Abstract
Presenilins mediate an unusual intramembranous proteolytic activity known as γ-secretase, two substrates of which are the Notch receptor (Notch) and the β-amyloid precursor protein (APP). γ-Secretase-mediated cleavage of APP, like that of Notch, yields an intracellular fragment [APP intracellular domain (AICD)] that forms a transcriptively active complex. We now demonstrate a functional role for AICD in regulating phosphoinositide-mediated calcium signaling. Genetic ablation of the presenilins or pharmacological inhibition of γ-secretase activity (and thereby AICD production) attenuated calcium signaling in a dose-dependent and reversible manner through a mechanism involving the modulation of endoplasmic reticulum calcium stores. Cells lacking APP (and hence AICD) exhibited similar calcium signaling deficits, and—notably—these disturbances could be reversed by transfection with APP constructs containing an intact AICD, but not by constructs lacking this domain. Our findings indicate that the AICD regulates phosphoinositide-mediated calcium signaling through a γ-secretase-dependent signaling pathway, suggesting that the intramembranous proteolysis of APP may play a signaling role analogous to that of Notch.
The majority of early-onset, autosomal-dominant familial cases of Alzheimer's disease are caused by mutations in the presenilin genes (PS1, PS2) (1). The presenilins are essential components of an intramembranous proteolytic activity known as γ-secretase (1). Several type-I integral membrane proteins have been identified as substrates for γ-secretase, including the Notch receptor (Notch) and the β-amyloid precursor protein (APP) (2). Notch is a signaling molecule that regulates cell-fate determination during development (3). Signaling through Notch is triggered by the binding of ligands such as Delta and Jagged, which induces cleavage of Notch by TACE (4). Subsequent cleavage by γ-secretase releases the Notch intracellular domain, which binds to transcription factors (e.g., Suppressor of Hairless) and translocates to the nucleus, where it regulates transcription of Enhancer of Split complex genes (5).
Similarities between the processing of Notch and APP have prompted speculation that APP may play an analogous signaling role. Cleavage of APP by γ-secretase, the final step in the generation of β-amyloid (Aβ), liberates a fragment analogous to Notch intracellular domain, the APP intracellular domain (AICD), which interacts with the nuclear adaptor protein Fe65 (6–8). Fe65, in turn, interacts with the transcription factor CP2/LSF/LBP1 (9, 10) and Tip60, a histone acetyltransferase (8). Recently, AICD complexed with Fe65 and Tip60 has been shown to potently regulate the expression of artificial expression constructs in transfected cells (8). However, no physiologic signaling role has yet been ascribed to the AICD.
Mutations in the presenilins, in addition to their well documented effects on γ-secretase activity, also produce highly consistent alterations in intracellular calcium signaling pathways, which include a potentiation of the phosphoinositide/calcium signaling cascade (11–14) and deficits in capacitative calcium entry (14, 15). In the present study, we sought to elucidate the nature of the causal link between γ-secretase activity and intracellular calcium signaling pathways. By using fibroblasts from presenilin knockout mice and selective γ-secretase inhibitors, we show a remarkably tight link between γ-secretase activity and the amplitude of phosphoinositide-mediated calcium signals. The calcium signaling alterations present in PS1−/− cells were also evident in APP−/− cells and were reversed by transfection with APP constructs containing an intact AICD—and by AICD alone—but not by constructs lacking this domain. Collectively, our findings suggest that APP plays a physiologic signaling role analogous to Notch that regulates intracellular calcium signaling by way of its γ-secretase-derived intracellular domain.
Materials and Methods
Reagents.
Fura 2-AM and ionomycin were from Molecular Probes. pEGFP-N1 (EGFP, enhanced green fluorescent protein) was from CLONTECH. Cell culture reagents were purchased from Invitrogen. All other reagents were from Sigma unless otherwise noted.
Cell Culture and Calcium Imaging.
