Abstract
Vertebrate kinetochores contain over 50 different proteins organized into three distinct regions: the inner plate, outer plate, and fibrous corona. The present study characterizes numerous precursors of kinetochore assembly in a system free of centromeric chromatin, Xenopus extracts. Hydrodynamic analysis suggests there are a minimum of two monomeric proteins and six preassembled complexes that accumulate on centromeres to form the kinetochore. The inner and outer kinetochore assemble from at least two distinct kinetochore complexes containing the proteins Mis12, Zwint, and Ndc80, all of which interact by immunoprecipitation. There is also a network of interactions between the fibrous corona proteins that is dissociated by microtubules. We quantify the number of molecules of specific proteins assembled into a single kinetochore. There are between 800 and 1200 molecules of the measured inner and outer kinetochore proteins, demonstrating that the components in these regions are in similar stoichiometry. In contrast, the measured fibrous corona proteins are present at 250-300 molecules per kinetochore. Zwint, but not Mis12, requires the Ndc80 complex for assembly into the kinetochore. Further, Ndc80 requires Zwint for assembly, indicating a codependency for these two proteins. Our data provide a model for the structural architecture and assembly pathway of the vertebrate kinetochore.
INTRODUCTION
Accurate segregation of sister chromatids at anaphase is critical for maintaining genome integrity. Central to this process is the kinetochore (KT), a highly dynamic macro-molecular structure that assembles onto centromeric heterochromatin. The KT attaches sister chromatids to the mitotic spindle, mediates chromosome movements, and blocks mitotic progression into anaphase in the absence of bipolar microtubule attachment (reviewed in Cleveland et al., 2003).
An explanation of how proteins assemble onto centromeres to form a KT remains an undescribed phenomenon and should provide key insights into the mechanisms underlying its function. This has been difficult to address because KTs assemble from a large number of proteins (>60) into a structure that is bound to chromatin, precluding the use of standard biochemical techniques. Much of our understanding of KT structure and assembly is based on dependency experiments in which a protein is depleted and KT structure is subsequently examined by immunofluorescence. Although these techniques have yielded insight into the requirements for a specific protein's localization to the KT, they fail to address the architecture and interactions within an assembled KT. This will require defining the interactions between KT proteins, the number of specific components within a KT, and how these interactions are regulated both during KT assembly and in response to microtubule attachment.
Xenopus egg extracts provide an excellent system for studying the KT's structural architecture and biochemical interactions. These extracts contain stockpiles of soluble KT complexes in preparation for rapid, early embryonic divisions. The in vitro egg extracts are void of chromosomes, assembled KTs, and microtubule polymers. Moreover, extracts are capable of assembling fully functional KTs on demembranated sperm that send spindle checkpoint signals, align metaphase chromosomes, and segregate chromosomes in anaphase (Minshull et al., 1994; Murray et al., 1996; Desai et al., 1997). Because these in vitro assembled KTs appear to mirror those assembled in vivo, this provides a powerful and biochemically amenable system to study the interactions between KT proteins.
We have previously purified and characterized the Xenopus Ndc80 KT protein and found that it exists in a soluble, preassembled, four protein complex independent of its association with the KT (McCleland et al., 2003, 2004). This suggests that KTs assemble via a multistep process: groups of proteins first assemble into soluble complexes, and then given the proper cell cycle cues; these complexes assemble onto centromeric heterochromatin. Thus, an initial map of KT protein interactions can be generated by identifying the preassembled, stoichiometric KT complex interactions (intracomplex), and the weaker interactions between these complexes (intercomplex). The biochemical purification of human and worm KT proteins have provided an initial framework for this map (Cheeseman et al., 2004; Obuse et al., 2004). However, in these purifications the stoichiometry between components has not been quantified, and they have been performed using overexpressed, tagged proteins from lysates containing intact KTs. Therefore, these data cannot distinguish between intra- and intercomplex interactions, a critical parameter of the KT assembly process.
Electron microscopy (EM) of the KT reveals three distinct domains: the inner KT, outer KT, and fibrous corona (McEwen et al., 1998). In addition, some micrographs detect a region of low electron density between the inner and outer KT referred to as the interzone. The inner KT organizes centromeric DNA into a specialized structure that provides a platform for outer KT assembly and is composed of the constitutive centromere binding proteins Cenp-A, Cenp-C, and Mis12 (Palmer et al., 1987; Saitoh et al., 1992; Goshima et al., 2003). The outer KT assembles during prophase and prometaphase and electron micrographs indicate that it is an electron-dense plate capable of binding up to 29 microtubules in vertebrate cells (Rieder, 1981). Outer plate components remain bound to the KT from prometaphase through anaphase, are required for mature microtubule attachment and include the proteins Ndc80/Hec1 and Zwint (Starr et al., 2000; McCleland et al., 2003; Deluca et al., 2004). The fibrous corona is distal to the centromere, where it extends from the outer KT. It assembles after nuclear envelope break-down and provides an interface for initial microtubule capture. After microtubule attachment, the corona region is no longer visible by EM (McEwen et al., 1998). Many proteins exhibit a localization pattern consistent with this region, including the microtubule motor proteins Cenp-E and Dynein, their regulatory proteins, CLIP-170, Dynactin, LIS1, and the Rod complex, and the spindle check-point proteins, Mad2, Cdc20, and BubR1 (reviewed in Maiato et al., 2004).
We have generated a collection of antibodies allowing us to systematically examine proteins and complexes in the inner, outer, and fibrous corona regions of the Xenopus KT. The number of molecules of specific proteins assembled into a KT was calculated. We estimate that an individual KT contains between 800 and 1200 molecules of each measured inner and outer KT protein and 200-300 molecules of each measured fibrous corona protein. The stoichiometry between proteins in the inner and outer plates, and the large number of molecules present for the formation of only 30 microtubule binding sites, is further evidence that the KT is assembled from repeated units (Zinkowski et al., 1991), each containing numerous copies of specific proteins. Hydrodynamic analysis demonstrates that the inner and outer KT domains are formed from soluble, preassembled complexes containing the proteins Mis12, Zwint, and Ndc80. Similarly, we found in the fibrous corona that CLIP-170, Dynein, Dynactin, LIS1, MAST, and Rod exist as distinct proteins or in distinct complexes. However, a subfraction of CLIP-170 interacts with many of the fibrous corona proteins in a manner that is regulated by microtubules. Additionally, a subfraction of Zwint interacts with Ndc80, CLIP-170, Dynein, LIS1, and Dynactin, suggesting that this complex may act as a molecular bridge within the KT. Our data provide both the stoichiometry and a physical interaction map for much of the known KT. We conclude that vertebrate KTs are assembled from a core of soluble, preassembled complexes that interact at centromeres during mitosis.
