Abstract
H2AX is a core histone H2A variant that contains an absolutely conserved serine/glutamine (SQ) motif within an extended carboxy-terminal tail. H2AX phosphorylation at the SQ motif (γ-H2AX) has been shown to increase dramatically upon exogenously introduced DNA double-strand breaks (DSBs). In this study, we use quantitative in situ approaches to investigate the spatial patterning and cell cycle dynamics of γ-H2AX in a panel of normally growing (unirradiated) mammalian cell lines and cultures. We provide the first evidence for the existence of two distinct yet highly discernible γ-H2AX focal populations: a small population of large amorphous foci that colocalize with numerous DNA DSB repair proteins and previously undescribed but much more abundant small foci. These small foci do not recruit proteins involved in DNA DSB repair. Cell cycle analyses reveal unexpected dynamics for γ-H2AX in unirradiated mammalian cells that include an ATM-dependent phosphorylation that is maximal during M phase. Based upon similarities drawn from other histone posttranslational modifications and previous observations in haploinsufficient (H2AX-/+) and null mice (H2AX-/-), γ-H2AX may contribute to the fidelity of the mitotic process, even in the absence of DNA damage, thereby ensuring the faithful transmission of genetic information from one generation to the next.
INTRODUCTION
H2AX is a histone H2A variant that is highly conserved from Giardia intestinalis to humans (reviewed in Pilch et al., 2003). In humans, H2AX comprises ∼2-25% of the total H2A population (Rogakou et al., 1998), whereas in Saccharomyces cerevisiae it is much more prevalent and represents ∼95% of the total H2A population (Celeste et al., 2002). H2AX is distinct from all other H2A variants in that it harbors a carboxy (C)-terminal extension that includes an invariant serine/glutamine (SQ) motif located four amino acid residues from the C terminus (Rogakou et al., 1998). There has been considerable research into the role(s) H2AX and its C-terminal phosphorylation play in regulating DNA DSB repair activities and targeting. For example, after ionizing radiation (IR) H2AX is rapidly phosphorylated at the γ-serine of the SQ motif. It is this phosphorylated form that is commonly referred to as γ-H2AX. Members of the phosphatidyl-inositol-3-kinase (PI3-K) protein kinase (PIKK) family, including ataxia telangiectasia mutated (ATM), DNA-PKCS (DNA-protein kinase, catalytic subunit) and ATR (ATM and Rad3-related) are all thought to contribute to H2AX phosphorylation levels in vivo (Motoyama and Naka, 2004; Stiff et al., 2004). It is now generally accepted that ATM and ATR induce H2AX phosphorylation in response to DNA DSBs in mutually exclusive pathways that are dependent on the underlying insult. ATM induces H2AX phosphorylation in response to γ-irradiation, whereas ATR induces phosphorylation in response to UV damage (reviewed in Zhou and Elledge, 2000; Nyberg et al., 2002). In either case, initial γ-H2AX increases after exogenous DNA DSB is extremely rapid, occurring within minutes (Rogakou et al., 1999). H2AX phosphorylation continues to increase until maximal phosphorylation is achieved within 30-60 min (Rogakou et al., 1998; MacPhail et al., 2003a). After DNA DSB induction, the amount of γ-H2AX induced is proportional to the number of DSBs incurred. It is estimated that there are ∼2000 H2AX molecules phosphorylated per DSB (Rogakou et al., 1999). In haploinsufficient mice (H2AX-/+) or mice lacking functional H2AX (H2AX-/- or H2AXΔ/Δ), the efficacy of DSB repair is dramatically impeded, the DNA damage-induced G2/M checkpoint fails and numerous morphological abnormalities, including chromosome- and mitotic-defects, are observed (Bassing et al., 2002; Celeste et al., 2002; Fernandez-Capetillo et al., 2002). More recently, H2AX has been shown to function as a dosage-dependent suppressor of oncogenic translocations and tumors in mice (Bassing et al., 2003), whereas in humans H2AX maps to a cytogenetic region frequently altered in human cancers. These observations are consistent with a role for altered or mutated H2AX expression in the onset and progression of at least some human tumors. Collectively, the above-mentioned data demonstrate that H2AX performs a vital role in the identification and efficient repair of damaged genomes, thereby circumventing certain genomic instabilities that could manifest as specific cancers.
In the absence of experimentally applied radiation, the steady-state levels of γ-H2AX is expected to reflect the sum of naturally occurring DNA DSBs, arising from both biological and environmental sources. Because natural exogenous DNA damage sources are largely cell cycle independent, any global differences observed in the abundance of γ-H2AX is anticipated to reflect an underlying requirement for DNA DSB and/or repair at specific cell cycle stages. However, as with most posttranslational histone modifications that have ascribed nuclear functions, it is possible that a subset of γ-H2AX may exist that is independent of DNA DSB repair. Recently, Olive and colleagues (MacPhail et al., 2003a) detailed the relationship between cell cycle and γ-H2AX production in mammalian cells after exposure to DNA DSB-inducing agents. They concluded that γ-H2AX levels increase as cells progress from G1 to S phase and exhibit maximal signal intensities in S phase. However, little is known about the cell cycle dependence of γ-H2AX that occurs naturally or in the absence of artificially induced DNA damage.
In this study, we examine the qualitative and quantitative differences of γ-H2AX foci in untreated mammalian cell lines and cultures and document their cell cycle dynamics. By quantifying data obtained by digital imaging microscopy (DIM), we provide the first description of two readily distinguished γ-H2AX focal populations—a novel and predominant population of small foci that do not colocalize with DNA DSB repair proteins, and a small population of large foci that colocalize with many repair proteins and are reminiscent of IR-induced foci (IRIF). Furthermore, by using DIM, flow cytometry, immunoblotting, and quantitative imaging microscopy (QIM), we identify novel cell cycle associated dynamics for γ-H2AX in untreated mammalian cells. Finally, we demonstrate that the mitosis-associated increase in γ-H2AX is dependent on functional ATM expression by exploiting cell lines and cultures defective or deficient in ATM or DNA-PKCS expression.
MATERIALS AND METHODS
Cell Culture
HeLa (human epithelioid cervical carcinoma cell line), SK-N-SH (human neuroblastoma cell line), COS-7 (African green monkey, SV40 transform kidney cell line), 10T1/2 (C3H mouse embryo cell line), and IM (Indian muntjac skin cells, primary culture) were propagated in DMEM, DMEM, RPMI 1640 medium, Ham's F10 medium, and α-minimal essential medium (MEM), respectively. All media contained 10% fetal bovine serum (FBS), except for IM, which contained 20%. Seven additional human cell cultures were included in this study to examine potential contributions ATM and DNA-PKCS may have in the temporal progression pattern of γ-H2AX in unirradiated cells. AT2BE (Barfknecht and Little, 1982) and AT5BI (Simons, 1979) are primary human cultures derived from ataxia telangiectasia (AT) patients that are ATM defective, whereas GM38 is a normal adult human fibroblast control culture. M059J (lacks DNA-PKCS) and M059K (control) cells were derived from a malignant glioma isolated from a single human patient (Lees-Miller et al., 1995). Finally, the pEBS7 (EBS) and pEBS7-YZ5 (YZ5) cells are SV40-transformed cells from a single AT-patient where EBS are ATM defective, but YZ5 stably express full-length ATM (Ziv et al., 1997), and are therefore isogenic. These seven supplemental cell cultures/lines were propagated in DMEM/F12 (1:1) containing 10% FBS supplemented with 1 mM glutamine. All cells were grown in a 37°C humidified atmosphere containing 5% CO2.