Mouse embryonic fibroblasts were immortalized by transfection with the large-T antigen of SV40. N2a cells were maintained in a 1:1 mixture of DMEM/10% FBS and Optimem I supplemented with 200 μg/ml of G418; all other cell lines were maintained in DMEM/10% FBS supplemented with 200 μg/ml of G418. Calcium imaging was performed as described (14). Transient transfections were carried out with LipofectAMINE 2000 according to manufacturer's recommendations (Invitrogen).
Enzyme-Linked Immunosorbent Assays.
Human and mouse Aβ ELISAs were performed as described (16, 17). Mouse Aβ was measured from the conditioned medium of fibroblasts maintained for 16 h in Optimem I low-serum medium (Life Technologies). Human Aβ was measured from the conditioned medium of N2a cells incubated for 3–4 h in Hepes-buffered control solution (HCSS) containing 120 mM NaCl, 5.4 mM KCl, 0.8 mM MgCl2, 2 mM CaCl2, 15 mM glucose, 20 mM Hepes (pH 7.3), and 0.5% phenol red.
Results and Discussion
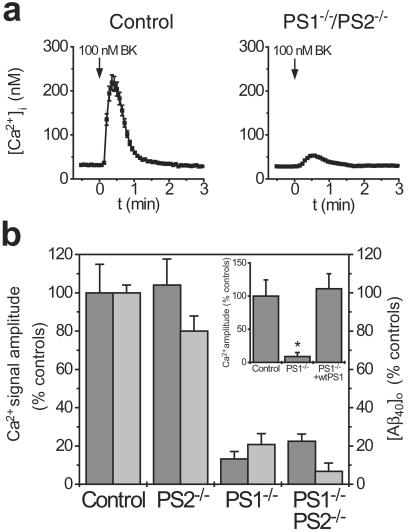
To investigate the role of γ-secretase activity in modulating phosphoinositide-linked calcium signaling, we studied calcium signaling in primary and immortalized fibroblasts from mice lacking one or both of the presenilin genes, which display varying degrees of impairment in γ-secretase activity (18, 19). Cytosolic calcium signals were recorded in Fura-2-loaded cells after stimulation with 100 nM bradykinin (BK), an agonist that stimulates the phosphoinositide signaling cascade and triggers release of calcium from intracellular stores. To monitor γ-secretase activity, we also measured Aβ1–40 production in the conditioned medium from these same cells by using a highly sensitive ELISA (20). Relative to controls, calcium responses were markedly diminished in cells lacking either PS1 alone or both PS1 and PS2, but not in cells lacking PS2 (Fig. 1). Notably, the extent of the deficits in calcium signaling correlated strongly with the amount of γ-secretase activity in the same cells (Fig. 1b). To establish whether these changes were reversible, PS1−/− fibroblasts were cotransfected with enhanced green fluorescent protein together with either wild-type PS1 or empty vector. Expressing cells were identified by using a standard FITC filter cube before calcium imaging. The deficits in calcium signaling present in PS1−/− cells were completely restored by transfection with PS1 (Fig. 1b Inset) and thus were not attributable to nonspecific effects of ablation of the PS1 gene. These findings indicate that the presenilins play a physiologic role in modulating the phosphoinositide/calcium signaling pathway, and suggest that the potentiation in calcium signaling conferred by Alzheimer's disease-linked presenilin mutations (11–14) represents a gain of function in this normal modulatory role. This finding is analogous to the discovery that increased Aβ1–42 production represents a pathological gain of function in the normal function of γ-secretase (18).
Figure 1.
Fibroblasts from presenilin knockout mice show impairments in calcium signaling that correlate with Aβ production. (a) Representative calcium signals evoked by BK (100 nM) in fibroblasts from mice lacking both presenilin genes or controls. (b) Comparison of calcium signal amplitude (dark bars) and [Aβ1–40] in the conditioned medium (light bars) of fibroblasts from presenilin knockout mice. (Inset) The deficits in calcium signaling in PS1−/− mice are completely reversed by transfection with wild-type PS1. Calcium data are mean ± SE for 8–24 experiments per condition. ELISA data are mean ± SE of triplicate determinations. *, P < 0 01.