MATERIALS AND METHODS
Isolation of xMis12, xZwint, xCLIP-170, xP27, xMAST, and xZwilch Clones
Full-length or fragments for Xenopus Mis12, Zwint, CLIP-170, p27, MAST, and Zwilch genes were cloned from either their respective EST or a Xenopus stage 11.5-14 cDNA library using the following PCR primers: xMis12 5′ (AGCGGATCCCATATGTCTGTTCGTGCAATGTG); xMis12 3′ (AGCGGATCCCATATGTCTGTTCGTGCAATGTG), xZwint 5′ (GCGGGATCCCATATGGCGGAGGCAGCTGGTCG); xZwint 3′ (GGACTCGAGCGGCCGCCTAACCACCGAGACTCCATG), xCLIP-170 5′ (GGGGGATCCCATATGAGTTCCCTGAAGCCTAGTG); xCLIP-170 3′ (CCCGGATCCAAGCTTTTAAGAATCGAGTTCACGATTGTG), xP27 5′ (CCGGATCCATATGGCGGCTCCTCTGAGGC); xP27 3′ (GGCGAGCTCGAATTCCAACACTCAGCTTTTCATTGGG), xMAST 5′ (CGGGATCCCATATGTCCATAGACCACTCAG); xMAST 3′ (GGCGGATCCTAACTGTGCGTGGAGACGTCC); xZwilch 5′ (CCCGAATTCCATATGTGGGCTGAAAGGCATCG); and xZwilch 3′ (T7 primer). All PCR products were cloned into pET28 (Novagen, Madison, WI) via NdeI/NotI restriction sites to yield an amino-terminal 6-His fusion protein.
Protein Purification, Antibodies, Immunofluorescence, and Immunoblotting
6His-Mis12, Zwint, CLIP-170, p27, MAST, and Zwilch were expressed in the Escherichia coli strain BL21 (DE3 pLysS; Novagen), purified using Ni-NTA resin (Qiagen, Valencia, CA), and injected into rabbits to generate polyclonal sera (Covance, Denver, PA). Antibodies were affinity purified over corresponding immunizing protein coupled to Sepharose (Amersham Biosciences, Piscataway, NJ) as described in Harlow and Lane (1988). All antibodies were dialyzed into phosphate-buffered saline. Immunofluorescence and immunoblotting was performed as previously described (McCleland et al., 2003). Purchased antibodies included α-LIS1 (AbCam, Cambridge, United Kingdom), α-p150Glued (BD Transduction Laboratories, Lexington, KY), α-tubulin DM1α (Sigma, St. Louis, MO) and α-H2B (Upstate Biotechnology, Lake Placid, NY). An antibody to Dynein Heavy Chain was a generous gift from Kevin Pfister (University of Virginia).
Egg Extract Manipulations
CSF-arrested egg extracts and sperm nuclei were prepared as described by Murray (1991). IP experiments were done from either CSF extract or CSF extract supplemented with 9000 sperm/μl. Spindle checkpoint signaling was induced through addition of nocodazole (10 μg/ml). Ran-mediated asters were assembled by the addition of 25 μM constitutively active RanQ69L (Kalab et al., 1999; Ohba et al., 1999; Wilde and Zheng, 1999). Cycled extracts and immunofluorescence on chromatin were performed as described by Desai et al. (1999). In Zwint antibody addition experiments, a final concentration of 4 mg/ml antibody was incubated in extract for 30 min (on ice) to inactivate the protein, before the addition of sperm.
Immunoprecipitation and Immunodepletion
IPs and depletions of extracts were performed as described previously (McCleland et al., 2003). Briefly, 20 μg of rabbit IgG (Sigma) or 20 μg of affinity-purified antibodies were covalently coupled to 20 μl of Affi-Prep Protein-A beads (Bio-Rad, Hercules, CA) using dimethylpimelimidate (Pierce, Rockford, IL). For IP, beads were rotated in extracts at either 4°C or room temperature for 1-3 h. Depletions were done in successive rounds for no more than 2 h at 4°C. Beads were washed, eluted with glycine, pH 2.5 (see above), TCA precipitated, and analyzed by immunoblot.
Gel Filtration Chromatography and Sucrose Gradient Sedimentation
Mature oocyte extract (MOE; McCleland and Stukenberg, 2003) was clarified by ultracentrifugation in a S120-AT2 (Sorvall, Newton, CT) at 40,000 rpm for 1 h (4°C). The clarified extracts were syringe filtered (Fisher, Pittsburgh, PA) and loaded onto a Superose 6 Gel Filtration column (Amersham Biosciences). The column was run in 10 mM HEPES, pH 7.7, 300 mM NaCl, 1 mM MgCl2, 50 mM sucrose, 1 mM dithiothreitol, collecting 0.5-ml fractions. Similarly, clarified and filtered MOE was layered over a 5-30% sucrose gradient prepared in 10 mM K-HEPES, 100 mM NaCl, 1 mM MgCl2, 0.1 mM EDTA, 0.1 mM EGTA, pH 7.7, and centrifuged at 30,000 rpm for 18 h at 4°C in a SW-40 rotor. Fractions, 0.5 ml, were collected and TCA precipitated for immunoblot analysis.
The calculations performed for Table 1, utilize the Siegel and Monty Equation to estimate native molecular weight from the empirically determined stokes radius and diffusional coefficient. The frictional coefficient was calculated as a ratio of the empirically determined stokes radius compared with the minimum stokes radius (Rmin) of protein or complex with a known molecular weight. The Rmin is determined using the formula Rmin = (3V/4π)1/3, where V is volume of a protein or complex determined mathematically as M (Da) = 837 V (nm3).
Table 1.
Hydrodynamic analysis of vertebrate kinetochore proteins
| Subcomplex | Xenopus protein analyzed | Individual protein MW (kDa) | Svedberg coeff (S) | Stokes radius (Å) | Measured complex MW (kDa) | Frictional coefficient | Concentration in extract (nM) | Molecules per kinetochore |
|---|---|---|---|---|---|---|---|---|
| Mis 12/Zwint complex | Mis12 | 26 | 15.6 | >200 | >1200 | >2.9 | 9.6 | 1000 ± 700 |
| Zwint | 42 | 30.0 | 750 ± 300 | |||||
| Ndc80 complex | Ndc80 | 75 | 4.3 | 114 | 200 | 3.0 | 66.0 | 930 ± 300 |
| Nuf2 | 54 | 50.0 | 1200 ± 300 | |||||
| Spc24 | 22 | n.d. | n.d. | |||||
| Spc25 | 26 | 28.4 | 800 ± 300 | |||||
| Rod complex | Rod | 250 | 20 | 114 | 940 | 1.8 | n.d. | |
| Zw10 | 82 | n.d. | ||||||
| Zwilch | 70 | 20.5 | 300 ± 200 | |||||
| Dynein complex | Dynein IC | 74 | 20 | >200 | n.d. | n.d. | n.d. | n.d. |
| Dynactin complex | p150/Glued | 150 | 20 | 162 | 1300 | 2.3 | n.d. | n.d. |
| p27 | 27 | 55 | 250 ± 100 | |||||
| CLIP-170 | CLIP-170 | ∼275 | 16.8 | >200 | n.d. | n.d. | n.d. | n.d. |
| MAST | MAST | 165 | 4.3 | 92 | 170 | 2.5 | n.d. | n.d. |
| LIS1 | LIS1 | 46 | 15.6 | n.d.a | n.d. | n.d. | n.d. | n.d. |
Native MW was calculated from the experimentally measured S value and Stokes radii using the Siegel and Monty equation. n.d., not determined.
