Abstract
Antipsychotic drugs have been shown to suppress tumor growth and induce cell death, but their clinical application remains limited. Pimozide, an FDA-approved antipsychotic, holds significant potential for cancer treatment. However, the mechanisms underlying tumor cell responses to Pimozide remain unclear. In this study, we identify a critical role for peroxisomes in mediating tumor cell resistance to Pimozide. Our findings demonstrate that Pimozide increases peroxisome numbers and that peroxisomal deficiency significantly enhances Pimozide-induced cell death. We show that peroxisomes mitigate Pimozide-induced apoptosis primarily through fatty acid oxidation and ether lipid synthesis, rather than reactive oxygen species (ROS) metabolism. Moreover, Pimozide treatment upregulates peroxisomal lipid-metabolizing enzymes in tumor cells. As key metabolic hubs interconnected with mitochondrial metabolism, peroxisomes support energy homeostasis, thereby preventing Pimozide-induced cell death. These findings underscore the importance of peroxisomes in maintaining mitochondrial morphology and cellular energy homeostasis, offering novel insights into the potential therapeutic applications of Pimozide in cancer treatment.

Subject terms: Cancer metabolism, Target validation
Introduction
Many antipsychotics have demonstrated tumor-suppressive properties, with Pimozide serving as a representative example [1, 2]. Pimozide is a well-established antipsychotic that primarily targets dopamine receptors for the treatment of psychiatric disorders [3]. Interestingly, some studies suggest that individuals using antipsychotics may have a lower risk of developing cancer compared to the general population, indicating a potential role for Pimozide in cancer therapy [1]. Several studies have shown that Pimozide inhibits the proliferation and metastasis of various cancer types [4–6], often acting synergistically with chemotherapy or radiotherapy to enhance tumor cell suppression [7–9]. As an FDA-approved drug with a well-documented safety profile, Pimozide holds significant promise for cancer treatment. However, a previous clinical trial reported that its therapeutic efficacy was suboptimal [10]. The precise mechanisms underlying its anti-tumor effects remain to be fully elucidated.
Recent studies have revealed that Pimozide is closely linked to lipid metabolism in tumor cells. It has been shown to inhibit lysosomal function, leading to lipid accumulation within tumor cells and promoting cell death through lipotoxicity. Tumor cells also synthesize free fatty acids via glutamine metabolism, which helps maintain lipid homeostasis. Fatty acids and cholesterol support mitochondrial function and cell survival [6, 11]. Despite these insights, the full scope of Pimozide’s anti-tumor mechanisms remains to be elucidated. Lipid metabolism plays a critical role in tumor metastasis and chemotherapy resistance [12]. Peroxisomes, central to lipid metabolism, facilitate key processes such as fatty acid oxidation, ether lipid synthesis, polyamine oxidation, and bile acid synthesis [13]. They also take up lipids stored in lipid droplets and process cholesterol derived from lysosomes [14–16]. These functions are essential for maintaining intracellular lipid homeostasis [17]. In tumor cells, several metabolic pathways within peroxisomes are upregulated, promoting tumor progression [18]. Disruption of key proteins involved in peroxisome biogenesis impairs lipid metabolism and may hinder tumor progression [19, 20]. Recent research has emphasized the role of peroxisomes in supporting the metabolic demands of tumor cells and contributing to cellular stress resistance [15, 21]. Peroxisomes are known to respond to various stressors, including amino acid starvation [22], hypoxia [23], and reactive oxygen species (ROS) [24], and are implicated in chemoresistance [25]. Peroxisomes are highly dynamic organelles, exhibiting significant changes under stress conditions in tumor cells. For instance, under amino acid starvation, hypoxia, or oxidative stress (ROS), peroxisomes are degraded via pexophagy [26]. Conversely, treatment with chemotherapeutic agents such as HDAC inhibitors (Daci) induces peroxisome proliferation, contributing to drug resistance in tumor cells [25]. This dynamism is a key feature of peroxisomes, playing an essential role in cellular stress responses. Alterations in peroxisome levels are often accompanied by changes in metabolic enzyme levels, which regulate various cellular metabolic pathways [27]. Therefore, we hypothesize that peroxisomes may play a crucial role in regulating the anti-tumor effects of Pimozide.
In this study, we employed CRISPR-Cas9 technology to delete a key gene involved in peroxisome biogenesis, effectively eliminating peroxisomes from tumor cells. Our initial mechanistic studies were performed in HeLa cells, a well-established cervical cancer cell line. To ensure the broader relevance of our findings, we further extended our experiments to additional tumor cell lines, including breast, liver, and other cervical cancer-derived cells. Our results demonstrate that Pimozide induces an increase in peroxisome numbers and that peroxisome-deficient cells show increased sensitivity to Pimozide, highlighting the critical role of peroxisomes in modulating Pimozide-induced tumor cell death. Importantly, we found that peroxisomes inhibit Pimozide-induced cell death mainly through fatty acid oxidation and ether lipid synthesis pathways rather than through reactive oxygen species metabolism. Pimozide treatment upregulated enzymes involved in these lipid metabolic pathways, which are essential for maintaining mitochondrial morphology and cellular energy homeostasis. Furthermore, ATP supplementation effectively rescued cells from Pimozide-induced death. Together, these findings reveal a crucial role for peroxisomal lipid metabolism in tumor cell resistance to Pimozide and suggest that targeting peroxisomal pathways could provide novel opportunities for combination cancer therapy.
Results
Pimozide treatment increases the number of peroxisomes
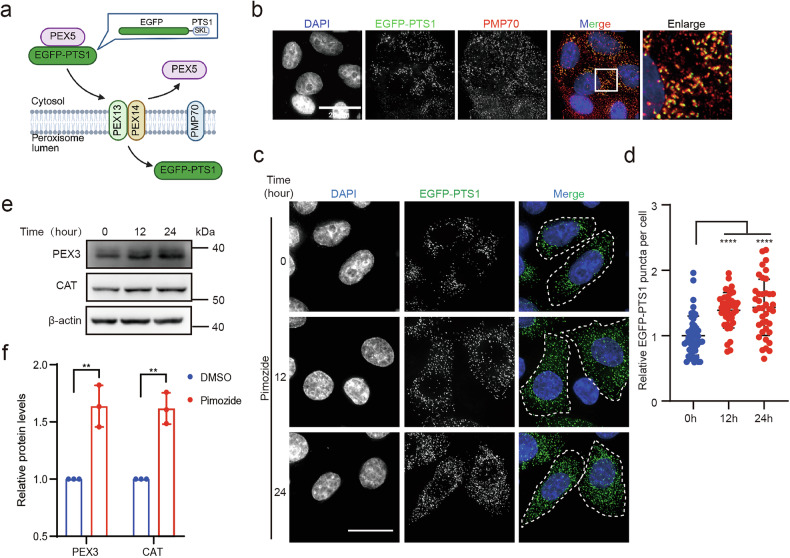
To explore the potential role of peroxisomes in the anti-tumor effects of Pimozide, we first examined whether Pimozide could affect peroxisome dynamics. Peroxisomal matrix proteins, which contain peroxisomal targeting signals (PTS) at the C-terminus, are recognized and imported into the peroxisome [28]. According to this, we constructed the EGFP-PTS1, a fluorescent protein that can be imported into peroxisomes, to visualize the dynamics of peroxisomes in cells (Fig. 1a). Our results showed that EGFP-PTS1 colocalized with the peroxisomal membrane protein PMP70 (Fig. 1b and Supplementary Fig. 1a), confirming that EGFP-PTS1 can effectively visualize peroxisomes in cells. Then, we quantified the number of intracellular peroxisomes under Pimozide treatment, the results showed that the number of intracellular peroxisomes significantly increased after treatment (Fig. 1c, d and Supplementary Fig. 1b, c). Additionally, Western blot analysis revealed that Pimozide treatment upregulated the expression of peroxisomal proteins, including PEX3 and catalase (CAT) (Fig. 1e, f). Altogether, our results demonstrated that peroxisome number is increased under Pimozide treatment.