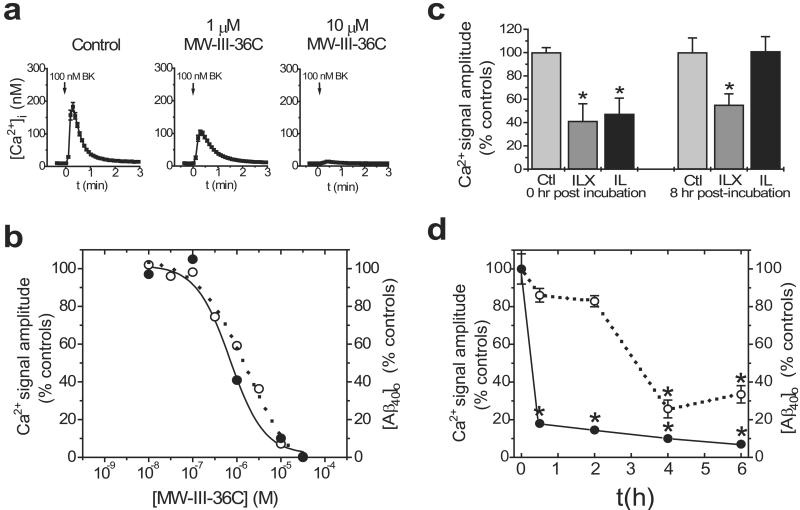
The disturbances in calcium signaling observed in cells lacking presenilins could be a consequence of the presenilins' role in γ-secretase activity or because of other unrelated activities. To discriminate between these possibilities, we tested the effects of direct pharmacological inhibition of γ-secretase on calcium signaling. To that end, BK-evoked calcium signals were recorded in N2a cells stably overexpressing human Swedish APP [N2aWt11 cells (21)] after a 3- to 4-h incubation in various concentrations of γ-secretase inhibitors (Fig. 2). We tested numerous different inhibitors comprising a variety of chemical classes (Table 1), including several transition-state mimics (i.e., directed at the active site of γ-secretase) and one inhibitor (MW-III-36C) that is structurally similar to compounds shown to interact directly with the presenilin proteins (22). Pharmacological inhibition of γ-secretase markedly diminished the amplitude of BK-evoked calcium responses in a dose-dependent fashion (Fig. 2 a and b), whereas structurally related but functionally inert compounds were without effect (Table 1). Similar results were obtained in other cell types (e.g., Chinese hamster ovary, HEK-293, M-17, fibroblasts, and primary cortical neurons) and in response to other agonists (e.g., bombesin and ATP) that activate the phosphoinositide/calcium-signaling pathway (data not shown). To quantify γ-secretase activity in the same cells used for calcium experiments, the conditioned medium was collected immediately before imaging and subjected to a sensitive Aβ ELISA (16). In all cases, a remarkably strong correlation was observed between γ-secretase activity and calcium responsiveness (Fig. 2b). Moreover, the rank order of the IC50 values for inhibition of calcium responsiveness and those for inhibition of Aβ production were closely similar across all inhibitors tested (Table 1).
Figure 2.
Pharmacological inhibition of γ-secretase impairs calcium signaling. (a) Typical BK-evoked calcium signals in N2aWt11 cells treated with various concentrations of MW-III-36C. (b) Effects of a representative γ-secretase inhibitor on both BK-evoked calcium signal amplitude (open circles) and [Aβ1–40] in the conditioned medium (closed circles) after incubation in various concentrations of compound for 3–4 h. Calcium data are mean of quadruplicate determinations. ELISA data are mean of triplicate determinations. (c) The calcium deficits produced by γ-secretase inhibitors are reversible (see text for details). Data are mean ± SE of 6 experiments per condition. (d) Time course of effectiveness of IL on calcium signal amplitude (open circles) and [Aβ1–40] in the conditioned medium (closed circles). Calcium data are mean ± SE of 4 experiments per condition; ELISA data are mean of triplicate determinations *, P < 0.01.