LIS1 did not elute from a gel filtration column.
Estimating the Number of Protein Molecules per KT
Calculating the number of specific protein molecules per kinetochore first required measuring the concentration of each protein in egg extract. The full-length genes of KT proteins were expressed in bacteria and purified. The amount of undegraded, full-length protein in these preps was determined by comparing serial dilutions of purified protein to dilutions of a bovine serum albumin standard run on the same Coomassie-stained gel. To subsequently measure the concentration of a specific protein in extract, serial-diluted CSF extract was compared with the full-length band of its purified, recombinant protein using quantitative Western blot. Because transfer efficiency is a major concern when Western blotting, this analysis can only be preformed on proteins for which we have a full-length polypeptide.
To assay the number of molecules at KTs, sperm was added (10,000 sperm/μl) to 100 μl of extract and incubated in the presence of nocodazole (10 μg/ml) to assemble KTs. Extracts were diluted 1:10 in BRB80 + 20% glycerol + 0.5% Triton X-100, layered onto a glycerol cushion (BRB80 + 40% glycerol) and centrifuged in a RP55S rotor (7000 × g, 25 min, 18°C). Cushions were washed with BRB80, and the pellet was analyzed by Western blot. The amount of protein on fully assembled KTs was compared with the known levels in extract. The protein levels on chromatin purified from Aurora B depleted extracts prepared in the same way was subtracted as background. Rabbit polyclonal H2B antibody was used to show equivalent amounts of chromatin were purified (Upstate Biotechnology).
RESULTS
Generation of Xenopus KT Reagents
A number of affinity-purified antibodies were generated against Xenopus KT proteins. Xenopus homologues were identified by sequence homology to human Mis12, Zwint, Rod, Zw10, CLIP-170, Clasp/MAST/Orbit (hereafter referred to as MAST), and the p27 subunit of Dynactin. Full-length or partial genes were cloned, expressed as recombinant proteins in E. coli, and injected into rabbits to generate polyclonal antibodies. Mis12 and Zwint antibodies recognize expected 25- and 42-kDa proteins by immunoblot, respectively (Figure 1). Antibodies generated against fragments of Rod and Zw10 recognize expected 230 kDa and 80 kDa proteins by immunoblot, respectively (Figure 1) and stain KTs in Xenopus extracts (McCleland et al., 2003). Using affinity-purified Rod antibodies, we purified the Rod complex and identified stoichiometric complex members Zw10 and Zwilch by mass spectrometry (unpublished data). We subsequently cloned full-length Xenopus Zwilch and generated an antibody that recognizes a 70-kDa protein by immunoblot (Figure 1). We also generated an antibody to the amino terminal 774 amino acids of Xenopus CLIP-170, which shares 57% protein sequence identity to its human homolog. Affinity-purified CLIP-170 antibody recognized a 275- and a 115-kDa protein in Xenopus interphase egg extract (Figure 1). Purification and subsequent mass spectrometry revealed that the 275-kDa protein is an alternatively spliced embryonic form of CLIP-170 (unpublished data). The 115-kDa band is likely to be a homolog of the previously characterized CLIP-115. Antibodies against a fragment of Xenopus MAST recognized a predicted 170-kDa protein and antibodies to the p27 subunit of Dynactin recognized a ∼25-kDa protein (Figure 1).
Figure 1.
Antibody characterizations of inner, outer and fibrous corona kinetochore proteins. The Xenopus homologues of human KT proteins were identified by sequence similarity. The genes were cloned and bacterially expressed recombinant proteins were used to generate antibodies in rabbits. Affinity-purified antibodies against Mis12, Zwint, Rod, Zw10, Zwilch, CLIP-170, MAST, and p27 proteins were used to immunoblot Xenopus egg extract. (* indicates a cross-reacting band for the MAST antibody).
Measuring the Number of Individual Protein Molecules per KT
To better understand the architecture of the vertebrate KT, we developed an assay to measure the number of molecules of a given protein at individual KTs. We first calculated the endogenous cytoplasmic concentration of the KT proteins in Xenopus extracts using quantitative Western blots comparing serially diluted full-length recombinant protein to serially diluted CSF extract (run on the same gel). To estimate the degree of variability in this procedure, we determined the concentration of three different members of the Ndc80 complex, which contains four tightly bound subunits with 1:1:1:1 stoichiometry (Ndc80/Hec1, Nuf2, Spc24, and Spc25; McCleland et al., 2004). The measured concentrations ranged from 28 to 66 nM, suggesting an approximate twofold experimental variation. All other KT proteins examined had a concentration within a similar range (Table 1). Mis12 has the lowest protein concentration of those measured at ∼10 nM.
Knowing their concentration in extracts allowed us to measure the copy number of specific proteins assembled onto a single KT. By this method, a known concentration of sperm nuclei are added to Xenopus extracts and incubated to assemble KTs. The chromatin is then purified through a glycerol cushion and analyzed by immunoblot (Figure 2B). Because the fibrous corona is disassembled after KT-microtubule attachment, nocodazole is also added to the reaction to retain members of this region on KTs. Nocodazole addition has no effect on the amount of the outer plate protein Ndc80 present at KTs in both extracts and tissue culture cells (Deluca et al., 2004; unpublished data). Purified mitotic chromosomes are quantitatively immunoblotted with KT protein antibodies and are compared with serially diluted CSF extracts to calculate the amount of protein bound to isolated chromosomes. Knowing the endogenous protein concentration, the number of sperm added and the number of KTs assembled per sperm allows us to calculate the number of protein molecules per chromosome. To account for nonspecific chromatin binding, sperm chromatin are also assembled in an Aurora B depleted extract. Aurora B depleted Xenopus extracts assemble Cenp-A onto KTs but fail to assemble the outer KT and fibrous corona, indicated by a loss of Nuf2, p150Glued, Bub1, Rod, and Zw10 staining (Figure 2A and unpublished data). Specific KT binding was determined by subtracting the amount of protein bound to Aurora B depleted chromatin from chromatin assembled in mock depleted extracts.
Figure 2.
Calculation of the number of individual protein molecules per kinetochore. (A) Depletion of the Aurora B complex from Xenopus CSF extracts inhibits KT assembly. CSF extracts were depleted of the Aurora B complex (shown by Western blot), incubated with sperm and nocodazole, and KT assembly was assayed by immunofluorescence on purified chromatin. Staining of Cenp-A, Nuf2, Zw10, and p150Glued are shown in red and DNA is in blue. (B) The number of molecules per KT for several proteins was determined by Western blotting purified chromatin after KT assembly. The flow chart implicates the steps used in purifying chromatin to measure the number of specific proteins at individual KTs. Using antibodies to several KT proteins, purified chromatin was blotted and compared with serial dilutions of extract for densitometric comparison. The concentration of protein in extract was determined for each protein analyzed. Aurora B depleted extracts serve as a background control for the amount of nonspecific protein being purified with chromatin in the absence of KTs. Purified chromatin was probed with an H2B antibody to ensure equal amounts were obtained from each reaction.