Indirect Immunofluorescence Staining for Digital Imaging Microscopy
Cells were plated onto sterilized glass coverslips and immunofluorescently labeled as described previously (McManus and Hendzel, 2003). Primary antibodies included throughout these studies include a mouse monoclonal antibody, anti-γ-H2AX (1:4000; Upstate Biotechnology, Lake Placid, NY [JBW301]), and nine affinity-purified rabbit polyclonal antibodies; anti-γ-H2AX (1:400; Abcam, Cambridge, United Kingdom [ab2893]), anti-γ-H2AX (1:200; Abcam [ab11174]), anti-Brca1 (1:200; Abcam [ab2956]), anti-53BP1 (1: 1000; Abcam [ab4565]), anti-Mre11 (1:500), anti-Rad51 (1:1000), anti-NBS1 (1:200), anti-Ku80 (1:7500), anti-XRCC4 (1:200; Abcam [ab2857]), anti-DNA ligase IV (1:200; Abcam [ab6145]), anti-ATM (1:200), and anti-phosphorylated serine 10 (PhosS10) of histone H3 (1:500) (Hendzel et al., 1997). The Mre11, NBS1, Rad51, and Ku80 antibodies were gifts from Dr. J. Y. Masson (l'Université Laval, Quebec City, Quebec, Canada), whereas Dr. G. K Chan (University of Alberta, Edmonton, Alberta, Canada) generously provided the ATM antibody (Gately et al., 1998). In addition, activated ATM was investigated with an anti-ATM phosphorylated at serine 1981 antibody (1:500; Abcam [ab2888]). Primary mouse monoclonal antibodies were detected with an anti-mouse AlexaFluor 488 antibody (1:200; Molecular Probes, Eugene OR), whereas primary rabbit polyclonal antibodies were detected with either and anti-rabbit AlexaFluor 488 antibody (1:200; Molecular Probes) or an anti-rabbit cyanin-3 (Cy-3) antibody (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA).
γ-Irradiation
GM38, SK-N-SH, HeLa, and IM cells were exposed to 2 Gy in ambient air using a model CS-600 137CS irradiator (Picker, Glendale, CA). Cells were permitted to recover for 30 min in a 37°C humidified atmosphere containing 5% CO2 before 4% paraformaldehyde fixation and indirect immunofluorescent (IIF) staining as detailed above.
Calf Intestinal Alkaline Phosphatase (CIP) Assay
Asynchronous 10T1/2 cells were seeded onto sterilized glass coverslips as described above. 10T1/2 fibroblasts were purposely selected for this assay because they contain prominent pericentromeric heterochromatic regions that are easily detectable with 4,6-diamidino-2-phenylindole (DAPI) counterstaining. It is within these pericentromeric heterochromatin regions where anti-PhosS10 (positive control) immunoreactivity is greatest within prophase and prometaphase cells (Hendzel et al., 1997; McManus and Hendzel, unpublished data). The next day, cells were washed once with phosphate-buffered saline (PBS) and twice with saponin buffer (138 mM KCl, 4 mM MgCl2, 3 mM EGTA, and 20 mM HEPES, pH 7.5). Coverslips were gently inverted onto Parafilm containing a 25-μl aliquot of 25 μg/ml saponin (BioChemika, Oakville, Ontario, Canada) diluted in saponin buffer. Cells were permeabilized for exactly 1 min, carefully washed three times with saponin buffer and gently inverted onto a 25-μl aliquot of either 12.5 U of CIP diluted buffer (GE Healthcare, Piscataway, NJ), or buffer alone (control). Cells were incubated in a humidified atmosphere at 37°C for 1 h and fixed immediately with 4% paraformaldehyde as described above. IIF was conducted as above with both anti-PhosS10 and anti-γ-H2AX (JBW301). High-resolution (100×) DIM images were collected (see below) and montages were assembled in Photoshop 6.0 (Adobe Systems, Mountain View, CA).
In Situ Indirect immunofluorescence Peptide Competition Assay
Based on the extensive use of IIF techniques throughout this study, an IIF peptide competition assay was developed that could be performed in situ on paraformaldehyde fixed cells. Briefly, anti-γ-H2AX antibodies (JBW301 and ab2893) were diluted in 25 μl of PBS as described above, to which 7.5 μg (or 1.5 μl of a 5 mg/ml stock) of peptide was added. Two H2AX peptides were used—one corresponding to amino acid residues 133-142 of phosphorylated H2AX (CKATQASphosQEY-H2AXp), and the other corresponding to amino acid residues 131-142 of unphosphorylated H2AX (GGKKATQASQEY-H2AXnp)—and were provided by Abcam. Peptide-antibody preparations were mixed gently and incubated at room temperature for 1 h. IIF labeling of cells was performed as detailed above. High-resolution (100×) DIM images were collected and montage images were assembled in Photoshop.
Image Acquisition and Image Deconvolution
DIM was performed using an Axioplan 2 (Carl Zeiss, Jena, Germany) microscope equipped with a 12-bit Coolsnap HQ cooled charge-coupled device camera and a z-stage motor. All field micrographs used for QIM were collected with a 40× oil-immersion fluar lens (numerical aperture [NA] 1.3), whereas high-resolution three-dimensional (3D) images of individual nuclei were collected at 200-nm intervals with a 100× oil-immersion plan apochromat lens (NA 1.4).
High-resolution images were processed by maximum-likelihood-expectation deconvolution in SoftWoRx (Thermo Electron, Waltham, MA) using a constrained iterative algorithm and theoretical optical transfer files generated for 460 nm (DAPI), 505 nm (Alexa Fluor 488), and 565 nm (Cy3) emission wavelengths. Deconvolved images were imported into Imaris (Bitplane AG, Zurich, Switzerland) where γ-H2AX focal volumes were determined by generating 3D reconstructions under Surpass. 3D rotations and movies were generated from deconvolved stack files in MetaMorph (Molecular Devices, Sunnyvale, CA). Figures were assembled in Photoshop.
Flow Cytometry
Asynchronous and subconfluent cells were harvested using 0.53 mM EDTA. Cells were washed three times in PBS, aliquoted as 2 × 106 cells per tube, and fixed and permeabilized in 70% ice-cold ethanol. Fixed cells were maintained at 4°C for up to 1 wk. Cells were immunofluroescently labeled with anti-γ-H2AX (1:2000 [JBW301]) for 30 min, washed three times in PBS, and incubated with anti-mouse Alexa Fluor 488 (1:200) for 30 min. Cells were stained for DNA content with 60 μM propidium iodide (PI; Sigma-Aldrich, St. Louis, MO) containing 25 μM RNase A (Sigma-Aldrich) for 30 min at 37°C. Cells were resuspended in 500 μl of PBS, and signal intensities were examined using a FACSort (BD Biosciences, San Jose, CA) and compared with controls (unstained, PI only, primary with secondary, and mouse IgG1 isotype control; 1:200, Sigma-Aldrich). Graphs were exported as Tif images and assembled in Photoshop.
Cell Synchronization
Cell lines were synchronized or enriched at three specific stages of the cell cycle, namely, prophase/prometaphase (P/PM), G1/S phase boundary, and metaphase (M). Cell populations were enriched at P/PM by 12-h incubation with 15 nM nocodazole (Sigma-Aldrich). G1/S phase-enriched populations were generated through standard double thymidine treatments with 2.5 mM thymidine (Sigma). To generate mitosis-enriched populations, double thymidine-treated cells were washed three times with prewarmed PBS. Fresh medium was added, and cells were permitted to progress for 5 h in the absence of thymidine before the addition of nocodazole (15 nM) or N-acetyl-L-leucyl-L-leucyl-L-norleucinal (ALLN) (40 μg/ml; Calbiochem, San Diego, CA) for 4 h, which produced populations enriched at either P/PM or M respectively, as confirmed visually by DIM. In our assay systems, no qualitative differences were observed between cells treated with nocodazole for 12 h or double thymidine treated cells released and incubated with nocodazole or ALLN for 4 h.
Immunoblotting
Approximately 1 × 106 mitotically-enriched (see above) or asynchronously growing cells were lysed in nuclei buffer (250 mM sucrose, 200 mM NaCl, 10 mM Tris-HCl, pH 8.0, 2 mM MgCl2, 1 mM CaCl2, and 1% Triton X-100) containing 1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich). Nuclei were isolated and proteins were acid extracted on ice with 0.4 N sulfuric acid for 20 min. Equivalent protein amounts from 2.0 × 105 cells were resolved along with 5 μl of prestained standards (Kaleidoscope; Bio-Rad, Hercules, CA) on 15% SDS polyacrylamide denaturing gels. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Immun-Blot; Bio-Rad) in transfer buffer (48 mM Tris-base, 39 mM glycine, 0.04% SDS, and 20% methanol) at 100 V for 1 h. Equivalent loading of protein from asynchronous and nocodazole arrested cells were confirmed by either copper phthalocyanine 3,4′,4″,4‴-tetrasulfonic acid tetrasodium salt (CPTS) staining of the PVDF membranes as described by Bickar and Reid (1992) or by Coomassie Blue staining of parallel gels. PVDF membranes were washed twice in PBS to remove CPTS dye before blocking the PVDF membranes for 1 h at room temperature in 5% skim milk in Tris-buffered saline (TBS; pH 7.4) containing 0.1% Tween-20 (TBST). Membranes were immunostained with anti-γH2AX (1:2000 [JBW301]) diluted in 5% nonfat milk containing 0.1% Tween 20 overnight at 4°C. After three 10-min washes with TBST, membranes were incubated for 1 h with an appropriate secondary antibody conjugated with horse-radish peroxidase (anti-mouse at 1:10,000; Jackson ImmunoResearch Laboratories) diluted in 5% nonfat milk in TBST. Subsequently, membranes were washed three times (10 min) with TBST before incubation with Western blotting luminescence detection reagent (GE Healthcare) for 5 min.
