Neuropharmacology. 1995;34:615–620. doi: 10.1016/0028-3908(95)00038-8. [DOI] [PubMed] [Google Scholar]
- 40.de Boer T H, Nefkens F, van Helvoirt A, van Delft A M. J Pharmacol Exp Ther. 1996;277:852–860. [PubMed] [Google Scholar]
- 41.Sugden D. J Neurochem. 1990;55:1655–1658. doi: 10.1111/j.1471-4159.1990.tb04952.x. [DOI] [PubMed] [Google Scholar]
- 42.Olcese J, Munker M. Brain Res. 1994;643:150–154. doi: 10.1016/0006-8993(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 43.Miguez J M, Simonneaux V, Pevet P. J Pineal Res. 1997;23:63–71. doi: 10.1111/j.1600-079x.1997.tb00337.x. [DOI] [PubMed] [Google Scholar]