A number of control experiments were performed to confirm that fully assembled KTs were being measured. Similar amounts of KT proteins were found on chromosomes whether or not they were fixed with formaldehyde before purification (unpublished data), ensuring that KTs were not disassembled during the isolation process. We also measured the kinetics of KT assembly and found that the reaction is completed by 15 min (unpublished data). Additionally, to ensure that equivalent amounts of total chromatin were purified from Aurora B and mock-depleted samples, purified chromatin was blotted for histone H2B (Figure 2B).
Data from a representative experiment are shown in Figure 2B. Quantitative immunoblots comparing the amount of KT components on purified chromosomes to dilutions of Xenopus extract are shown in Figure 2B and the data from these experiments are displayed in Table 1. The inner and outer KT proteins Mis12, Zwint, and Ndc80 were each present in strikingly similar amounts at 1000, 750, and 930 copies per KT, respectively (Table 1). Two components of the fibrous corona were also present in amounts similar to one another, Zwilch at 300 copies and the p27 subunit of Dynactin at 250 copies per KT. To estimate the variability in this assay, we measured the amount of three Ndc80 complex subunits at KTs and calculated similar numbers for each (Table 1). We conclude that fully assembled KTs contain ∼750-1200 copies of each inner and outer KT protein measured and ∼300 copies of each fibrous corona protein measured.
Hydrodynamic Analysis of KT Subcomplexes
To explore the physical characteristics of proteins and preassembled KT complexes, we analyzed the hydrodynamic properties of each of the KT proteins in Table 1. By analyzing a proteins sedimentation and diffusional properties one can determine whether they exist as monomers or in larger molecular weight complexes. Clarified Xenopus mitotic extracts were fractionated over a Superose 6 gel filtration column or sedimented through a 5-30% sucrose gradient, and the resulting fractions were immunoblotted with our KT antibodies (Figure 3, A and B, and Table 1). The Svedberg coefficients (S) and Stokes radii were empirically determined from the sucrose gradient and gel filtration data, respectively, and the native molecular weight and the frictional coefficient (fo/fmin; a measurement describing a proteins degree of elongation) for each protein/complex were calculated (Table 1). As the frictional coefficient increases it indicates an increased degree of elongation within a protein or complex. Our data indicate that the complexes containing Mis12, Zwint, and Ndc80 are all highly elongated. The Rod complex does not show the same degree of elongation, but is clearly not spherical. As previously demonstrated by EM (Scheel et al., 1999), CLIP-170 is highly elongated and the hydrodynamics of MAST suggests that it is as well. Our analysis shows that most known proteins and complexes of the vertebrate KT are elongated. This is consistent with KT proteins containing large coiled coil regions, as predicted by sequence analysis.
Figure 3.
Gel filtration and sucrose gradient analysis of kinetochore protein subcomplexes. (A) Xenopus mitotic high-speed supernatants were run over a Superose 6 gel filtration column. Alternate fractions were combined, TCA-precipitated, and immunoblotted with a panel of KT antibodies. (B) Xenopus mitotic high-speed supernatants were sedimented through a 10 ml, 5-30% sucrose gradient in a Sw50.2Ti rotor. Fractions were TCA precipitated and immunoblotted with the same panel of KT protein antibodies.
Mis12 and Zwint both eluted in the void fractions of our gel filtration column. A portion of Zwint was included in the column and, despite comigrating with Mis12 in the void, clearly did not share a common peak with Mis12. Over sucrose gradient, Mis12 and Zwint partially cosediment, but do not overlap completely. The sedimentation and diffusional properties of Mis12 and Zwint suggest that they may be in a complex with one another, although this alone is not definitive. Whether it is a single complex or separate complexes, our data are consistent with these proteins interacting with stoichiometric binding partners (Cheeseman et al., 2004; Obuse et al., 2004; Kops et al., 2005), because the calculated native molecular weight of a single complex of that size would be at least 1280 kDa (Table 1). We have previously shown that members of the Ndc80 complex (Ndc80, Nuf2, Spc24, and Spc25) comigrate as a stoichiometric, ∼200-kDa complex (McCleland et al., 2003, 2004). Ndc80 migrated independent of both Mis12 and Zwint by gel filtration and sucrose gradient, clearly demonstrating it is not in a stoichiometric complex with either of these proteins (or any other KT proteins tested; Figure 3).
The fibrous corona proteins Rod, Zw10, and Zwilch comigrate on sucrose gradients and over gel filtration and their complex has a predicted molecular weight of 940 kDa (Figure 3 and Table 1), suggesting that either it is a dimer or there are still unidentified components. Dynein's propensity to aggregate over gel filtration explains its appearance in the void fraction (K. Pfister, personal communication). It has been previously demonstrated that Dynein and Dynactin sediment as distinct 20S complexes (Paschal et al., 1987; Collins and Vallee, 1989). p150Glued and p27, members of the 11 subunit Dynactin complex, also comigrated and coimmunoprecipitated (unpublished data). The fibrous corona proteins CLIP-170, MAST, and LIS1 did not comigrate with any tested proteins or complexes. LIS1 migrated at 15.6S and has been previously shown to interact with NudE and NudC (Morris et al., 1998; Kitagawa et al., 2000). LIS1 failed to elute from a gel filtration column so we could not determine its Stokes radius. MAST's calculated native molecular weight indicates it is a monomer in solution (Table 1). Our hydrodynamic data indicate that before assembly onto centromeres there are at least two proteins and six preassembled complexes that contain vertebrate KT proteins.
Intercomplex Interactions of the KT
Gradient sedimentation and gel filtration are not equilibrium techniques and therefore fail to detect transient interactions. To measure the interactions between KT proteins and complexes, Ndc80, Zwint, Mis12, Zwilch, CLIP-170, p27, and MAST were immunoprecipitated from CSF extracts and the resulting immunoprecipitations and supernatants were immunoblotted with a panel of KT antibodies (Figure 4). These experiments were done in Xenopus extracts, which lack centromeric DNA so that we can detect interactions between the precursors of KT assembly and eliminate the possibility that entire KTs are being pulled down.
Figure 4.
Coimmunoprecipitation of proteins from the inner, outer, and fibrous corona domains of the kinetochore. CSF extract was immunoprecipitated with affinity-purified antibodies to Ndc80, Zwint, Mis12, Zwilch, CLIP-170, p27 (Dynactin), and MAST. The immunoprecipitates (left panel) and supernatants after IP (right panel) were probed with a panel of KT protein antibodies.
Mis12, Zwint, and Ndc80 all coimmunoprecipitate (Figure 4). Interestingly, IP of Ndc80 and Zwint significantly reduced the level of Mis12 in the supernatant. However, IP of Mis12 did not alter the levels of Zwint or Ndc80 in the supernatant, consistent with the lower concentration of Mis12 in the extract. These results suggest that these interactions are substoichiometric. Ndc80 and Mis12 did not exhibit any interactions with members of the fibrous corona region, suggesting that their main roles lie within the inner and outer KT. However, a subfraction of Zwint coimmunoprecipitated with the fibrous corona proteins CLIP-170, Dynein, Dynactin (p27 and p150Glued), and LIS1 (Figure 4). Confirming this interaction, CLIP-170 IP pulled down trace amounts of Zwint and longer exposures of p27 and Zwilch IPs also show low levels of interaction with Zwint (Figure 4 and unpublished data). The relative amounts of Zwint in the various IPs varied, but Zwint consistently interacted strongly with the inner and outer KT components and weakly with the fibrous corona components. We conclude that Zwint's main function lies within the outer KT, but propose a role for the Zwint complex in establishing a molecular bridge for the attachment of the fibrous corona to the outer KT.