Fig. 1. Pimozide induces increased peroxisome numbers.
a The process of EGFP-PTS1 being recognized and imported into the peroxisomal matrix. EGFP-PTS1: Fusion of peroxisome targeting sequence 1(PTS1) at the C-terminus of the EGFP, SKL Ser-Lys-Leu. b EGFP-PTS1 co-localizes with the peroxisomal membrane protein PMP70 in HeLa (WT) cells, Scale bar: 20 μm. c, d EGFP-PTS1 puncta in HeLa (EGFP-PTS1) stable cell line after Pimozide treatment for a different time, Pimozide:10 μM. Scale bar: 20 μm. Each dot represents one cell, n ≥ 30. e Levels of PEX3 and CAT after Pimozide treatment for different time in HeLa (WT) cells, Pimozide: 10 μM. f Quantification of peroxisomal protein levels relative to β-actin by western blot following Pimozide treatment. HeLa (WT) cells were treated with 10 μM Pimozide for 24 h.
Selective autophagy is a critical mechanism for regulating peroxisome dynamics [26]. It has been reported that Pimozide can induce an increase in intracellular autophagic flux [6]. Our results demonstrated a significant increase in intracellular p62 puncta, indicating that Pimozide stimulates autophagy. However, no evident co-localization was observed between autophagosomes and peroxisomes, suggesting that pexophagy did not occur in these cells (Supplementary Fig. 2a, b).
Peroxisomes inhibit pimozide-induced cell death in tumor cells
Next, we sought to determine whether increased peroxisome numbers play a role in regulating Pimozide-induced cell death. Given that PEX3 is essential for peroxisome de novo synthesis [29], we generated peroxisome-deficient cells by knocking out the PEX3 gene (Fig. 2a and Supplementary Fig. 3a). Upon expressing EGFP-PTS1 in PEX3 knockout cells, we observed no significant intracellular fluorescent puncta, confirming the absence of peroxisomal vesicles in these cells (Fig. 2b, c and Supplementary Fig. 3b, c). Consistent with previous studies, PEX3 knockout also resulted in mitotic arrest [30] (Supplementary Fig. 4a–d). Together, these results demonstrate the successful generation of a peroxisome-deficient cell line.
Fig. 2. Pimozide promotes peroxisome-deficient tumor cell death.
a PEX3 knockout in HeLa cells. b, c EGFP-PTS1 puncta in HeLa cells after PEX3 knockout, Scale bar: 10 μm. Each dot represents one cell, n ≥ 30. d, e Cell death in HeLa (WT) and HeLa (PEX3-KO) cells, Pimozide: 10 μM, 48 h. Scale bar: 100 μm. f Apoptosis pathway proteins in HeLa (WT) and HeLa (PEX3-KO) cells after pimozide treatment, Pimozide: 10 μM, 48 h. g Cell death in HeLa (WT) and HeLa (PEX3-KO) cells, Pimozide: 10 μM, 48 h; ABT263: 20 μM, 48 h; Z-VAD-FMK: 50 μM, 48 h.
We treated both peroxisome-deficient and normal tumor cells with Pimozide and evaluated cell death percentages using SYTOX Green. The results showed a significant increase in cell death in peroxisome-deficient tumor cells compared to peroxisome-normal tumor cells (Fig. 2d, e and Supplementary Fig. 5a–c), indicating that peroxisomes play a critical role in mediating resistance to Pimozide-induced tumor cell death. Previous studies have shown that Pimozide can promote apoptosis in tumor cells [31]. To further investigate, we assessed the levels of apoptosis-related proteins, PARP1 and caspase-3, via western blotting. A substantial increase in cleaved PARP1 and cleaved caspase-3 was observed in Pimozide-treated peroxisome-deficient tumor cells, corroborating the role of peroxisomes in mediating resistance to Pimozide-induced cell death (Fig. 2f).
To investigate whether apoptosis is the primary form of cell death in peroxisome-deficient tumor cells following Pimozide treatment, we treated the cells with Z-VAD-FMK, a specific inhibitor of apoptosis, and analyzed the cell death rates of peroxisome-deficient and peroxisome-normal tumor cells. The results demonstrated that Z-VAD-FMK only partially decreased the percentage of cell death in both wild-type and peroxisome-deficient tumor cells treated with Pimozide (Fig. 2g). These findings suggest that Pimozide-induced cell death is only partially dependent on apoptosis.
Resistance to Pimozide mediated by peroxisomes is independent of ROS
Next, we investigated how peroxisomes mediated cell death resistance to Pimozide. Pimozide has been shown to promote tumor cell death by generating ROS [5], while ROS metabolism is a key function of peroxisomes [32]. Thus, we hypothesized that peroxisome-mediated resistance to Pimozide-induced cell death depends on ROS generation. We first examined the intracellular ROS levels. Although Pimozide induced an increase in intracellular ROS levels, no significant difference in ROS levels was observed between wild-type and peroxisome-deficient cells. Furthermore, we treated cells with N-acetylcysteine (NAC), a potent antioxidant, in combination with Pimozide (Fig. 3a). NAC effectively inhibited the ROS increase induced by Pimozide in both wild-type and peroxisome-deficient cells (Fig. 3a). We then assessed the percentage of cell death under these conditions. The results showed no significant difference in cell death between the Pimozide group and the Pimozide-plus-NAC group in peroxisome-deficient cells, although NAC reduced cell death induced by H2O2 (Fig. 3b, c).