Quantitative Imaging Microscopy and Statistical Analyses
Asynchronous cells were fixed and immunofluorescently labeled with anti-γ-H2AX (JBW301) as detailed above with 40× field images acquired by DIM. QIM was performed in MetaMorph (Molecular Devices) as detailed previously (McManus and Hendzel, 2003, 2005). For each individual cell line examined, the exposure times for each individual channel were first optimized and then maintained constant throughout the entire acquisition phase. The nuclear signal from each individual cell from each image was then quantified in MetaMorph and intensity values and volumes were exported into Excel (Microsoft, Redmond, WA). In addition, each cell was manually classified as either interphase or mitotic (prophase through to telophase) based on chromosome morphologies of the corresponding DAPI channel. Finally, to account for histone variability that occurs as cells progress through the cell cycle, normalized γ-H2AX signal intensities (Norm-γ-H2AX) were generated. Calculating the ratio between the total γ-H2AX signal intensity and the total DAPI signal intensity (e.g., total γ-H2AX signal/total DAPI signal) generated the norm γ-H2AX signal intensities for each individual cell. Finally, to statistically evaluate the differences between the mean Norm-γ-H2AX signal intensities from interphase and mitotic populations for each cell line, student t tests were performed in Prism (GraphPad Software, San Diego, CA).
RESULTS
γ-H2AX Foci in Unirradiated Nuclei Exhibit Distinctive Localization Patterns
To investigate the spatial patterning of γ-H2AX, a panel of mammalian cell lines and cultures were used that were subjected to either IR (see Materials and Methods) or grown under normal growth conditions (untreated) and immunofluorescently labeled with a commonly used anti-γ-H2AX antibody (Upstate Biotechnology [JBW301]). As anticipated, γ-H2AX foci were observed in all IR-treated cells, but surprisingly they were also identified in all untreated cells and at all stages of the cell cycle. The existence of γ-H2AX foci in all unirradiated cells was further confirmed with a second anti-γ-H2AX antibody (polyclonal) obtained from a different source (see below). However, qualitative differences in the overall γ-H2AX signal intensities were striking: the IR-treated cells exhibited dramatically elevated signal intensities compared with those of the untreated cells. In general, the overall γ-H2AX signal intensities of IR-treated nuclei were at least fivefold greater than the signal intensities from corresponding untreated nuclei (our unpublished data).
On closer inspection of both the IR-treated and untreated cells, two distinct γ-H2AX focal populations were evident—a large population of small foci and a small population of large amorphous foci (Figure 1 and Supplemental Movies 1-4). The larger foci are morphologically similar to foci generated in the IR-treated cells and have been well studied as H2A.X phosphorylation that is associated with DSBs. The smaller foci, however, have not been studied before. These foci are present in both IR-treated and untreated cells. We verified that the small foci, which were typically of lower intensity staining than the large foci, did not reflect cross-reactivity with unphosphorylated histone H2A.X or another nuclear protein. Data presented as Supplemental Figures 1, 2, 3 demonstrate that the detection of these foci is not dependent on the γ-H2AX antibody used and has an absolute requirement for phosphorylation. Hence, all foci detected were regions of chromatin enriched in γ-H2AX.
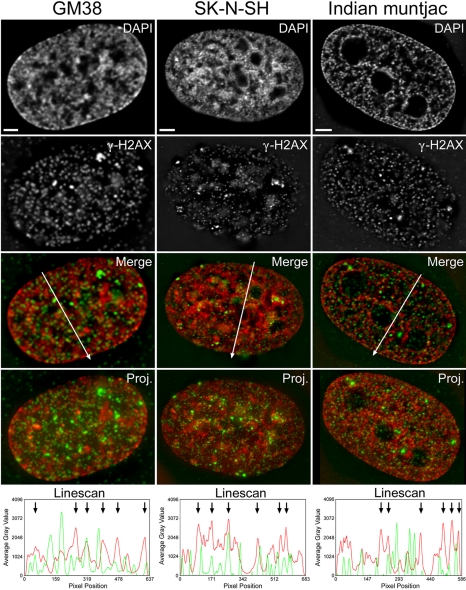
Figure 1.
γ-H2AX foci partition in both heterochromatin and euchromatin. Depicted here are representative deconvolved, high-resolution (100×) DIM images of unirradiated GM38, SK-N-SH, and IM cells immunofluorescently labeled with anti-γ-H2AX (green) and counterstained with DAPI (red) to reveal DNA. The merged image is a single plane from the 3D deconvolved series and is the plane used for the linescan analyses shown in the bottom panels. The entire 3D image is presented as a deconvolved projection in the fourth row (Proj.). Linescan analyses were performed with the arrows in the Merge panel indicating the regions and directions of the scans. Bars, 3 μm. A graph depicting the average gray level (y-axis) versus pixel position (x-axis) is shown in the fifth row with the DAPI channel information shown in red and the γ-H2AX channel information shown in green. Peak DAPI intensities identify heterochromatic regions and are identified by black arrows in the linescans, whereas euchromatic regions are identified by less intense DAPI staining.
Figure 2.
Spatial localization of the small γ-H2AX foci with DNA repair proteins. Asynchronous and untreated SK-N-SH cells were immunofluorescently labeled with anti-γ-H2AX and individual components of either homologous recombination repair (e.g., Mre11, Rad51, NBS1, and Brca1; A) or nonhomologous end-joining (e.g., Ku80, 53BP1, XRCC4, and DNA ligase IV; B). The DNA was counterstained with DAPI. Depicted here are single planes from representative high-resolution (100×) 3D series that were subjected to software-driven deconvolution. Note the lack of colocalization (absence of yellow signal) between the small γ-H2AX foci (red) and all repair proteins (green) investigated. Bars, 2 μm.
Figure 3.
G2/M-specific increases in γ-H2AX signal intensities in unirradiated mammalian cells. The temporal progression pattern of γ-H2AX in nine different unirradiated mammalian cell lines or cultures was investigated (A-I); AT2BE are ATM-defective, whereas M059J are DNA-PKCS deficient. Each panel contains a representative flow cytometric analysis dot plot depicting γ-H2AX (FL1-H, y-axis) signal intensity versus PI (FL2-A, x-axis) signal intensity of asynchronous populations. The G0/G1, S phase and G2/M populations are indicated for HeLa cells (panel A) and can be extrapolated to all other graphs. The G2/M populations exhibiting increased γ-H2AX signal intensities have been identified by a black circle. Each panel also contains a representative DIM image (40×) containing interphase and at least one mitotic cell (white arrow) to demonstrate the dramatic differences γ-H2AX signal intensities in those cells. In the left image, the DNA is counterstained with DAPI, whereas corresponding γ-H2AX-immunofluorescently labeled image is shown on the right. Note that the images were collected to best represent the signal intensities of the mitotic cells and were not acquired to best represent the small γ-H2AX foci in the interphase cells. Note the dramatic difference in signal intensities in all cell lines except the AT2BE cells. Bars, 2 μm.
We quantified the total number of foci from 3D image series collected of interphase (G1) and mitotic (prophase) primary fibroblasts isolated from IM that were either IR-treated or untreated (Table 1). Comparisons of the mean number of γ-H2AX foci per nucleus at G1 and prophase indicate that only slight increases occurred within either the untreated and IR-treated populations (Table 1). For example, untreated IM cells at G1 and prophase average 348.8 ± 67.3 and 387.9 ± 87.4 foci/nucleus, respectively. After IR-treatment however, the average number of foci per nucleus decreased by approximately half within G1 or prophase (Table 1). In IM cells in G1, the number mean of γ-H2AX foci per nucleus decreased from 348.8 ± 67.3 to 179.6 ± 68.9 after IR-treatment. Similar quantitative results were observed in human SK-N-SH neuroblastoma cells (our unpublished data). These results indicate that a large change or redistribution in γ-H2AX accompanies IR-induced DNA damage.