We similarly examined interactions between fibrous corona proteins. Zwilch antibodies IP both Rod and Zw10 and examination of the supernatants suggest that this IP removes the majority of these proteins (Figure 4). Taken together, our concentration measurements, IP and hydrodynamic data suggest that Rod, Zw10, and Zwilch exist in a preassembled, stoichiometric complex. Zwilch IPs did not pull down significant amounts of any other KT protein examined. Rod IPs also failed to IP any other KT proteins (unpublished data).
CLIP-170 immunoprecipitated Rod, LIS1, Dynein, and Dynactin. Examination of the supernatants after IP suggests that only a small fraction of these proteins interact. IP of p27 (Dynactin) pulled down p150Glued. Longer p27 IPs significantly reduce the amount of both p27 and p150Glued in the supernatant, confirming their existence in a stoichiometric complex (unpublished data). p27 also IPs substoichiometric amounts of CLIP-170, Dynein, and LIS1 (Figure 4). MAST was not detected in IPs of any of the tested KT proteins.
Dependencies of Outer KT Components for Assembly
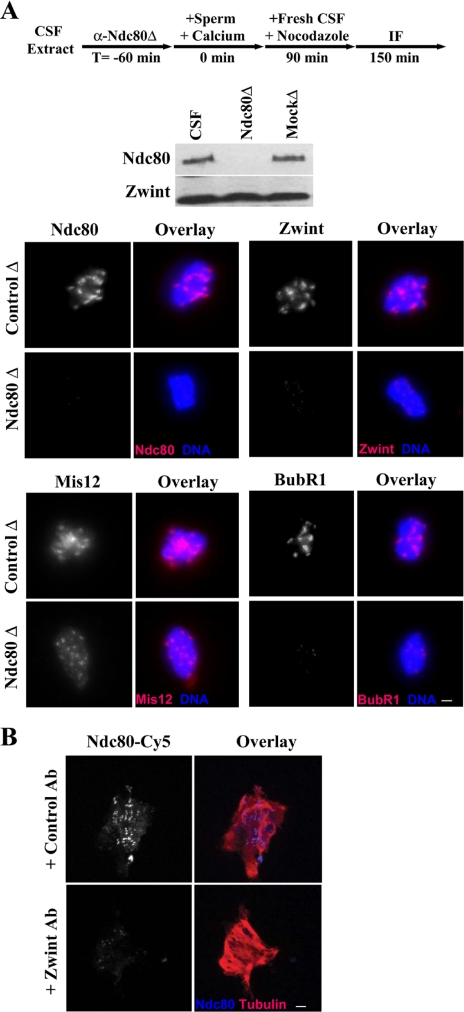
We have previously shown that Ndc80 is required for a number of fibrous corona proteins to assemble on KTs in Xenopus extracts, including Rod, Zw10, and Dynactin (McCleland et al., 2003). To further explore the role of outer plate proteins in KT assembly, we tested whether Ndc80 is required for the KT localization of inner and outer plate proteins, as well as fibrous corona proteins that have not been tested yet. Ndc80 was depleted from a CSF extract and sperm was added to initiate KT assembly. The extract was then cycled through S-phase with the addition of calcium and arrested at the subsequent mitosis by the addition of fresh CSF. The experiment was performed in the presence of nocodazole to prevent the microtubule dependent disassembly of the fibrous corona. KT assembly was assayed by immunofluorescence on purified chromatin using antibodies to Mis12, Zwint, Ndc80, and BubR1.
Depletion of Ndc80 inhibited the assembly of Zwint onto KTs, indicated by its absence in Ndc80 depleted extracts (Figure 5A). The inner plate protein Mis12 assembled onto KTs normally, indicating that Ndc80 is not required for assembly of the inner plate (Figure 5A). There was a small, but consistent, decrease in Mis12 levels at KTs in Ndc80 depleted extracts, presumably due to the knockdown of its protein levels during Ndc80 depletion (Figure 4). In addition, Cenp-A localized normally in these assembly experiments, confirming that Ndc80 is not required for inner plate construction onto centromeres (McCleland et al., 2003). The fibrous corona and checkpoint protein BubR1 is also unable to assemble in Ndc80 depleted extracts, as indicated by a loss of staining at KTs (Figure 5A). We further tested CLIP-170 and Zwilch and found that these proteins also assemble onto KTs in an Ndc80-dependent manner (unpublished data).
Figure 5.
Ndc80 and Zwint are codependent for assembly of the outer kinetochore. (A) Depletion of the Ndc80 complex from Xenopus extracts inhibits outer KT and fibrous corona assembly. CSF extracts were depleted of the Ndc80 complex (shown by Western blot). Sperm and calcium were added to cycle the extract through S-phase. Fresh CSF extract and nocodazole were added to rearrest extracts at metaphase without microtubules. KT assembly was assayed by immunofluorescence on purified chromatin with antibodies to Ndc80, Mis12, Zwint, and Zwilch. KT proteins (red), DNA (blue); bar, 2 μM. (B) Zwint is required for the association of Ndc80 with KTs. Anti-Zwint antibodies were incubated in a CSF extract. Sperm and calcium were added to cycle the extract through S-phase. Fresh CSF extract was added to rearrest extracts at metaphase. The assembly of Ndc80 onto KTs was tested by probing purified chromatin with a Cy5-conjugated Ndc80 antibody and the DM1α microtubule antibody. Microtubules (red), Ndc80 (blue); bar, 2 μM.
Because Zwint localization is consistent with that of the outer plate, we sought to test its role in KT assembly as well. Unfortunately, our Zwint antibody is unable to deplete the protein from extract. Therefore, we knocked out its function by adding Zwint antibodies to the extract and then localized Ndc80 to KTs with a Cy5-conjugated α-Ndc80 antibody. Addition of the Zwint antibody prevented the assembly of Ndc80 onto the KT (Figure 5B). We conclude that in Xenopus extracts: 1) the inner KT can assemble independent of the outer KT, 2) there is an interdependence of the Ndc80 complex and Zwint protein for outer KT assembly, and 3) the localization of fibrous corona proteins tested depends on outer KT assembly.