Fig. 3. ROS are not responsible for Pimozide-promoted peroxisome-deficient cell death.
a ROS levels in HeLa (WT) and HeLa (PEX3-KO) cells, Pimozide: 10 μM, 48 h; H2O2: 1500 μM, 1 h; NAC: 5 mM, 48 h. b, c Cell death in HeLa (PEX3-KO) cells, Pimozide: 10 μM, 48 h; H2O2: 1500 μM, 24 h; NAC: 5 mM. Scale bar: 100 μm. d PRDX5 and CAT are ROS-degrading enzymes in peroxisome. e Knock down PRDX5 and CAT in HeLa (WT) cells. f Cell death in PRDX5 and CAT knockdown HeLa (WT) cells, Pimozide: 10 μM, 48 h.
Additionally, as PRDX5 and catalase (CAT) are key ROS-metabolizing enzymes in peroxisomes [32] (Fig. 3d), we constructed stable cell lines with knockdowns of these two genes, respectively, to detect cell death percentage under Pimozide treatment. Our results showed that Pimozide treatment did not induce significant cell death after knocking down PRDX5 and CAT (Fig. 3e, f). These findings suggest that ROS is not responsible for Pimozide-induced cell death in peroxisome-deficient tumor cells.
Peroxisomal lipid metabolism promote resistance to Pimozide
Peroxisomes serve as critical platforms for intracellular signaling and essential metabolic functions. Dysfunctional peroxisomes impair peroxisomal metabolism, leading to inefficiencies in various cellular processes [27]. We hypothesize that the deficiency of functional peroxisomes impairs peroxisomal metabolic pathways, which subsequently contributes to cell death during Pimozide treatment.
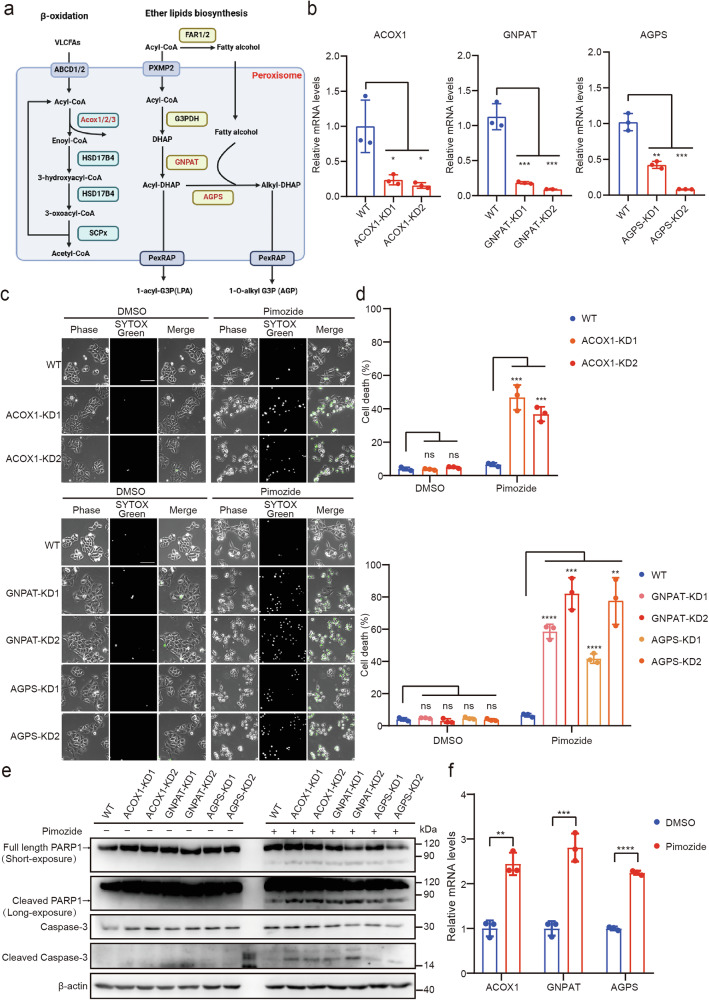
Lipid metabolism represents a crucial cellular pathway and significantly contributes to tumor metastasis and therapy resistance [33]. Pimozide has been shown to disrupt lipid metabolic homeostasis in tumor cells [11], whereas peroxisomes play a critical role in maintaining metabolic homeostasis [17]. To investigate the mechanism underlying Pimozide-induced cell death in peroxisome-deficient cells, we explored the involvement of peroxisomal lipid metabolic processes, specifically fatty acid oxidation and ether lipid synthesis. These two pathways are fundamental to lipid metabolism, with key enzymes such as ACOX1, GNPAT, and AGPS playing central roles (Fig. 4a).
Fig. 4. Peroxisomal fatty acid oxidation and ether lipid synthesis pathway inhibit Pimozide-induced cell death.
a Peroxisomal fatty acid oxidation and ether lipid synthesis pathway, Red: key enzyme. b ACOX1, GNPAT, and AGPS are knocked down in HeLa cells. c, d Cell death in HeLa (WT) and HeLa (ACOX1-KD, GNPAT-KD, AGPS-KD) after Pimozide treatment, Pimozide: 10 μM, 48 h. Scale bar: 100 μm. e Apoptosis pathway proteins in HeLa (WT) and HeLa (ACOX1-KD, GNPAT-KD, AGPS-KD) cells after Pimozide treatment, Pimozide: 10 μM, 48 h. f Relative mRNA level of ACOX1, GNPAT, and AGPS in HeLa (WT) cells after Pimozide treatment, Pimozide: 10 μM, 24 h.
We knocked down ACOX1, GNPAT, and AGPS and subsequently treated these knockdown cells with Pimozide (Fig. 4b and Supplementary Fig. 6a–c). The results showed that Pimozide induced cell death in ACOX1, GNPAT, and AGPS knockdown cells, comparable to the observations in PEX3 knockout cells (Fig. 4c, d and Supplementary Fig. 6d–f). Additionally, the levels of cleaved PARP1 and cleaved caspase-3 were elevated after Pimozide treatment in these knockdown cells, providing further evidence for apoptosis induction (Fig. 4e).
Previous data have demonstrated that Pimozide increases peroxisome levels. To further explore this, we examined the expression of key enzymes involved in fatty acid oxidation and ether lipid synthesis. Our results indicated that Pimozide treatment led to an elevation in the mRNA levels of ACOX1, GNPAT, and AGPS (Fig. 4f). These findings suggest that peroxisomes play a protective role against Pimozide-induced cell death by enhancing fatty acid oxidation and ether lipid synthesis pathways.
Peroxisomes inhibit pimozide-induced cell death via regulating ATP homeostasis
Peroxisomes are key metabolic hubs within cells, and their metabolites interact with various organelles, particularly mitochondria. The lipid metabolism of peroxisomes is closely linked to mitochondrial function, as peroxisomal lipid metabolism is essential for maintaining mitochondrial morphology and function [34, 35]. In tumor cells, peroxisomal fatty acid oxidation contributes to mitochondrial oxidative phosphorylation, while ACOX1 knockdown disrupts this process, resulting in decreased intracellular ATP levels [36, 37]. Ether lipid synthesis, which produces ether lipids crucial for cell membranes, also impacts mitochondrial function. These ether lipids are incorporated into mitochondrial membranes, facilitating respiratory chain assembly and enhancing ATP production. Furthermore, plasmalogens, a specific type of ether lipid, play a role in regulating mitochondrial dynamics and maintaining energy homeostasis [38–40].