Table 1.
A wide variety of γ-secretase inhibitors have effects on calcium signaling and Aβ production that are similar in rank-order potency
| Compound | Chemical class | IC50Ca, μM | IC50Aβtot, μM | Notes | Refs. |
|---|---|---|---|---|---|
| IL | Peptide aldehyde | 10 | 8 | Reversible | 17 |
| ILX | Peptide epoxide | 10 | 10 | Irreversible analogue of IL | 17 |
| YIL | Peptide aldehyde | 1 | 0.5 | ELISA data are for Aβ1–40 only | |
| CIL | Peptide aldehyde | 1 | 0.1 | Potent analogue of IL | 17 |
| PIA | Peptide aldehyde | >100 | >100 | Structurally related to IL but inactive | |
| MW-III-36C | (Hydroxyethyl) urea | 2 | 0.5 | Similar to L-685,458 | 22, † |
| MW-III-26A | (Hydroxyethyl) urea | 40 | 25* | Structurally related to MW-III-36C | † |
| CM-265 | Peptide difluoroketone | 30 | 8–25* | Potency varies among different cell types | |
| JT-326 | Peptide | >100 | >100* | Structurally related to CM-265 but inactive |
Determined in Chinese hamster ovary cells overexpressing wild-type APP incubated with compound for 6 h; all other data are from N2aWt11 cells incubated with compound for 3–4 h.
Wolfe, M. S., Esler, W. P., Kimberly, W. T., Diehl, T. S., Rahmati, T. & Selkoe, D. J. (2000) Soc. Neurosci. 26, 298.9 (abstr.).
To determine whether pharmacological inhibition of γ-secretase was reversible, we compared IL, a highly soluble peptide aldehyde shown to act reversibly on γ-secretase in in vitro assays (17), and ILX, a structurally related peptide epoxide that cannot be washed out and hence acts irreversibly (see Table 1). Whereas IL and ILX (50 μM) were both effective immediately after a 4-h incubation period, the effects of IL but not ILX were completely reversed 8 h after removal of the compound (Fig. 2c). Thus, genetic ablation of the presenilin genes and pharmacological inhibition of γ-secretase both inhibit calcium signaling in a dose-dependent and reversible manner.
To examine the time course of pharmacologic inhibition of γ-secretase, we performed calcium imaging experiments in N2aWt11 cells after incubation with IL (Fig. 2d) and other γ-secretase inhibitors (data not shown) for various periods of time. Whereas Aβ production was inhibited by >80% by IL by 1 h, significant inhibition of calcium responses was detected only after incubations of 4 h or longer (Fig. 2d).
That γ-secretase inhibition produces effects on Aβ before effects on calcium signaling is significant for two reasons. First, it suggests that γ-secretase activity is upstream of calcium signaling pathways, rather than downstream as suggested (15). Consistent with this observation, we have found that Aβ production measured in a cell-free assay is not affected by addition of excess calcium or EGTA (ref. 17; T. Golde, unpublished observations). Second, and most importantly, these findings raise the possibility that proteolytic products of γ-secretase activity may mediate a signaling pathway that in turn affects intracellular calcium signaling.