Fibrous Corona Protein Interactions Are Regulated by the Cell Cycle and Microtubules
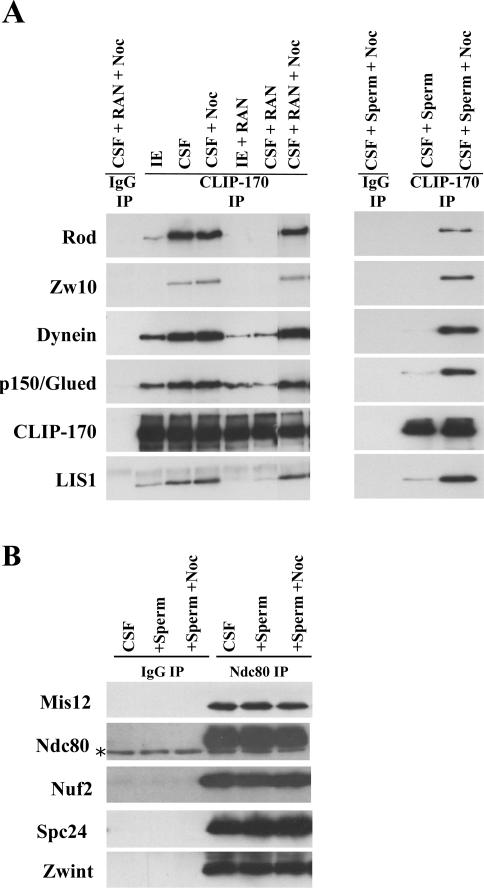
Numerous protein-protein interactions are regulated by changes in cell cycle state. To test whether KT protein interactions are regulated in this manner, we compared IPs from CSF extracts to those driven into interphase through the addition of calcium. Because CLIP-170 interacts with a number of proteins in the fibrous corona, we immunoprecipitated it from extracts and found a dramatic increase in its association with Rod, Zw10, p150Glued, Dynein, and LIS1 in CSF extracts when compared with interphase extracts (Figure 6A, left panel). This demonstrates that the interaction between CLIP-170 and the fibrous corona proteins are stronger in mitosis than interphase.
Figure 6.
Fibrous corona interactions are regulated by cell cycle state and microtubules. (A) CLIP-170 interaction with Rod, Dynein, Dynactin, and LIS1 is disrupted in Interphase extracts and by microtubules. Left, CSF extracts were driven into interphase with the addition of calcium (IE), treated with the microtubule poison nocodazole to disrupt microtubules, and supplemented with RanQ69L to nucleate microtubules. Extracts were then immunoprecipitated with CLIP-170 antibodies, and precipitates were blotted with a panel of KT antibodies. Right, CSF extracts were incubated with sperm with or without nocodazole. CLIP-170 was subsequently immunoprecipitated, and the precipitates were immunoblotted with the panel of KT antibodies. (B) Interactions between the inner and outer KT proteins are not affected by the addition of sperm or microtubules. After the addition of sperm or sperm and nocodazole, extracts were immunoprecipitated with Ndc80 antibodies and assayed for interactions with a panel of antibodies representing inner and outer plate proteins. (* indicates cross-linked IgG in the Ndc80 blot)
The rapid assembly of the KT in extracts after sperm addition suggests that some unknown signal is driving protein interactions at the centromere. We therefore wanted to examine whether the presence of sperm could up-regulate the interactions between KT proteins and complexes. To test this, CLIP-170 was immunoprecipitated from extracts that had been incubated with a high concentration of sperm (9000/μl) for 30 min. The amount of the fibrous corona proteins Rod, Zw10, Dynein, p150Glued, CLIP-170, and Lis1 in the IPs was measured by immunoblot. Surprisingly, rather than drive these interactions, the addition of sperm inhibited them in CSF extracts (Figure 6A, right panel). The addition of sperm to extracts not only contributes chromatin, but also centrosomes, which nucleate microtubules. Because fibrous corona proteins only localize together at the KT in the absence of MT attachment, we reasoned that the generation of dynamic microtubules could potentially dissociate these soluble interactions. To test if the microtubules generated by sperm addition were disrupting these interactions, we repeated the above experiment in the presence of the microtubule poison nocodazole. The addition of nocodazole rescued the interaction between CLIP-170 and the proteins in the fibrous corona (Figure 6A, right panel).
RanQ69L (constitutively active) has been shown to drive microtubule aster assembly in mitotic extracts (Kalab et al., 1999; Ohba et al., 1999; Wilde and Zheng, 1999). To confirm our results which suggest that generating microtubules inhibits the interaction between proteins in the fibrous corona, CLIP-170 was immunoprecipitated 30 min after addition of RanQ69L. We found that in the presence of RanQ69L alone the interaction between proteins in the fibrous corona was inhibited. Adding nocodazole to the extracts containing RanQ69L rescued the interaction between CLIP-170 and the other fibrous corona proteins (Figure 6A, left panel). This result demonstrates that the microtubules generated by RanQ69L regulate these interactions. Taken together, these results confirm that microtubules regulate the interactions between CLIP-170 and the fibrous corona proteins Rod, Zw10, Dynein, p150Glued, and Lis1.
The addition of sperm to extracts (with and without nocodazole) did not generate a detectable difference in the interactions between inner and outer KT proteins because similar amounts of Mis12 and Zwint were measured in Ndc80 IPs under these conditions (Figure 6B). This result suggests that the interactions between inner and outer KT proteins are not regulated by a readily diffusible signal that is generated by chromatin or centromeres. In addition, in contrast to the fibrous corona, microtubules do not effect the interaction between proteins in the inner and outer plate.
DISCUSSION
Using Xenopus extracts we have taken a systematic approach to biochemically dissect the proteins and complexes of the vertebrate KT, and 1) show that the vertebrate KT forms from a number (≥8) of soluble proteins and complexes that preassemble outside of their centromeric context, 2) calculate the number of molecules per KT for several components, 3) map biochemical interactions between these proteins and complexes, 4) determine the dependencies for assembly of the outer KT, and 5) show that protein interactions in the fibrous corona are regulated by microtubules.
KT Structure
We are the first to measure the stoichiometry of KT subunits assembled onto KTs. Our calculation that ∼1000 molecules of Mis12, Zwint, and the Ndc80 complex members assemble into KTs provides significant insight into KT structure. Because a vertebrate KT can bind up to 30 microtubules (Rieder, 1981), this suggests that ∼30 molecules of each protein assemble into a structure that binds a single microtubule. The calculation that there are ∼30 molecules of an individual protein per MT binding site is consistent with a repeated subunit structure of the KT, as has been proposed by others (Zinkowski et al., 1991). We favor a model wherein Ndc80 complexes create a cylindrical binding pocket at the KT, interacting with one another along the long face of the complex, encircling an incoming microtubule. Strikingly, it would take ∼30 Ndc80 complexes to encircle a single microtubule (circumference of ∼790 Å) in this manner, given its measured length of 570 Å, and an approximate axial ratio of 20:1 (De Wulf et al., 2003;Wei et al., 2005).
This model assumes that the Ndc80 complex is positioned in the kinetochore lengthwise pointing from the centromere out. If the complexes examined here also align in such a manner, this could account for the large size of the KT plates. Hydrodynamics indicate that complexes containing Zwint and Mis12 could easily span their respective regions because they are both longer than Ndc80. CLIP-170 has been measured by EM to be ∼1350 Å long (Scheel et al., 1999) and an embryonic form may be even longer, potentially allowing CLIP-170 to span the entire length of the fibrous corona. CLIP-170's length, localization and ability to interact in a regulated manner with several KT proteins suggest it may act as a scaffold within the fibrous corona.