Pimozide, known to impair glucose uptake, ATP synthesis, and lactate production, may induce cell death in peroxisome-deficient tumor cells due to ATP deficiency [41, 42]. To test this hypothesis, we assessed ATP levels in tumor cells. Our results demonstrated a significant reduction in ATP levels in peroxisome-deficient cells following Pimozide treatment (Fig. 5a). Importantly, supplementation with ATP rescued Pimozide-induced cell death (Fig. 5b–d and Supplementary Fig. 7a–c), indicating that ATP depletion is a critical factor contributing to the observed cell death. Similarly, ATP supplementation alleviated cell death in ACOX1, GNPAT, and AGPS knockdown cells (Fig. 5e), further corroborating the link between energy homeostasis and Pimozide-induced cell death.
Fig. 5. Peroxisomes contribute to mitochondrial energy metabolism inhibits Pimozide-induced cell death.
a ATP levels in HeLa (WT) and HeLa (PEX3-KO) cells, Pimozide: 10 μM, 48 h, ATP: 250 μM, 24 h. b, c Cell death in HeLa (WT) and HeLa (PEX3-KO) cells after treatment, Pimozide: 10 μM, 48 h, ATP: 250 μM, 48 h. Scale bar: 100 μm. d Apoptosis pathway proteins in HeLa (WT) and HeLa (PEX3-KO) cells after treatment, Pimozide: 10 μM, 48 h, ATP: 250 μM, 48 h. e Cell death in WT, and ACOX1, GNPAT, AGPS knockdown HeLa cells, Pimozide: 10 μM, 48 h, ATP: 250 μM, 48 h. f, g Mitochondrial morphology of HeLa (WT) and HeLa (PEX3-KO) cells after pimozide treatment, the area, perimeter, branch length, and form factor of mitochondria were analyzed. Scale bar: 10 μm. Each dot represents one cell, n ≥ 20.
Mitochondrial morphology is closely linked to energy metabolism. Elongated mitochondria are typically more bioenergetically efficient, whereas fragmented mitochondria are indicative of impaired energy metabolism [43]. Peroxisomal lipid metabolism is known to influence mitochondrial morphology [20, 36]. We found that Pimozide treatment caused an increase in mitochondrial fragmentation in peroxisome-deficient cells (Fig. 5f, g), supporting the role of peroxisomes in preserving mitochondrial function and energy metabolism.
Since tumor cells rely heavily on glucose metabolism to generate ATP and support lipid metabolism [44], we investigated how glucose availability influenced our results. To address this, we increased the glucose concentration and observed that higher glucose levels significantly mitigated Pimozide-induced cell death (Supplementary Fig. 8a, b). These findings emphasize the critical role of peroxisomes in maintaining cellular energy homeostasis, highlighting their importance in ATP production and resistance to Pimozide-induced cell death.
Discussion
In this study, we demonstrated that peroxisomes are crucial in inhibiting Pimozide-induced cell death in tumor cells. While Pimozide induces an increase in ROS levels, our findings suggest that peroxisomes do not act as ROS degraders in this context. Instead, they support survival of tumor cells through peroxisomal fatty acid oxidation and ether lipid synthesis pathways. This conclusion is further supported by the increased peroxisome levels and upregulation of enzymes involved in these metabolic processes. Additionally, ATP levels were found to be essential for the survival of tumor cells treated with Pimozide, with peroxisomal lipid metabolism contributing to the maintenance of mitochondrial morphology and energy homeostasis in tumor cells.
The proliferation of peroxisomes is regulated by intracellular peroxisome proliferator-activated receptors (PPARs), which induce the expression of peroxisome-related genes and promote peroxisome proliferation [45]. Previous studies have shown that Pimozide inhibits FABP4 (fatty acid-binding protein 4), leading to reduced degradation of Peroxisome proliferator-activated receptor gamma (PPARγ) and promoting adipogenesis in adipocytes [46]. Our results show that Pimozide induces an increase in peroxisome levels in tumor cells, potentially through the inhibition of PPAR degradation, thus enhancing peroxisome proliferation. Although Pimozide is commonly recognized as a dopamine receptor inhibitor, our experiments with Haloperidol, another dopamine receptor antagonist, did not yield the same results, suggesting that Pimozide may exert its effects through alternative mechanisms (Supplementary Fig. 9a). Specifically, Pimozide has been reported to inhibit STAT3 and STAT5, key transcription factors involved in the regulation of tumor cell metabolism, including glucose metabolism, lipid metabolism, and oxidative phosphorylation [31, 47]. Through suppression of STAT3/5 signaling, Pimozide may indirectly promote peroxisome proliferation and support tumor cell survival under metabolic stress. In addition to STAT3/5, Pimozide is also known to inhibit acid sphingomyelinase (ASM), an enzyme critical for lysosomal function and sphingolipid metabolism. Inhibition of ASM by Pimozide can disrupt lysosomal integrity and trafficking, which has been implicated in its cytotoxic effects in tumor cells. Therefore, the antitumor activity of Pimozide likely arises from the combined impact on multiple pathways, including STAT3/5-mediated metabolic regulation and ASM-mediated lysosomal homeostasis [31, 47, 48]. Given this complexity, it is important to consider the expression levels and activity of these targets across different cancer types. Caution should be exercised in interpreting Pimozide’s therapeutic potential, as the variability in STAT3/5 and ASM expression may significantly influence its efficacy and safety profile in a tumor type–specific manner.
Peroxisomes have been implicated in ferroptosis [49, 50], but their role in other modes of cell death, such as apoptosis, remains largely unexplored. In this study, we observed that Pimozide increases peroxisome levels in tumor cells, a phenomenon that occurs before the onset of apoptosis. This temporal relationship suggests that peroxisomes may have a protective role in the cell death process. The precise function of peroxisomes in this context requires further investigation. Our data suggest that Pimozide-induced cell death in peroxisome-deficient tumor cells is not limited to apoptosis, implying that other cell death mechanisms may be involved. Previous studies have indicated that Pimozide can induce autophagic cell death, which may align with our findings [6, 51].
We also observed that glucose concentration plays a pivotal role in Pimozide-induced cell death in peroxisome-deficient tumor cells. At lower glucose concentrations, cells are more likely to undergo cell death, highlighting the importance of ATP in this process. While tumor cells can utilize various metabolic pathways to survive under glucose limitation [52], our study suggests that peroxisomes play a novel role in supporting energy metabolism during such conditions, helping tumor cells cope with limited glucose availability.