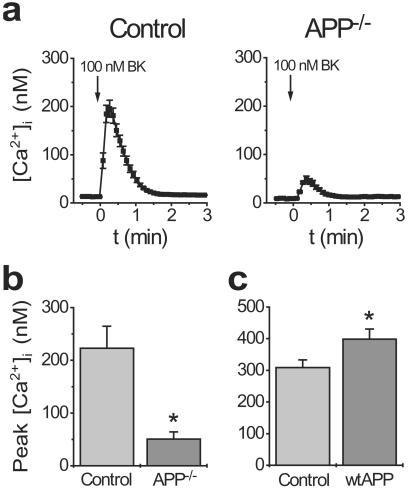
Because the effects of diminished γ-secretase activity on calcium signaling correlated so strongly with Aβ production, we postulated that derivates of APP proteolysis might mediate the effects on calcium signaling. We therefore studied calcium signaling in immortalized fibroblasts from APP−/− mice (23). As illustrated in Fig. 3, calcium signaling was markedly disrupted in APP−/− cells in a manner closely resembling PS1−/− cells (cf. Fig. 1 a and b). Intriguingly, the opposite was true in Chinese hamster ovary cells overexpressing wild-type APP: these cells, which produce increased amounts of Aβ (24) and also AICD, showed a significant increase in the amplitude of BK-evoked calcium signals relative to untransfected controls (Fig. 3c). Although observed in different cell types, these results are consistent with the possible involvement of γ-secretase-mediated proteolytic fragments of APP in the modulation of intracellular calcium signaling.
Figure 3.
APP−/− fibroblasts exhibit deficits in calcium signaling resembling PS1−/− cells. (a) Representative BK-evoked calcium signals from APP−/− fibroblasts and controls. The amplitude of calcium signals is diminished in APP−/− cells (b) but increased in Chinese hamster ovary cells overexpressing APP (c). Data are mean ± SE of 6 experiments per condition. *, P < 0.01.
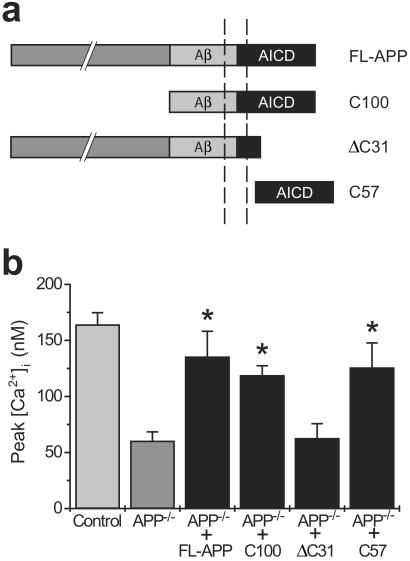
To ascertain which proteolytic fragment(s) of APP is sufficient to rescue the calcium deficits present in APP−/− cells, we cotransfected these cells with cDNAs encoding either full-length APP or one of several deletion mutants (Fig. 4) together with enhanced green fluorescent protein as an expression control. Calcium imaging was performed on the transfected cells 48 h later. As illustrated in Fig. 4b, rescue of the calcium impairments was achieved only with APP constructs expressing an intact γ-secretase-derived intracellular fragment (i.e., AICD). Notably, no evidence of rescue was apparent after transfection with APPΔC31 (25), a construct that lacks critical residues required for the binding of Fe65 (26) (and, hence, transcription factors), although expression of this construct was verified by Western blot (data not shown). In contrast, expression of the free AICD alone (C57) successfully rescued the calcium deficits.
Figure 4.
The calcium deficits in APP−/− cells can be rescued by transfection with constructs containing the AICD. (a) Schematic illustration of the APP constructs used for transfections. (b) Rescue of the calcium impairments in APP−/− cells was achieved after transfection with constructs containing an intact AICD or AICD alone, but not by constructs lacking this domain. Data are mean ± SE of 8 experiments per condition. *, P < 0.05 relative to APP−/− cells.
Several observations support the idea that the proteolytic fragment responsible for rescuing the calcium effects is the AICD rather than extracellular secreted fragments of APP (i.e., soluble APP or Aβ). First, secreted products of APP are expected to be very low in the calcium imaging experiments because of low transfection efficiency (3–5%) and because cells were washed several times before loading with calcium indicator and again before imaging. Moreover, Aβ levels in particular are expected to be low because fibroblasts do not express high levels of β-secretase. Furthermore, we directly tested for possible effects of soluble APP fragments by incubating APP−/− fibroblasts in conditioned medium from Chinese hamster ovary cells overexpressing abundant wild-type APP; we observed no effects on BK-evoked calcium signals after either short- (30-min) or long-term (16-h) incubations (data not shown). These results, together with the observation that AICD alone is sufficient to rescue the calcium signaling deficits in APP−/− cells (Fig. 4b), suggest that the AICD is the domain responsible for modulating calcium signaling. Although we were unable to show biochemical evidence for the generation of the AICD in these experiments, this is likely attributable both to low transfection efficiency in fibroblasts and to the extremely short half-life of the AICD (6–8). It is worth noting that functional activity of the Notch intracellular domain was described well before it could be detected at the biochemical level (27).