Table 1.
Volumetric distribution of γ-H2AX foci in unirradiated and irradiated IM fibroblasts
| Mean volume ± SD (×10−2 μm3)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Stagea | IRb | n | Foci/cell ± SD | mVol ± SDc (×10−2 μm3) | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
| G1 | − | 15 | 348.8 ± 67.3 | 16.20 ± 68.45 | 1.599 ± 0.3990 | 3.415 ± 0.6812 | 6.919 ± 1.535 | 52.90 ± 130.2 |
| M | − | 15 | 387.9 ± 87.4 | 17.98 ± 189.8 | 1.382 ± 0.2758 | 2.656 ± 0.4850 | 5.198 ± 1.146 | 62.74 ± 376.2 |
| G1 | + | 15 | 179.6 ± 68.9 | 56.51 ± 253.4 | 1.517 ± 0.3649 | 3.168 ± 0.6328 | 7.391 ± 2.545 | 212.7 ± 473.8 |
| M | + | 15 | 183.9 ± 58.2 | 75.73 ± 359.4 | 1.343 ± 0.2647 | 2.618 ± 0.5442 | 7.185 ± 3.024 | 292.2 ± 674.5 |
IM cells were purposely selected because they are ideally suited for quantitative cytological investigations due to their relatively large and flat nuclei and because they exhibit very pronounced changes in chromatin structure that accompany specific cell cycle stages.
Cell cycle stage (G1/S, G1/S boundary; M, prophase).
IR, γ-irradiation (− is unirradiated, and + is irradiated with 2 Gy and allowed to recover for 30 min).
mVol ± SD is mean focal volume ± SD.
To further characterize the γ-H2AX foci in unirradiated cells, we determined the mean volumes of γ-H2AX foci in both untreated and IR-treated cells (Table 1). IM cells were again selected for this analysis for the reasons indicated above. The mean γ-H2AX focal volumes in unirradiated G1 (0.16 ± 0.68 μm3) and prophase (0.18 ± 1.9 μm3) cells were similar with only a slight increase associated with the prophase cell (1.11-fold). In IR-treated cells, slightly larger increases in mean volumes were calculated compared with the untreated cells (Table 1). The mean γ-H2AX focal volumes G1 (0.57 ± 2.5 μm3) and prophase (0.76 ± 3.6 μm3) cells identified a 1.34-fold increase in mean γ-H2AX focal volumes. Finally, comparisons between untreated and IR-treated cells at the same cell cycle stage identified a 3.5- and 4.2-fold increase in mean γ-H2AX focal volumes in the IR-treated cells. These differences differ significantly with previously published focal diameters (0.04-0.16 μm) determined for the large γ-H2AX foci (IRIF) that were determined from two-dimensional projections of 3D data after IR-treatment (MacPhail et al., 2003a,b). Nevertheless, our results are completely consistent with previous studies that demonstrate an overall increase in the number of large γ-H2AX foci that accompanies IR-treatment. Furthermore, when comparing cells at the same cell cycle stage by subdividing the volumetric data into quartiles, it is evident that the majority of the variation between untreated and IR-treated cells is confined to the fourth quartile. In contrast, comparisons within the first three quartiles fail to reveal significant differences between the respective G1/S and prophase pairings. These data suggest that the differences could be accounted for by either increases in the number or size of the largest γ-H2AX foci.
Finally, we investigated the average number of foci per nucleus and noted that it was markedly different between unirradiated and irradiated cells irrespective of cell cycle stage (e.g., G1 or prophase) (Table 1 and Supplemental Movies 1-4). Quantification determined that there is an approximately twofold decrease in the total number of foci in the IR-treated population.
Small and Large γ-H2AX Foci Partition in Both Heterochromatin and Euchromatin
It has previously been shown that changes in γ-H2AX signal intensities accompany changes in chromatin structure (Schwartz et al., 1993; Park et al., 2003; Reitsema et al., 2004). Therefore, we reasoned that γ-H2AX foci in untreated cells might preferentially partition within specific types of chromatin in a manner that reflects the different levels of higher order chromatin compaction. Accordingly, we investigated the partitioning of the small and large γ-H2AX foci within euchromatin (actively transcribing chromatin) and heterocharomatin (transcriptionally silent chromatin) based on DAPI staining—euchromatin stains “poorly” with DAPI, whereas heterochromatin stains intensely. Surprisingly, in untreated cells both the small and large focal populations partition within euchromatin and heterochromatin with no overwhelming preference for either (Figure 1; our unpublished data). However, the most intensely staining γ-H2AX foci (i.e., the large γ-H2AX foci) were frequently observed juxtaposed to, rather than superimposed over, highly compacted heterochromatin as identified by the sharp peaks in the DAPI channel (Figure 1; our unpublished data). The more abundant small γ-H2AX foci seemed to partition equally within euchromatin and heterochromatin (Figure 1; our unpublished data). These observations suggest that the alterations in the abundance of γ-H2AX that correlate with premature chromatin condensation or relaxation of the chromatin structure accompanying hypotonic treatments, must differ significantly from the changes in chromatin folding associated specifically with the assembly and maintenance of euchromatin or heterochromatin.
Small γ-H2AX Foci Are Distinguishable from Large γ-H2AX DNA Repair Foci
Currently, there is strong evidence demonstrating spatial relationships (i.e., colocalization) between the large repair γ-H2AX foci (e.g., IRIF) and numerous repair proteins, including Mre11, NBS1 (MRN complex), Brca1, Rad51, Ku80, 53BP1, XRCC4, and DNA ligase IV (Paull et al., 2000; Schultz et al., 2000; Rappold et al., 2001; Fernandez-Capetillo et al., 2003). Based on these observations, if all phosphorylated regions of H2A.X are involved in DSB repair, the small γ-H2AX foci should colocalize with DNA DSB repair proteins involved in either homologous recombination repair (e.g., Mre11, NBS1, Rad51, or Brca1) or nonhomologous end-joining (e.g., Ku80, 53BP1, XRCC4, or DNA ligase IV), even in the absence of externally applied sources of DNA damage. To determine whether proteins involved in DSB repair were recruited to these smaller foci, 3D image series were acquired from unirradiated interphase human cells (e.g., SK-N-SH, HeLa and GM38) immunofluorescently labeled with anti-γ-H2AX and an antibody recognizing each of the repair proteins listed above. Figure 2 presents a representative image of each repair protein immunofluorescently labeled in SK-N-SH cells in combination with anti-γ-H2AX—similar results were observed in HeLa and GM38 (our unpublished data). Due to species specificities of the repair protein antibodies, we could not investigate these spatial relationships in 10T1/2, COS-7, or IM cells. Nevertheless, despite the observation that in human cells the large γ-H2AX foci exhibit extensive colocalization with these repair proteins after IR, very little, if any colocalization is observed between the small γ-H2AX foci and the repair protein foci in unirradiated cells (Figure 2). Cells immunofluorescently labeled with anti-Rad51 or anti-53BP1, however, did exhibit rare instances of colocalization (Figure 2), but this was restricted to less than five foci per nucleus (our unpublished data). Colocalization was frequently observed in the large γ-H2AX foci (Supplemental Figure 8). This is consistent with the large γ-H2AX foci arising from endogenous or naturally occurring DNA DSBs. The small γ-H2AX foci, in contrast, represent a novel and unique population of γ-H2AX that can be distinguished from the large DNA DSB foci (e.g., IRIF) based on the absence of recruitment of DNA DSB repair proteins.
The ATM, ATR, and DNA-PK kinases, which are known to have H2A.X kinase activity, are differentially sensitive to caffeine. Therefore, we treated cells with different concentrations of caffeine to determine whether a specific kinase could be assigned to the generation of the small foci that are unrelated to DSBs. The individual cell lines showed the same trends in their response to caffeine. After exposure to 3 mM caffeine for 4 h, a marked decrease was evident throughout the culture. Quantification of fluorescence intensity differences within the cultures revealed a 60.6% reduction in γ-H2AX staining. Nonetheless, although attenuated, both the mitotic chromosomes and interphase nuclei showed the presence of a smaller number of large foci amid a large number of small foci. After exposure to 10 mM caffeine for 4 h, mitotic cells were not detectably stained (our unpublished data). Although the interphase signals were further attenuated and fewer cells generated staining above the background, some interphase cells clearly retained both small and larger foci. The response of GM38 cells to caffeine treatment is illustrated in Supplemental Figure 5. Overall, the fluorescence intensity of these cells was reduced 81.6% relative to untreated cells.