Clinically, pimozide is primarily prescribed as an antipsychotic agent. However, its clinical application is limited by dose-dependent side effects, including extrapyramidal symptoms and cardiac toxicity, with a recommended maximum daily dose of 10 mg. At this dosage, the plasma concentrations achieved in patients are significantly lower than the concentrations commonly used in preclinical anticancer studies, highlighting the challenge of achieving therapeutically relevant levels in vivo. Additionally, adverse effects such as somnolence, arrhythmias, and QT interval prolongation further restrict the potential for dose escalation. Despite these limitations, the established mechanisms of action and promising preclinical antitumor activity of pimozide suggest that it may still hold therapeutic potential, particularly as part of a combination therapy or targeted treatment strategy. Optimization of drug delivery systems and mitigation of systemic toxicity may enhance its clinical applicability. Furthermore, other resistance mechanisms—such as limited tumor penetration, glycolysis-related metabolic flexibility, and upregulation of drug efflux transporters—may also contribute to the suboptimal efficacy of pimozide in vivo and merit further investigation [1, 11]. Therefore, further investigation into efficient drug delivery systems and resistance mechanisms is essential to facilitate the clinical use of pimozide in cancer therapy.
Peroxisomes are essential organelles in cellular metabolism, yet their full role in tumor progression remains incompletely understood. While recent studies have highlighted peroxisomes’ involvement in cellular stress responses, their functions in different stages of tumor progression warrant further exploration. Our work underscores the importance of peroxisomes in maintaining energetic homeostasis in tumor cells and provides insights that may guide the development of targeted cancer therapies.
Methods
Cell lines culturing and treatment
HEK293T (ATCC, CRL-3216), HeLa (CAS, Shanghai, TCHu187), MDA-MB-231 (CAS, Shanghai, TCHu227), HepG2 (CAS, Shanghai, TCHu72) and SiHa (CAS, Shanghai, TCHu113) cells were cultured in DMEM (Gibco, 12800017) supplemented with 10% FBS (Gibco,10099-141) and 1% penicillin-streptomycin (Beyotime Biotechnology, C0222) in a humidified incubator at 37 °C with 5% CO2. In experiments, HeLa, MDA-MB-231, HepG2 and SiHa cells were cultured in medium (Gibco, A1443001) containing 5 mM glucose, 4 mM glutamine, 1 mM pyruvate, 10% FBS, and 1% penicillin-streptomycin during treatment, except for the conditions described in Supplementary Fig. 8, which used medium with 25 mM or 5 mM glucose, 4 mM glutamine, 1 mM pyruvate, 10% FBS, and 1% penicillin-streptomycin.
Plasmids
For the PEX3 knockout plasmid, sgPEX3 was inserted into lentiGuide-Puro (Addgene, 52963) and used in combination with lentiCas9-Blast (Addgene, 52962). The sequence of sgPEX3 was used:
5′-CATGCTTCCAACACTGAGAG-3′.
All shRNAs targeting PRDX5, CAT, ACOX1, GNPAT, and AGPS, inserted into the PLKO vector, were obtained from commercial (Sigma). The following shRNAs were used:
shPRDX5-1: 5′-CCGGGCCCTGAATGTGGAACCAGATCTCGAGATCTGGTTCCACATTCAGGGCTTTTTG-3′;
shPRDX5-2:5′-CCGGGAGACAGACTTATTACTAGATCTCGAGATCTAGTAATAAGTCTGTCTCTTTTTG-3′;
shCAT-1:5′-CCGGCGCTCATCACTGGATGAAGATCTCGAGATCTTCATCCAGTGATGAGCGTTTTTG-3′
shCAT-2:5′-CCGGCGGAGATTCAACACTGCCAATCTCGAGATTGGCAGTGTTGAATCTCCGTTTTTG-3′
shACOX1-1:5′-CCGGCGAATCTTACAAGCACCTGAACTCGAGTTCAGGTGCTTGTAAGATTCGTTTTTG-3′
shACOX1-2:5′-CCGGGCAGCCAGATTAGTAGAAATTCTCGAGAATTTCTACTAATCTGGCTGCTTTTTG-3′
shGNPAT-1:5′-CCGGGCCAAGACATTGACTCCTAAACTCGAGTTTAGGAGTCAATGTCTTGGCTTTTTG-3′
shGNPAT-2:5′-CCGGCCAGAAAGATTCTCTCTGAAACTCGAGTTTCAGAGAGAATCTTTCTGGTTTTTG-3′
shAGPS-1:5′-CCGGGCATCCTTAAATCCTAGTGATCTCGAGATCACTAGGATTTAAGGATGCTTTTTG-3’
shAGPS-2:5′-CCGGCCTCAGGTTTCCTCTATCTTTCTCGAGAAAGATAGAGGAAACCTGAGGTTTTTG-3′
EGFP-PTS1 was cloned from the EGFP sequence, and the peroxisomal targeting sequence (PTS1) was added to C-terminus. The PTS1 is composed of the amino acids serine (Ser), lysine (Lys), and leucine (Leu) at the C-terminus of proteins targeted to peroxisomes. EGFP-PTS1 was inserted into the pLVX (Clontech) vector through the EcoRI and BamHI sites.
For plasmids with overexpression of GFP-H2B, GFP-H2B was inserted into the pLVX (Clontech) vector through the EcoRI and BamHI sites.
Construction of knockdown and knockout cells
The PEX3 knockout cells were generated using the CRISPR-Cas9 system. LentiCas9-Blast (Addgene, 52962) and modified lentiGuide-Puro (Addgene, 52963) were sequentially transfected into the cells. The plasmid, pMD2.G, and psPAX2 were co-transfected into HEK293T cells at a ratio of 2:1:1. Following a 48 h incubation period, the lentivirus encapsulating the sgRNA sequence was then transduced into the target cells. PEX3 knockout cells were selected using 10 μg/mL blasticidin and 1 μg/mL puromycin. Western blotting analysis was employed to confirm the efficiency of PEX3 knockout. For PRDX5, CAT, ACOX1, GNPAT, and AGPS knockdown, plasmids containing shRNA were transfected into the HEK293T cells with pMD2.G and psPAX2 at a ratio of 2:1:1. Following a 48 h incubation period, the lentivirus encapsulating the shRNA sequence was then transduced into the target cells. PRDX5, CAT, ACOX1, GNPAT, and AGPS knockdown cells were selected using 1 μg/mL puromycin. Either Western blotting or quantitative polymerase chain reaction (qPCR) was used to assess the efficiency of the gene knockdown.
Cell death assay
The cells were seeded in 24-well plates at a density of 2 × 10⁴ cells per well. Once the cells were fully adherent, they were treated with a drug-containing medium at a specific concentration, and SYTOX™ Green (Thermo, S7020) was added to the culture medium. Cells were imaged using an Olympus microscope, and fluorescence from SYTOX™ Green was collected. The nuclei of dead cells emitted green fluorescence. Use ImageJ to perform statistics on images or analyze using flow cytometry. In the flow cytometry experiment, cells were digested with trypsin, and the digestion was stopped with PBS. The collected cells were washed with PBS. Subsequently, signals were obtained using CytoFLEX S CytExpert software and analyzed through FlowJo. FSC-A and SSC-A gates were used to remove cell fragments, while the FITC gate was used to detect dead cells. Instrument gain and amplifier settings remained unchanged throughout. The following compounds are used in cell death assay: DMSO (Sangon Biotech, A600163-0250), Pimozide (Selleck, S4358), ABT263 (Selleck, S1001), Z-VAD-FMK (Selleck, S7023), NAC (Sigma, A9165), H2O2 (Sigma, 323381), ATP (Sigma, A2383), Haloperidol (Medchemexpress, HY-14538).