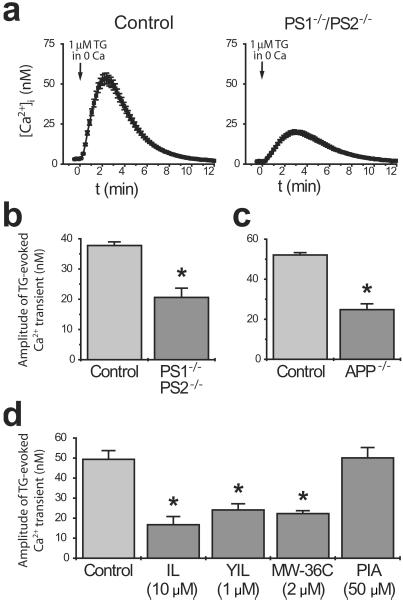
Mutations in the presenilins are associated with increased levels of calcium in the endoplasmic reticulum (ER) (14, 28, 29), and these alterations are likely to represent the cellular mechanism by which presenilin mutations potentiate phosphoinositide/calcium signaling and reduce capacitative calcium entry (14). We therefore sought to determine whether a related mechanism also underlies the calcium signaling deficits observed in the present study. To this end, ER calcium stores were depleted by treating cells with the ER calcium ATPase inhibitor thapsigargin (1 μM) in calcium-free medium; the amplitude of the resulting calcium transient was taken as a measure of ER calcium content (Fig. 5a). Relative to controls, significant reductions in ER calcium content were evident in all cells having impaired AICD production, including PS1−/−/PS2−/− and APP−/− fibroblasts and N2a cells treated with various γ-secretase inhibitors (Fig. 5 b–d). Thus, the cellular mechanism by which AICD modulates phosphoinositide calcium signaling involves the modulation of ER calcium levels.
Figure 5.
Cells with impaired AICD production exhibit reduced ER calcium levels. To quantify ER calcium content, calcium stores were depleted with 1 μM thapsigargin in calcium-free medium. (a) Representative traces from control and PS1−/−/PS2−/− cells. (b–d) Amplitude of thapsigargin-evoked calcium transients in (b) PS1−/−/PS2−/− cells, (c) APP−/− cells, and (d) in cells treated with various γ-secretase inhibitors (or PIA, an inactive control). Data are mean + SE of 4–6 experiments per condition. *, P < 0.01.
Here we demonstrate that blockade of AICD production (through genetic ablation of APP or through genetic or pharmacological inhibition of γ-secretase) leads to impairments in phosphoinositide-mediated calcium signaling. Notably, these impairments are dose-dependent and can be reversed by restoration of AICD production or expression of the AICD alone. Although AICD could modulate calcium signaling via a number of possible mechanisms, recent data showing that AICD forms a transcriptively active complex (8) suggest that AICD might affect calcium signaling by regulating the expression of genes involved in calcium homeostasis. Consistent with this possibility, a blast search revealed that CP2/LSF/LBP1 response elements are present in the regulatory region of an ER calcium ATPase responsible for transporting calcium from the cytosol into the lumen of the ER (data not shown). The regulation of genes affecting ER calcium levels could constitute a “master switch” to modulate the sensitivity of the phosphoinositide calcium signaling pathway. In this way, it is possible to envision that signaling through APP could influence calcium-dependent processes such as cellular differentiation, neurite outgrowth, and synaptic plasticity.