Dynamic Changes in the Abundance of γ-H2AX Accompany Cell Cycle Progression in Unirradiated Mammalian Cells
Previous studies by Olive and colleagues (MacPhail et al., 2003a,b) demonstrated that γ-H2AX foci in irradiated cells exhibit cell cycle-dependent increases. Therefore, we reasoned that in untreated/nonirradiated mammalian cells, γ-H2AX foci might exhibit temporal kinetics reflecting the underlying functional requirement for increases in γ-H2AX at specific cell cycle stages. To further explore this possibility and to define any temporal progression of γ-H2AX, flow cytometry and DIM were performed. By coupling γ-H2AX immunofluorescent labeling with either PI (flow cytometry) or DAPI (DIM) staining of the DNA, we were able to easily discern distinct cell cycle stages.
In five unirradiated cell lines/cultures (HeLa, SK-N-SH, Cos-7, 10T1/2, and IM) examined by flow cytometry, a general increase in γ-H2AX signal intensity was observed as cells progressed through the cell cycle (see below; Figure 3, A-E). There was an initial increase in γ-H2AX signal intensities as cells entered early S phase that continued to increase as cells progressed into mid- and late-S phase. Surprisingly however, as cells entered G2/M, γ-H2AX signal intensities continued to increase. In G2/M, two populations became evident based on γ-H2AX signal intensities—a small subpopulation of cells that exhibited increased γ-H2AX signal intensities over the predominant subpopulation that exhibited lower γ-H2AX signal intensities (Figure 3, A-E). On cytokinesis or entry into G1, γ-H2AX signal intensities rapidly returned to basal steady-state levels. These temporal dynamics demonstrate that in untreated mammalian cells the abundance of γ-H2AX increases in a cell cycle-dependent manner and that maximal levels occur in G2/M.
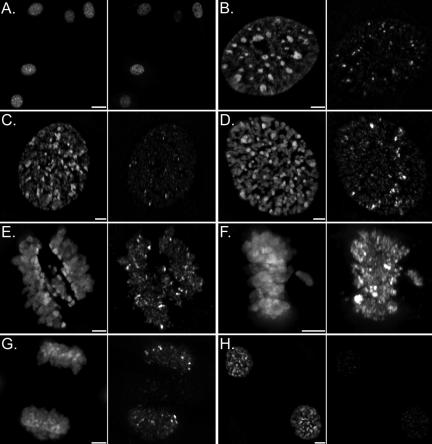
To more precisely define the temporal dynamics of γ-H2AX specifically during mitosis, high-resolution DIM was performed on unirradiated HeLa, SK-N-SH, Cos-7, 10T1/2, and IM immunofluorescently labeled with anti-γ-H2AX. Visually appreciable differences in γ-H2AX signal intensities were observed between interphase and mitotic populations in all five cell lines examined (Figure 3, A-E). On closer inspection, a general temporal progression pattern was observed that paralleled the results characterized by flow cytometry. As with flow cytometry, some heterogeneity within each cell cycle stage was observed by DIM (Figure 4A). However, the general trend was that γ-H2AX signal intensities increased as cells progressed from G1 into and S phase G2 and reached maximal intensities during mitosis. The most dramatic γ-H2AX dynamics occurred between prophase and telophase/cytokinesis (Figure 4, B-H). As cells progressed through the initial stages of mitosis (prophase to prometaphase) γ-H2AX signal intensities increased until maximal levels were reached at metaphase (Figure 4F). Increased signal intensities occurred along the entire length of chromosome arms and were not excluded from highly compacted regions such as pericentromeric heterochromatin (Figure 4F), which is easily identifiable in mouse 10T1/2 cells throughout the cell cycle as DAPI-intense staining regions (Figure 4). The increased γ-H2AX signal intensities that accompanied entry into mitosis were accounted for by slight increases in focal volumes (e.g., 0.16 versus 0.18 μm3) and numbers (e.g., 349 versus 388) (Table 1). Once cells transitioned into anaphase, γ-H2AX signal intensities began to decrease until basal steady-state levels were observed immediately after cytokinesis. These DIM data confirm that in untreated mammalian cells γ-H2AX does exhibit temporal dynamics that are particularly pronounced during the brief period of mitosis, which takes ∼1 h to complete. Furthermore, these data demonstrate that maximal γ-H2AX signal intensities occur at or near metaphase. Immunoblotting confirmed that this was not due to a change in epitope accessibility (Supplemental Figure 6).
Figure 4.
Typical γ-H2AX signal intensity variability observed throughout the cell cycle. Asynchronous and untreated mouse 10T1/2 fibroblasts were fixed and immunofluorescently labeled with anti-γ-H2AX (right) and counterstained with DAPI (left). 10T1/2 cells were specifically chosen to investigate the spatial localization of γ-H2AX foci relative to the DAPI-rich staining regions such as pericentromeric heterochromatin. Panel A is a 40× field micrograph depicting the γ-H2AX signal intensity variability routinely observed in interphase nuclei that is independent of G1, S phase, or G2 (our unpublished data). B-H are 3D projections of deconvolved high-resolution (100×) images at specific cell cycle stages: early G2 (B), late G2 (C), prophase (D), prometaphase (E), metaphase (F), telophase (G), and early G1 (H). The exposure time of only the γ-H2AX channel in panels B-H were maintained constant and depicts the typical γ-H2AX signal intensity dynamics specifically during mitosis. Bars, 20 μm (A) and 3 μm (B-H).
Functional ATM Expression Correlates with Mitosis-specific H2AX Phosphorylation
Previous biochemical studies have demonstrated that PIKK family members regulate γ-H2AX kinetics in response to DNA DSBs (Burma et al., 2001; Ward and Chen, 2001; Park et al., 2003; Stiff et al., 2004). Specifically, independent roles for ATM and DNA-PKCS kinase activities in regulating H2AX phosphorylation have been established (Burma et al., 2001; Park et al., 2003; Stiff et al., 2004). Therefore, it stands to reason that either, or both, of these kinase activities may underlie the mitosis-associated increases in γ-H2AX detailed above. To investigate these possibilities we examined γ-H2AX dynamics in cell lines or cultures deficient or defective for either ATM or DNA-PKCS. Using ATM-defective AT2BE (Barfknecht and Little, 1982) and DNA-PKCS-deficient M059J (Lees-Miller et al., 1995), human primary cell cultures, and their respective controls, GM38 and M059K, we performed identical experiments to those detailed above, including DIM, flow cytometry, and immunoblot analyses.
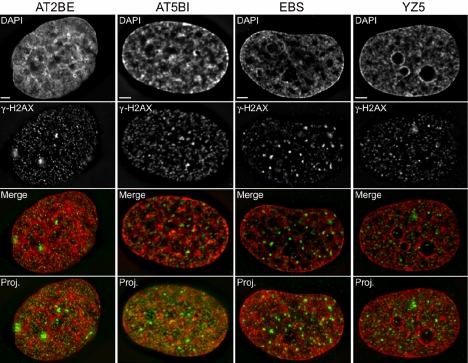
DIM investigations performed in AT2BE, GM38, M059J, and M059K revealed similar γ-H2AX localization patterns to those detailed above. However, it was noted that the AT2BE cells exhibited lower immunoreactivity toward γ-H2AX. This is not unexpected as ATM has prominent role in regulating H2AX phosphorylation. Nevertheless, both small and large focal populations were clearly evident in AT2BE cells (Figure 5). This is most likely due to the presence and activities of other upstream kinases (Burma et al., 2001). GM38, M059J, and M059K cells all exhibited similar temporal dynamics as those described above: γ-H2AX signal intensities were minimal in interphase and maximal in mitosis (Figure 3, F-H). In contrast however, AT2BE cells did not exhibit the mitosis-specific increase in γ-H2AX (Figure 3I).
Figure 5.
Localization of γ-H2AX in AT-deficient and AT-corrected cells. Depicted here are single representative deconvolved, high-resolution (100×) DIM images of unirradiated AT-defective (AT2BE, AT5BI, and EBS) and AT-corrected (YZ5) nuclei immunofluorescently labeled with anti-γ-H2AX (green) and counterstained with DAPI (red) to reveal DNA. The signal intensity of each channel was independently optimized and cannot be directly compared between cell lines/cultures. The merged image is a single plane from the 3D deconvolved series, whereas the entire 3D image is presented as a deconvolved projection in the fourth row (Proj.). Bars, 2 μm.