Western blot analysis
A cell lysis buffer containing 50 mM Tris-HCl (pH 7.4) (Sangon Biotech, B548138), 150 mM NaCl (Sinopharm, 10019318), 50 mM NaF(Sangon Biotech, A500850), 1 mM EDTA (Sinopharm, 10009617), 1 mM Na4P2O7 (Sangon Biotech, A500883), 1 mM Na₃VO₄ (Sangon Biotech, A600869), 1 mM PMSF (Sangon Biotech, A610425), and a proteinase inhibitor cocktail (Sigma, P8340) was used to obtain whole-cell lysates. The lysate was boiled at 95 °C for 5 min. The proteins were then separated by electrophoresis using a 10–15% polyacrylamide gel and transferred to PVDF membranes (Millipore, IPVH00010). The membrane was blocked with 5% defatted milk dissolved in Tris-buffered saline containing 0.1% Tween-20(TBST) for 1 h. The primary antibody was incubated at 4 °C for 12 h, and the secondary antibody was incubated at room temperature for 1 h. After washing the membrane, the TanonTM High-sig ECL Western Blotting Substrate (Tanon, 180–501) was used to generate chemiluminescence signals, and Western Lightning Chemiluminescence Reagent Plus ChemiScope software was used to obtain signals. Protein banding was quantified using ImageJ plugin Gels. The following antibodies were used: β-actin (Proteintech, 60008-1-Ig, 1:5000); PEX3 (ABclonal, A7352, 1:1000); PRDX5 (Proteintech, 17724-1-AP, 1:5000); CAT (Proteintech, 21260-1-AP, 1:6000); PARP1 (Proteintech, 13371-1-AP, 1:2000); Cleaved Caspase-3 (Cell Signaling Technology, #9662, 1:1000); Caspase-3 (Cell Signaling Technology, #9661, 1:1000); HRP-conjugated anti-rabbit secondary antibody(Jackson, 111-035-003, 1:5000); HRP-conjugated anti-mouse secondary antibody(Jackson, 115-035-003, 1:5000).
Immunofluorescence
The adherent cells were fixed with 4% paraformaldehyde (Sigma, P6148) at room temperature for 15 min, followed by permeabilization with 0.2% Triton X-100 (Sigma, 93443) in PBS for 5 min and blocking with 1% BSA (Biosharp, BS114-100g) in TBST for 30 min. The primary antibody was incubated at 4 °C for 12 h, and the secondary antibody was incubated at room temperature for 1 h, followed by 0.5 μg/mL DAPI (Sigma, D9542) staining. The cell-loaded slides were mounted using Dako Fluorescence Mounting Medium (Dako North America, S302380-2). Data were collected and processed using deconvolution and z-stack projection with DeltaVision softWoRx software, and ImageJ was used for data analysis. The following antibody was used: PMP70 (Sigma, SAB4200181, 1:1000); TOM20 (Proteintech, 11802-1-AP, 1:500); p62 (Proteintech, 18420-1-AP, 1:500); Alexa 594 goat anti-mouse secondary antibody (Jackson, 115-585-146, 1:500); Alexa 594 goat anti-rabbit secondary antibody (Jackson, 111-585-144, 1:500).
Quantitative PCR
Total RNA was extracted using an RNA extraction kit (TransZol, ET101-01-V2). First-strand cDNA synthesis was performed using the HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, R212-02). Quantitative PCR was conducted using AceQ qPCR SYBR Green Master Mix (Vazyme, Q111-02). PCR data were collected using PikoReal software. The following primers are used:
Actin-F:5′-GGACTTCGAGCAAGAGATGG-3′
Actin-R:5′-AGCACTGTGTTGGCGTACAG-3′
ACOX1-F:5′-CGCCGAGAGATCGAGAACATG-3′
ACOX1-R:5′-GCCAAACTCCCTCATCTTCTTCAC-3′
GNPAT-F:5′-TGAGCTTCTAGGGGTTCCTA-3′
GNPAT-R:5′-CATGTCCTCAGACTGTTTCTG-3′
AGPS-F:5′-GGCTGGCATAACAGGACAAGAG-3′
AGPS-R:5′-CTTCATGCCTGATGCGCGAGTA-3′
Time-lapse imaging of mitotic progression
HeLa (H2B-GFP) stable cell lines were seeded onto an eight-chambered coverglass (Ibidi, 80826). Imaging was performed using a Nikon Eclipse Ti-E microscope with NIS-Elements AR software, capturing data every 5–10 min. The exposure time was set to 0.1 s, and the imaging session lasted for 36 h. The temperature was maintained at 37 °C throughout the experiment. The FITC channel was used to collect fluorescence from the cell nucleus. ImageJ was used to observe and analyze the mitotic phenotype. The following compounds are used in experiments: Taxol (Selleck, S1150).
Cell cycle assay
Adherent cells in the culture dish were digested with trypsin and stained using the Cell Cycle and Apoptosis Analysis Kit (Beyotime, C1052). Add 1 mL of pre-cooled 70% ethanol to the cells and fix at 4 °C for 30 min. After washing with pre-cooled PBS, add Propidium iodide staining solution and incubate at 37 °C in the dark for 30 min The data was analyzed using FlowJo. Signals were obtained using CytoFLEX S CytExpert software. PE gating was employed to detect signals from the nucleus, while FSC-A and SSC-A gates were applied to exclude data from cell debris. The following compounds are used in experiments: Taxol (Selleck, S1150).
ATP level assay
ATP levels were measured using the ATP Assay Kit (Beyotime, S0026). Adherent cells were digested with trypsin, and ATP detection lysate was added and centrifuged to obtain the supernatant. The supernatant and ATP detection working solution were added to the detection Wells, and the signal was detected using Luminometer. The following compounds are used in experiments: ATP (Sigma, A2383).
Reactive oxygen species detection
Adherent cells in the culture dish were digested with trypsin and subsequently suspended in PBS. The cells were stained with CM-H2DCFDA (Thermo, C6827) according to the manufacturer’s instructions, using a concentration of 1 μM CM-H2DCFDA for 30 min. The stained cells were washed with PBS for signal detection. Signals were obtained using CytoFLEX S CytExpert software. The data was analyzed using FlowJo. FITC gating was employed to detect ROS in the cells. FSC-A and SSC-A gates were used to remove cell fragments. Instrument gain and amplifier settings remained unchanged throughout. The following compounds are used in experiments: Pimozide (Selleck, S4358), NAC (Sigma, A9165), H2O2 (Sigma, 323381).