The fibrous corona is the most dynamic region of the KT. Cells incubated in nocodazole for several hours generate “collars” of fibrous corona proteins (specifically Cenp-E) around sister chromatid centromeres (Thrower et al., 1996), whereas after microtubule attachment these components are no longer present at the KT. Ndc80 does not show an increase under these conditions, suggesting that this size increase is fibrous corona specific (Deluca et al., 2004). We measured the number of specific protein molecules present at a single KT in the presence of nocodazole to estimate a maximum number of molecules assembled in the fibrous corona. Under these conditions the fibrous corona proteins are present in a one-to-four stoichiometry with those assembled onto the inner and outer plates. Because of the dynamic nature of the proteins in this region, in the future it will be interesting to measure the numbers of proteins present at KTs under various conditions.
A model diagramming the mapped physical interactions between KT proteins is shown in Figure 7. Lines drawn between proteins and complexes indicate interactions we identified by IP. The region within the KT where these proteins have been localized is indicated, as well as their approximate length compared with that region. We conclude that vertebrate KTs form from a series of preassembled, soluble subcomplexes. Additionally, we suggest that the inner KT assembles independent of the outer plate, the outer KT requires both Ndc80 and Zwint protein function and the fibrous corona assembly requires an intact outer KT.
Figure 7.
Model of kinetochore architecture and protein interactions. (A) A representation of KT assembly and protein-protein interactions are shown. Arrows indicate the numerous known protein-protein interactions reported in this study. The size of the different KT regions is indicated. The inner KT, interzone and outer KT have been measured by electron microscopy to be ∼400 Å and the fibrous corona is ∼1500 Å. The minimal length of each KT subcomplex is drawn approximately to scale compared with the depth of the KT and to each other.
We have demonstrated novel interactions between Zwint and CLIP-170, Rod, Dynein, Dynactin, and LIS1. This article extends the previously described interactions between Mis12, Zwint, and Ndc80 by examining endogenous complexes and relative stoichiometry. Ndc80 migrates independent of both Mis12 and Zwint by both fractionation methods tested (Figure 3), demonstrating it is clearly in a distinct complex. Mis12 and Zwint partially cosediment and comigrate over sucrose gradient and gel filtration (Figure 3) and IP one another (Figure 4), suggesting that they are in the same complex, in agreement with the recently published affinity tag purifications of these proteins (Cheeseman et al., 2004; Obuse et al., 2004; Kops et al., 2005). Immunofluorescence localizes Mis12 to the KTs inner plate (Goshima et al., 2003; Obuse et al., 2004) and immuno-EM localizes Ndc80 to the outer plate (Deluca et al., 2004). Zwint's localization pattern is consistent with that of Ndc80 (Starr et al., 2000), suggesting it is also an outer plate component (Figure 7). The spatial separation of Zwint and Mis12 within the KT, their ability to assemble onto centromeres independent of one another, and the fact that after Zwint IP, the amount of Mis12 in the supernatant remains unchanged (Figure 5 and 3, respectively; Cheeseman et al., 2004) suggest that these proteins are in separate complexes. We conclude that Ndc80 assembles onto KTs in a distinct, soluble complex, but currently there are ambiguous data on whether Zwint and Mis12 are in separate large molecular-weight complexes, or in the same complex.
Regulation of KT interactions
One apparent difference between somatic and embryonic KT assembly is the complete requirement for the Aurora B complex for assembly of the outer-plate proteins. Although it has not been directly tested, it is unlikely that Ndc80 localization to KTs requires the Aurora B complex is somatic cells. In somatic systems, knockdown of Aurora B by dominant negative expression, siRNA or pharmacological inhibitors leads to a phenotype wherein KTs can still bind microtubules. However, the loss of Ndc80 leads to complete inability to congress chromosomes and form KT fibers (Deluca et al., 2002; McCleland et al., 2004). The requirement of Aurora B to localize Ndc80 may reflect a reaction specific to the de novo KT assembly of embryos. Alternatively the differences between cells and extracts may reflect the degree of knockdown, as there are hints for Aurora B requirements for the localization of Dynein, Cenp-E, and BubR1 in somatic systems (Murata-Hori and Wang, 2002; Ditchfield et al., 2003; Hauf et al., 2003).
It has been postulated that the fibrous corona forms initial, side-on attachments with dynamic microtubules and that these interactions mature into stable, end-on attachments coincident with corona displacement (Rieder and Alexander, 1990). It is unclear how the fibrous corona binds microtubules and dissociates from the KT. We show that the fibrous corona protein CLIP-170's interaction with Dynein, Dynactin, LIS1, and Rod increases under mitotic conditions suggesting a specific role for this interaction in spindle formation. Moreover, the interaction is disassembled by microtubules. Recent work demonstrated the ability of CLIP-170 to fold back on itself allowing for an intramolecular interaction between its N- and C-termini. It was further shown that microtubules drove it toward an unfolded, open conformation (Lansbergen et al., 2004). Combined with our data, this suggests that the closed (folded) conformation of CLIP-170 is able to interact with the fibrous corona proteins. Further, when in contact with microtubules, CLIP-170 unfolds and releases these proteins, which are then loaded onto microtubules and undergo Dynein-mediated translocation to centrosomes.
Acknowledgments
We thank Dan Burke and other members of the Burke and Stukenberg laboratories for their insight into this project. Special thanks to Kevin Pfister for the Dynein antibody and to Margaret Bolton, Cortney Kestner, Rebekka Sprouse, and Anne K. McCullough for generation and characterization of antibodies and for help with numerous reagents. We also would like to thank Gary Gorbsky for critical revisions of the manuscript. This work was supported by grants from the American Cancer Society (RSG-04-021-01-CCG) and the National Institute of Health (GM63045). P.T.S. is a Pew Scholar. D.L.S. and M.J.E. are supported by training grant HD07528 for Developmental Biology at the University of Virginia.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-03-0239) on August 3, 2005.