Our results, together with the finding that the AICD is transcriptively active (8), suggest that the released AICD could be a signaling molecule analogous to the Notch intracellular domain (NICD) of the Notch pathway. Both APP and Notch are processed by γ-secretase within their transmembrane domains, adjacent to a conserved valine (27, 30), to release the intracellular domains. Notch proteolysis and NICD release is triggered at the cell surface by interaction with cognate ligands such as Delta and Jagged (4). Perhaps APP proteolysis is likewise initiated by ligands that have yet to be identified. Calcium signaling might constitute a valuable physiological marker that could be exploited in the search for ligands of APP.
Because γ-secretase is considered a prime therapeutic target in Alzheimer's disease, our studies indicate that this approach will alter phosphoinositide-mediated calcium signaling. Given the universal importance of calcium as the major intracellular signaling cation, careful evaluation of potential side effects arising from γ-secretase inhibition is warranted. However, in light of the tight link we observe between γ-secretase activity and calcium signaling, we expect that therapeutic doses of γ-secretase inhibitors that aim only to partially inhibit γ-secretase activity (e.g., 20–30%) should have only moderate effects on calcium signaling.
Acknowledgments
We thank Dr. Sangram Sisodia (Univ. of Chicago) for the N2a cells, Dr. Eddie Koo (Univ. of California, San Diego) for several APP constructs, Tritia Yamasaki and Salvatore Oddo for excellent technical assistance, and members of the Selkoe laboratory for helpful comments. We thank Drs. Abdul Fauq and Chewki Ziani-Cherif for the synthesis of inhibitors, and Dr. Eckman and colleagues for ELISA plate preparation. This work was supported by National Institutes of Health Grant P50-AG16573, American Health Assistance Foundation Grant A20000005, American Federation for Aging Research Grant P00101 (all to F.M.L.), a Beeson Award from the American Federation for Aging Research, an Ellison Medical Foundation New Scholars award, and by National Institutes of Health Grant NS39072 (all to T.E.G.).
Abbreviations
- APP
β-amyloid precursor protein
- Notch
Notch receptor
- AICD
APP intracellular domain
- BK
bradykinin
- Aβ
β-amyloid
- ER
endoplasmic reticulum
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wolfe M S, Haass C. J Biol Chem. 2001;276:5413–5416. doi: 10.1074/jbc.R000026200. [DOI] [PubMed] [Google Scholar]
- 2.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm J S, Schroeter E H, Schrijvers V, Wolfe M S, Ray W J, et al. Nature (London) 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas S, Rand M D, Lake R J. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 4.Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens J R, Cumano A, Roux P, Black R A, Israël A. Mol Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 5.Greenwald I. Genes Dev. 1998;12:1751–1762. doi: 10.1101/gad.12.12.1751. [DOI] [PubMed] [Google Scholar]
- 6.Cupers P, Orlans I, Craessaerts K, Annaert W, De Strooper B. J Neurochem. 2001;78:1168–1178. doi: 10.1046/j.1471-4159.2001.00516.x. [DOI] [PubMed] [Google Scholar]
- 7.Kimberly W T, Zheng J B, Guenette S Y, Selkoe D J. J Biol Chem. 2001;276:40288–40292. doi: 10.1074/jbc.C100447200. [DOI] [PubMed] [Google Scholar]
- 8.Cao X, Sudhof T C. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 9.Fiore F, Zambrano N, Minopoli G, Donini V, Duilio A, Russo T. J Biol Chem. 1995;270:30853–30866. doi: 10.1074/jbc.270.52.30853. [DOI] [PubMed] [Google Scholar]