Peroxisome number analysis
To visualize peroxisomes, cells were stably transfected with EGFP-PTS1, which targets the peroxisomal matrix. Immunofluorescence microscopy was performed to capture images of EGFP-PTS1-labeled peroxisomes. For image quantification, the “Threshold” function in ImageJ was applied to isolate EGFP-PTS1 puncta within individual cells. Subsequently, the “Analyze Particles” plugin in ImageJ was used to count the number of puncta per cell.
Mitochondrial morphological analysis
After drug treatment, mitochondria were stained with TOM20 during immunofluorescence. Data were collected and processed using deconvolution and z-stack projection with DeltaVision softWoRx software. After acquiring images, circle all the mitochondria of a single cell in the images. 2D commands of the ImageJ plugin Mitochondria Analyzer were used to analyze the area, perimeter, branch length, and form factor of mitochondria in individual cells.
Colocalization analysis
Following image acquisition, circle a single cell in the images; the different channels of the single-cell image were separated and analyzed using the ImageJ plugin Coloc2. The Pearson correlation coefficient was employed to quantify colocalization. The Pearson correlation coefficient ranges from −1 to 1. A higher absolute value indicates a stronger correlation between the variables.
Statistical analysis
Each experiment was independently repeated at least three times. Data are presented as mean ± standard deviation (s.d.). Statistical analyses were performed using unpaired two-tailed Student’s t-test. The consistency of variances among the different groups was assessed to ensure the validity of the statistical analysis. All statistical analyses were conducted using GraphPad Prism 8 software. Statistical significance is indicated as follows: ns (not significant), *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001.
Supplementary information
Acknowledgements
This work is supported by: National Science Foundation of China 92357301, 32370776, 32170736; The Strategic Priority Research Program of the Chinese Academy of Sciences, Grant XDB0940101; National Key R&D Program of China 2022YFA1303100, Noncommunicable Chronic Diseases-National Science and Technology Major Project 2023ZD0507500; Research Funds of Center for Advanced Interdisciplinary Science and Biomedicine of IHM of USTC QYPY20220017.
Author contributions
ZP conducted the majority of the experiments and data analysis. FY contributed to experiment design and manuscript writing. WY assisted with experiments and data analysis. FL, FC, JG, and ZY supervised the experiments and participated in manuscript writing. All authors approved the final manuscript submission.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study does not involve data requiring ethical approval. This study only conducted in vitro experiments using commercially purchased, ethically unrestricted standard tumor cell lines and did not involve any human participants, human tissue samples, or live vertebrates.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jing Guo, Email: jguo2013@ustc.edu.cn.
Zhenye Yang, Email: zhenye@ustc.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41389-025-00575-0.
References
- 1.Shaw V, Srivastava S, Srivastava SK. Repurposing antipsychotics of the diphenylbutylpiperidine class for cancer therapy. Semin Cancer Biol. 2021;68:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang J, Zhao DW, Liu ZX, Liu FK. Repurposing psychiatric drugs as anti-cancer agents. Cancer Lett. 2018;419:257–65. [DOI] [PubMed] [Google Scholar]
- 3.Elmaci I, Altinoz MA. Targeting the cellular schizophrenia. Likely employment of the antipsychotic agent pimozide in treatment of refractory cancers and glioblastoma. Crit Rev Oncol Hemat. 2018;128:96–109. [DOI] [PubMed] [Google Scholar]
- 4.Jiang G, Zhou XZ, Hu Y, Tan XY, Wang D, Yang LA, et al. The antipsychotic drug pimozide promotes apoptosis through the RAF/ERK pathway and enhances autophagy in breast cancer cells. Cancer Biol Ther. 2024;25:2302413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim U, Kim CY, Lee JM, Ryu B, Kim J, Shin C, et al. Pimozide inhibits the human prostate cancer cells through the generation of reactive oxygen species. Front Pharmacol. 2020;10:1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer N, Henkel L, Linder B, Zielke S, Tascher G, Trautmann S, et al. Autophagy activation, lipotoxicity and lysosomal membrane permeabilization synergize to promote pimozide- and loperamide-induced glioma cell death. Autophagy. 2021;17:3424–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rath BH, Camphausen K, Tofilon PJ. Glioblastoma radiosensitization by pimozide. Transl Cancer Res. 2016;5:S1029–S32. [DOI] [PMC free article] [PubMed]
- 8.Liu ACH, Cathelin S, Yang YT, Dai DL, Ayyathan DM, Hosseini M, et al. Targeting STAT5 signaling overcomes resistance to idh inhibitors in acute myeloid leukemia through suppression of stemness. Cancer Res. 2022;82:4325–39. [DOI] [PubMed] [Google Scholar]
- 9.Li ZQ, Chen C, Chen LN, Hu DD, Yang XQ, Zhuo WY, et al. STAT5a confers doxorubicin resistance to breast cancer by regulating ABCB1. Front Oncol. 2021;11:697950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neifeld JP, Tormey DC, Baker MA, Meyskens FL, Taub RN. Phase-II trial of the dopaminergic inhibitor pimozide in previously treated melanoma patients. Cancer Treat Rep. 1983;67:155–7. [PubMed] [Google Scholar]
- 11.Zhong YG, Geng F, Mazik L, Yin XM, Becker AP, Mohammed S, et al. Combinatorial targeting of glutamine metabolism and lysosomal-based lipid metabolism effectively suppresses glioblastoma. Cell Rep Med. 2024;5:101706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasseur S, Guillaumond F. Lipids in cancer: a global view of the contribution of lipid pathways to metastatic formation and treatment resistance. Oncogenesis. 2022;11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JA. Peroxisome metabolism in cancer. Cells. 2020;9:1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CL, Weigel AV, Ioannou MS, Pasolli HA, Xu CS, Peale DR, et al. Spastin tethers lipid droplets to peroxisomes and directs fatty acid trafficking through ESCRT-III. J Cell Biol. 2019;218:2583–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Bai H. Peroxisomal stress response and inter-organelle communication in cellular homeostasis and aging. Antioxidants. 2022;11:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sargsyan Y, Thoms S. Staying in healthy contact: how peroxisomes interact with other cell organelles. Trends Mol Med. 2020;26:201–14. [DOI] [PubMed] [Google Scholar]
- 17.Lodhi IJ, Semenkovich CF. Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metab. 2014;19:380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahabieh MS, Di Pietro E, Jangal M, Goncalves C, Witcher M, Braverman NE, et al. Peroxisomes and cancer: the role of a metabolic specialist in a disease of aberrant metabolism. BBA Rev Cancer. 2018;1870:103–21. [DOI] [PubMed] [Google Scholar]