To further investigate the roles ATM and DNA-PKCS might have in the mitosis-specific H2AX phosphorylation, flow cytometry was performed as described above (Figure 3, F-I). In M059J, M059K and GM38 cells, γ-H2AX fluorescent signal intensities increased as cells progressed from G0/G1 to S phase and continued to increase as cells entered G2/M. The two G2/M subpopulations observed above in HeLa, SK-N-SH, COS-7, 10T1/2, and IM were also apparent, but less obvious. In addition, DNA content analysis demonstrated that the M059J cell line exhibits two distinct cell populations that differ with respect to their ploidy levels and was confirmed using ModFit LT software (Verity Software House, Topsham, ME) (our unpublished data). AT2BE cells, however, exhibited unique temporal dynamics. As with all other cell lines or cultures, γ-H2AX signal intensities initially increased from G1 to mid-S phase. However, as AT2BE cells entered mid-S phase, γ-H2AX levels reached a plateau and subsequently decreased during late S phase. As cells continued into G2/M, γ-H2AX signal intensities decreased yielding only a single G2/M population. These results suggest that the mitosis-specific increase in γ-H2AX that occurs in normal unirradiated mammalian cells may require functional ATM expression and be independent of DNA-PKCS expression. Fluorescence microscopy verified that activated ATM, detected with an antibody specific for an activated form of ATM, was present in mitotic cells (Supplemental Figure 4).
Quantitative Imaging Microscopy Reveals that Functional ATM Is Correlated with the Mitosis-specific Increase in H2AX Phosphorylation
To specifically investigate the role(s) ATM and DNA-PKCS have in γ-H2AX dynamics, quantitative analysis of individual cells in images collected by fluorescence microscopy (QIM) was performed on five different asynchronous cell lines or cultures (HeLa, M059K, M059J, GM38, and AT2BE). Because of the variability in γ-H2AX TSI between cell lines, it was necessary to independently optimize the γ-H2AX exposure times for each cell line or culture. Accordingly, qualitative comparisons cannot be made between cell types when visually examining the figures. In four cell lines, HeLa, M059K, M059J, and GM38, visually apparent differences between interphase and mitotic mean norm-γ-H2AX fluorescent TSI were observed (Figure 6, A-D). Student's t tests (Table 2) comparing the mean normalized γ-H2AX TSI (Norm γ-H2AX) of the interphase and mitotic populations confirmed that these differences were all statistically significant (p < 0.0001). Moreover, the mean Norm γ-H2AX TSI of the mitotic populations were between 3.3-fold (HeLa) and 5.5-fold (M059K) greater than those of the corresponding interphase populations, whereas the M059J and GM38 were 3.5- and 4.5-fold, respectively. In contrast, quantitative comparisons made between the mean norm-γ-H2AX fluorescent intensities from AT2BE cells failed to reveal the large differences in abundance (Figure 6E). A Student's t test determined that the difference in mean Norm γ-H2AX TSI was not statistically significant (p value = 0.848; Table 2). The difference between mean Norm-γ-H2AX TSI from interphase and mitotic populations was only 1.1-fold (Figure 6E). These data corroborate those of the previous sections and demonstrate that functional ATM expression correlates with mitosis-specific H2AX phosphorylation.
Figure 6.
QIM reveals a mitotic-specific phosphorylation event that correlates with functional ATM expression. Eight unirradiated mammalian cell lines or cultures were independently investigated by QIM; HeLa (A), M059K (B), M059J (C [DNA-PKCS deficient]), GM38 (D), AT2BE (E [ATM defective]), AT5BI (F [ATM defective]), EBS (G [ATM defective]), and YZ5 (H [ATM corrected]). Depicted here are the mean Norm-γ-H2AX total signal intensities (TSI) of interphase (I) and mitotic (M) populations (prophase through telophase). Student's t tests identified highly statistically significant differences (p < 0.0001) in mean Norm-γ-H2AX TSI between I and M populations for HeLa, M059K, M059J, GM38, and YZ5 (Table 2). The remaining cell lines/cultures do not exhibit statistically significant p-values (p value > 0.05; Table 2). The fold increase in mean Norm-γ-H2AX TSI intensity between the I and M populations was calculated for each cell line or culture and is indicated on the M column.
Table 2.
Quantitative analysis of normalized γ-H2AX signal intensity in mitotic and interphase cells
| Interphase population
|
Mitotic population
|
||||
|---|---|---|---|---|---|
| Cell linea | Mean ± SEMb | nc | Mean ± SEM | n | p Valued |
| HeLa | 0.120 ± 0.006 | 226 | 0.397 ± 0.040 | 36 | <0.0001 |
| M059K | 0.130 ± 0.006 | 483 | 0.721 ± 0.100 | 19 | <0.0001 |
| M059J | 0.185 ± 0.006 | 400 | 0.644 ± 0.053 | 27 | <0.0001 |
| GM38 | 0.085 ± 0.005 | 182 | 0.385 ± 0.069 | 20 | <0.0001 |
| AT2BE | 0.308 ± 0.050 | 141 | 0.340 ± 0.066 | 13 | 0.848 |
| AT5BI | 0.648 ± 0.012 | 305 | 0.698 ± 0.054 | 26 | 0.257 |
| EBS | 0.254 ± 0.050 | 392 | 0.278 ± 0.048 | 27 | 0.292 |
| YZ5 | 0.205 ± 0.013 | 457 | 0.654 ± 0.123 | 31 | <0.0001 |
M059J are DNA-PKcs deficient, AT2BE,AT5BI and EBS are ATM defective, and YZ5 are ATM corrected.
Mean Norm-γ-H2AX signal intensity ± SEM.
n, number.
A p value <0.05 is considered to be statistically significant.
The Role of ATM in Mitosis-associated Increases in γ-H2AX
To further explore the role ATM may have in regulating γ-H2AX dynamics during mitosis we performed similar experiments to those outlined above using a second ATM-defective cell culture (AT5BI) derived from an AT patient (Simons, 1979). We also made use of the SV40 transformed EBS and YZ5 cell lines. These isogenic cell lines were originally derived from a single AT patient and only differ through the introduction of ATM cDNA into the YZ5 cell line so that only it stably expresses functional and full-length ATM (Ziv et al., 1997). Like AT2BE cells, DIM demonstrated that AT5BI (Supplemental Figure 7) and EBS cell lines exhibited lower immunoreactivity toward the anti-γ-H2AX antibody but still presented both small and large γ-H2AX foci (Figure 5). Relative to the matched cell line YZ5, the relative intensity of γ-H2AX was 28% lower. When QIM was performed on these cell lines (Figure 6, F-H), Student's t tests failed to identify a statistically significant difference between the mean Norm-γ-H2AX TSI for the AT2BI and EBS cell lines but did show a highly significant change in the YZ5 cell line (Supplemental Table 2). As with the AT2BE cells, the mitotic population of the EBS cells was only 1.1-fold greater than that of the interphase population (Figure 6F). Together, these data along with the data of the previous sections strongly support a functional role for ATM in regulating the mitosis-associated increase in γ-H2AX.
Although these results are consistent with a requirement for ATM in the generation of a robust mitotic phosphorylation of H2A.X, we noted that the absence of ATM did not eliminate H2A.X phosphorylation during mitosis. To further test whether other kinases may participate in the mitosis-specific phosphorylation of H2A.X, we examined the EBS and YZ5 cell lines for the effect of caffeine treatment on the presence or absence of phosphorylation during mitosis. Figure 7 shows images collected under identical conditions to allow comparisons between the cell lines and between the different drug treatments. In the absence of caffeine, the mean fluorescence intensity of the EBS cell line was 72% of that measured in the YZ5 cell line. Both cell lines showed a reduction in staining upon treatment with either 3 or 10 mM caffeine treatment. The EBS cell line was reduced to 47 and 34%, respectively, of the signal intensity of the untreated YZ5 cell line, whereas the staining of the YZ5 cell line was reduced to 59 and 58% at 3 and 10 mM caffeine, respectively. Figure 7 illustrates that in the absence of caffeine, mitotic YZ5 cells are stained robustly throughout the chromosomes. In contrast, the EBS line has a small number of prominent foci associated with the chromosomes but the overall staining of the chromosomes is comparatively weak. At 3 mM caffeine, very little change is seen in the staining of mitotic chromosomes in the EBS cell line. In contrast, the staining of the mitotic chromosomes in the YZ5 cell line is noticeably attenuated but not eliminated. However, at 10 mM caffeine, all staining of the mitotic chromosomes are lost in both cell lines. This occurred, despite the maintenance of a small amount of interphase phosphorylation.