- 10.Minopoli G, de Candia P, Bonetti A, Faraonio R, Zambrano N, Russo T. J Biol Chem. 2001;276:6545–6550. doi: 10.1074/jbc.M007340200. [DOI] [PubMed] [Google Scholar]
- 11.Guo Q, Furukawa K, Sopher B L, Pham D G, Xie J, Robinson N, Martin G M, Mattson M P. NeuroReport. 1996;8:379–383. doi: 10.1097/00001756-199612200-00074. [DOI] [PubMed] [Google Scholar]
- 12.Leissring M A, Paul B A, Parker I, Cotman C W, LaFerla F M. J Neurochem. 1999;72:1061–1068. doi: 10.1046/j.1471-4159.1999.0721061.x. [DOI] [PubMed] [Google Scholar]
- 13.Leissring M A, Parker I, LaFerla F M. J Biol Chem. 1999;274:32535–32538. doi: 10.1074/jbc.274.46.32535. [DOI] [PubMed] [Google Scholar]
- 14.Leissring M A, Akbari Y, Fanger C M, Cahalan M D, Mattson M P, LaFerla F M. J Cell Biol. 2000;149:793–798. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo A S, Cheng I, Chung S, Grenfell T Z, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, et al. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki N, Cheung T T, Cai X D, Odaka A, Otvos L, Jr, Eckman C, Golde T E, Younkin S G. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 17.McLendon C, Xin T, Ziani-Cherif C, Murphy M P, Findlay K A, Lewis P A, Pinnix I, Sambamurti K, Wang R, Fauq A, Golde T E. FASEB J. 2000;14:2383–2386. doi: 10.1096/fj.00-0286fje. [DOI] [PubMed] [Google Scholar]
- 18.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Nature (London) 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 19.Herreman A, Hartmann D, Annaert W, Saftig P, Craessaerts K, Serneels L, Umans L, Schrijvers V, Checler F, Vanderstichele H, et al. Proc Natl Acad Sci USA. 1999;96:11872–11877. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawarabayashi T, Younkin L, Saido T, Shoji M, Ashe K, Younkin S. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thinakaran G, Harris C L, Ratovitski T, Davenport F, Slunt H H, Price D L, Borchelt D R, Sisodia S S. J Biol Chem. 1997;272:28415–28422. doi: 10.1074/jbc.272.45.28415. [DOI] [PubMed] [Google Scholar]
- 22.Li Y M, Xu M, Lai M T, Huang Q, Castro J L, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil J G, et al. Nature (London) 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- 23.Heber S, Herms J, Gajic V, Hainfellner J, Aguzzi A, Rulicke T, von Kretzschmar H, von Koch C, Sisodia S, Tremml P, et al. J Neurosci. 2000;20:7951–7963. doi: 10.1523/JNEUROSCI.20-21-07951.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koo E H, Squazzo S L. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- 25.Lu D C, Rabizadeh S, Chandra S, Shayya R F, Ellerby L M, Ye X, Salvesen G S, Koo E H, Bredesen D E. Nat Med. 2000;6:397–404. doi: 10.1038/74656. [DOI] [PubMed] [Google Scholar]
- 26.Zambrano N, Buxbaum J D, Minopoli G, Fiore F, De Candia P, De Renzis S, Faraonio R, Sabo S, Cheetham J, Sudol M, Russo T. J Biol Chem. 1997;272:6399–6405. doi: 10.1074/jbc.272.10.6399. [DOI] [PubMed] [Google Scholar]
- 27.Schroeter E H, Kisslinger J A, Kopan R. Nature (London) 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 28.Leissring M A, LaFerla F M, Callamaras N, Parker I. Neurobiol Dis. 2001;8:469–478. doi: 10.1006/nbdi.2001.0382. [DOI] [PubMed] [Google Scholar]
- 29.Schneider I, Reverse D, Dewachter I, Ris L, Caluwaerts N, Kuiperi C, Gilis M, Geerts H, Kretzschmar H, Godaux E, et al. J Biol Chem. 2001;276:11539–11544. doi: 10.1074/jbc.M010977200. [DOI] [PubMed] [Google Scholar]
- 30.Sastre M, Steiner H, Fuchs K, Capell A, Multhaup G, Condron M M, Teplow D B, Haass C. EMBO Rep. 2001;2:835–841. doi: 10.1093/embo-reports/kve180. [DOI] [PMC free article] [PubMed] [Google Scholar]