- 19.Huang F, Cai FY, Dahabieh MS, Gunawardena K, Talebi A, Dehairs J, et al. Peroxisome disruption alters lipid metabolism and potentiates antitumor response with MAPK-targeted therapy in melanoma. J Clin Investig. 2023;133:e166644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee RG, Rudler DL, Raven SA, Peng L, Chopin A, Moh ESX, et al. Quantitative subcellular reconstruction reveals a lipid mediated inter-organelle biogenesis network. Nat Cell Biol. 2024;26:57–71. [DOI] [PubMed] [Google Scholar]
- 21.He AY, Dean JM, Lodhi IJ. Peroxisomes as cellular adaptors to metabolic and environmental stress. Trends Cell Biol. 2021;31:656–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sargent G, van Zutphen T, Shatseva T, Zhang L, Di Giovanni V, Bandsma R, et al. PEX2 is the E3 ubiquitin ligase required for pexophagy during starvation. J Cell Biol. 2016;214:677–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walter KM, Schönenberger MJ, Trötzmüller M, Horn M, Elsässer HP, Moser AB, et al. Hif-2α promotes degradation of mammalian peroxisomes by selective autophagy. Cell Metab. 2014;20:882–97. [DOI] [PubMed] [Google Scholar]
- 24.Zhang JW, Tripathi DN, Jing J, Alexander A, Kim J, Powell RT, et al. ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat Cell Biol. 2015;17:1259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahabieh MS, Ha Z, Di Pietro E, Nichol JN, Bolt AM, Goncalves C, et al. Peroxisomes protect lymphoma cells from HDAC inhibitor-mediated apoptosis. Cell Death Differ. 2017;24:1912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller DR, Thorburn A. Autophagy and organelle homeostasis in cancer. Dev Cell. 2021;56:906–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waterham HR, Ferdinandusse S, Wanders RJ. Human disorders of peroxisome metabolism and biogenesis. Biochim Biophys Acta. 2016;1863:922–33. [DOI] [PubMed] [Google Scholar]
- 28.Mahalingam SS, Shukla N, Farré JC, Zientara-Rytter K, Subramani S. Balancing the opposing principles that govern peroxisome homeostasis. Trends Biochem Sci. 2021;46:200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugiura A, Mattie S, Prudent J, McBride HM. Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature. 2017;542:251–4. [DOI] [PubMed] [Google Scholar]
- 30.Asare A, Levorse J, Fuchs E. Coupling organelle inheritance with mitosis to balance growth and differentiation. Science. 2017;355:eaah4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ranjan A, Kaushik I, Srivastava SK. Pimozide suppresses the growth of brain tumors by targeting STAT3-mediated autophagy. Cells. 2020;9:2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fransen M, Nordgren M, Wang B, Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim Biophys Acta. 2012;1822:1363–73. [DOI] [PubMed] [Google Scholar]
- 33.Hoy AJ, Nagarajan SR, Butler LM. Tumour fatty acid metabolism in the context of therapy resistance and obesity. Nat Rev Cancer. 2021;21:753–66. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka H, Okazaki T, Aoyama S, Yokota M, Koike M, Okada Y, et al. Peroxisomes control mitochondrial dynamics and the mitochondrion-dependent apoptosis pathway. J Cell Sci. 2019;132:jcs224766. [DOI] [PubMed] [Google Scholar]
- 35.Schrader M, Costello J, Godinho LF, Islinger M. Peroxisome-mitochondria interplay and disease. J Inherit Metab Dis. 2015;38:681–702. [DOI] [PubMed] [Google Scholar]
- 36.Shen S, Faouzi S, Souquere S, Roy S, Routier E, Libenciuc C, et al. Melanoma persister cells are tolerant to BRAF/MEK inhibitors via ACOX1-mediated fatty acid oxidation. Cell Rep. 2020;33:108421. [DOI] [PubMed] [Google Scholar]
- 37.Tannoury M, Ayoub M, Dehgane L, Nemazanyy I, Dubois K, Izabelle C, et al. ACOX1-mediated peroxisomal fatty acid oxidation contributes to metabolic reprogramming and survival in chronic lymphocytic leukemia. Leukemia. 2024;38:302–17. [DOI] [PubMed] [Google Scholar]
- 38.Bennett CF, O’Malley KE, Perry EA, Balsa E, Latorre-Muro P, Riley CL, et al. Peroxisomal-derived ether phospholipids link nucleotides to respirasome assembly. Nat Chem Biol. 2021;17:703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Z, Ho IL, Soeung M, Yen EY, Liu J, Yan L, et al. Ether phospholipids are required for mitochondrial reactive oxygen species homeostasis. Nat Commun. 2023;14:2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu DH, Tan M, Lu DL, Kleiboeker B, Liu XJ, Park H, et al. TMEM135 links peroxisomes to the regulation of brown fat mitochondrial fission and energy homeostasis. Nat Commun. 2023;14:6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Qu P, Zhou XZ, Ji YX, Yuan S, Liu SP, et al. Pimozide inhibits the growth of breast cancer cells by alleviating the Warburg effect through the P53 signaling pathway. Biomed Pharmacother. 2022;150:113063. [DOI] [PubMed] [Google Scholar]
- 42.Tsujimoto Y. Apoptosis and necrosis: intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997;4:429–34. [DOI] [PubMed] [Google Scholar]
- 43.Giacomello M, Pyakurel A, Glytsou C, Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020;21:204–24. [DOI] [PubMed] [Google Scholar]
- 44.Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16:635–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schrader M, Costello JL, Godinho LF, Azadi AS, Islinger M. Proliferation and fission of peroxisomes - an update. BBA Mol Cell Res. 2016;1863:971–83. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Lin HQ, Law WK, Liang WC, Zhang JF, Hu JS, et al. Pimozide, a novel fatty acid binding protein 4 inhibitor, promotes adipogenesis of 3T3-L1 cells by activating PPARγ. Acs Chem Neurosci. 2015;6:211–8. [DOI] [PubMed]
- 47.Li YJ, Zhang C, Martincuks A, Herrmann A, Yu H. STAT proteins in cancer: orchestration of metabolism. Nat Rev Cancer. 2023;23:115–34. [DOI] [PubMed] [Google Scholar]
- 48.Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, et al. Identification of novel functional inhibitors of acid sphingomyelinase. PLoS ONE. 2011;6:e23852. [DOI] [PMC free article] [PubMed]
- 49.Zou YL, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P, et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. 2020;585:603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui WW, Liu D, Gu W, Chu B. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 2021;28:2536–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zielke S, Meyer N, Mari M, Abou-El-Ardat K, Reggiori F, van Wijk SJL, et al. Loperamide, pimozide, and STF-62247 trigger autophagy-dependent cell death in glioblastoma cells. Cell Death Dis. 2018;9:994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levy T, Voeltzke K, Hruby L, Alasad K, Bas Z, Snaebjörnsson M, et al. mTORC1 regulates cell survival under glucose starvation through 4EBP1/2-mediated translational reprogramming of fatty acid metabolism. Nat Commun. 2024;15:4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.