Figure 7.
Effect of caffeine on the phosphorylation of H2A.X. Top, staining of EBS (ATM-/- cells) after 4-h incubation with the indicated concentration of caffeine. Bottom, corresponding image sets from the ATM restored YZ5 cell line. The arrows indicate cells that are in mitosis. The insets on the bottom of each panel show corresponding DAPI images for each field of cells shown. The inset at the top right corner of the 3 mM caffeine-treated EBS panel is a 1.5× magnification view of a contrast-enhanced image of the prometaphase cell highlighted with the arrow. The purpose is to illustrate the presence of staining along the chromosome arms of the mitotic chromosomes.
DISCUSSION
Histone H2A.X phosphorylation has a well known association with DNA DSBs. It was therefore expected that histone H2A.X contributes to genomic stability. Celeste et al. (2002) demonstrated that H2AX is not essential for viability but that phenotypes reminiscent of proliferation and mitotic defects, such as low mitotic indices and elevated chromosomal aberrations, were readily apparent in cells lacking H2AX. Similarly increased genomic instability occurs in haploinsufficient H2AX mice (H2AX-/+) and mice lacking H2AX (H2AX-/- or H2AXΔ/Δ) (Bassing et al., 2002; Celeste et al., 2002, 2003). Whereas all of these defects can be explained by defects in DSB repair, in demonstrating a cycle of H2A.X phosphorylation that exists independent of DSBs, our study introduces the possibility that other functions for histone H2A.X phosphorylation exist and may impact upon genomic stability independent of DNA damage.
In this study, we demonstrate that, in the absence of exogenous or artificial sources of DNA damage, hundreds of sites γ-H2AX phosphorylation exists throughout the genome of normally growing mammalian cell lines and primary cultures. A small proportion of these sties are large amorphous foci, morphologically similar to the foci induced by ionizing irradiation induced DSBs, and these sites often were observed to recruit DSB repair proteins. These sites likely represent “naturally occurring” DNA DSBs. The majority of the sites enriched in γ-H2AX were not associated with the recruitment of DSB repair proteins. These abundant but smaller domains partitioned equally between euchromatin and heterochromatin. Based upon the ability of caffeine, at concentrations of 3 mM, where ATM is completely and ATR is partially inhibited, and at 10 mM, where ATM and ATR but not DNA-PK, are completely inhibited, both ATM and ATR contribute to, but are not exclusively responsible for the accumulation of phosphorylated H2A.X in these numerous smaller foci. The small amount of H2A.X kinase activity that is resistant to caffeine could be contributed by DNA PK. Alternatively, there may be novel H2A.X kinases that contribute to steady-state H2A.X phosphorylation during interphase.
We found that the steady-state phosphorylation of histone H2A.X was under cell cycle regulation. We demonstrate that cell cycle-dependent changes in the abundance of γ-H2AX occur with the minimum abundance of sites being associated with G1. As cells progress through S phase, there is a normal, but gradual increase in γ-H2AX. The initial increase in γ-H2AX correlates well with previously described DNA DSBs that occur during DNA replication as a result of stalled and collapsed replication forks and the activities of topoisomerases I and II (Limoli et al., 2002; Furuta et al., 2003; Huang et al., 2003, 2004). Unexpectedly, however, we found that γ-H2AX signal intensities do not plateau in S phase but rather continue to increase until maximal levels are attained in G2/M. A major conclusion from this temporal progression pattern is that the upstream kinases are regulated with respect to their total nuclear activity but remain active throughout the cell cycle.
Based upon the specific increase of steady-state H2A.X phosphorylation during entry into mitosis, it can be concluded that there is a kinase activation event or events occurring as cells enter into mitosis. In several ATM null cell lines, we failed to observe this mitosis-specific increase in the homogeneous staining of chromosomes observed when ATM was present within the cell. Therefore, our results are most consistent with the ATM kinase activity being particularly active during the initial stages of mitosis and responsible for most of the mitosis-specific increase in γ-H2AX levels. Consistent with the presence of active ATM during mitosis, immunofluorescence detection of the activated form of ATM (Bakkenist and Kastan, 2003), based upon anti-ATM phospho-serine 1981 immunofluorescent staining, activated ATM is readily detectable at all stages of the cell cycle including the early stages of mitosis (Supplemental Figure 4). Experiments performed with caffeine, which can inhibit both ATM and ATR kinases at millimolar concentrations, revealed, however, that for a complete ablation of the staining of metaphase chromosomes, both ATM and ATR kinases must be inhibited. Thus, our results do not indicate that ATM is exclusively responsible for the homogeneous staining of chromosomes that is observed during mitosis. Rather, ATM seems to be the primary kinase but some phosphorylation occurs in an ATM-independent manner that is most likely contributed by ATR. The ability to ablate mitotic chromosome staining with 10 mM caffeine distinguished mitotic H2A.X phosphorylation from the phosphorylation of small foci during interphase.
Although the exact function for the mitosis-specific increase in H2AX phosphorylation remains to be elucidated, its existence within all unirradiated mammalian (ATM+/+) cells examined suggests it may be involved in a conserved mitotic function. The phosphorylation cycle mirrors that reported previously for other posttranslational histone modifications (Wei et al., 1999; Peters et al., 2001; Petersen et al., 2001; Bui et al., 2004; Hendzel et al., 1997). Serine 10 phosphorylation of histone H3 presents an interesting parallel. This phosphorylation is associated with gene activation during interphase, whereas during mitosis, it is phosphorylated en masse and is functionally required for normal mitosis. Notably, ATM-deficient cells are genetically unstable and this has been assumed to reflect deficiencies in DNA repair and/or telomere stability. Our results introduce an additional possibility that mitotic defects may also arise from participation of ATM-dependent mitotic phosphorylations in the fidelity of the chromosome segregation process.
Supplementary Material
Acknowledgments
We thank Drs. J. Allalunis-Turner (University of Alberta) and R. Mirzayans (University of Alberta) for generously providing the M059J/K and AT2BE/AT5BI/GM38 cells, respectively, and for helpful discussions; Dr. X. Sun (Cell Imaging Facility) for microscopy assistance and helpful discussions; and J. Wizniak for technical assistance with flow cytometry. We thank Dr. Y. Shiloh (Sackler School of Medicine, Tel Aviv, Israel) for permission to use the EBS and YZ5 cells. We thank Abcam for generously providing the rabbit polyclonal anti-γ-H2AX (ab11174 and ab2983), anti-Brca1, anti-53BP1 antibodies, and H2AX peptides and are extremely grateful to Drs. J. Y. Masson (l'Université Laval) and G. K. Chan (University of Alberta) for generously providing the Mre11, NBS1, Rad51, and Ku80, and ATM antibodies, respectively. We thank the Canadian Institutes of Health Research (CIHR) and the Alberta Heritage Foundation for Medical Research (AHFMR) for operational funding support. During the course of this study, K.J.M. was supported by studentships CIHR and AHFMR. M.J.H. is supported by scholarships from the CIHR and AHFMR.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-01-0065) on July 19, 2005.