References
- Cheeseman, I. M., Niessen, S., Anderson, S., Hyndman, F., Yates, J. R., III, Oegema, K., Desai, A. (2004). A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 18, 2255-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland, D. W., Mao, Y., and Sullivan, K. F. (2003). Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112, 407-421. [DOI] [PubMed] [Google Scholar]
- Collins, C. A., and Vallee, R. B. (1989). Preparation of microtubules from rat liver and testis: cytoplasmic dynein is a major microtubule associated protein. Cell Motil. Cytoskelet. 14, 491-500. [DOI] [PubMed] [Google Scholar]
- De Wulf, P., McAinsh, A. D., and Sorger, P. K. (2003). Hierarchical assembly of the budding yeast kinetochore from multiple subcomplexes. Genes Dev. 17, 2902-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca, J. G., Dong, Y., Hergert, P., Strauss, J., Hickey, J. M., Salmon, E. D., and McEwen, B. F. (2004). Hec1 and Nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol. Biol. Cell 16, 519-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca, J. G., Moree, B., Hickey, J. M., Kilmartin, J. V., and Salmon, E. D. (2002). hNuf2 inhibition blocks stable kinetochore-microtubule attachment and induces mitotic cell death in HeLa cells. J. Cell Biol. 159, 549-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, A., Deacon, H. W., Walczak, C. E., and Mitchison, T. J. (1997). A method that allows the assembly of kinetochore components onto chromosomes condensed in clarified Xenopus egg extracts. Proc. Natl. Acad. Sci. USA 94, 12378-12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, A., Murray, A., Mitchison, T. J., and Walczak, C. E. (1999). The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61, 385-412. [DOI] [PubMed] [Google Scholar]
- Ditchfield, C., Johnson, V. L., Tighe, A., Ellston, R., Haworth, C., Johnson, T., Mortlock, A., Keen, N., and Taylor, S. S. (2003). Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161, 267-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G., Kiyomitsu, T., Yoda, K., and Yanagida, M. (2003). Human centromere chromatin protein hMis12, essential for equal segregation, is independent of CENP-A loading pathway. J. Cell Biol. 160, 25-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Hauf, S., Cole, R. W., LaTerra, S., Zimmer, C., Schnapp, G., Walter, R., Heckel, A., van Meel, J., Rieder, C. L., Peters, J. M. (2003). The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161, 281-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalab, P., Pu, R. T., and Dasso, M. (1999). The ran GTPase regulates mitotic spindle assembly. Curr. Biol. 9, 481-484. [DOI] [PubMed] [Google Scholar]
- Kitagawa, M., Umezu, M., Aoki, J., Koizumi, H., Arai, H., and Inoue, K. (2000). Direct association of LIS1, the lissencephaly gene product, with a mammalian homologue of a fungal nuclear distribution protein, rNUDE. FEBS Lett. 479, 57-62. [DOI] [PubMed] [Google Scholar]
- Kops, G. J., Kim, Y., Weaver, B. A., Mao, Y., McLeod, I., Yates, J. R., III, Tagaya, M., and Cleveland, D. W. (2005). ZW10 links mitotic checkpoint signaling to the structural kinetochore. J. Cell Biol. 169, 49-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen, G. et al. (2004). Conformational changes in CLIP-170 regulate its binding to microtubules and dynactin localization. J. Cell Biol. 166, 1003-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato, H., DeLuca, J., Salmon, E. D., and Earnshaw, W. C. (2004). The dynamic kinetochore-microtubule interface. J. Cell Sci. 117, 5461-5477. [DOI] [PubMed] [Google Scholar]
- McCleland, M. L., Gardner, R. D., Kallio, M. J., Daum, J. R., Gorbsky, G. J., Burke, D. J., and Stukenberg, P. T. (2003). The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev. 17, 101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleland, M. L., Kallio, M. J., Barrett-Wilt, G. A., Kestner, C. A., Shabanowitz, J., Hunt, D. F., Gorbsky, G. J., and Stukenberg, P. T. (2004). The vertebrate Ndc80 complex contains Spc24 and Spc25 homologs, which are required to establish and maintain kinetochore-microtubule attachment. Curr. Biol. 14, 131-137. [DOI] [PubMed] [Google Scholar]
- McCleland, M. L., and Stukenberg, P. T. (2003). Purification of the Ndc80 kinetochore subcomplex from Xenopus eggs. Methods Mol. Biol. 296, 383-391. [DOI] [PubMed] [Google Scholar]
- McEwen, B. F., Hsieh, C. E., Mattheyses, A. L., and Rieder, C. L. (1998). A new look at kinetochore structure in vertebrate somatic cells using high-pressure freezing and freeze substitution. Chromosoma 107, 366-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull, J., Sun, H., Tonks, N. K., and Murray, A. W. (1994). A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell 79, 475-486. [DOI] [PubMed] [Google Scholar]
- Morris, S. M., Albrecht, U., Reiner, O., Eichele, G., and Yu-Lee, L. Y. (1998). The lissencephaly gene product Lis1, a protein involved in neuronal migration, interacts with a nuclear movement protein, NudC. Curr. Biol. 8, 603-606. [DOI] [PubMed] [Google Scholar]
- Murata-Hori, M., and Wang, Y. L. (2002). The kinase activity of aurora B is required for kinetochore-microtubule interactions during mitosis. Curr. Biol. 12, 894-899. [DOI] [PubMed] [Google Scholar]
- Murray, A. W. (1991). Cell cycle extracts. Methods Cell Biol. 36, 581-605. [PubMed] [Google Scholar]
- Murray, A. W., Desai, A. B., and Salmon, E. D. (1996). Real time observation of anaphase in vitro. Proc. Natl. Acad. Sci. USA 93, 12327-12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuse, C., Iwasaki, O., Kiyomitsu, T., Goshima, G., Toyoda, Y., and Yanagida, M. (2004). A conserved Mis12 centromere complex is linked to heterochromatic HP1 and outer kinetochore protein Zwint-1. Nat. Cell Biol. 6, 1135-1141. [DOI] [PubMed] [Google Scholar]
- Ohba, T., Nakamura, M., Nishitani, H., and Nishimoto, T. (1999). Self-organization of microtubule asters induced in Xenopus egg extracts by GTP-bound Ran. Science 284, 1356-1358. [DOI] [PubMed] [Google Scholar]
- Palmer, D. K., O'Day, K., Wener, M. H., Andrews, B. S., and Margolis, R. L. (1987). A 17-kD centromere protein (CENP-A) copurifies with nucleosome core particles and with histones. J. Cell Biol. 104, 805-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal, B. M., Shpetner, H. S., and Vallee, R. B. (1987). MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J. Cell Biol. 105, 1273-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C. L. (1981). The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma 84, 145-158. [DOI] [PubMed] [Google Scholar]
- Rieder, C. L., and Alexander, S. P. (1990). Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J. Cell Biol. 110, 81-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh, H., Tomkiel, J., Cooke, C. A., Ratrie, H., III, Maurer, M., Rothfield, N. F., Earnshaw, W. C. (1992). CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell 70, 115-125. [DOI] [PubMed] [Google Scholar]
- Scheel, J., Pierre, P., Rickard, J. E., Diamantopoulos, G. S., Valetti, C., van der Goot, F. G., Haner, M., Aebi, U., and Kreis, T. E. (1999). Purification and analysis of authentic CLIP-170 and recombinant fragments. J. Biol. Chem. 274, 25883-25891. [DOI] [PubMed] [Google Scholar]
- Starr, D. A., Saffery, R., Li, Z., Simpson, A. E., Choo, K. H., Yen, T. J., and Goldberg, M. L. (2000). HZwint-1, a novel human kinetochore component that interacts with HZW10. J. Cell Sci. 113(Pt 11), 1939-1950. [DOI] [PubMed] [Google Scholar]
- Thrower, D. A., Jordan, M. A., and Wilson, L. (1996). Modulation of CENP-E organization at kinetochores by spindle microtubule attachment. Cell Motil. Cytoskelet. 35, 121-133. [DOI] [PubMed] [Google Scholar]
- Wei, R. R., Sorger, P. K., and Harrison, S. C. (2005). Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc. Natl. Acad. Sci. USA 102, 5363-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde, A., and Zheng, Y. (1999). Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science 284, 1359-1362. [DOI] [PubMed] [Google Scholar]
- Zinkowski, R. P., Meyne, J., and Brinkley, B. R. (1991). The centromere-kinetochore complex: a repeat subunit model. J. Cell Biol. 113, 1091-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]