Abbreviations used: ATM, ataxia telangiectasia mutated; DNA DSB, DNA double-strand break; DIM, digital imaging microscopy; DNA-PKCS, DNA protein kinase, catalytic subunit; M, metaphase; P/PM, prophase/prometaphase; QIM, quantitative imaging microscopy.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Bakkenist, C. J., and Kastan, M. B. (2003). DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499-506. [DOI] [PubMed] [Google Scholar]
- Barfknecht, T. R., and Little, J. B. (1982). Hypersensitivity of ataxia telangiectasia skin fibroblasts to DNA alkylating agents. Mutat. Res. 94, 369-382. [DOI] [PubMed] [Google Scholar]
- Bassing, C. H., et al. (2002). Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc. Natl. Acad. Sci. USA 99, 8173-8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassing, C. H., Suh, H., Ferguson, D. O., Chua, K. F., Manis, J., Eckersdorff, M., Gleason, M., Bronson, R., Lee, C., and Alt, F. W. (2003). Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell 114, 359-370. [DOI] [PubMed] [Google Scholar]
- Bickar, D., and Reid, P. D. (1992). A high-affinity protein stain for western blots, tissue prints, and electrophoretic gels. Anal. Biochem. 203, 109-115. [DOI] [PubMed] [Google Scholar]
- Bui, H. T., Yamaoka, E., and Miyano, T. (2004). Involvement of histone H3 (Ser10) phosphorylation in chromosome condensation without CDC2 kinase and mitogen-activated protein kinase activation in pig oocytes. Biol. Reprod. 70, 1843-1851. [DOI] [PubMed] [Google Scholar]
- Burma, S., Chen, B. P., Murphy, M., Kurimasa, A., and Chen, D. J. (2001). ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276, 42462-42467. [DOI] [PubMed] [Google Scholar]
- Celeste, A., Difilippantonio, S., Difilippantonio, M. J., Fernandez-Capetillo, O., Pilch, D. R., Sedelnikova, O. A., Eckhaus, M., Ried, T., Bonner, W. M., and Nussenzweig, A. (2003). H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell 114, 371-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste, A., et al. (2002). Genomic instability in mice lacking histone H2AX. Science 296, 922-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Capetillo, O., Celeste, A., and Nussenzweig, A. (2003). Focusing on foci: H2AX and the recruitment of DNA-damage response factors. Cell Cycle 2, 426-427. [PubMed] [Google Scholar]
- Fernandez-Capetillo, O., et al. (2002). DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat. Cell Biol. 4, 993-997. [DOI] [PubMed] [Google Scholar]
- Furuta, T., et al. (2003). Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J. Biol. Chem. 278, 20303-20312. [DOI] [PubMed] [Google Scholar]
- Gately, D. P., Hittle, J. C., Chan, G. K., and Yen, T. J. (1998). Characterization of ATM expression, localization, and associated DNA-dependent protein kinase activity. Mol. Biol. Cell 9, 2361-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel, M. J., Wei, Y., Mancini, M. A., Van Hooser, A., Ranalli, T., Brinkley, B. R., Bazett-Jones, D. P., and Allis, C. D. (1997). Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106, 348-360. [DOI] [PubMed] [Google Scholar]
- Huang, X., Okafuji, M., Traganos, F., Luther, E., Holden, E., and Darzynkiewicz, Z. (2004). Assessment of histone H2AX phosphorylation induced by DNA topoisomerase I and II inhibitors topotecan and mitoxantrone and by the DNA cross-linking agent cisplatin. Cytometry 58A, 99-110. [DOI] [PubMed] [Google Scholar]
- Huang, X., Traganos, F., and Darzynkiewicz, Z. (2003). DNA damage induced by DNA topoisomerase I- and topoisomerase II-inhibitors detected by histone H2AX phosphorylation in relation to the cell cycle phase and apoptosis. Cell Cycle 2, 614-619. [PubMed] [Google Scholar]
- Lees-Miller, S. P., Godbout, R., Chan, D. W., Weinfeld, M., Day, R. S., 3rd, Barron, G. M., and Allalunis-Turner, J. (1995). Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science 267, 1183-1185. [DOI] [PubMed] [Google Scholar]
- Limoli, C. L., Giedzinski, E., Bonner, W. M., and Cleaver, J. E. (2002). UV-induced replication arrest in the xeroderma pigmentosum variant leads to DNA double-strand breaks, γ-H2AX formation, and Mre11 relocalization. Proc. Natl. Acad. Sci. USA 99, 233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhail, S. H., Banath, J. P., Yu, T. Y., Chu, E. H., Lambur, H., and Olive, P. L. (2003a). Expression of phosphorylated histone H2AX in cultured cell lines following exposure to X-rays. Int. J. Radiat. Biol. 79, 351-358. [DOI] [PubMed] [Google Scholar]
- MacPhail, S. H., Banath, J. P., Yu, Y., Chu, E., and Olive, P. L. (2003b). Cell cycle-dependent expression of phosphorylated histone H2AX: reduced expression in unirradiated but not X-irradiated G1-phase cells. Radiat. Res. 159, 759-767. [DOI] [PubMed] [Google Scholar]
- McManus, K. J., and Hendzel, M. J. (2003). Quantitative analysis of CBP- and P300-induced histone acetylations in vivo using native chromatin. Mol. Cell. Biol. 23, 7611-7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus, K. J., and Hendzel, M. J. (2005). Using quantitative imaging microscopy to define the target substrate specificities of histone post-translational-modifying enzymes. Methods 36, 351-361. [DOI] [PubMed] [Google Scholar]
- Motoyama, N., and Naka, K. (2004). DNA damage tumor suppressor genes and genomic instability. Curr. Opin. Genet. Dev. 14, 11-16. [DOI] [PubMed] [Google Scholar]
- Nyberg, K. A., Michelson, R. J., Putnam, C. W., and Weinert, T. A. (2002). Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet 36, 617-656. [DOI] [PubMed] [Google Scholar]
- Park, E. J., Chan, D. W., Park, J. H., Oettinger, M. A., and Kwon, J. (2003). DNA-PK is activated by nucleosomes and phosphorylates H2AX within the nucleosomes in an acetylation-dependent manner. Nucleic Acids Res. 31, 6819-6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull, T. T., Rogakou, E. P., Yamazaki, V., Kirchgessner, C. U., Gellert, M., and Bonner, W. M. (2000). A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10, 886-895. [DOI] [PubMed] [Google Scholar]
- Peters, A. H., et al. (2001). Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323-337. [DOI] [PubMed] [Google Scholar]
- Petersen, J., Paris, J., Willer, M., Philippe, M., and Hagan, I. M. (2001). The S. pombe aurora-related kinase Ark1 associates with mitotic structures in a stage dependent manner and is required for chromosome segregation. J. Cell Sci. 114, 4371-4384. [DOI] [PubMed] [Google Scholar]
- Pilch, D. R., Sedelnikova, O. A., Redon, C., Celeste, A., Nussenzweig, A., and Bonner, W. M. (2003). Characteristics of γ-H2AX foci at DNA double-strand breaks sites. Biochem. Cell Biol. 81, 123-129. [DOI] [PubMed] [Google Scholar]
- Rappold, I., Iwabuchi, K., Date, T., and Chen, J. (2001). Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA damage-signaling pathways. J. Cell Biol. 153, 613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsema, T. J., Banath, J. P., MacPhail, S. H., and Olive, P. L. (2004). Hypertonic saline enhances expression of phosphorylated histone H2AX after irradiation. Radiat. Res. 161, 402-408. [DOI] [PubMed] [Google Scholar]
- Rogakou, E. P., Boon, C., Redon, C., and Bonner, W. M. (1999). Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 146, 905-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou, E. P., Pilch, D. R., Orr, A. H., Ivanova, V. S., and Bonner, W. M. (1998). DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858-5868. [DOI] [PubMed] [Google Scholar]
- Schultz, L. B., Chehab, N. H., Malikzay, A., and Halazonetis, T. D. (2000). p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J. Cell Biol. 151, 1381-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, J. L., Cowen, J. M., Moan, E., Sedita, B. A., Stephens, J., and Vaughan, A. T. (1993). Metaphase chromosome and nucleoid differences between Chinese hamster ovary-K1 and its radiosensitive derivative xrs-5. Mutagenesis 8, 105-108. [DOI] [PubMed] [Google Scholar]
- Simons, J. W. (1979). Development of a liquid-holding technique for the study of DNA-repair in human diploid fibroblasts. Mutat. Res. 59, 273-283. [DOI] [PubMed] [Google Scholar]
- Stiff, T., O'Driscoll, M., Rief, N., Iwabuchi, K., Lobrich, M., and Jeggo, P. A. (2004). ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 64, 2390-2396. [DOI] [PubMed] [Google Scholar]
- Ward, I. M., and Chen, J. (2001). Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276, 47759-47762. [DOI] [PubMed] [Google Scholar]
- Wei, Y., Yu, L., Bowen, J., Gorovsky, M. A., and Allis, C. D. (1999). Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell 97, 99-109. [DOI] [PubMed] [Google Scholar]
- Zhou, B. B., and Elledge, S. J. (2000). The DNA damage response: putting checkpoints in perspective. Nature 408, 433-439. [DOI] [PubMed] [Google Scholar]
- Ziv, Y., Bar-Shira, A., Pecker, I., Russell, P., Jorgensen, T. J., Tsarfati, I., and Shiloh, Y. (1997). Recombinant ATM protein complements the cellular A-T phenotype. Oncogene 15, 159-167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.